Water Vapor Blending Ratio Effects on Combustion Thermal Performance and Emission of Hydrogen Homogeneous Charge Compression Ignition
Abstract
1. Introduction
2. Simulation Method and Verification
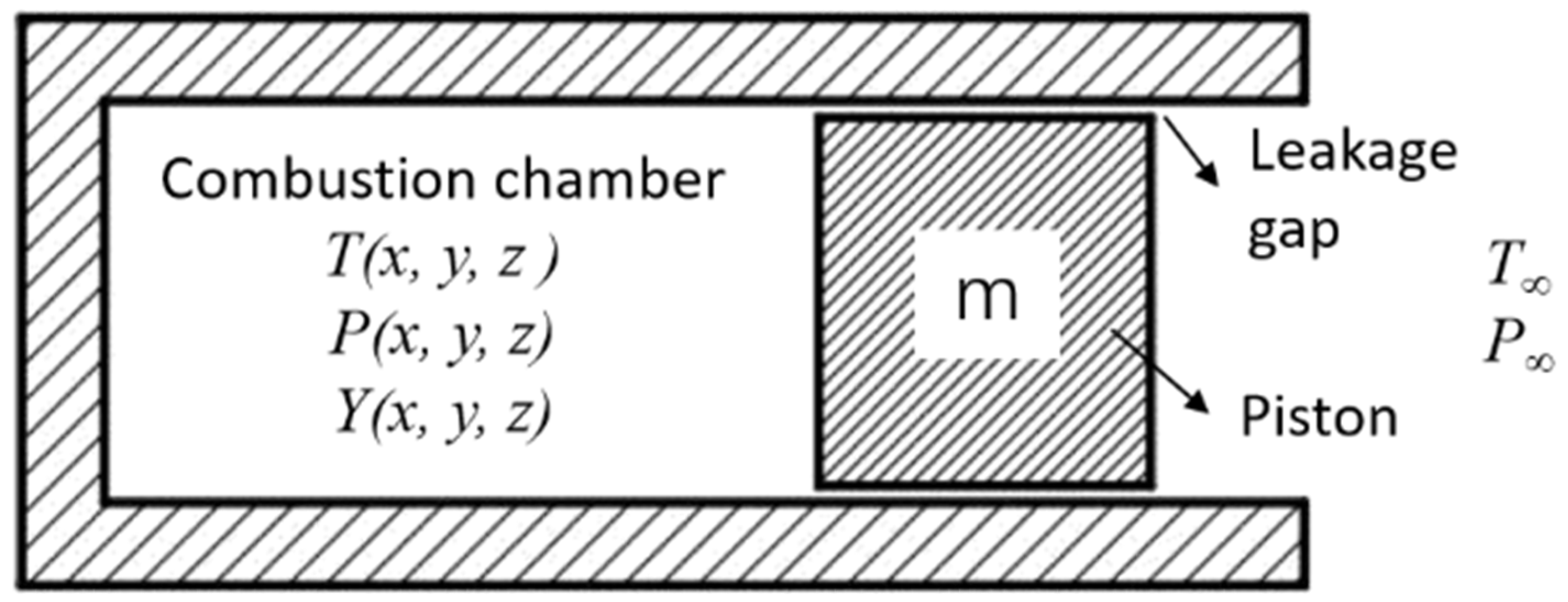
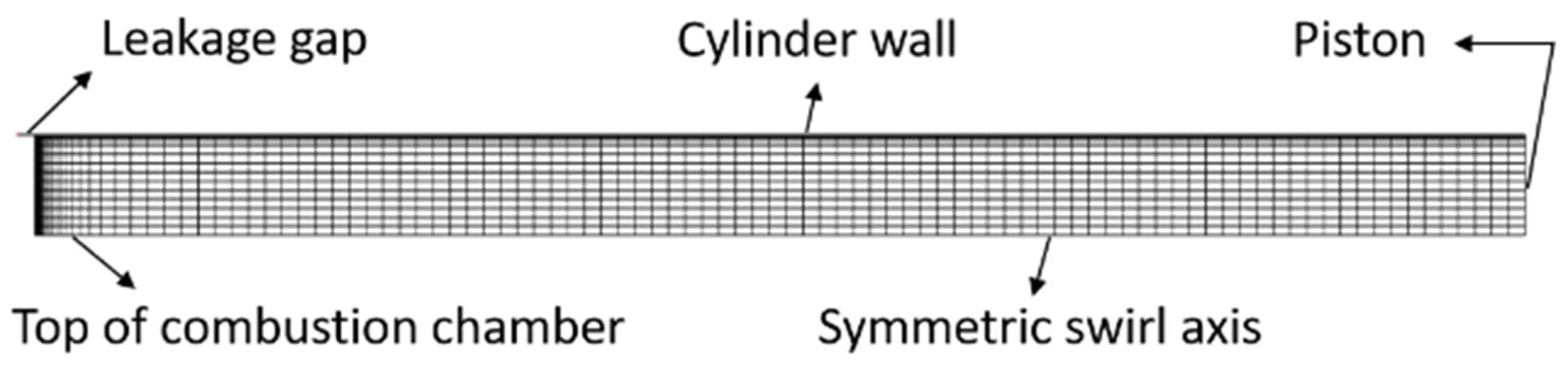
2.1. Physical Model and Grid
2.2. Mathematical Model
2.3. Mesh Independence Analysis
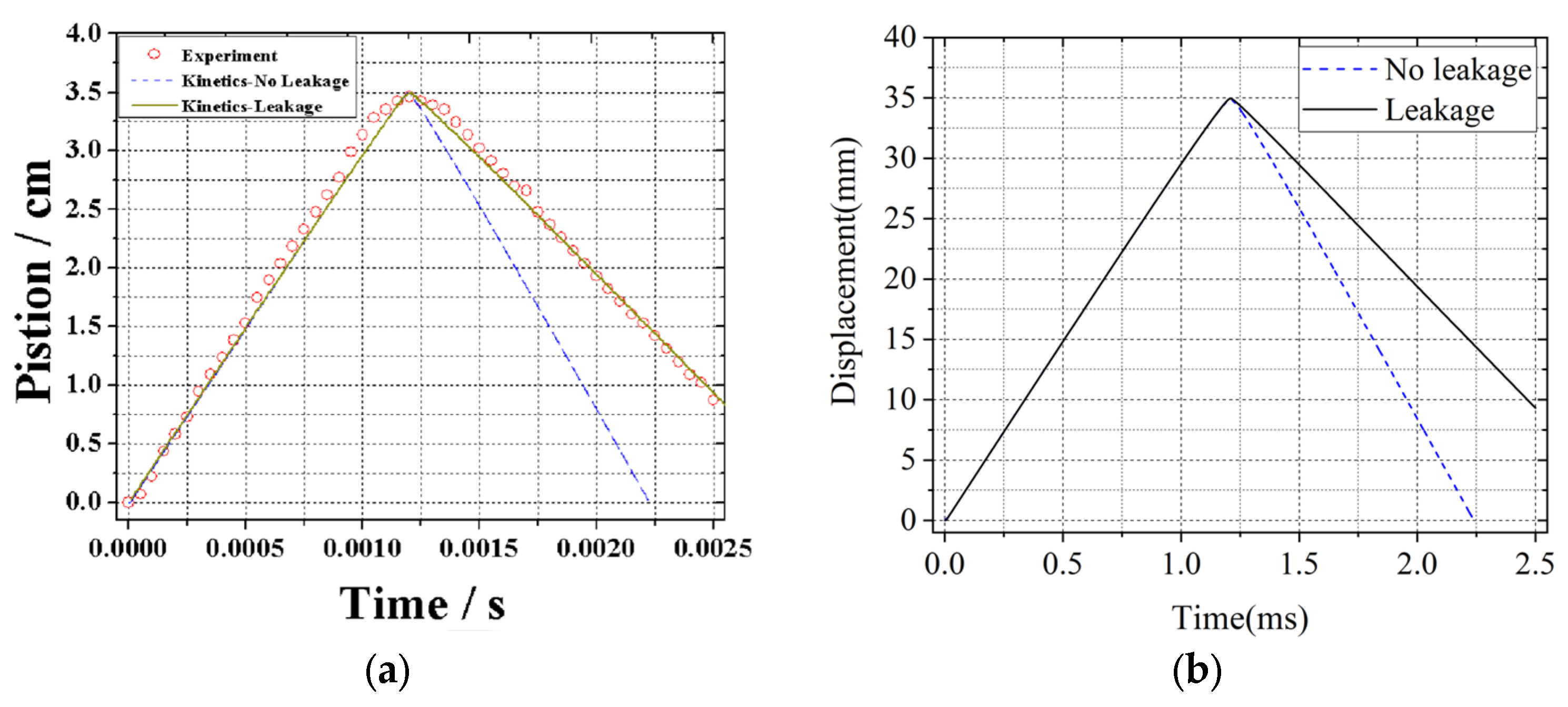
2.4. Model Validation
2.5. Simulation Conditions
3. Results and Discussion
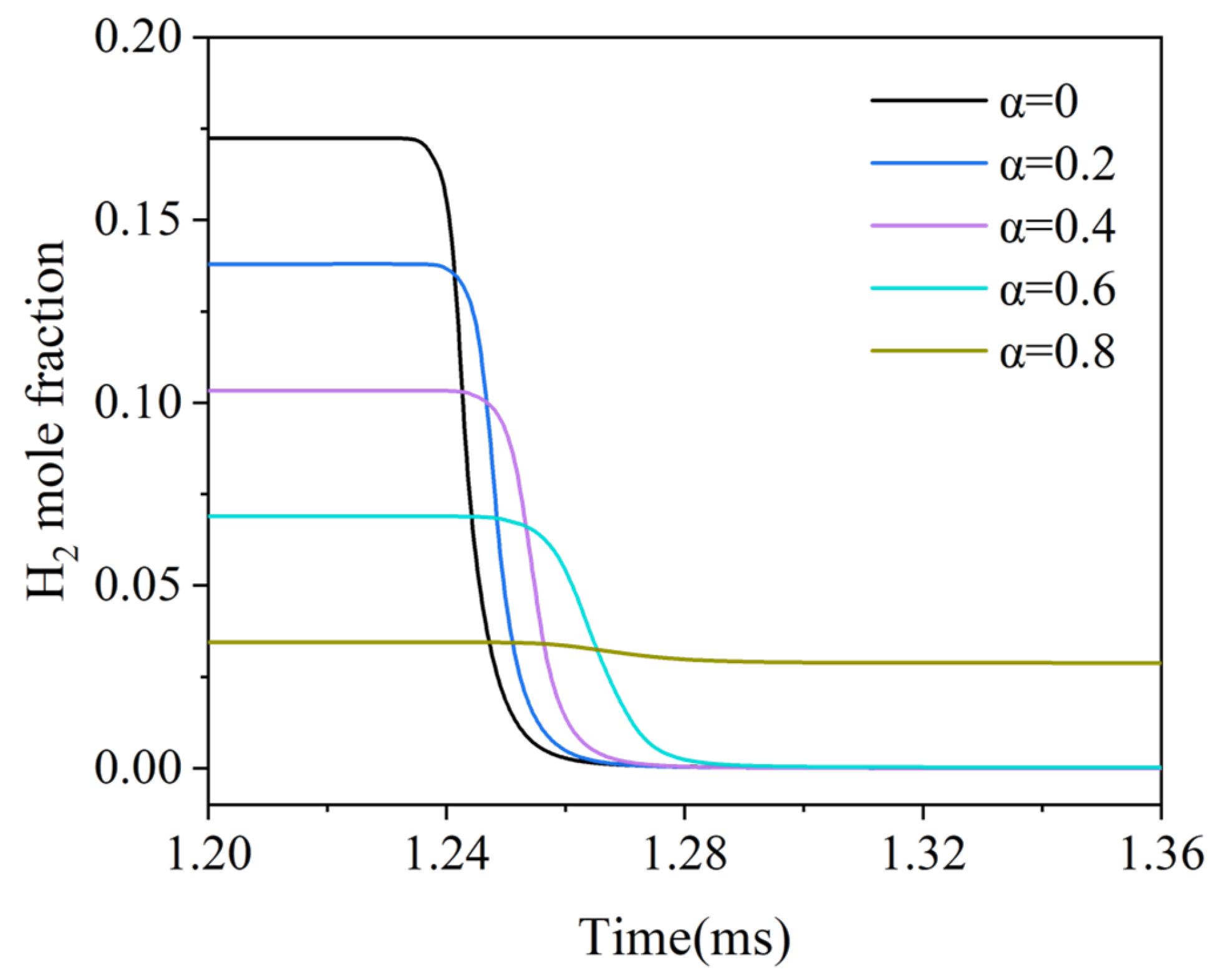
3.1. The H2 Ignition Process
3.2. The Combustion Temperature and Pressure
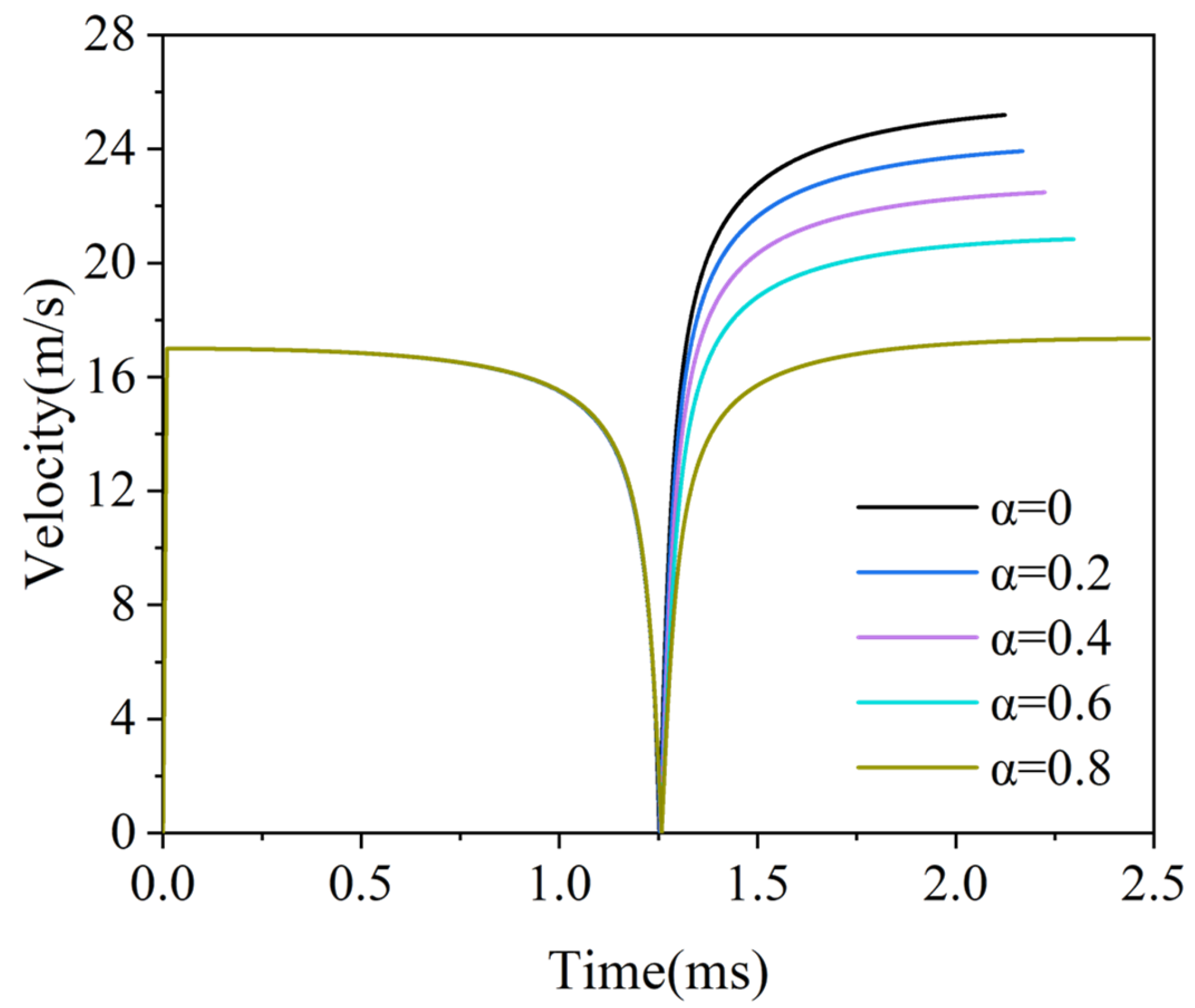
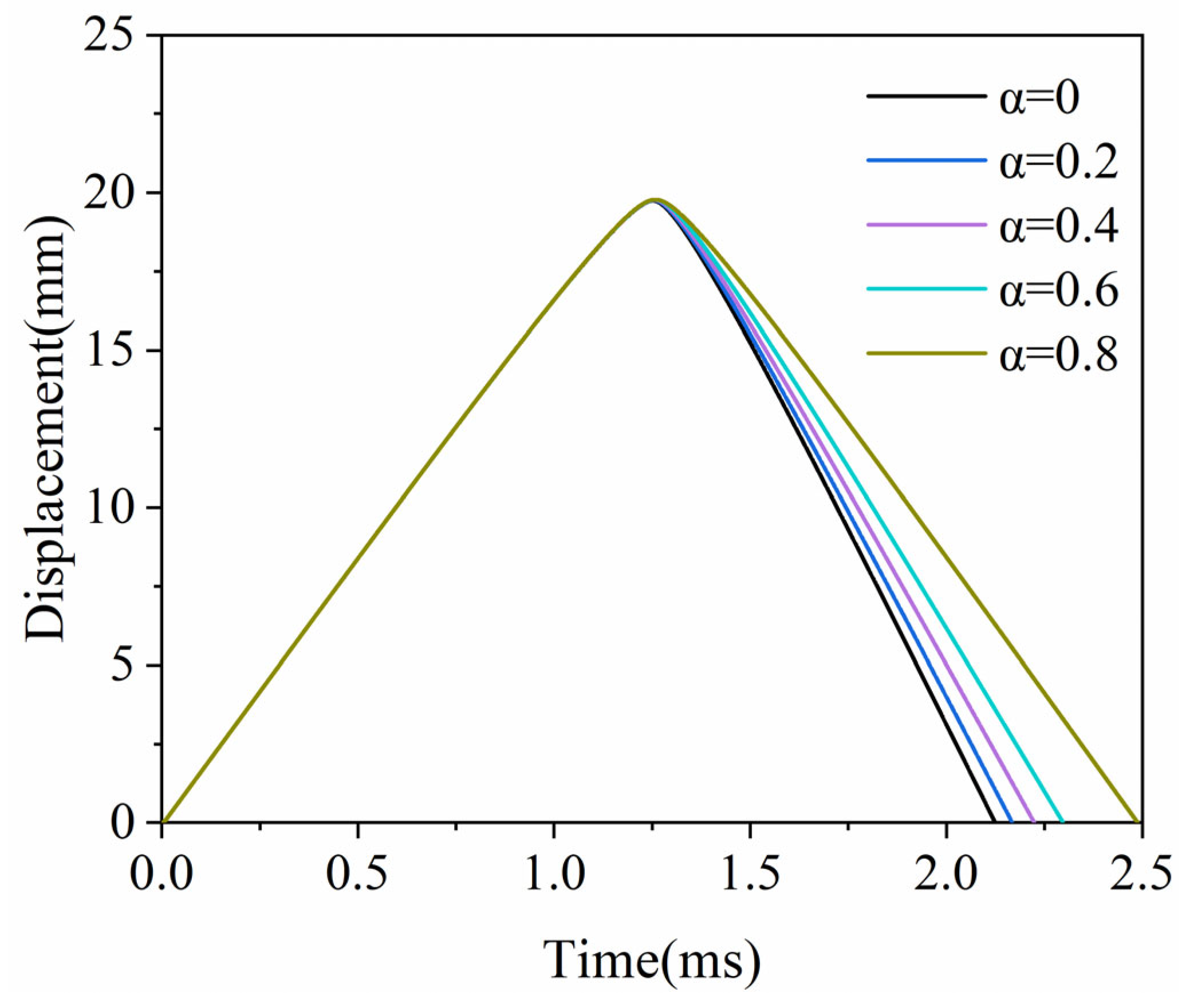
3.3. The Free Piston Movement Process
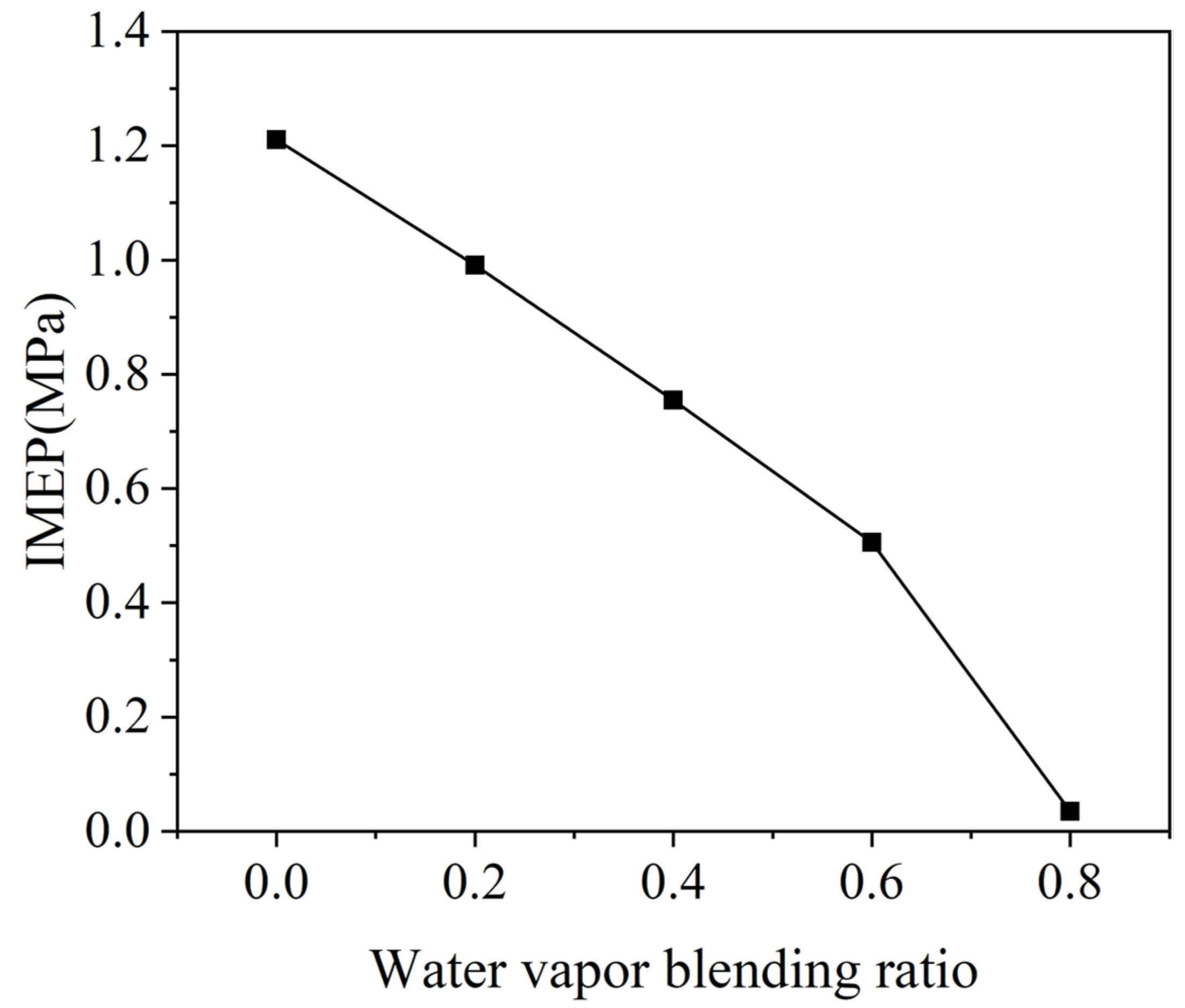
3.4. The Power Capacity of the Micro-Free-Piston Engine
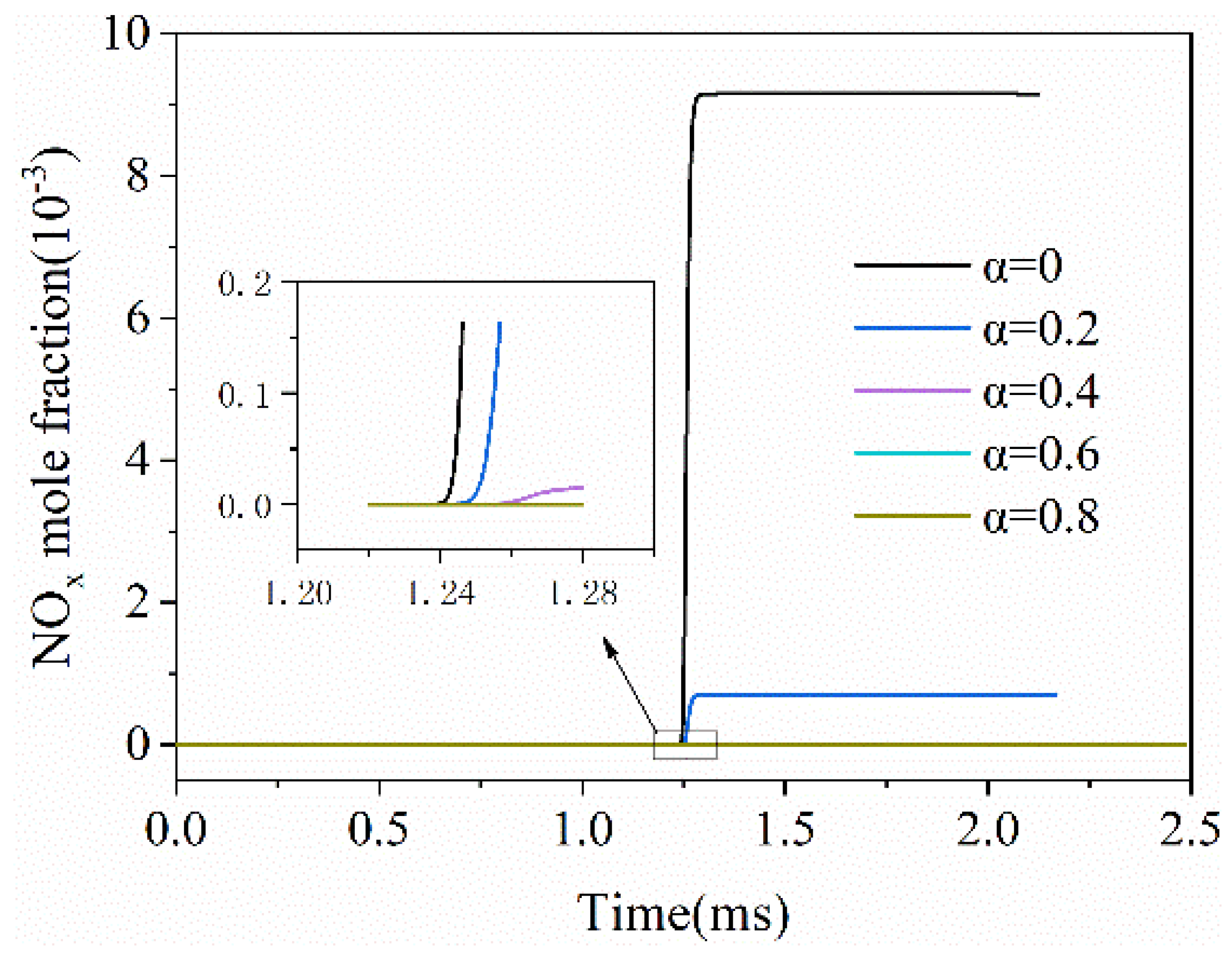
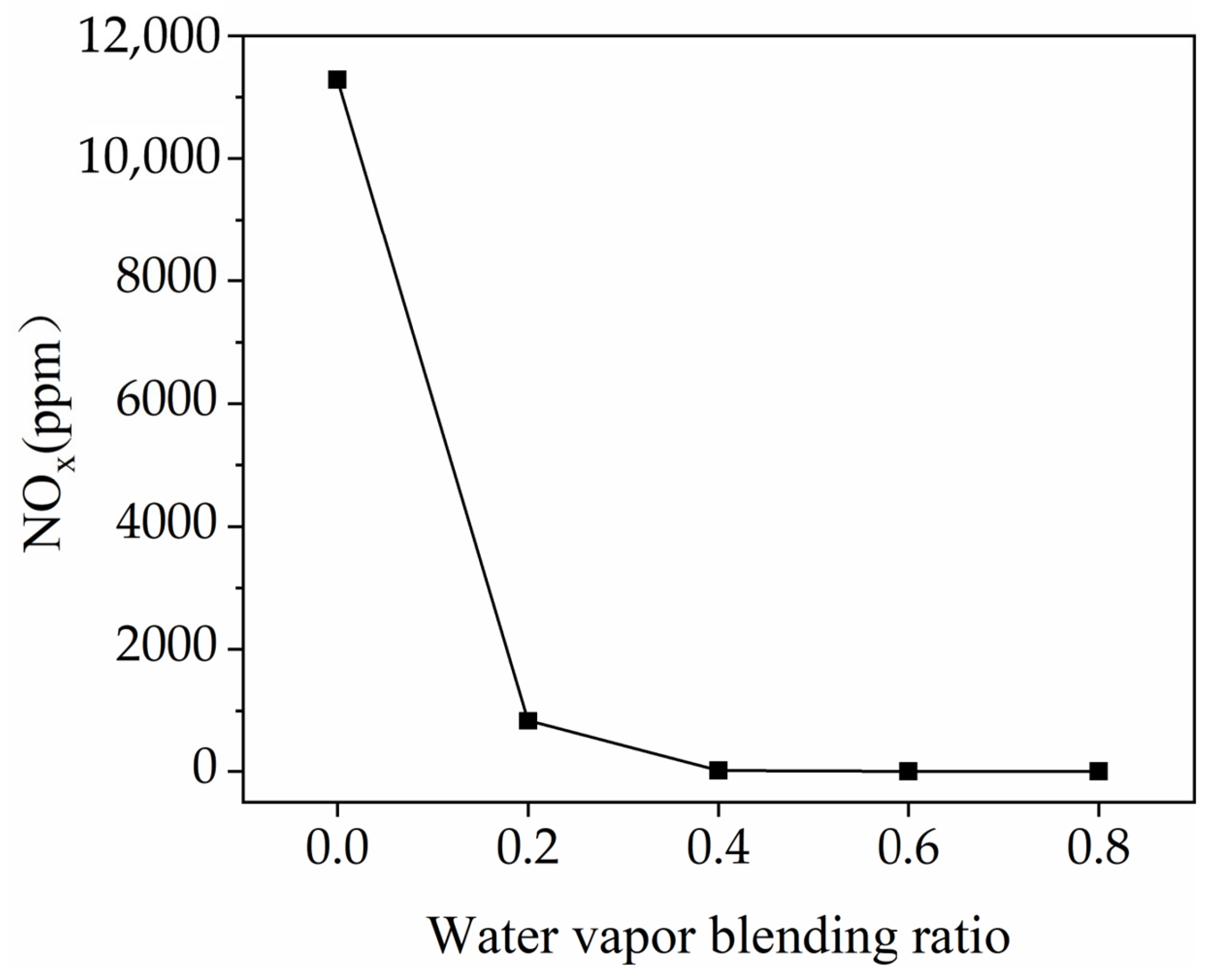
3.5. The NOx Emissions
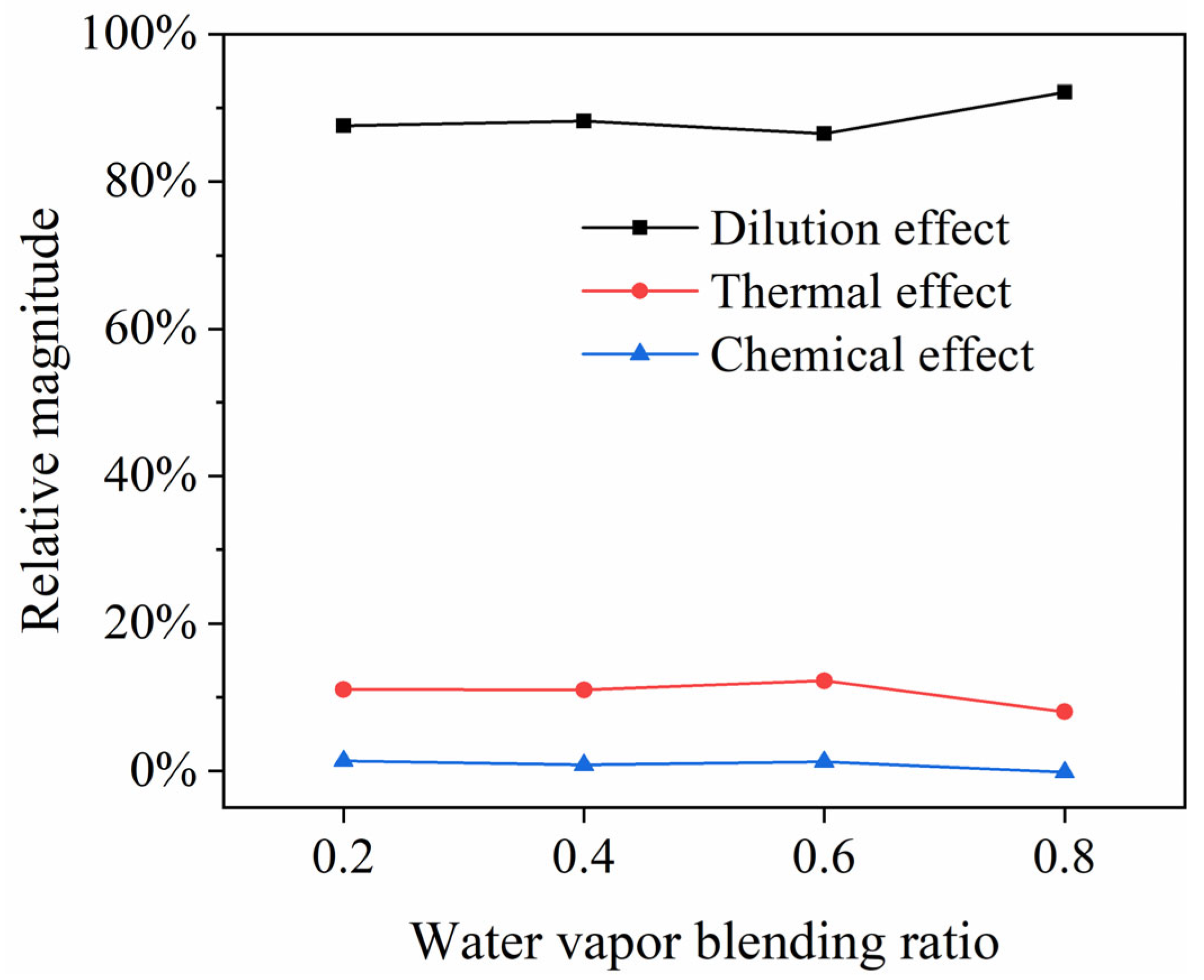
3.6. The Analysis of the Effects of Water Vapor on Combustion Temperature
4. Conclusions
- (1)
- After blending H2 with water vapor, the ignition time of H2 HCCI delays, and the power output capacity of the micro-free-piston engine declines. With the increase in α, the combustion of the mixture gas continues to deteriorate. When α reaches 0.8, there is no obvious ignition in the combustion chamber.
- (2)
- The maximum temperature and pressure in the combustor decrease with the increase in α. The rate of pressure increase declines after blending H2 with water vapor, which is beneficial to alleviate detonation in the combustor.
- (3)
- Blending H2 with water vapor is an efficient means of reducing NOx emissions. When α increases from 0 to 0.2, NOx emissions decrease by 93%.
- (4)
- The dilution effect, thermal effect and chemical effect of water vapor can all reduce the combustion temperature. Among those three kinds of effects above, the dilution effect has the most significant impact on the reduction in the combustion temperature, accounting for about 87.4% of all effects, followed by the thermal effect and the chemical effect. When H2 does not burn fully in the combustion chamber, the dilution effect increases, while the thermal effect and chemistry effect decrease.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Onishi, S.; Jo, S.H.; Shoda, K.; Jo, P.D.; Kato, S. Active Thermo-Atmosphere Combustion (ATAC)—A NEW Combustion Prosess for Internal Combustion Engines. SAE Int. 1979, 88, 1851–1860. [Google Scholar]
- Duan, X.; Lai, M.-C.; Jansons, M.; Guo, G.; Liu, J. A review of controlling strategies of the ignition timing and combustion phase in homogeneous charge compression ignition (HCCI) engine. Fuel 2021, 285, 119142. [Google Scholar] [CrossRef]
- Aichlmayr, H.T.; Kittelson, D.B.; Zachariah, M.R. Miniature free-piston homogeneous charge compression ignition engine-compressor concept—Part I: Performance estimation and design considerations unique to small dimensions. Chem. Eng. Sci. 2002, 57, 4161–4171. [Google Scholar] [CrossRef]
- Aichlmayr, H.T.; Kittelson, D.B.; Zachariah, M.R. Micro-HCCI combustion: Experimental characterization and development of a detailed chemical kinetic model with coupled piston motion. Combust. Flame 2003, 135, 227–248. [Google Scholar] [CrossRef]
- Zhou, Y.; Sofianopoulos, A.; Lawler, B.; Mamalis, S. Advanced combustion free-piston engines: A comprehensive review. Int. J Engine Res. 2020, 21, 1205–1230. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Q.; He, Z.X.; Zhang, P.G. Influence of Piston Initial State on HCCI Combustion in Micro Free-Piston Engine Using Experiment Images and CFD Analysis. Adv. Mater. Res. 2013, 724–725, 1350–1354. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, Q.; Bai, J.; Zhang, P.G.; Liu, Y.; Zhang, J.B. Effect of mixture initial conditions on HCCI combustion in micro combustion chamber. Chin. Intern. Combust. Engine Eng. 2014, 35, 18–24. [Google Scholar]
- Bai, J.; Wang, Q.; He, Z.; Li, C.; Pan, J. Study on methane HCCI combustion process of micro free-piston power device. Appl. Therm. Eng. 2014, 73, 1066–1075. [Google Scholar] [CrossRef]
- Yu, L.L.; Wang, Q.; Huang, R.; Zhao, Y.; Bai, J. Combustion Properties of Hydrogen-Enriched Methane in Micro-Power Plants. J. Henan Univ. Sci. Technol. 2019, 40, 30–36. [Google Scholar]
- Wang, Q.; Zhao, Y.; Wu, F.; Bai, J. Study on the combustion characteristics and ignition limits of the methane homogeneous charge compression ignition with hydrogen addition in micro-power devices. Fuel 2019, 236, 354–364. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, F.; Zhao, Y.; Bai, J.; Huang, R. Study on combustion characteristics and ignition limits extending of micro free-piston engines. Energy 2019, 179, 805–814. [Google Scholar] [CrossRef]
- Mohammed, S.E.; Aziz, A.R.A.; Baharom, M.B.; Jaffry, A.; Ismael, M.A. Effect of aspect ratio on the performance characteristics of free piston linear generator engine fueled by hydrogen. Int. J. Hydrogen Energy 2021, 46, 10506–10517. [Google Scholar]
- Bai, J.; Sun, S.N.; Wang, Q. Numerical Simulation Study on the Influence of Intake and Exhaust Structure Parameters on Scavenging Process of Micro Free-Piston Engine. Chin. Intern. Combust. Engine Eng. 2021, 42, 42–51. [Google Scholar]
- Zhao, F.; Zhang, H.J. Analysis of Application Prospect for Hydrogen Combustion Engines. China Resour. Compr. Util. 2020, 38, 72–74. [Google Scholar]
- Xi, D.; Wang, Y.; Liu, J.Z.; Zhou, J.H. Current Status in the Study of the Humidified Combustion. J. Eng. Therm. Energy Power 2014, 29, 1–6+103. [Google Scholar]
- Du, G.; Wang, Z.; Wang, D.; Wang, X.; Fu, X. Study on the effect of water addition on combustion characteristics of a HCCI engine fueled with natural gas. Fuel 2020, 270, 117547. [Google Scholar] [CrossRef]
- Lu, H.B.; Yao, Q.H. Numerical Simulation of Hydrogen Engine Cylinder’s Combustion with Water Injection. Phys. Gases 2021, 6, 37–47. [Google Scholar]
- Dhyani, V.; Subramanian, K.A. Control of backfire and NOx emission reduction in a hydrogen fueled multi-cylinder spark ignition engine using cooled EGR and water injection strategies. Int. J. Hydrogen Energy 2019, 44, 6287–6298. [Google Scholar] [CrossRef]
- Xu, P.; Ji, C.; Wang, S.; Cong, X.; Ma, Z.; Tang, C.; Meng, H.; Shi, C. Effects of direct water injection on engine performance in a hydrogen (H2)-fueled engine at varied amounts of injected water and water injection timing. Int. J. Hydrogen Energy 2020, 45, 13523–13534. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Q.; Xie, Q.; Zhang, P.G. Effect of leakage on working process of micro free-piston engine. J. Jiangsu Univ. 2014, 35, 14–19. [Google Scholar]
- Jiang, Y.; Qiu, R. Reduction and optimization of methane combustion mechanism based on PCAS and genetic algorithm. Fire Saf. Sci. 2014, 23, 41–49. [Google Scholar]
- Ó Conaire, M.; Curran, H.J.; Simmie, J.M.; Pitz, W.J.; Westbrook, C.K. A comprehensive modeling study of hydrogen oxidation. Int. J. Chem. Kinet. 2004, 36, 603–622. [Google Scholar] [CrossRef]
- Curran, H.J.; Gaffuri, P.; Pitz, W.J.; Westbrook, C.K. A Comprehensive Modeling Study of n-Heptane Oxidation. Combust. Flame 1998, 114, 149–177. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Xu, N.; Yu, S.; Zhang, M.; Huang, Z. Thermal and Chemical Effects of Water Addition on Laminar Burning Velocity of Syngas. Energy Fuels 2014, 28, 3391–3398. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Xu, N.; Yu, S.; Huang, Z. Comparative study on the effect of CO2 and H2O dilution on laminar burning characteristics of CO/H2/air mixtures. Int. J. Hydrogen Energy 2014, 39, 3450–3458. [Google Scholar] [CrossRef]
- Xu, P.; Ji, C.; Wang, S.; Cong, X.; Ma, Z.; Tang, C.; Meng, H.; Shi, C. Effects of direct water injection on engine performance in engine fueled with hydrogen at varied excess air ratios and spark timing. Fuel 2020, 269, 117209. [Google Scholar] [CrossRef]
- Hu, E.; Jiang, X.; Huang, Z.; Iida, N. Numerical Study on the Effects of Diluents on the Laminar Burning Velocity of Methane–Air Mixtures. Energy Fuels 2012, 26, 4242–4252. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Wu, F.; Bai, J. Effect of the Blending Ratio of CO2 on Combustion Characteristics of Methane Homogeneous Charge Compression Ignition. Trans. CSICE 2019, 37, 385–392. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; Huang, Y.; Liu, D.; Duan, J.; Guo, S.; Qin, Z. The effect of hydrogen injection parameters on the quality of hydrogen–air mixture formation for a PFI hydrogen internal combustion engine. Int. J. Hydrogen Energy 2017, 42, 23832–23845. [Google Scholar] [CrossRef]
- Sun, X.; Liu, H.; Duan, X.; Guo, H.; Li, Y.; Qiao, J.; Liu, Q.; Liu, J. Effect of hydrogen enrichment on the flame propagation, emissions formation and energy balance of the natural gas spark ignition engine. Fuel 2022, 307, 121843. [Google Scholar] [CrossRef]
- Soloiu, V.; Duggan, M.; Harp, S.; Vlcek, B.; Williams, D. PFI (port fuel injection) of n-butanol and direct injection of biodiesel to attain LTC (low-temperature combustion) for low-emissions idling in a compression engine. Energy 2013, 52, 143–154. [Google Scholar] [CrossRef]
- Veza, I.; Irianto; Tuan Hoang, A.; Yusuf, A.A.; Herawan, S.G.; Soudagar, M.E.M.; Samuel, O.D.; Said, M.F.M.; Silitonga, A.S. Effects of Acetone-Butanol-Ethanol (ABE) addition on HCCI-DI engine performance, combustion and emission. Fuel 2023, 333, 126377. [Google Scholar] [CrossRef]
- Wang, G.; Guiberti, T.F.; Cardona, S.; Jimenez, C.A.; Roberts, W.L. Effects of residence time on the NOx emissions of premixed ammonia-methane-air swirling flames at elevated pressure. Proc. Combust. Inst. 2022, in press. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Ment, S.; Qian, Y.J.; Tang, F.; Bian, S.; Zhuang, Y. Effects of different initial temperature and water vapor dilution ratios on laminar flame characteristics of ethanol/isooctane mixture. Chin. Intern. Combust. Engine Eng. 2021, 42, 16–23. [Google Scholar]
- Wang, S.; Zhai, Y.; Wang, Z.; Hou, R.; Zhang, T.; Ji, C. Comparison of air and EGR with different water fractions dilutions on the combustion of hydrogen-air mixtures. Fuel 2022, 324, 124686. [Google Scholar] [CrossRef]






| Nodes of the Length Direction | Nodes of the Width Direction | Number of Grids | Peak Temperature (K) | Peak Pressure (MPa) |
|---|---|---|---|---|
| 80 | 15 | 1106 | 2815.38 | 67.70 |
| 20 | 1501 | 2815.97 | 68.22 | |
| 25 | 1896 | 2814.16 | 68.24 | |
| 100 | 15 | 1386 | 2815.84 | 67.77 |
| 20 | 1881 | 2816.02 | 68.24 | |
| 25 | 2376 | 2814.77 | 68.42 | |
| 120 | 15 | 1666 | 2815.66 | 67.82 |
| 20 | 2261 | 2816.74 | 68.20 | |
| 25 | 2856 | 2814.63 | 68.62 |
| Parameters | Value |
|---|---|
| Length of combustor (mm) | 35 |
| Diameter of the piston (mm) | 3 |
| Leakage clearance (μm) | 5 |
| Mass of piston (g) | 1 |
| Initial velocity of the piston (m/s) | 30 |
| Initial temperature of mixture gas (K) | 300 |
| Initial pressure in the cylinder (MPa) | 0.1 |
| Gas | Methane |
| Equivalent ratio | 0.5 |
| Temperature of walls (K) | 300 |
| Parameters | Value |
|---|---|
| Length of combustor (mm) | 20 |
| Diameter of the piston (mm) | 3 |
| Mass of piston (g) | 1 |
| Initial velocity of the piston (m/s) | 17 |
| Initial temperature of mixture gas (K) | 300 |
| Initial pressure in combustor (Pa) | 10, 1325 |
| Gas | H2 |
| Equivalent ratio | 0.5 |
| Temperature of walls (K) | 300 |
| α | 0, 0.2, 0.4, 0.6, 0.8 |
| Turbulence model | RNG κ-ε model |
| Reaction model | EDC model |
| Spatial discretization | Second-order upwind scheme |
| Algorithm | PISO |
| α | Peak Temperature (K) | O2 Mole Fraction before Combustion | Residence Time above 1800 K (ms) | NOx Mole Fraction (10−3) | NOx (ppm) |
|---|---|---|---|---|---|
| 0 | 2806.92 | 0.17 | 0.12 | 9.15 | 11,283.79 |
| 0.2 | 2511.46 | 0.17 | 0.08 | 0.67 | 793.65 |
| 0.4 | 2200.11 | 0.17 | 0.05 | 0.02 | 17.22 |
| 0.6 | 1835.68 | 0.17 | 0.01 | 0.00 | 0.05 |
| 0.8 | 1386.22 | 0.17 | 0.00 | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, W.; Huang, X.; Fu, J.; Ma, Y.; Li, G.; Huang, Q. Water Vapor Blending Ratio Effects on Combustion Thermal Performance and Emission of Hydrogen Homogeneous Charge Compression Ignition. Energies 2022, 15, 9055. https://doi.org/10.3390/en15239055
Yuan W, Huang X, Fu J, Ma Y, Li G, Huang Q. Water Vapor Blending Ratio Effects on Combustion Thermal Performance and Emission of Hydrogen Homogeneous Charge Compression Ignition. Energies. 2022; 15(23):9055. https://doi.org/10.3390/en15239055
Chicago/Turabian StyleYuan, Wenhua, Xueliang Huang, Jun Fu, Yi Ma, Guangming Li, and Qike Huang. 2022. "Water Vapor Blending Ratio Effects on Combustion Thermal Performance and Emission of Hydrogen Homogeneous Charge Compression Ignition" Energies 15, no. 23: 9055. https://doi.org/10.3390/en15239055
APA StyleYuan, W., Huang, X., Fu, J., Ma, Y., Li, G., & Huang, Q. (2022). Water Vapor Blending Ratio Effects on Combustion Thermal Performance and Emission of Hydrogen Homogeneous Charge Compression Ignition. Energies, 15(23), 9055. https://doi.org/10.3390/en15239055






