Performance Analysis of Thermal Energy Storage System Integrated with a Cooking Unit
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Method
2.1.1. Design and Construction of the TES System
2.1.2. Experimental Setup
2.2. Thermal Performance Analysis of the TES System
2.2.1. Total Energy Stored
2.2.2. Heat Energy Retained
2.3. Analysis of Cooking Performance
2.3.1. Efficiency of Heat Extraction
2.3.2. Rate of Energy Consumption during Cooking While Charging the TES System
2.4. Uncertainty Treatment
3. Results and Discussion
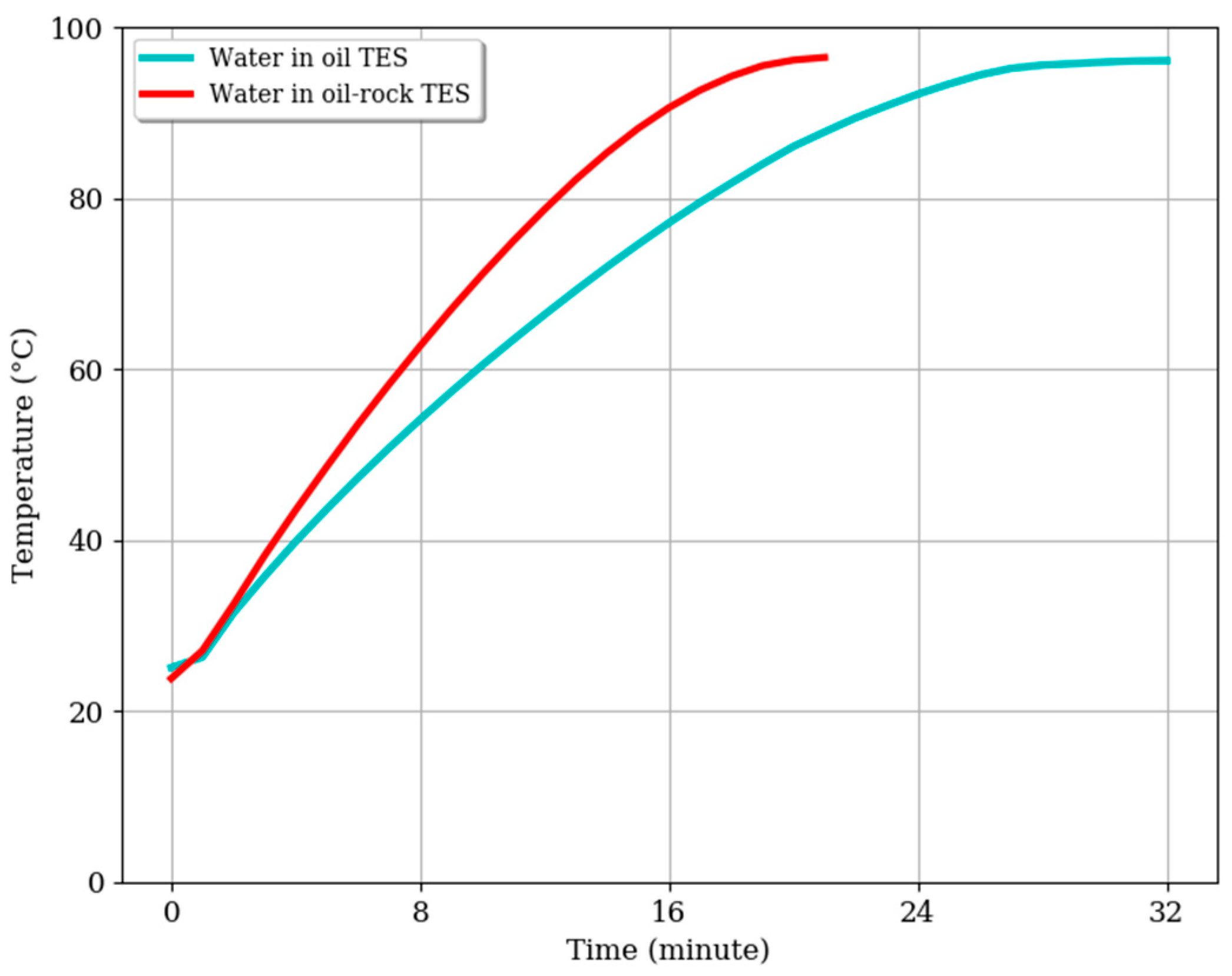
3.1. Temperature Profile of the Oil-Only TES System during Charging
3.2. Temperature Profile of the Oil–Rock Pebble TES System during Charging
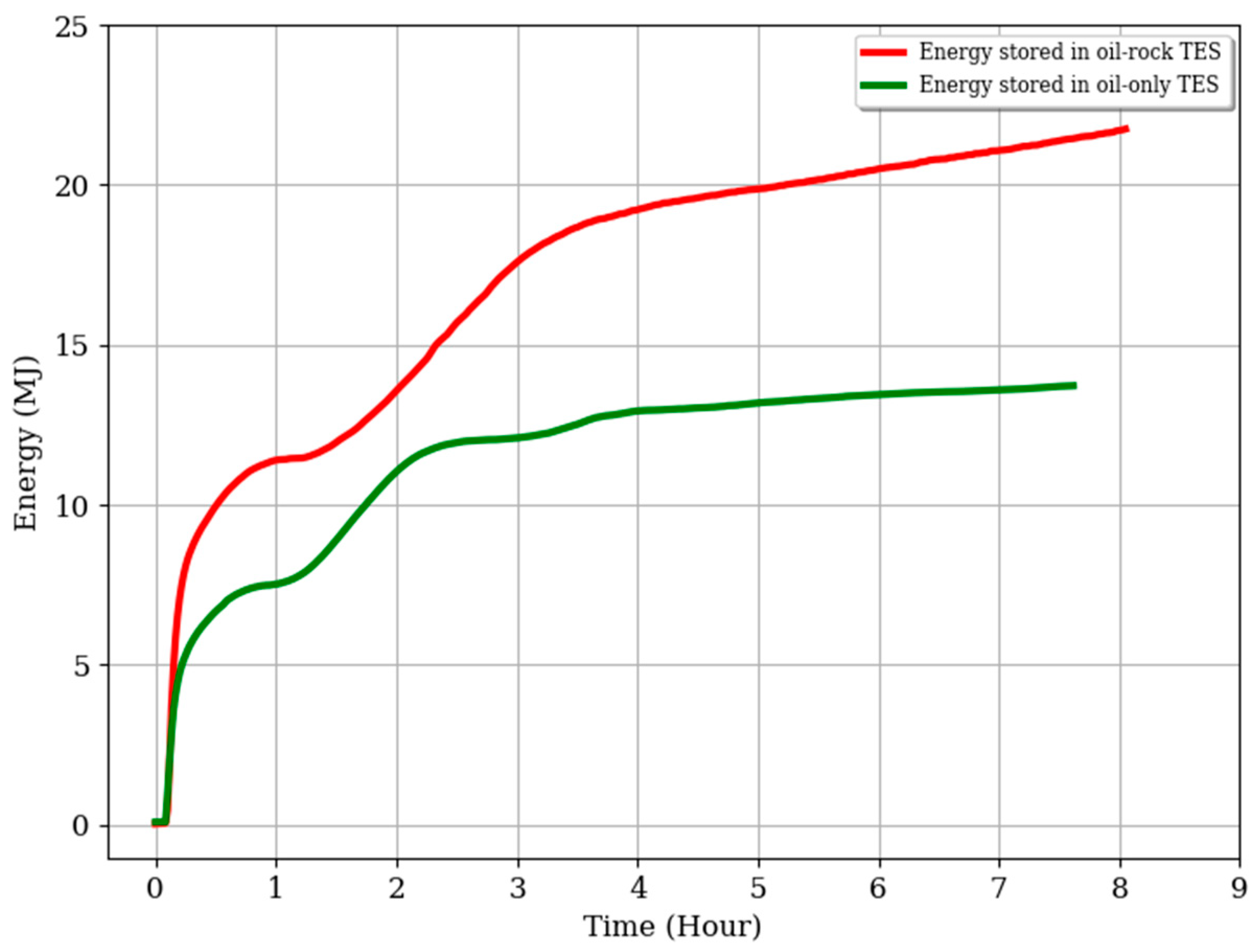
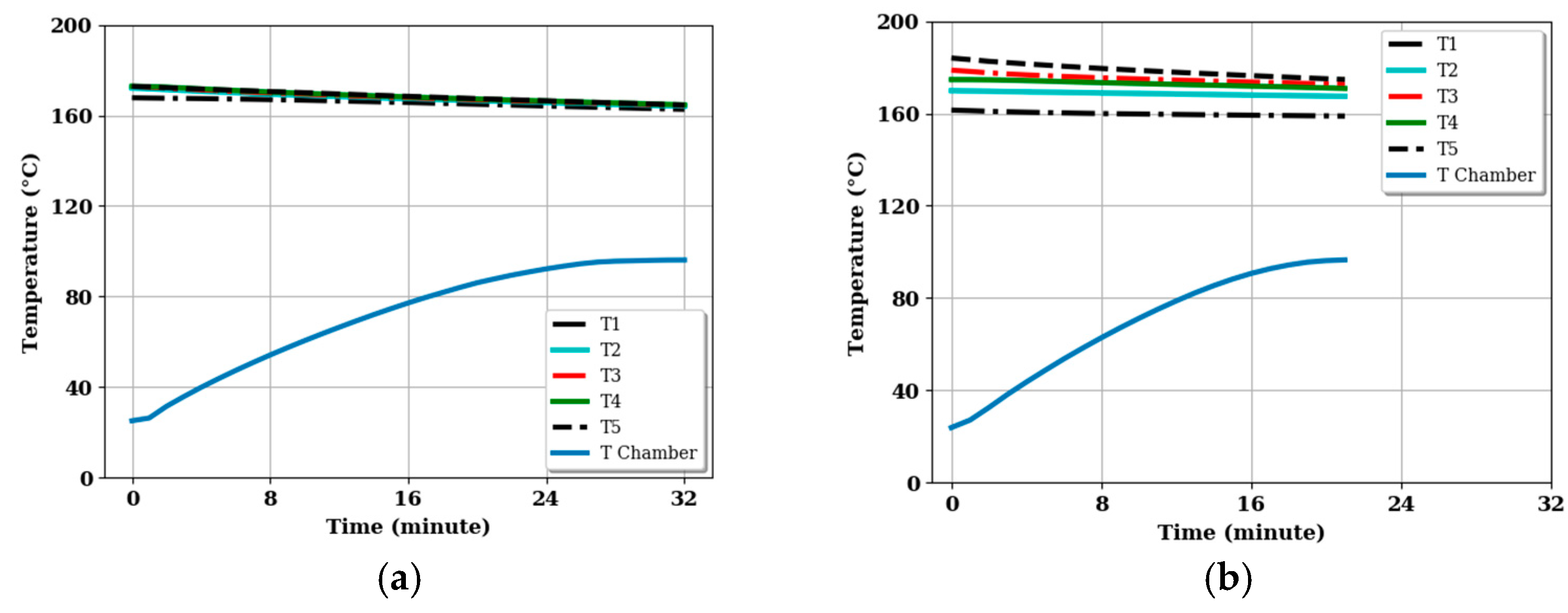
3.3. Energy Stored
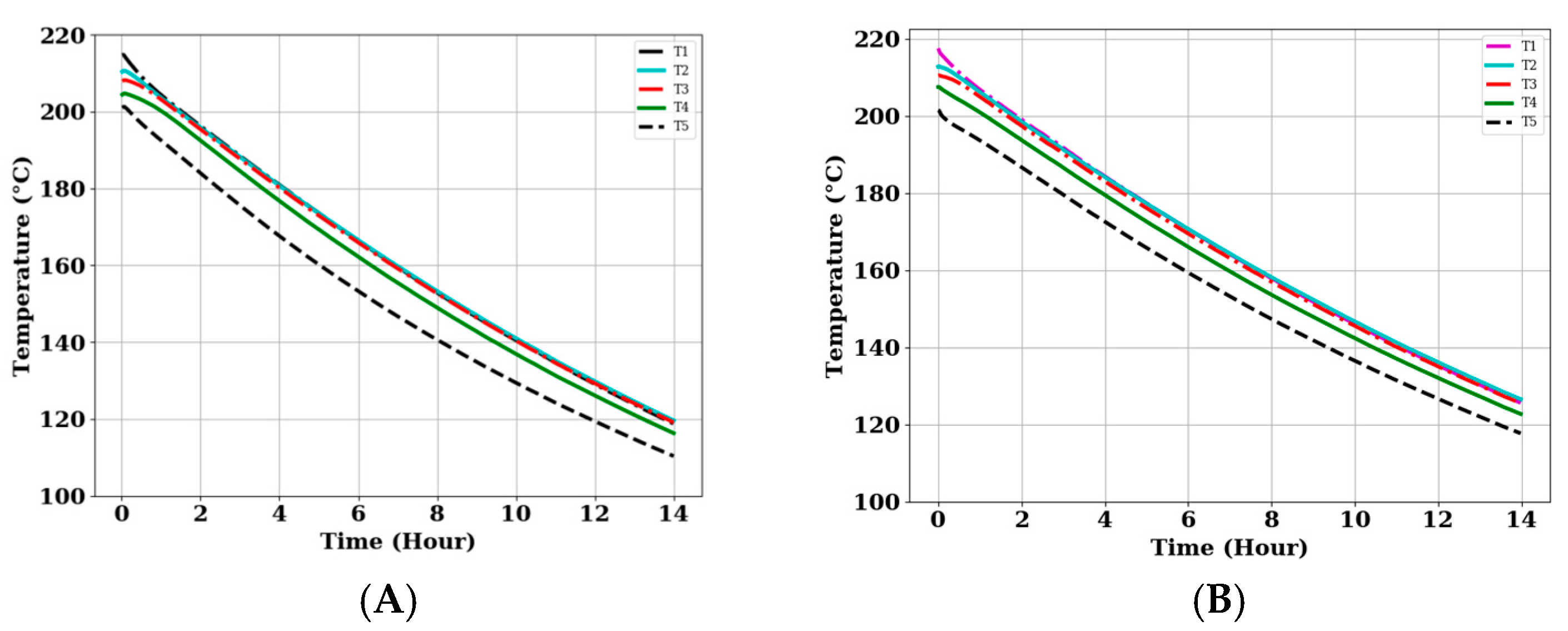
3.4. Heat Retention Capacity
3.5. Cooking Performance of the TES Systems
3.6. Efficiency of Heat Extraction of the TES Systems
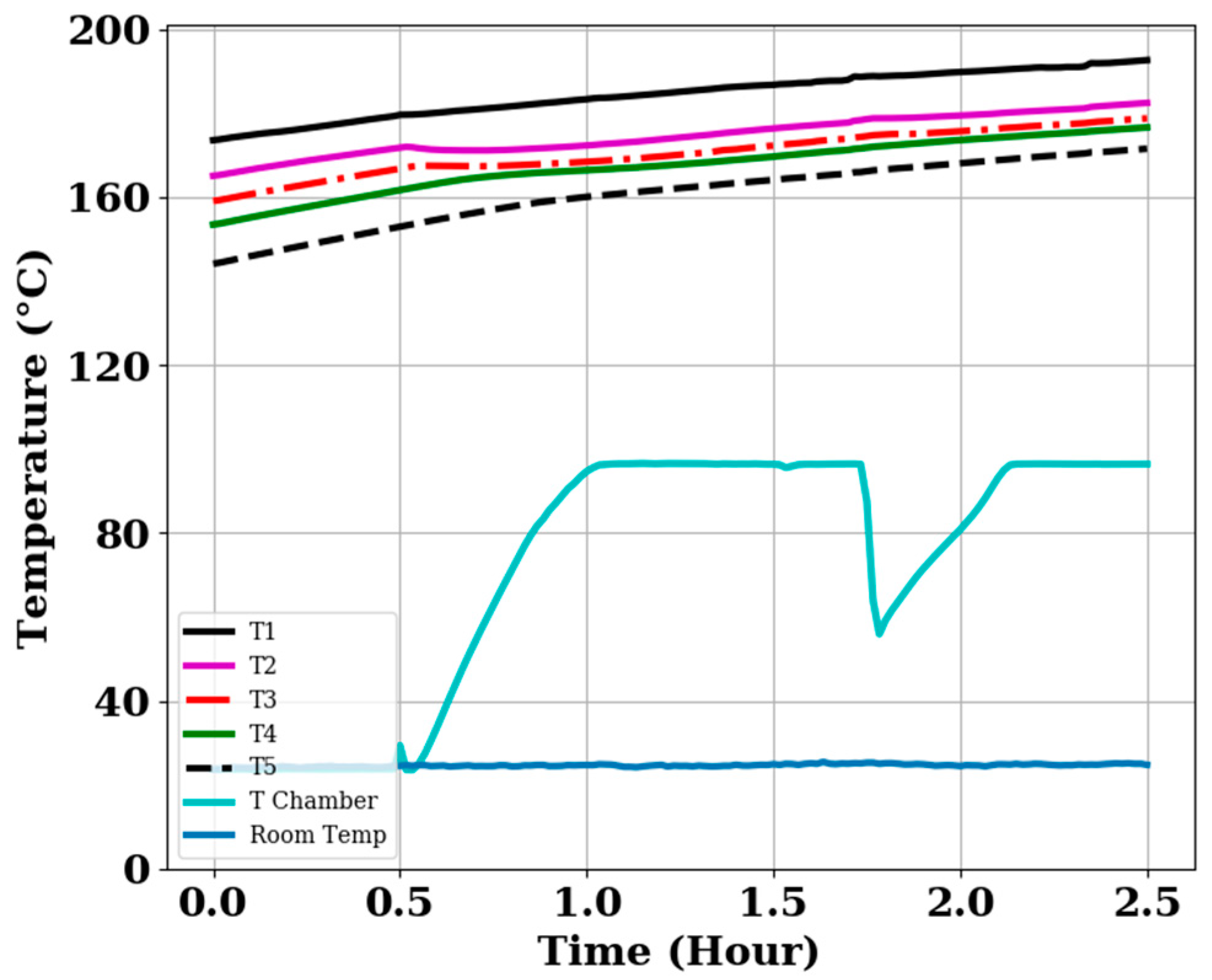
3.7. Temperature Profiles of the TES Systems during Cooking While Charging
3.7.1. Cooking while Charging Completely Discharge Storage
3.7.2. Cooking While Charging a Partially Charged Storage Unit
3.8. Overall Heat Transfer Coefficient
3.9. Cooking after the Heat Retention Period
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| As | surface area of storage unit, m2 |
| cav | average specific heat capacity, J kg−1K−1 |
| cp | specific heat capacity at constant pressure, J kg−1K−1 |
| cp,b | specific heat capacity of beans, J kg−1K−1 |
| cp,w | specific heat capacity of water, J kg−1K−1 |
| Eret | heat energy retained, J |
| Et | total heat energy stored, J |
| ETC | effective thermal conductivity |
| HTF | heat transfer fluid |
| LMTD | log mean temperature difference |
| m | mass, kg |
| mb | mass of beans, kg |
| Qabs | rate of heat absorption, W |
| Qloss | rate of heat loss, W |
| Qrel | rate of heat release, W |
| T | temperature, °C |
| ΔT | temperature difference in a segment, °C |
| Tamb | ambient temperature, °C |
| Tboil | boiling temperature, °C |
| TES | thermal energy storage |
| Tf | final temperature, °C |
| Ts,f | final temperature of storage, °C |
| Ts,i | initial temperature of storage, °C |
| Tw,i | initial temperature of water, °C |
| Tw,max | maximum temperature of water, °C |
| UCT | uncontrolled cooking test |
| Us | overall heat transfer coefficient of storage, W m−2K−1 |
| V | volume, L |
| Greek letter | |
| void fraction | |
| density, kg m−3 | |
| efficiency, | |
| Subscript | |
| av | average |
| j | segment |
| o | oil |
| r | rock |
| amb | ambient |
References
- Rehfuess, E.; World Health Organization. Fuel for Life: Household Energy and Health. World Health Organization, 2006. Available online: https://apps.who.int/iris/handle/10665/43421 (accessed on 8 August 2022).
- Joshi, S.B.; Jani, A.R. Design, development and testing of a small-scale hybrid solar cooker. Sol. Energy 2015, 122, 148–155. [Google Scholar] [CrossRef]
- Mehetre, S.A.; Panwar, N.L.; Sharma, D.; Kumar, H. Improved biomass cookstoves for sustainable development: A review. Renew. Sustain. Energy Rev. 2017, 73, 672–687. [Google Scholar] [CrossRef]
- Tabuti, J.R.S.; Dhillion, S.S.; Lye, K.A. Firewood use in Bulamogi County, Uganda: Species selection, harvesting and consumption patterns. Biomass Bioenergy 2003, 25, 581–596. [Google Scholar] [CrossRef]
- MWE. Water and Environment Sector Performance Report 2018. Available online: https://www.mwe.go.ug/library/sector-performance-report-2018 (accessed on 1 August 2022).
- De, D.K.; De, N.N.; Nathaniel, M.; Olawole, K. Minimizing energy usage in cooking to protect environments and health. Int. J. Energy Environ. Res. 2014, 2, 20–44. [Google Scholar]
- Mubiru, J.; Banda, E.J.K.B.; D’Ujanga, F.; Senyonga, T. Assessing the distribution of monthly mean hourly solar irradiation at an African Equatorial site. Energy Convers. Manag. 2007, 48, 380–383. [Google Scholar] [CrossRef]
- Muthusivagami, R.M.; Velraj, R.; Sethumadhavan, R. Solar cookers with and without thermal storage—A review. Renew. Sustain. Energy Rev. 2010, 14, 691–701. [Google Scholar] [CrossRef]
- Lentswe, K.; Mawire, A.; Owusu, P.; Shobo, A. A review of parabolic solar cookers with thermal energy storage. Heliyon 2021, 7, e08226. [Google Scholar]
- Nahar, N.M. Performance and testing of a hot box storage solar cooker. Energy Convers. Manag. 2003, 44, 1323–1331. [Google Scholar] [CrossRef]
- Nkhonjera, L.; Bello-Ochende, T.; John, G.; King’ondu, C.K. A review of thermal energy storage designs, heat storage materials and cooking performance of solar cookers with heat storage. Renew. Sustain. Energy Rev. 2017, 75, 157–167. [Google Scholar] [CrossRef]
- Lentswe, K.; Mawire, A.; Owusu, P. Experimental Energetic and Exergetic Performance of a Combined Solar Cooking and Thermal Energy Storage System. Energies 2022, 15, 8334. [Google Scholar] [CrossRef]
- Soria-Verdugo, A. Experimental analysis and simulation of the performance of a box-type solar cooker. Energy Sustain. Dev. 2015, 29, 65–71. [Google Scholar] [CrossRef]
- Sharma, S.D.; Buddhi, D.; Sawhney, R.L.; Sharma, A. Design, development and performance evaluation of a latent heat storage unit for evening cooking in a solar cooker. Energy Convers. Manag. 2000, 41, 1497–1508. [Google Scholar] [CrossRef]
- Okello, D.; Nydal, O.J.; Banda, E.J.K. Experimental investigation of thermal de-stratification in rock bed TES systems for high temperature applications. Energy Convers. Manag. 2014, 86, 125–131. [Google Scholar] [CrossRef]
- Buddhi, D.; Sharma, S.D.; Sharma, A. Thermal performance evaluation of a latent heat storage unit for late evening cooking in a solar cooker having three reflectors. Energy Convers. Manag. 2003, 44, 809–817. [Google Scholar] [CrossRef]
- Schwarzer, K.; da Silva, M.E.V. Solar cooking system with or without heat storage for families and institutions. Sol. Energy 2003, 75, 35–41. [Google Scholar] [CrossRef]
- Mussard, M.; Nydal, O.J. Charging of a heat storage coupled with a low-cost small-scale solar parabolic trough for cooking purposes. Sol. Energy 2013, 95, 144–154. [Google Scholar] [CrossRef]
- Mawire, A.; Lentswe, K.A.; Okello, D.; Nyeinga, K.; Lugolole, R. Energy and exergy performance of three sensible heat storage systems during charging. In Proceedings of the 2018 6th International Renewable and Sustainable Energy Conference (IRSEC), Rabat, Morocco, 5–8 December 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–6. [Google Scholar]
- Kajumba, P.K.; Okello, D.; Nyeinga, K.; Nydal, O.J. Experimental investigation of a cooking unit integrated with thermal energy storage system. J. Energy Storage 2020, 32, 101949. [Google Scholar] [CrossRef]
- Gautam, A.; Saini, R.P. A review on sensible heat based packed bed solar thermal energy storage system for low temperature applications. Sol. Energy 2020, 207, 937–956. [Google Scholar] [CrossRef]
- Prieto, C.; Cooper, P.; Fernández, A.I.; Cabeza, L.F. Review of technology: Thermochemical energy storage for concentrated solar power plants. Renew. Sustain. Energy Rev. 2016, 60, 909–929. [Google Scholar] [CrossRef]
- Okello, D.; Nydal, O.J.; Nyeinga, K.; Banda, E.J. Experimental investigation on heat extraction from a rock bed heat storage system for high temperature applications. J. Energy South. Afr. 2016, 27, 30–37. [Google Scholar] [CrossRef]
- Kumaresan, G.; Vigneswaran, V.S.; Esakkimuthu, S.; Velraj, R. Performance assessment of a solar domestic cooking unit integrated with thermal energy storage system. J. Energy Storage 2016, 6, 70–79. [Google Scholar] [CrossRef]
- Kajumba, P.K.; Okello, D.; Nyeinga, K.; Nydal, O.J. Assessment of the energy needs for cooking local food in Uganda: A strategy for sizing thermal energy storage with cooker system. Energy Sustain. Dev. 2022, 67, 67–80. [Google Scholar] [CrossRef]
- Tabu, B.; Nyeinga, K.; Chaciga, J.; Okello, D. Thermal Performance of Selected Oils in Uganda for Indirect Solar Domestic Cooking Applications. Tanzan. J. Sci. 2018, 44, 77–90. [Google Scholar]
- Okello, D.; Foong, C.W.; Nydal, O.J.; Banda, E.J. An experimental investigation on the combined use of phase change material and rock particles for high temperature (350 °C) heat storage. Energy Convers. Manag. 2014, 79, 1–8. [Google Scholar] [CrossRef]
- Mawire, A.; Taole, S.H. Experimental energy and exergy performance of a solar receiver for a domestic parabolic dish concentrator for teaching purposes. Energy Sustain. Dev. 2014, 19, 162–169. [Google Scholar] [CrossRef]
- Karunanithy, C.; Shafer, K. Heat transfer characteristics and cooking efficiency of different sauce pans on various cooktops. Appl. Therm. Eng. 2016, 93, 1202–1215. [Google Scholar] [CrossRef]
- Rismanchi, B.; Saidur, R.; Boroumandjazi, G.; Ahmed, S. Energy, exergy and environmental analysis of cold thermal energy storage (CTES) systems. Renew. Sustain. Energy Rev. 2012, 16, 5741–5746. [Google Scholar] [CrossRef]
- Velraj, R. Sensible heat storage for solar heating and cooling systems. In Advances in Solar Heating and Cooling; Elsevier Ltd.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Dirisu, J.O.; Oyedepo, S.O.; Fayomi, O.S.I. Thermal energy assessment of oil bean stalk as a novel additive to building ceilings. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2190. [Google Scholar]
- Marques, B.; Tadeu, A.; Almeida, J.; António, J.; de Brito, J. Characterisation of sustainable building walls made from rice straw bales. J. Build. Eng. 2020, 28, 101041. [Google Scholar] [CrossRef]
- Pico TC-08. Available online: https://www.picotech.com/download/datasheets/usb-tc-08-thermocouple-data-logger-data-sheet.pdf (accessed on 7 October 2022).
- Schlipf, D.; Schicktanz, P.; Maier, H.; Schneider, G. Using sand and other small grained materials as heat storage medium in a packed bed HTTESS. Energy Procedia 2015, 69, 1029–1038. [Google Scholar] [CrossRef]
- UNDP. Cooking with Electricity in Uganda: Barriers and Opportunities. 2020. Available online: https://mecs.org.uk (accessed on 6 September 2022).
- Robinson, J.; Ibraimo, M.; Pemberton-Pigott, C. The Uncontrolled Cooking Test: Measuring Three-Stone Fire Performance in Northern Mozambique. Available online: http://cleancookstoves.org/binary-data/DOCUMENT/file/000/000/82-1.pdf (accessed on 26 October 2022).
- Villasmil, W.; Fischer, L.J.; Worlitschek, J. A review and evaluation of thermal insulation materials and methods for thermal energy storage systems. Renew. Sustain. Energy Rev. 2019, 103, 71–84. [Google Scholar] [CrossRef]
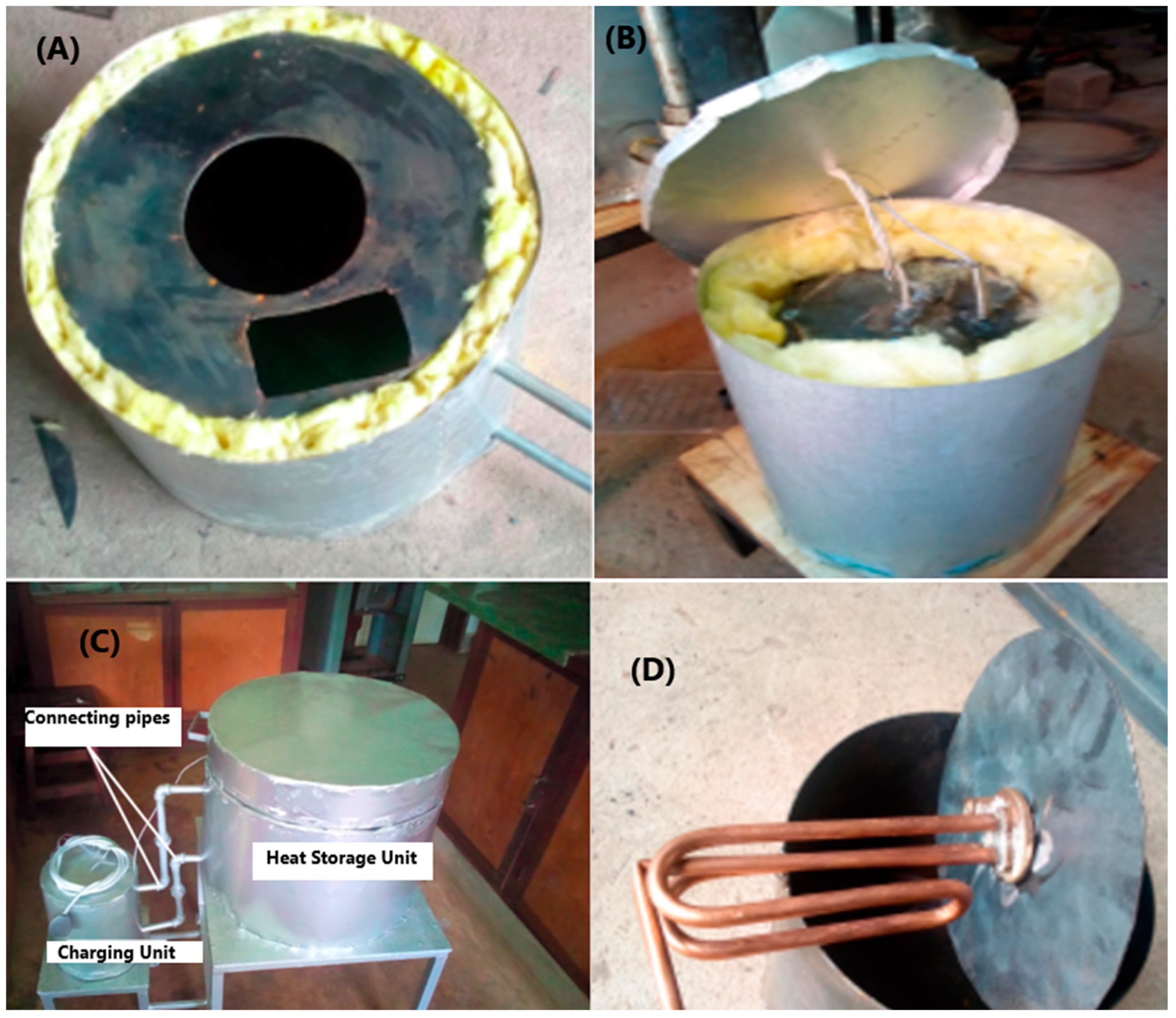

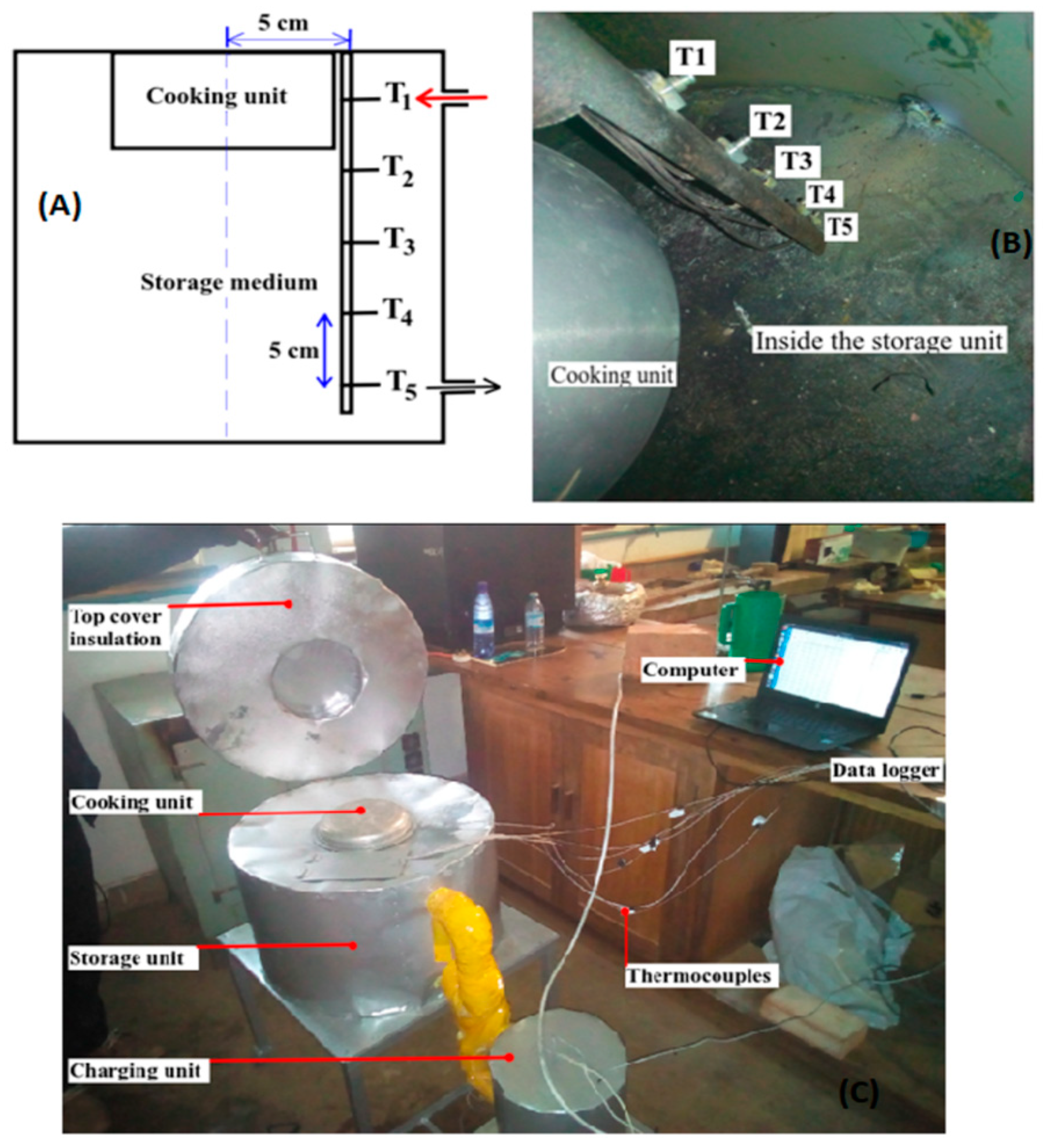
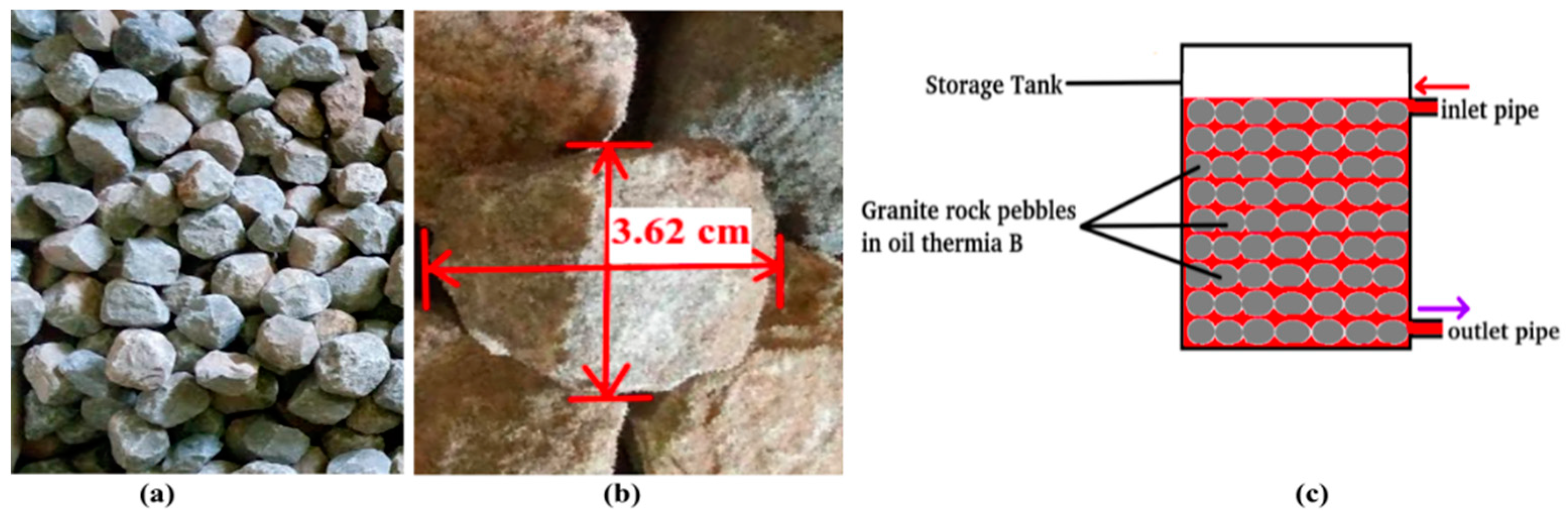



| Section | Part | Internal Diameter (cm) | Height/Length (cm) |
|---|---|---|---|
| Storage Unit | Outer cylinder | 62.0 | 35.0 |
| Inner cylinder | 52.0 | 30.0 | |
| Cooking Unit | 20.0 | 11.5 | |
| Charging Unit | Outer cylinder | 28.0 | 30.0 |
| Inner cylinder | 18.0 | 20.0 | |
| Connecting pipes | Outlet from storage unit | 1.6 | 51.0 |
| Inlet to storage unit | 1.6 | 58.0 |
| Quantity | Value | Unit |
|---|---|---|
| Density, ρ | 875 − 0.604 T | kg m−3 |
| Specific heat capacity, c | 1.8087 + 0.0036 T | kJ kg−1K−1 |
| Flash point | 220 | °C |
| Boiling point | 355 | °C |
| TES System | Time (min) | Qabs (W) | Qloss (W) | Qrel (W) | η (%) |
|---|---|---|---|---|---|
| Oil-only | 32 | 157.8 | 104.0 | 261.8 | 60.3 |
| Oil–rock | 21 | 241.7 | 130.6 | 372.3 | 64.9 |
| TES System | Time (mins) | Efficiency of Heat Extraction (%) |
|---|---|---|
| Oil-only | 116 | 42 |
| Oil–rock pebble | 106 | 49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okello, D.; Omony, R.; Nyeinga, K.; Chaciga, J. Performance Analysis of Thermal Energy Storage System Integrated with a Cooking Unit. Energies 2022, 15, 9092. https://doi.org/10.3390/en15239092
Okello D, Omony R, Nyeinga K, Chaciga J. Performance Analysis of Thermal Energy Storage System Integrated with a Cooking Unit. Energies. 2022; 15(23):9092. https://doi.org/10.3390/en15239092
Chicago/Turabian StyleOkello, Denis, Robinson Omony, Karidewa Nyeinga, and Jimmy Chaciga. 2022. "Performance Analysis of Thermal Energy Storage System Integrated with a Cooking Unit" Energies 15, no. 23: 9092. https://doi.org/10.3390/en15239092
APA StyleOkello, D., Omony, R., Nyeinga, K., & Chaciga, J. (2022). Performance Analysis of Thermal Energy Storage System Integrated with a Cooking Unit. Energies, 15(23), 9092. https://doi.org/10.3390/en15239092








