Recent Advances in the Synthesis, Application and Economic Feasibility of Ionic Liquids and Deep Eutectic Solvents for CO2 Capture: A Review
Abstract
:1. Introduction
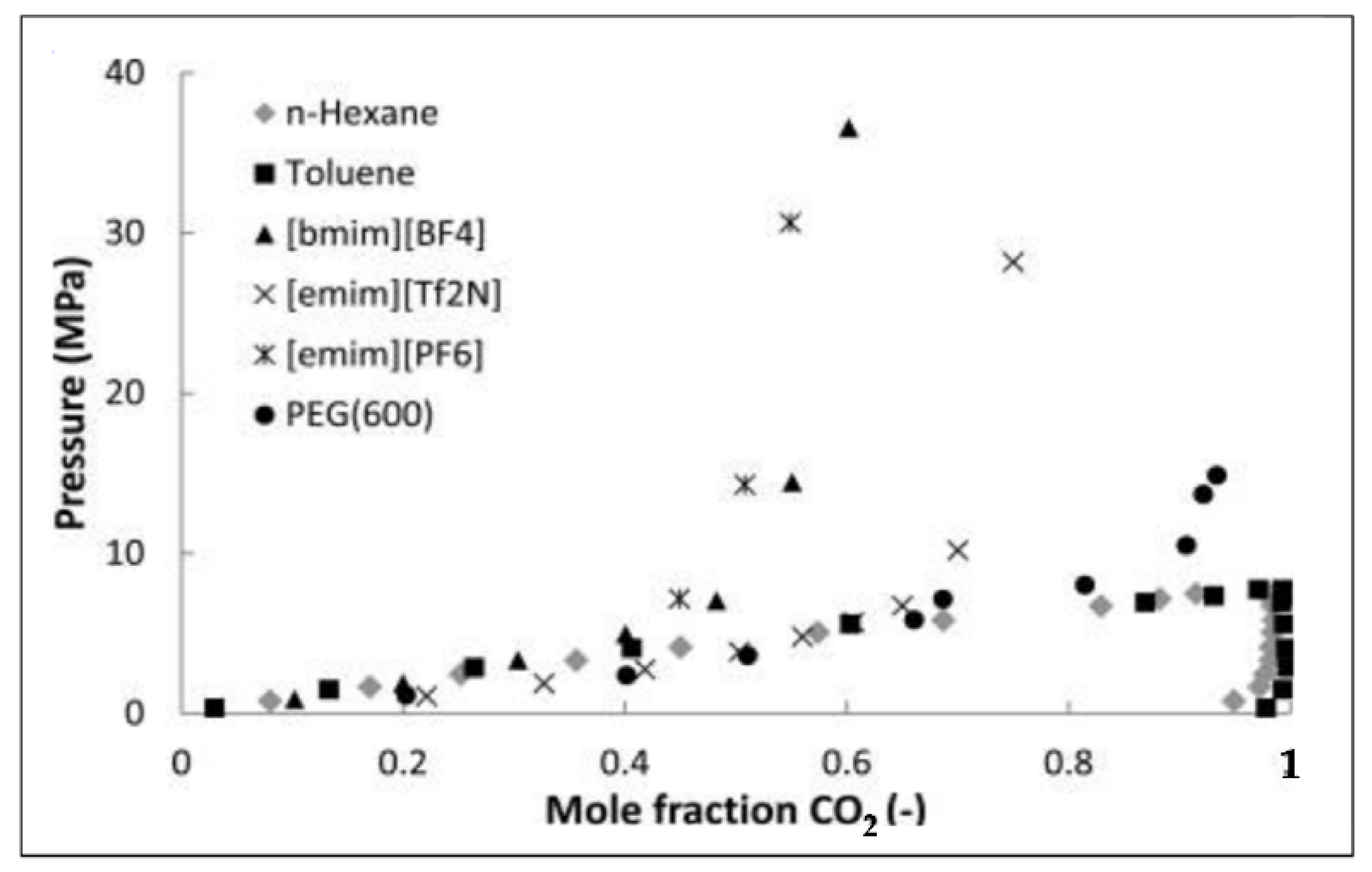
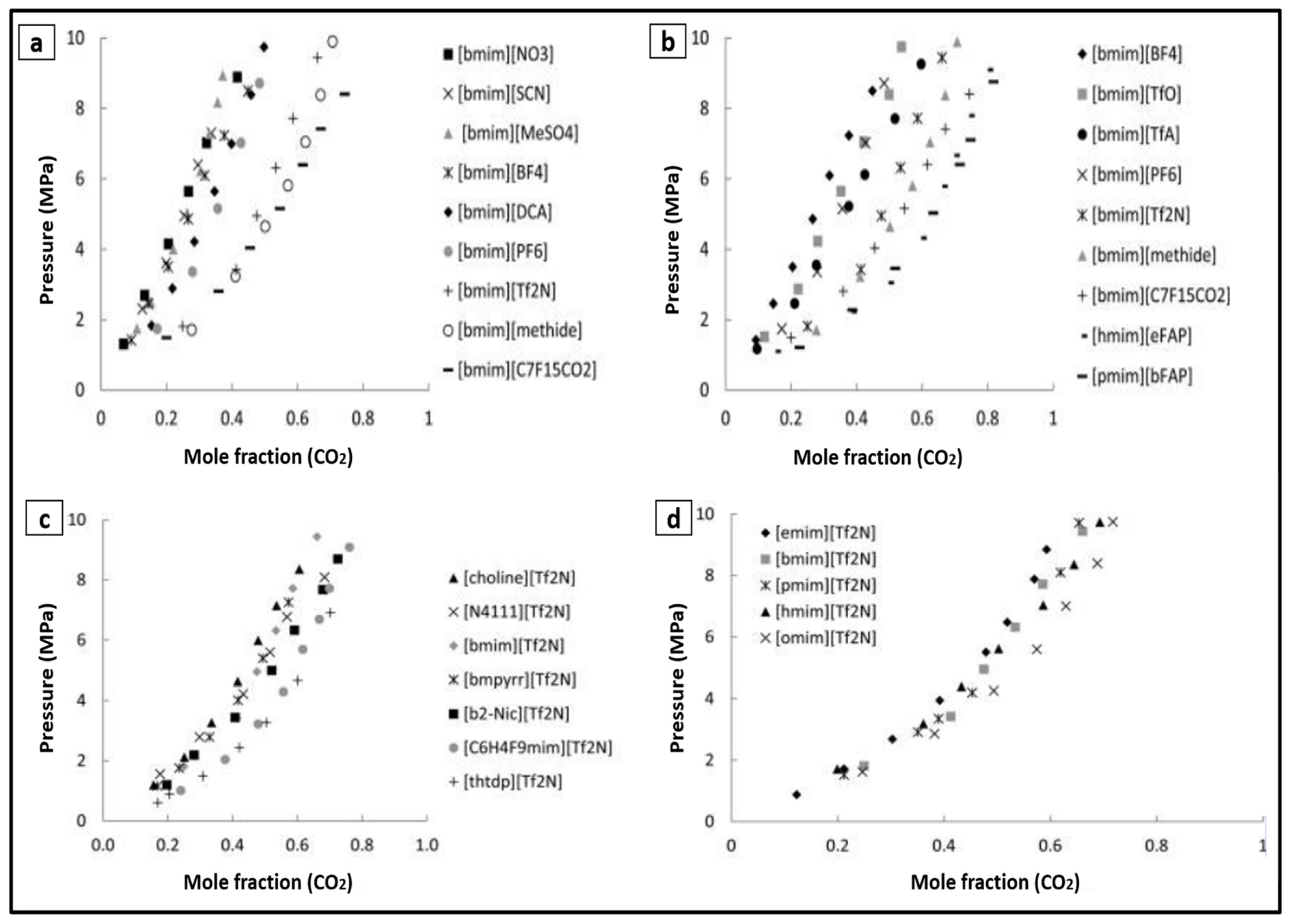
2. Ionic Liquids
Functionalization of ILs for CO2 Capture
3. ILs Used for CO2 Capture in the Last Five Years
| Ionic Liquid | Mw (g mol−1) | T (K) | P (kPa) | CO2 Solubility (mol kg−1) | References |
|---|---|---|---|---|---|
| [EMIM] [Ac] | 170.2 | 313.2 | 100 | 1.65 | [57] |
| [EMIM] [Ala] | 200.26 | 313.2 | 100 | 1.89 | [57] |
| [EMIM] [Gly] | 186.3 | 313.2 | 100 | 2.32 | [57] |
| [TETAH] [Lys] | 266.43 | 313.15 | 100 | 9.72 | [59] |
| [DETAH] [Py] | 171.25 | 313.15 | 100 | 11.91 | [65] |
| [DETAH] [Im] | 171.25 | 313.15 | 100 | 11.39 | [65] |
| [DETAH] [Lys] | 277.39 | 313.15 | 100 | 7.68 | [59] |
| [DETAH] [Gly] | 178.24 | 313.15 | 100 | 10.15 | [65] |
| [DETAH] [Tz] | 172.23 | 313.15 | 100 | 10.1 | [65] |
| [DMAPAH] [2F-PhO] | 214.4 | 303.2 | 100 | 3.12 | [61] |
| [DMAPAH] [3F-PhO] | 21 4.4 | 303.2 | 100 | 3.39 | [61] |
| [DMAPAH] [3,5F-PhO] | 232.3 | 303.2 | 100 | 3.52 | [61] |
| [DMAPAH] [4F-PhO] | 214.4 | 303.2 | 100 | 3.99 | [61] |
| [N1111] [Lys] | 219 | 303 | 100 | 1.84 | [64] |
| [P2228] [6-BrBnIm] | 428.42 | 333.15 | 0–149.9 | 0–2.01 | [62] |
| [P2228] [BnIm] | 349.52 | 298.15 | 0–99.8 | 0–2.78 | [62] |
| [P2228] [2CNPyr] | 323.48 | 295.15 | 0–99.8 | 0–2.84 | [62] |
| [BMIM] [2-Op] | 233.31 | 303.15 | 100 | 4.37 | [66] |
| [BMmim] [2-Op] | 247.34 | 303.15 | 100 | 4.29 | [66] |
| [BMPyr] [2-Op] | 236.36 | 303.15 | 100 | 4.95 | [66] |
| [N4442] [2-Op] | 308.51 | 303.15 | 100 | 4.02 | [66] |
| [P4442OH] [2-Op] | 341.47 | 303.15 | 100 | 2.75 | [66] |
| [P4442] [2-OP] | 325.47 | 303.15 | 100 | 4.3 | [66] |
| [Ph-C8eim] [2-Op] | 379.54 | 293.15 | 100 | 4.45 | [66] |
| [P4442] [DAA] | 331.48 | 293.15 | 10 | 3.78 | [63] |
| [P4442] [Suc] | 329.46 | 293.15 | 10 | 5, 3.4 | [63] |
| [P4442] [Ph-Suc] | 329.47 | 293.15 | 100 | 4.25 | [64] |
| [P4442] [Suc] | 329.46 | 293.15 | 100 | 5.62 | [64] |
| [P4442] [Cy-Suc] | 383.56 | 293.15 | 100 | 5.76 | [64] |
| [P66614] [Beta-Ala] | 572.96 | 303.15 | 100 | 1.74 | [60] |
| [P66614]2[Asp] | 1068.83 | 303.15 | 100 | 1.83 | [60] |
| [P66614] [MA-Tetz] | 582.96 | 303.15 | 100 | 1.94 | [60] |
| [P66614] [Gly] | 558.93 | 303.15 | 100 | 2.15 | [60] |
| [TMGH] [PhO] | 209.29 | 313.15 | 100 | 0.24 | [64] |
| [TMGH] [Im] | 183.26 | 313.15 | 100 | 3.49 | [64] |
| [TMGH] [Pyrr] | 182.27 | 313.15 | 100 | 3.62 | [64] |
| [VBTMA] [Ala] | 294.44 | 298 | 100 | 0.98 | [67] |
| [VBTMA] [Pro] | 320.47 | 298 | 100 | 1.19 | [67] |
| [VBTMA] [Ser] | 310.44 | 298 | 100 | 1.26 | [67] |
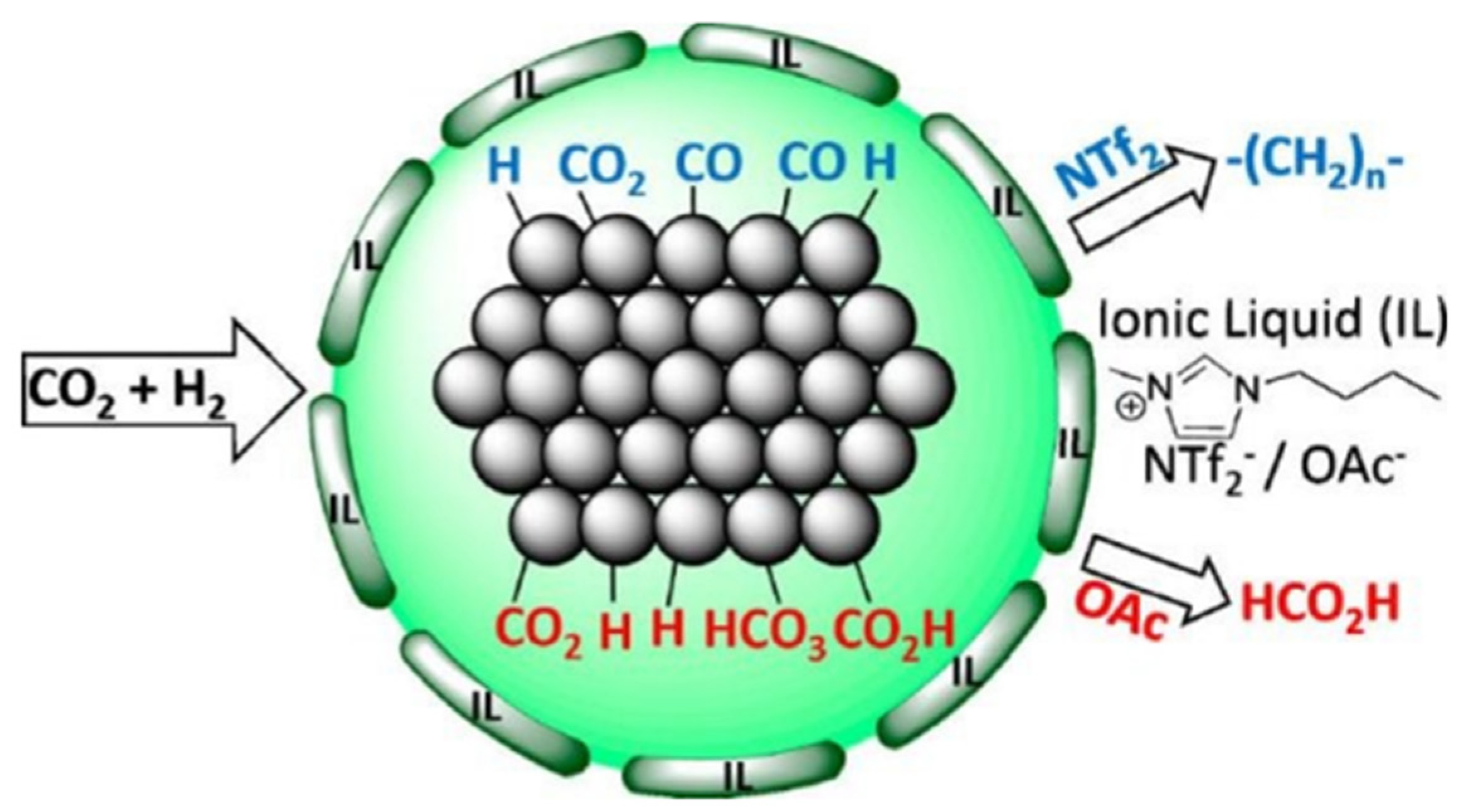
4. Conversion of CO2 into Valuable Products Using ILs
5. Economics of IL-Based CO2 Capture
6. Difficulties and Drawbacks of Using ILs to Capture CO2
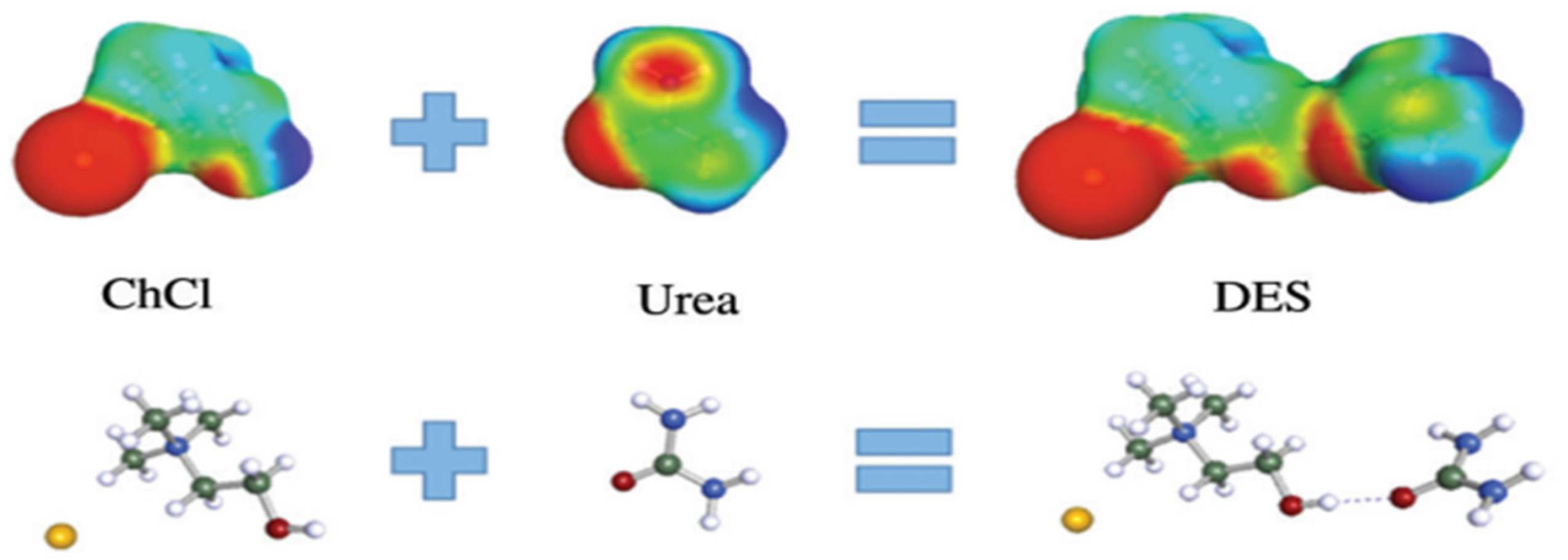
7. Deep Eutectic Solvents
Functionalization of DESs for CO2 Capture
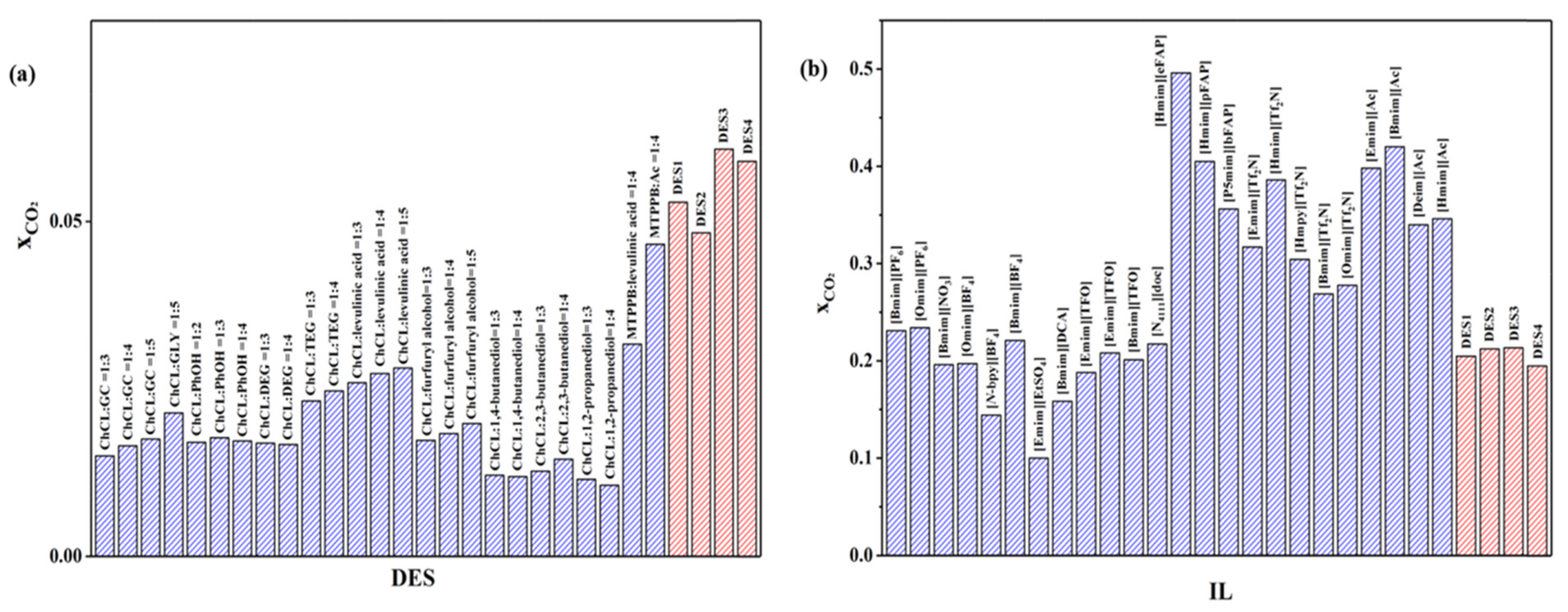
8. DESs Used for CO2 Capture in the Last Five Years
| HBD | Salt | Molar Ratio | Solubility | Pressure (MPa) | Temperature (K) | References |
|---|---|---|---|---|---|---|
| 1,4-Butanediol | ChCl | 1:3 | 0.1624/mol·kg−1 | 0.5097 | 293.15 | [145] |
| 1,4-Butanediol | ChCl | 1:4 | 0.1560/mol.kg−1 | 0.5134 | 293.15 | [145] |
| 2,3-Butanediol | ChCl | 1:3 | 0.1501/mol.kg−1 | 0.5113 | 293.15 | [145] |
| 2,3-Butanediol | ChCl | 1:4 | 0.1915/mol.kg−1 | 0.5085 | 293.15 | [145] |
| 1,2-Butanediol | ChCl | 1:3 | 0.1827/mol.kg−1 | 0.5145 | 293.15 | [145] |
| 1,2-Butanediol | ChCl | 1:4 | 0.1884/mol.kg−1 | 0.5015 | 293.15 | [145] |
| Levulinic acid | ChCl | 1:3 | 0.2549/mol.kg−1 | 0.57 | 303.15 | [146] |
| Levulinic acid | ChCl | 1:4 | 0.2700/mol.kg−1 | 0.5749 | 303.15 | [146] |
| Levulinic acid | ChCl | 1:5 | 0.2869/mol.kg−1 | 0.5667 | 303.15 | [146] |
| Furfuryl alcohol | ChCl | 1:3 | 0.1856/mol.kg−1 | 0.5828 | 303.15 | [146] |
| Furfuryl alcohol | ChCl | 1:4 | 0.2196/mol.kg−1 | 0.5815 | 303.15 | [146] |
| Furfuryl alcohol | ChCl | 1:5 | 0.2276/mol.kg−1 | 0.5774 | 303.15 | [146] |
| Ethylene glycol | ChCl | 1:2 | 3.1265/mol.kg−1 | 5.863 | 303.15 | [137] |
| Phenol | ChCl | 1:2 | 0.1945/mol.kg−1 | 0.4945 | 293.15 | [147] |
| Phenol | ChCl | 1:3 | 0.2052/mol.kg−1 | 0.5085 | 293.15 | [147] |
| Phenol | ChCl | 1:4 | 0.2108/mol.kg−1 | 0.5092 | 293.15 | [147] |
| Diethylene glycol | ChCl | 1:3 | 0.1687/mol.kg−1 | 0.5129 | 293.15 | [147] |
| Diethylene glycol | ChCl | 1:4 | 0.1852/mol.kg−1 | 0.5088 | 293.15 | [147] |
| Triethylene glycol | ChCl | 1:3 | 0.1909/mol.kg−1 | 0.504 | 293.15 | [147] |
| Triethylene glycol | ChCl | 1:4 | 0.1941/mol.kg−1 | 0.5135 | 293.15 | [147] |
| Urea | ChCl | 1:2 | 3.5592/mol.kg−1 | 5.654 | 303.15 | [148] |
| Glycerol | ChCl | 1:2 | 3.6929/mol.kg−1 | 5.863 | 303.15 | [137,148] |
| Urea | ChCl | 1:1.5 | 0.2010/mol.kg−1 | 11.84 | 313.15 | [136] |
| Urea | ChCl | 1:2 | 0.3090/mol.kg−1 | 12.5 | 313.15 | [136] |
| Urea | ChCl | 1:2.5 | 0.2030/mol.kg−1 | 12.45 | 313.15 | [136] |
| Triethylene glycol | ChCl | 1:4 | 0.0419/mol.kg−1 | 1 | 298.15 | [149] |
| Ethylene glycol | ChCl | 1:4 | 0.0230/mol.kg−1 | 1 | 298.15 | [149] |
| Ethylene glycol | ChCl | 1:8 | 0.0262/mol.kg−1 | 1 | 298.15 | [149] |
| Urea | ChCl | 1:4 | 0.0240/mol.kg−1 | 1 | 298.15 | [149] |
| Urea | ChCl | 1:2:5 | 0.0211/mol.kg−1 | 1 | 298.15 | [149] |
| Glycerol | ChCl | 1:3 | 0.0454/mol.kg−1 | 1 | 298.15 | [149] |
| Glycerol | ChCl | 1:8 | 0.0306/mol.kg−1 | 1 | 298.15 | [149] |
| Ethanol amine | ChCl | 1:6 | 0.1096/mol.kg−1 | 1 | 298.15 | [149] |
| Diethanol amine | ChCl | 1:6 | 0.0925/mol.kg−1 | 1 | 298.15 | [149] |
| Glycerol | ChCl | 1:12 | 0.0511/mol.kg−1 | 1 | 298.15 | [149] |
| Ethylene glycol | BTPPC | 1:12 | 0.0503/mol.kg−1 | 1 | 298.15 | [149] |
| Ethanol amine | BTPPB | 1:6 | 0.1441/mol.kg−1 | 1 | 298.15 | [149] |
| Ethanol amine | MTPPB | 1:7 | 0.1254/mol.kg−1 | 1 | 298.15 | [149] |
| Ethanol amine | MTPPB | 1:8 | 0.1189/mol.kg−1 | 1 | 298.15 | [149] |
| Ethanol amine | MTPPB | 1:6 | 0.1168/mol.kg−1 | 1 | 298.15 | [149] |
| Diethanol amine | TBAB | 1:6 | 0.1036/mol.kg−1 | 1 | 298.15 | [149] |
| Triethanol amine | TBAB | 1:3 | 0.0830/mol.kg−1 | 1 | 298.15 | [149] |
| Lactic acid | TMACl | 1:02 | 0.0588/mol.kg−1 | 1.992 | 308.15 | [150] |
| Lactic acid | TEACl | 1:02 | 0.0725/mol.kg−1 | 1.993 | 308.15 | [150] |
| Lactic acid | TBACl | 1:02 | 0.1272/mol.kg−1 | 1.992 | 308.15 | [150] |
| Glycerol + DBN | ChCl | 1:02:06 CH-Cl: gly:DBN | 2.3−2.4 mol.kg−1 | Ambient | [130] | |
| Urea | ChCl | 1:2 | 3.559 mol.kg−1 | 6 | 303.15 | [148] |
| Ethylene glycol | ChCl | 1:02 | 3.1265 mol.kg−1 | 5.863 | 303.15 | [137] |
| Ethanolamine | ChCl | 1:06 | 0.0749 mol.kg−1 | 1 | 298 | [149] |
| Ethanolamine | MTPP_Br | 0.0716 mol.kg−1 | 1 | 298 | [149] | |
| Ethanolamine | TBA_Br | 0.0591 mol.kg−1 | 1 | 298 | [149] | |
| Triethylene glycol | ChCl | 4:01 | 0.1941 mol.kg−1 | 0.5 | 293 | [147] |
| Phenol | ChCl | 4:01 | 0.2108 mol.kg−1 | 0.5 | 293 | [147] |
| Diethylene glycol | ChCl | 4:01 | 0.1852 mol.kg−1 | 0.5 | 293 | [147] |
9. Conversion of CO2 into Valuable Products Using DESs
10. Economics of DES-Based CO2 Capture
11. Difficulties and Drawbacks of DESs for CO2 Capture
12. Conclusions and Future Perspectives
- ❖
- From the studied literature, it is concluded that various ILs and DESs, due to their attractive properties, are suitable candidates for CO2 capture. Some researchers have reported ILs with better performance for CO2 capture but reported DESs are more economical, especially on the basis of synthesis.
- ❖
- Several functional groups, such as carbonyl, amine, and fluorine, as well as alkyl chain lengths, are incorporated in ILs because they improve the adsorption affinity of CO2 toward ILs. In the case of DESs, some factors, such as temperature, pressure, and alkyl chain length, are important to examine. However, the amine functional group in DESs has drawn a lot of interest for CO2 capture.
- ❖
- From the reported literature, it is concluded that CO2 could not only be captured through ILs and DESs from various resources but also possibly be converted into various valuable products.
- ❖
- It is also concluded that the available data are not sufficient, and more ILs need to be explored, especially DESs, for CO2 capture. The viscosity of ILs is the main problem for CO2 capture, and highly viscous ILs are not desirable for such applications. Similarly, the IL purity is also very important. However, the lower viscosity of DESs is also favorable for CO2 capture, and the literature reports that DESs with lower viscosity obtain good results for CO2 capture. The hygroscopic nature of DESs has been referred to as a drawback of these mixtures, as it has a considerable impact on CO2 solubility at low pressures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| Abbreviation | Full Name |
| AC | Acetate |
| [Ala] | Alanine |
| Arg | Arginine |
| Asp | Aspartic acid |
| ArgGly | Arginine glycerol |
| [2-Op] | 2-Hydroxyl pyridium |
| [BF4]− | Tetrafluoroborate |
| [Bmim]+ | 1-Butyl-3-methylimidazolium |
| [BMmim]+ | 1-Butyl-2,3-dimethylimidazolium |
| [BMPyr]+ | 1-Butyl-1-methylpyrrolidinium |
| [Bmim] [Ac] | 1-Butyl-3-methylimidazolium acetate |
| [Bmim] [BF4] | 1-Butyl-3-methylimidazolium tetrafluoroborate |
| [Bmim] [DCA] | 1-Butyl-3-methylimidazolium dicyanamide |
| [Bmim] [i-but] | 1-Butyl-3-methylimidazoliumisobutyrate |
| [Bmim] [GLY] | 1-Butyl-3-methylimidazolium glycinate |
| [Bmim] [OH] | 1-Butyl-3-methylimidazolium hydroxide |
| [Bmim] [PF6] | 1-Butyl-3-methylimidazolium hexafluorophosphate |
| [Bmim] [PF6] | 1-Butyl-3-methylimidazolium hexafluorophosphate |
| [Bmim] [PRO] | 1-Butyl-3-methylimidazolium prolinate |
| [Bmim] [Tf2N] | 1-Butyl-3-methylimidazolium bis[trifluoromethyl)sulfonyl] imide |
| [BMIM] [2-Op] | 1-Butyl-3-methylimidazolium 2-hydroxyl pyridium |
| [BMmim] [2-Op] | 1-Butyl-2,3-dimethylimidazolium 2-hydroxyl pyridium |
| [BMPyr] [2-Op] | 1-Butyl-1-methylpyrrolidinium 2-hydroxyl pyridium |
| [Bpy] [BF4] | 1-Butylpyridinium tetrafluoroborate |
| BTEA | Benzyl triethyl ammonium |
| BTMA | Benzyl trimethyl ammonium |
| CO2 | Carbon dioxide |
| [C2mim] [Tf2N] | 1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide |
| [C2mim] [Ac] | 1-Ethyl-3-methylimidazolium acetate |
| [C4mim] [Ac] | 1-Butyl-3-methylimidazolium acetate |
| [C4mim] [IBS] | 1-Butyl-3-methylimidazolium isobutyrate |
| [C4mim] [LEV] | 1-Butyl-3-methylimidazolium levulinate |
| [C4mim] [PRO] | 1-Butyl-3-methylimidazolium prolinate |
| [C4mim] [TMA] | 1-Butyl-3-methylimidazolium trimethylacetate |
| [C6mim] [Tf2N] | 1-Hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide |
| ChCl | Chlorine chloride |
| Cl | Chlorine |
| [CNPyr] | Cyanopyrrole |
| CsOH | Cesium hydroxide |
| DBN | 1,5-Diazabicyclo[4.3.0]non-5-ene |
| [DBU] [AC] | 1,8-Diazabicyclo[5.4.0]undec-7-ene acetate |
| DESs | Deep eutectic solvents |
| [DETAH] | Diethylenetriamine |
| [DETAH] [Lys] | Diethylenetriamine lysinate |
| [DETAH] [Tz] | Diethylenetriamine triazoles |
| [DETAH] [Im] | Diethylenetriamine imidazole |
| [DETAH] [AHA] | Diethylenetriamine aprotic heterocyclic anion |
| [DETAH] [Gly] | Diethylenetriamine glycine |
| [DETAH] [Py] | Diethylenetriamine Pyrazole |
| [TETAH] [Lys] | Triethylenetetramine lysinate |
| [DMAPAH]+ | 3-(Dimethylamino)-1-propylamine |
| [DMAPAH] [2F-PhO] | N,N-Dimethyl-1,3-propane diamine 2-fluorophenolate |
| [DMAPAH] [3F-PhO] | N,N-Dimethyl-1,3-propane diamine 3-fluorophenolate |
| [DMAPAH] [3,5F-PhO] | N,N-Dimethyl-1,3-propane diamine 3,5-difluorophenolate |
| [DMAPAH] [4F-PhO | N,N-Dimethyl-1,3-propane diamine 3-fluorophenolate |
| DMSO | Dimethyl sulfoxide |
| EA | Ethyl acetate |
| [Emim] [Ala] | 1-Ethyl-3-methylimidazolium alanine |
| [Emim] [DCN] | 1-Ethyl-3-methylimidazolium dicyanamide |
| [Emim] [EtSO4] | 1-Ethyl-3-methylimidazolium ethyl sulfate |
| [Emim] [GLY] | 1-Ethyl-3-methylimidazolium glycine |
| [Emim] [Tf2N] | 1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide |
| [Emim] [2-CNpyr] | 1-Ethyl-3-methylimidazolium 2-cyanopyrolide |
| [GLY]GHGs | GlycineGreenhouse gases |
| GluGly | Glutamate glycerol |
| Gua-EA | Guanidium ethyl acetate |
| HBA | Hydrogen-bond acceptor |
| HBD | Hydrogen-bond donor |
| HDBU | 1,8-Diazabicyclo-[5.4.0]-undec-7-ene 2,2,3,3,4,4,5 |
| H2 | Hydrogen |
| H2O | Water |
| HIS | Histidinate |
| HisGly | Histidine glycerol |
| [Hmim] [NTf2] | 1-Hexyl-3-methylimidazolium bis(trifluoromethyl)-sulfonyl) imide |
| ILs | Ionic liquids |
| IndIPCC | IndiumIntergovernmental Panel on Climate Change |
| [LEU] | Leucine |
| LYS | Lysinate |
| MEA | Methylethylamine |
| [MET] | Methionine |
| MTBD | 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene |
| MTTP | Methyl triphenylphosphonium |
| MTTP-LV-AC | Methyl triphenylphosphonium levulinic acetate |
| N2 | Nitrogen |
| [N-Bpy] [BF4] | N-butylpyridinium tetrafluoroborate |
| NDESs | Natural deep eutectic solvents |
| NiCl2 | Nickel chloride |
| NO3 | Nitrate |
| [N4442] [2-Op] | Tributylethylammonium 2-hydroxypyridine |
| [Omim] [BF4] | 1-Methyl-3-octyl-imidazolium-tetrafluoroborate |
| [Omim] [Tf2N] | 1-Octyl-3-methyl-imidazolium bis(trifluoromethylsulfonyl) imide |
| [Ph-C8eim] | 1-N-Ethyl-3-N-octyl-2-phenylimidazolium |
| [Ph-C8eim] [2-Op] | 1-N-Ethyl-3-N-octyl-2-phenylimidazolium 2-hydroxypyridine |
| [PF6]− | Hexafluorophosphate |
| [P2228]+ | Triethyloctylphosphonium |
| [P4442]+ | Tributylethylphosphonium |
| [P4442] [DAA] | Tributylethylphosphonium diacetamide |
| [P4442] [Suc] | Tributylethylphosphonium succinimide |
| [P4442OH]+ | Tributyl(hydroxyethyl)phosphonium |
| [P4442] [Cy-Suc] | Tributylethylphosphonium cyclo succinimide |
| [P2228] [CNPyr] | Triethyloctylphosphonium-2-cyanopyrrole |
| [P66614]+ | Trihexyltetradecylphosphonium |
| [P66614] [Beta-Ala] | Trihexyltetradecylphosphonium beta-alanine |
| [P66614]2[MA-Tetz] | Trihexyltetradecylphosphonium 5-(aminomethyl)-2H -tetrazole |
| [P66614] [Gly] | Trihexyltetradecylphosphonium glycine |
| [P66614] [CNPyr] | Trihexyltetradecylphosphonium-2-cyanopyrrole |
| [P66614] [NTf2] | Trihexyltetradecylphosphonium bis(trifluoromethyl)-sulfonyl)imide |
| P[[VBTMA] [PF6] | Poly-vinyl benzyl trimethylammonium hexafluorophosphate |
| [PRO] | Prolinate |
| [Py] | Pyrazole |
| Ru-Fe | Ruthenium iron |
| SerGly | Serine glycerol |
| TBAB | Tetrabutylammonium bromide |
| TBAC | Tetrabutylammonium chloride |
| TBD | Triazabicyclodecene (1,5,7-triazabicyclo[4.4.0]dec-5-ene |
| TEAC | Tetraethylammonium chloride |
| [Tf2N]− | Bis(trifluoromethylsulfonyl)imide |
| [TFO]− | Trifluoromethanesulfonate |
| TMAC | Tetramethylammonium chloride |
| [TMGH]+ | Tetramethylguanidinium |
| [TMGH] [PhO] | Tetramethylguanidinium phenol |
| [TMGH] [Im] | Tetramethylguanidinium imidazole |
| [TMGH] [Pyrr] | Tetramethylguanidinium pyrrole |
| TPAC | Tetrapropyl ammonium chloride |
| Triz | Triazole |
| V-Ac | Vinyl acetate |
| [VAL] | Valine |
| [VBTMA]+ | Vinylbenzyl trimethylammonium |
| [VBTMA] [Ala] | Vinylbenzyl trimethylammonium alanine |
| [VBTMA] [Pro] | Vinylbenzyl trimethylammonium prolinate |
| [VBTMA] [Ser] | Vinylbenzyl trimethylammonium serine |
| ZnBr2 | Zinc bromide |
References
- Treut, H.L.; Somerville, R.; Cubasch, U.; Ding, Y.; Mauritzen, C.; Mokssit, A.; Peterson, T.; Prather, M.; Solomon, S. Historical Overview of Climate Change, the Physical Science Basis; Cambridge University Press: Cambridge, UK, 2007; pp. 93–127. [Google Scholar]
- Aggarwal, R.K.; Markanda, S. Effect of greenhouse gases and human population in global warming. Huanjing Gongcheng Jishu Xuebao 2013, 2, 13–16. [Google Scholar]
- Mitchell, J.F.B. The “greenhouse” effect and climate change. Rev. Geophys. 1989, 27, 115–139. [Google Scholar] [CrossRef]
- Wuebbles, D.J.; Jain, A.K. Concerns about climate change and the role of fossil fuel use. Fuel Process. Technol. 2001, 71, 99–119. [Google Scholar] [CrossRef]
- Mendelsohn, R.; Dinar, A.; Williams, L. The distributional impact of climate change on rich and poor countries. Environ. Dev. Econ. 2006, 11, 159–178. [Google Scholar] [CrossRef] [Green Version]
- Shukla, P.R.; Skea, J.; Slade, R.; Al Khourdajie, A.; van Diemen, R.; McCollum, D.; Pathak, M.; Some, S.; Vyas, P.; Fradera, R.J.C.; et al. Climate Change 2022: Mitigation of Climate Change; Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Quadrelli, R.; Peterson, S. The energy–climate challenge: Recent trends in CO2 emissions from fuel combustion. Energy Policy 2007, 35, 5938–5952. [Google Scholar] [CrossRef]
- Ali, S.A.; Shah, S.N.; Shah, M.U.H.; Younas, M. Synthesis and performance evaluation of copper and magnesium-based metal organic framework supported ionic liquid membrane for CO2/N2 separation. Chemosphere 2022, 311, 136913. [Google Scholar] [CrossRef]
- Metz, B.; Davidson, O.; De Coninck, H.; Loos, M.; Meyer, L. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Yu, K.M.K.; Curcic, I.; Gabriel, J.; Tsang, S.C.E. Recent advances in CO2 capture and utilization. ChemSusChem 2008, 1, 893–899. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon capture and utilization update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Koiwanit, J.; Piewkhaow, L.; Manuilova, A.; Chan, C.W.; Wilson, M.; Tontiwachwuthikul, P. A Comparative of Life Cycle Assessment of Post-combustion, Pre-combustion and Oxy-fuel CO2 Capture. Energy Procedia 2014, 63, 7452–7458. [Google Scholar] [CrossRef] [Green Version]
- Petrakopoulou, F.; Tsatsaronis, G. Production of hydrogen-rich fuels for pre-combustion carbon capture in power plants: A thermodynamic assessment. Int. J. Hydrogen Energy 2012, 37, 7554–7564. [Google Scholar] [CrossRef]
- Rj, B.S.; Loganathan, M.; Shantha, M.S. A review of the water gas shift reaction kinetics. Int. J. Chem. React. Eng. 2010, 8. [Google Scholar] [CrossRef]
- Ju, Y.; Oh, H.-T.; Lee, J.-C.; Lee, C.-H. Performance and dynamic behavior of sorption-enhanced water-gas shift reaction in a fluidized bed reactor for H2 production and CO2 capture. Chem. Eng. J. 2021, 410, 127414. [Google Scholar] [CrossRef]
- Dey, A.; Dash, S.K.; Mandal, B. Chapter 1—Introduction to carbon capture. In Emerging Carbon Capture Technologies; Khalid, M., Dharaskar, S.A., Sillanpää, M., Siddiqui, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–31. [Google Scholar]
- Babar, M.; Bustam, M.A.; Ali, A.; Shah Maulud, A.; Shafiq, U.; Mukhtar, A.; Shah, S.N.; Maqsood, K.; Mellon, N.; Shariff, A.M. Thermodynamic data for cryogenic carbon dioxide capture from natural gas: A review. Cryogenics 2019, 102, 85–104. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef] [Green Version]
- Spigarelli, B.P.; Kawatra, S.K. Opportunities and challenges in carbon dioxide capture. J. CO2 Util. 2013, 1, 69–87. [Google Scholar] [CrossRef]
- Shimekit, B.; Mukhtar, H. Natural gas purification technologies-major advances for CO2 separation and future directions. Adv. Nat. Gas Technol. 2012, 2012, 235–270. [Google Scholar]
- Lin, H.; White, L.S.; Lokhandwala, K.; Baker, R.W. Natural gas purification. Encycl. Membr. Sci. Technol. 2013, 1–25. [Google Scholar] [CrossRef]
- Zhang, F.; Gao, K.-X.; Meng, Y.-N.; Qi, M.; Geng, J.; Wu, Y.-T.; Zhang, Z.-B. Intensification of dimethyaminoethoxyethanol on CO2 absorption in ionic liquid of amino acid. Int. J. Greenh. Gas Control 2016, 51, 415–422. [Google Scholar] [CrossRef]
- Sarmad, S.; Mikkola, J.-P.; Ji, X. Carbon Dioxide Capture with Ionic Liquids and Deep Eutectic Solvents: A New Generation of Sorbents. ChemSusChem 2017, 10, 324–352. [Google Scholar] [CrossRef]
- Elhamarnah, Y.A.; Nasser, M.; Qiblawey, H.; Benamor, A.; Atilhan, M.; Aparicio, S. A comprehensive review on the rheological behavior of imidazolium based ionic liquids and natural deep eutectic solvents. J. Mol. Liq. 2019, 277, 932–958. [Google Scholar] [CrossRef]
- Sistla, Y.S.; Khanna, A. CO2 absorption studies in amino acid-anion based ionic liquids. Chem. Eng. J. 2015, 273, 268–276. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, G.; Wang, H.; Li, Z.; Wang, J. Tuning ionic liquids with imide-based anions for highly efficient CO2 capture through enhanced cooperations. J. CO2 Util. 2018, 28, 299–305. [Google Scholar] [CrossRef]
- Ren, H.; Lian, S.; Wang, X.; Zhang, Y.; Duan, E. Exploiting the hydrophilic role of natural deep eutectic solvents for greening CO2 capture. J. Clean. Prod. 2018, 193, 802–810. [Google Scholar] [CrossRef]
- Zhang, K.; Hou, Y.; Wang, Y.; Wang, K.; Ren, S.; Wu, W. Efficient and Reversible Absorption of CO2 by Functional Deep Eutectic Solvents. Energy Fuels 2018, 32, 7727–7733. [Google Scholar] [CrossRef]
- Hasib-ur-Rahman, M.; Siaj, M.; Larachi, F. CO2 capture in alkanolamine/room-temperature ionic liquid emulsions: A viable approach with carbamate crystallization and curbed corrosion behavior. Int. J. Greenh. Gas Control. 2012, 6, 246–252. [Google Scholar] [CrossRef]
- Lian, S.; Song, C.; Liu, Q.; Duan, E.; Ren, H.; Kitamura, Y. Recent advances in ionic liquids-based hybrid processes for CO2 capture and utilization. J. Environ. Sci. 2021, 99, 281–295. [Google Scholar] [CrossRef]
- Tang, X.; Lv, S.; Jiang, K.; Zhou, G.; Liu, X. Recent development of ionic liquid-based electrolytes in lithium-ion batteries. J. Power Sources 2022, 542, 231792. [Google Scholar] [CrossRef]
- Bardak, F.; Bardak, C.; Karaca, C.; Kose, E.; Bilgili, S.; Atac, A. Anionic dependency of electronic and nonlinear optical properties of ionic liquids. J. Mol. Liq. 2022, 345, 117030. [Google Scholar] [CrossRef]
- Richu; Sharmhal, A.; Kumar, A. Insights into the applications and prospects of ionic liquids towards the chemistry of biomolecules. J. Mol. Liq. 2022, 368, 120580. [Google Scholar] [CrossRef]
- Forsyth, S.A.; Pringle, J.M.; MacFarlane, D.R. Ionic liquids—An overview. Aust. J. Chem. 2004, 57, 113–119. [Google Scholar] [CrossRef]
- Lodge, T.P.; Ueki, T. Mechanically Tunable, Readily Processable Ion Gels by Self-Assembly of Block Copolymers in Ionic Liquids. Acc. Chem. Res. 2016, 49, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Anderson, J.L.; Smith, E.A. Determination of Infinite Dilution Activity Coefficients of Molecular Solutes in Ionic Liquids and Deep Eutectic Solvents by Factorization-Machine-Based Neural Networks. ACS Sustain. Chem. Eng. 2022, 10, 13927–13935. [Google Scholar] [CrossRef]
- Naz, S.; Uroos, M. Ionic Liquids Based Processing of Renewable and Sustainable Biopolymers. In Biofibers and Biopolymers for Biocomposites: Synthesis, Characterization and Properties; Khan, A., Mavinkere Rangappa, S., Siengchin, S., Asiri, A.M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 181–207. [Google Scholar]
- Tateishi-Karimata, H.; Sugimoto, N. Structure, stability and behaviour of nucleic acids in ionic liquids. Nucleic Acids Res. 2014, 42, 8831–8844. [Google Scholar] [CrossRef] [PubMed]
- Acidi, A.; Hasib-ur-Rahman, M.; Larachi, F.; Abbaci, A. Ionic liquids [EMIM] [BF4],[EMIM] [Otf] and [BMIM] [Otf] as corrosion inhibitors for CO2 capture applications. Korean J. Chem. Eng. 2014, 31, 1043–1048. [Google Scholar] [CrossRef]
- Ramdin, M.; de Loos, T.W.; Vlugt, T.J.H. State-of-the-art of CO2 capture with ionic liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Galán Sánchez, L.M.; Meindersma, G.W.; de Haan, A.B. Solvent Properties of Functionalized Ionic Liquids for CO2 Absorption. Chem. Eng. Res. Des. 2007, 85, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Pinto, A.M.; Rodríguez, H.; Arce, A.; Soto, A. Combined physical and chemical absorption of carbon dioxide in a mixture of ionic liquids. J. Chem. Thermodyn. 2014, 77, 197–205. [Google Scholar] [CrossRef]
- Gurkan, B.; Goodrich, B.F.; Mindrup, E.M.; Ficke, L.E.; Massel, M.; Seo, S.; Senftle, T.P.; Wu, H.; Glaser, M.F.; Shah, J.K.; et al. Molecular Design of High Capacity, Low Viscosity, Chemically Tunable Ionic Liquids for CO2 Capture. J. Phys. Chem. Lett. 2010, 1, 3494–3499. [Google Scholar] [CrossRef]
- Chaipojjana, K.; Kiatkitipong, W. Utilization of Sugarcane Bagasse as a Co-Catalyst with Potassium Iodide for CO2 Conversion to Cyclic Carbonate. Master’s Thesis, Silpakorn University, Bangkok, Thailand, 2018. [Google Scholar]
- Karadas, F.; Atilhan, M.; Aparicio, S. Review on the Use of Ionic Liquids (ILs) as Alternative Fluids for CO2 Capture and Natural Gas Sweetening. Energy Fuels 2010, 24, 5817–5828. [Google Scholar] [CrossRef]
- Raveendran, P.; Wallen, S.L. Exploring CO2-philicity: Effects of stepwise fluorination. J. Phys. Chem. B 2003, 107, 1473–1477. [Google Scholar] [CrossRef]
- Tonner, R.; Frenking, G. Divalent carbon (0) chemistry, part 2: Protonation and complexes with main group and transition metal Lewis acids. Chem.—Eur. J. 2008, 14, 3273–3289. [Google Scholar] [CrossRef] [PubMed]
- Kilic, S.; Michalik, S.; Wang, Y.; Johnson, J.K.; Enick, R.M.; Beckman, E.J. Effect of Grafted Lewis Base Groups on the Phase Behavior of Model Poly(dimethyl siloxanes) in CO2. Ind. Eng. Chem. Res. 2003, 42, 6415–6424. [Google Scholar] [CrossRef]
- Muldoon, M.J.; Aki, S.N.V.K.; Anderson, J.L.; Dixon, J.K.; Brennecke, J.F. Improving carbon dioxide solubility in ionic liquids. J. Phys. Chem. B 2007, 111, 9001–9009. [Google Scholar] [CrossRef] [PubMed]
- Yokozeki, A.; Shiflett, M.B.; Junk, C.P.; Grieco, L.M.; Foo, T. Physical and chemical absorptions of carbon dioxide in room-temperature ionic liquids. J. Phys. Chem. B 2008, 112, 16654–16663. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhang, X.; Bai, L.; Zhang, X.; Wang, H.; Wang, J.; Bao, D.; Li, M.; Liu, X.; Zhang, S. Ionic-Liquid-Based CO2 Capture Systems: Structure, Interaction and Process. Chem. Rev. 2017, 117, 9625–9673. [Google Scholar] [CrossRef]
- Puxty, G.; Rowland, R.; Allport, A.; Yang, Q.; Bown, M.; Burns, R.; Maeder, M.; Attalla, M. Carbon dioxide postcombustion capture: A novel screening study of the carbon dioxide absorption performance of 76 amines. Environ. Sci. Technol. 2009, 43, 6427–6433. [Google Scholar] [CrossRef] [PubMed]
- Orlov, A.A.; Valtz, A.; Coquelet, C.; Rozanska, X.; Wimmer, E.; Marcou, G.; Horvath, D.; Poulain, B.; Varnek, A.; de Meyer, F. Computational screening methodology identifies effective solvents for CO2 capture. Commun. Chem. 2022, 5, 37. [Google Scholar] [CrossRef]
- Zheng, S.; Zeng, S.; Li, Y.; Bai, L.; Bai, Y.; Zhang, X.; Liang, X.; Zhang, S. State of the art of ionic liquid-modified adsorbents for CO2 capture and separation. AIChE J. 2022, 68, e17500. [Google Scholar] [CrossRef]
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Palomar, J. Process analysis overview of ionic liquids on CO2 chemical capture. Chem. Eng. J. 2020, 390, 124509. [Google Scholar] [CrossRef]
- Chen, F.-F.; Huang, K.; Fan, J.-P.; Tao, D.-J. Chemical solvent in chemical solvent: A class of hybrid materials for effective capture of CO2. AIChE J. 2018, 64, 632–639. [Google Scholar] [CrossRef]
- Supasitmongkol, S.; Styring, P. High CO2 solubility in ionic liquids and a tetraalkylammonium-based poly(ionic liquid). Energy Environ. Sci. 2010, 3, 1961–1972. [Google Scholar] [CrossRef]
- Jing, G.; Qian, Y.; Zhou, X.; Lv, B.; Zhou, Z. Designing and Screening of Multi-Amino-Functionalized Ionic Liquid Solution for CO2 Capture by Quantum Chemical Simulation. ACS Sustain. Chem. Eng. 2018, 6, 1182–1191. [Google Scholar] [CrossRef]
- Luo, X.Y.; Lv, X.Y.; Shi, G.L.; Meng, Q.; Li, H.R.; Wang, C.M. Designing amino-based ionic liquids for improved carbon capture: One amine binds two CO2. AIChE J. 2019, 65, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Zhang, X.; Tu, Z.; Wu, Y.; Hu, X. Low-viscous diamino protic ionic liquids with fluorine-substituted phenolic anions for improving CO2 reversible capture. J. Mol. Liq. 2018, 268, 617–624. [Google Scholar] [CrossRef]
- Tangqiumei, S.; Bonilla, G.M.A.; Morales-Collazo, O.; Lubben, M.J.; Brennecke, J.F. Recyclability of Encapsulated Ionic Liquids for Post-Combustion CO₂ Capture. Ind. Eng. Chem. Process Des. Dev. 2019, 58, 4997–5007. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, G.; Zhao, Y.; Wang, H.; Li, Z.; Dai, S.; Wang, J. Preorganization and Cooperation for Highly Efficient and Reversible Capture of Low-Concentration CO2 by Ionic Liquids. Angew. Chem. Int. Ed. 2017, 56, 13293–13297. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, Z.; Zhang, Z.; Zeng, S.; Li, F.; Zhang, X.; Nie, Y.; Zhang, L.; Zhang, S.; Ji, X. Ionic liquids/deep eutectic solvents for CO2 capture: Reviewing and evaluating. Green Energy Environ. 2021, 6, 314–328. [Google Scholar] [CrossRef]
- Lv, B.; Wu, J.; Lin, C.; Zhou, Z.; Jing, G. Kinetic and heat duty study of aprotic heterocyclic anion-based dual functionalized ionic liquid solutions for carbon capture. Fuel 2020, 263, 116676. [Google Scholar] [CrossRef]
- Luo, X.-Y.; Chen, X.-Y.; Qiu, R.-X.; Pei, B.-Y.; Wei, Y.; Hu, M.; Lin, J.-Q.; Zhang, J.-Y.; Luo, G.-G. Enhanced CO2 capture by reducing cation–anion interactions in hydroxyl-pyridine anion-based ionic liquids. Dalton Trans. 2019, 48, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- Altamash, T.; Haimour, T.S.; Tarsad, M.A.; Anaya, B.; Ali, M.H.; Aparicio, S.; Atilhan, M. Carbon Dioxide Solubility in Phosphonium-, Ammonium-, Sulfonyl-, and Pyrrolidinium-Based Ionic Liquids and their Mixtures at Moderate Pressures up to 10 bar. J. Chem. Eng. Data 2017, 62, 1310–1317. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Scientific Basis for Integrating Carbon Resource Processing, Utilization, and Recycling. Angew. Chem. Int. Ed. 2013, 52, 9620–9633. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mu, T. Conversion of CO2 to value-added products mediated by ionic liquids. Green Chem. 2019, 21, 2544–2574. [Google Scholar] [CrossRef]
- Sun, L.; Ramesha, G.K.; Kamat, P.V.; Brennecke, J.F. Switching the reaction course of electrochemical CO2 reduction with ionic liquids. Langmuir 2014, 30, 6302–6308. [Google Scholar] [CrossRef]
- Lopes, E.J.C.; Ribeiro, A.P.C.; Martins, L.M. New trends in the conversion of CO2 to cyclic carbonates. Catalysts 2020, 10, 479. [Google Scholar] [CrossRef]
- Peng, J.; Deng, Y. Cycloaddition of carbon dioxide to propylene oxide catalyzed by ionic liquids. New J. Chem. 2001, 25, 639–641. [Google Scholar] [CrossRef]
- Kawanami, H.; Sasaki, A.; Matsui, K.; Ikushima, Y. A rapid and effective synthesis of propylene carbonate using a supercritical CO2–ionic liquid system. Chem. Commun. 2003, 896–897. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Q.; Yue, X.-D.; Cai, F.; He, L.-N. Solventless synthesis of cyclic carbonates from carbon dioxide and epoxides catalyzed by silica-supported ionic liquids under supercritical conditions. Catal. Commun. 2007, 8, 167–172. [Google Scholar] [CrossRef]
- Fatima, S.S.; Borhan, A.; Ayoub, M.; Abd Ghani, N. Development and progress of functionalized silica-based adsorbents for CO2 capture. J. Mol. Liq. 2021, 338, 116913. [Google Scholar] [CrossRef]
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of ionic liquids with membrane technology: A new approach for CO2 separation. J. Membr. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Zhu, J.; He, B.; Huang, J.; Li, C.; Ren, T. Effect of immobilization methods and the pore structure on CO2 separation performance in silica-supported ionic liquids. Microporous Mesoporous Mater. 2018, 260, 190–200. [Google Scholar] [CrossRef]
- Voskian, S.; Brown, P.; Halliday, C.; Rajczykowski, K.; Hatton, T.A. Amine-Based Ionic Liquid for CO2 Capture and Electrochemical or Thermal Regeneration. ACS Sustain. Chem. Eng. 2020, 8, 8356–8361. [Google Scholar] [CrossRef]
- Hu, J.; Ma, J.; Liu, H.; Qian, Q.; Xie, C.; Han, B. Dual-ionic liquid system: An efficient catalyst for chemical fixation of CO2 to cyclic carbonates under mild conditions. Green Chem. 2018, 20, 2990–2994. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, W.; Han, B.; Dong, Z.; Zhao, G.; Wang, J.; Jiang, T.; Yang, G. Study on the Phase Behaviors, Viscosities, and Thermodynamic Properties of CO2/[C4mim] [PF6]/Methanol System at Elevated Pressures. Chem.—Eur. J. 2003, 9, 3897–3903. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Li, Z.; Wang, H.; Fan, M.; Wang, J. Efficient Ionic-Liquid-Promoted Chemical Fixation of CO2 into α-Alkylidene Cyclic Carbonates. ChemSusChem 2017, 10, 1120–1127. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, H.-Y.; Fukaya, N.; Yasuda, H.; Choi, J.-C. Direct synthesis of carbamate from CO2 using a task-specific ionic liquid catalyst. Green Chem. 2017, 19, 5614–5624. [Google Scholar] [CrossRef]
- Qadir, M.I.; Weilhard, A.; Fernandes, J.A.; de Pedro, I.; Vieira, B.J.C.; Waerenborgh, J.C.; Dupont, J. Selective Carbon Dioxide Hydrogenation Driven by Ferromagnetic RuFe Nanoparticles in Ionic Liquids. ACS Catal. 2018, 8, 1621–1627. [Google Scholar] [CrossRef]
- Melo, C.I.; Szczepańska, A.; Bogel-Łukasik, E.; Nunes da Ponte, M.; Branco, L.C. Hydrogenation of Carbon Dioxide to Methane by Ruthenium Nanoparticles in Ionic Liquid. ChemSusChem 2016, 9, 1081–1084. [Google Scholar] [CrossRef]
- Shi, F.; Deng, Y.; SiMa, T.; Peng, J.; Gu, Y.; Qiao, B. Alternatives to phosgene and carbon monoxide: Synthesis of symmetric urea derivatives with carbon dioxide in ionic liquids. Angew. Chem. 2003, 115, 3379–3382. [Google Scholar] [CrossRef]
- Jiang, T.; Ma, X.; Zhou, Y.; Liang, S.; Zhang, J.; Han, B. Solvent-free synthesis of substituted ureas from CO2 and amines with a functional ionic liquid as the catalyst. Green Chem. 2008, 10, 465–469. [Google Scholar] [CrossRef]
- Huang, K.; Chen, F.-F.; Tao, D.-J.; Dai, S. Ionic liquid–formulated hybrid solvents for CO2 capture. Curr. Opin. Green Sustain. Chem. 2017, 5, 67–73. [Google Scholar] [CrossRef]
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Ferro, V.R.; Palomar, J. Techno-economic feasibility of ionic liquids-based CO2 chemical capture processes. Chem. Eng. J. 2021, 407, 127196. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, Z. Dicationic Imizadolium-Based Tetrafluoroborate Ionic Liquids: Synthesis and Hydrothermal Stability Study. ChemistrySelect 2022, 7, e202201799. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Reichert, W.M.; Swatloski, R.P.; Broker, G.A.; Pitner, W.R.; Seddon, K.R.; Rogers, R.D. Efficient, halide free synthesis of new, low cost ionic liquids: 1,3-dialkylimidazolium salts containing methyl- and ethyl-sulfate anions. Green Chem. 2002, 4, 407–413. [Google Scholar] [CrossRef]
- Sohaib, Q.; Vadillo, J.M.; Gómez-Coma, L.; Albo, J.; Druon-Bocquet, S.; Irabien, A.; Sanchez-Marcano, J. CO2 capture with room temperature ionic liquids; coupled absorption/desorption and single module absorption in membrane contactor. Chem. Eng. Sci. 2020, 223, 115719. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chem. Eng. 2021, 2, 174–186. [Google Scholar] [CrossRef]
- Blasucci, V.; Hart, R.; Mestre, V.L.; Hahne, D.J.; Burlager, M.; Huttenhower, H.; Thio, B.J.R.; Pollet, P.; Liotta, C.L.; Eckert, C.A. Single component, reversible ionic liquids for energy applications. Fuel 2010, 89, 1315–1319. [Google Scholar] [CrossRef]
- Hasib-ur-Rahman, M.; Siaj, M.; Larachi, F. Ionic liquids for CO2 capture—Development and progress. Chem. Eng. Process. Process Intensif. 2010, 49, 313–322. [Google Scholar] [CrossRef]
- Vega, F.; Baena-Moreno, F.M.; Gallego Fernández, L.M.; Portillo, E.; Navarrete, B.; Zhang, Z. Current status of CO2 chemical absorption research applied to CCS: Towards full deployment at industrial scale. Appl. Energy 2020, 260, 114313. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J.; Du, Z.; Abdeltawab, A.A.; Al-Enizi, A.M.; Chen, X.; Yu, G. A process simulation study of CO2 capture by ionic liquids. Int. J. Greenh. Gas Control 2017, 58, 223–231. [Google Scholar] [CrossRef]
- de Riva, J.; Suarez-Reyes, J.; Moreno, D.; Díaz, I.; Ferro, V.; Palomar, J. Ionic liquids for post-combustion CO2 capture by physical absorption: Thermodynamic, kinetic and process analysis. Int. J. Greenh. Gas Control 2017, 61, 61–70. [Google Scholar] [CrossRef]
- Mota-Martinez, M.T.; Brandl, P.; Hallett, J.P.; Mac Dowell, N. Challenges and opportunities for the utilisation of ionic liquids as solvents for CO2 capture. Mol. Syst. Des. Eng. 2018, 3, 560–571. [Google Scholar] [CrossRef]
- García-Gutiérrez, P.; Jacquemin, J.; McCrellis, C.; Dimitriou, I.; Taylor, S.F.R.; Hardacre, C.; Allen, R.W.K. Techno-Economic Feasibility of Selective CO2 Capture Processes from Biogas Streams Using Ionic Liquids as Physical Absorbents. Energy Fuels 2016, 30, 5052–5064. [Google Scholar] [CrossRef] [Green Version]
- Akinola, T.E.; Oko, E.; Wang, M. Study of CO2 removal in natural gas process using mixture of ionic liquid and MEA through process simulation. Fuel 2019, 236, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhang, X.; Zhang, X.; Dong, H.; Zhang, S. Thermodynamic Modeling and Assessment of Ionic Liquid-Based CO2 Capture Processes. Ind. Eng. Chem. Res. 2014, 53, 11805–11817. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Drew, D.W.; Cantini, R.A.; Yokozeki, A. Carbon Dioxide Capture Using Ionic Liquid 1-Butyl-3-methylimidazolium Acetate. Energy Fuels 2010, 24, 5781–5789. [Google Scholar] [CrossRef]
- Chen, L.; Sharifzadeh, M.; Mac Dowell, N.; Welton, T.; Shah, N.; Hallett, J.P. Inexpensive ionic liquids:[HSO 4]−-based solvent production at bulk scale. Green Chem. 2014, 16, 3098–3106. [Google Scholar] [CrossRef] [Green Version]
- Meindersma, G.W.; de Haan, A.B. Conceptual process design for aromatic/aliphatic separation with ionic liquids. Chem. Eng. Res. Des. 2008, 86, 745–752. [Google Scholar] [CrossRef]
- Liu, Y.; Han, W.; Xu, Z.; Fan, W.; Peng, W.; Luo, S. Comparative toxicity of pristine graphene oxide and its carboxyl, imidazole or polyethylene glycol functionalized products to Daphnia magna: A two generation study. Environ. Pollut. 2018, 237, 218–227. [Google Scholar] [CrossRef]
- Wan, R.; Xia, X.; Wang, P.; Huo, W.; Dong, H.; Chang, Z. Toxicity of imidazoles ionic liquid [C16mim]Cl to HepG2 cells. Toxicol. In Vitro 2018, 52, 1–7. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Ashraf, M.; AlMayef, T.; Chawla, M.; Poater, A.; Cavallo, L. Amino acid ionic liquids as potential candidates for CO2 capture: Combined density functional theory and molecular dynamics simulations. Chem. Phys. Lett. 2020, 745, 137239. [Google Scholar] [CrossRef]
- Baghban, A.; Mohammadi, A.H.; Taleghani, M.S. Rigorous modeling of CO2 equilibrium absorption in ionic liquids. Int. J. Greenh. Gas Control 2017, 58, 19–41. [Google Scholar] [CrossRef]
- Haider, J.; Saeed, S.; Qyyum, M.A.; Kazmi, B.; Ahmad, R.; Muhammad, A.; Lee, M. Simultaneous capture of acid gases from natural gas adopting ionic liquids: Challenges, recent developments, and prospects. Renew. Sustain. Energy Rev. 2020, 123, 109771. [Google Scholar] [CrossRef]
- Solangi, N.H.; Anjum, A.; Tanjung, F.A.; Mazari, S.A.; Mubarak, N.M. A review of recent trends and emerging perspectives of ionic liquid membranes for CO2 separation. J. Environ. Chem. Eng. 2021, 9, 105860. [Google Scholar] [CrossRef]
- Gür, T.M. Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Ma, C.; Sarmad, S.; Mikkola, J.-P.; Ji, X. Development of Low-Cost Deep Eutectic Solvents for CO2 Capture. Energy Procedia 2017, 142, 3320–3325. [Google Scholar] [CrossRef]
- Chen, W.; Xue, Z.; Wang, J.; Jiang, J.; Zhao, X.; Mu, T. Investigation on the thermal stability of deep eutectic solvents. Acta Phys. Chim. Sin. 2018, 34, 904–911. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Aissaoui, T.; AlNashef, I.M.; Qureshi, U.A.; Benguerba, Y. Potential applications of deep eutectic solvents in natural gas sweetening for CO2 capture. Rev. Chem. Eng. 2017, 33, 523–550. [Google Scholar] [CrossRef]
- Ghaedi, H.; Ayoub, M.; Sufian, S.; Shariff, A.M.; Hailegiorgis, S.M.; Khan, S.N. CO2 capture with the help of Phosphonium-based deep eutectic solvents. J. Mol. Liq. 2017, 243, 564–571. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Lee, J.H.; Lee, H.J.; Jeong, Y.K.; Choi, J.W. Deep eutectic solvents as attractive media for CO2 capture. Green Chem. 2016, 18, 2834–2842. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, S.; Hou, Y.; Wu, W. Capture of Acidic Gases from Flue Gas by Deep Eutectic Solvents. Processes 2021, 9, 1268. [Google Scholar] [CrossRef]
- Mukesh, C.; Khokarale, S.G.; Virtanen, P.; Mikkola, J.-P. Rapid desorption of CO2 from deep eutectic solvents based on polyamines at lower temperatures: An alternative technology with industrial potential. Sustain. Energy Fuels 2019, 3, 2125–2134. [Google Scholar] [CrossRef]
- Adeyemi, I.; Abu-Zahra, M.R.M.; Alnashef, I. Experimental Study of the Solubility of CO2 in Novel Amine Based Deep Eutectic Solvents. Energy Procedia 2017, 105, 1394–1400. [Google Scholar] [CrossRef]
- Sarmad, S.; Nikjoo, D.; Mikkola, J.-P. Amine functionalized deep eutectic solvent for CO2 capture: Measurements and modeling. J. Mol. Liq. 2020, 309, 113159. [Google Scholar] [CrossRef]
- Haider, M.B.; Jha, D.; Marriyappan Sivagnanam, B.; Kumar, R. Thermodynamic and Kinetic Studies of CO2 Capture by Glycol and Amine-Based Deep Eutectic Solvents. J. Chem. Eng. Data 2018, 63, 2671–2680. [Google Scholar] [CrossRef]
- Cao, L.; Huang, J.; Zhang, X.; Zhang, S.; Gao, J.; Zeng, S. Imidazole tailored deep eutectic solvents for CO2 capture enhanced by hydrogen bonds. Phys. Chem. Chem. Phys. 2015, 17, 27306–27316. [Google Scholar] [CrossRef]
- Cui, G.; Lv, M.; Yang, D. Efficient CO2 absorption by azolide-based deep eutectic solvents. Chem. Commun. 2019, 55, 1426–1429. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shen, Y.; Shen, L.; Sun, C.; Chen, L.; Wang, Q.; Li, S.; Li, W. Novel Amino-Functionalized Ionic Liquid/Organic Solvent with Low Viscosity for CO2 Capture. Environ. Sci. Technol. 2020, 54, 3520–3529. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-Y.; Penley, D.; Klemm, A.; Dean, W.; Gurkan, B. Deep Eutectic Solvent Formed by Imidazolium Cyanopyrrolide and Ethylene Glycol for Reactive CO2 Separations. ACS Sustain. Chem. Eng. 2021, 9, 1090–1098. [Google Scholar] [CrossRef]
- Kaljurand, I.; Koppel, I.A.; Kütt, A.; Rõõm, E.-I.; Rodima, T.; Koppel, I.; Mishima, M.; Leito, I. Experimental Gas-Phase Basicity Scale of Superbasic Phosphazenes. J. Phys. Chem. A 2007, 111, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Baker, G.A.; Lee, J.S.; Pagni, R.M.; Dai, S. Ultrastable Superbase-Derived Protic Ionic Liquids. J. Phys. Chem. B 2009, 113, 4181–4183. [Google Scholar] [CrossRef] [PubMed]
- Sze, L.L.; Pandey, S.; Ravula, S.; Pandey, S.; Zhao, H.; Baker, G.A.; Baker, S.N. Ternary Deep Eutectic Solvents Tasked for Carbon Dioxide Capture. ACS Sustain. Chem. Eng. 2014, 2, 2117–2123. [Google Scholar] [CrossRef]
- Jiang, B.; Ma, J.; Yang, N.; Huang, Z.; Zhang, N.; Tantai, X.; Sun, Y.; Zhang, L. Superbase/Acylamido-Based Deep Eutectic Solvents for Multiple-Site Efficient CO2 Absorption. Energy Fuels 2019, 33, 7569–7577. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, Z.; Zhang, H.; Ma, J.; Jiang, B.; Zhang, L. Highly Efficient and Reversible CO2 Capture by Task-Specific Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2019, 58, 13321–13329. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, L.; Bai, Y.; Li, F.; Dong, H.; Wang, H.; Zhang, X.; Zeng, S. Superbase Ionic Liquid-Based Deep Eutectic Solvents for Improving CO2 Absorption. ACS Sustain. Chem. Eng. 2020, 8, 2523–2530. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mikkola, J.-P. Intermolecular interactions upon carbon dioxide capture in deep-eutectic solvents. Phys. Chem. Chem. Phys. 2018, 20, 24591–24601. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hou, M.; Han, B.; Wang, X.; Zou, L. Solubility of CO2 in a Choline Chloride + Urea Eutectic Mixture. J. Chem. Eng. Data 2008, 53, 548–550. [Google Scholar] [CrossRef]
- Leron, R.B.; Li, M.-H. Solubility of carbon dioxide in a eutectic mixture of choline chloride and glycerol at moderate pressures. J. Chem. Thermodyn. 2013, 57, 131–136. [Google Scholar] [CrossRef]
- Sarmad, S.; Xie, Y.; Mikkola, J.-P.; Ji, X. Screening of deep eutectic solvents (DESs) as green CO2 sorbents: From solubility to viscosity. New J. Chem. 2017, 41, 290–301. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, H.; Song, Z.; Chen, L.; Deng, L.; Qi, Z. Carbon dioxide solubility in phosphonium-based deep eutectic solvents: An experimental and molecular dynamics study. Ind. Eng. Chem. Res. 2019, 58, 17514–17523. [Google Scholar] [CrossRef]
- Wilm, L.F.B.; Das, M.; Janssen-Müller, D.; Mück-Lichtenfeld, C.; Glorius, F.; Dielmann, F. Photoswitchable Nitrogen Superbases: Using Light for Reversible Carbon Dioxide Capture. Angew. Chem. Int. Ed. 2022, 61, e202112344. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Huang, X.; Yang, D.; Wu, C.; Chen, J. Deep eutectic solvents composed of bio-phenol-derived superbase ionic liquids and ethylene glycol for CO2 capture. Chem. Commun. 2022, 58, 2160–2163. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Luo, H.; Luo, X.; Li, H.; Dai, S. Equimolar CO2 capture by imidazolium-based ionic liquids and superbase systems. Green Chem. 2010, 12, 2019–2023. [Google Scholar] [CrossRef]
- Jędrzejczak, P.; Collins, M.N.; Jesionowski, T.; Klapiszewski, Ł. The role of lignin and lignin-based materials in sustainable construction—A comprehensive review. Int. J. Biol. Macromol. 2021, 187, 624–650. [Google Scholar] [CrossRef] [PubMed]
- Bhawna; Pandey, A.; Pandey, S. Superbase-Added Choline Chloride-Based Deep Eutectic Solvents for CO2 Capture and Sequestration. ChemistrySelect 2017, 2, 11422–11430. [Google Scholar] [CrossRef]
- Chen, Y.; Ai, N.; Li, G.; Shan, H.; Cui, Y.; Deng, D. Solubilities of Carbon Dioxide in Eutectic Mixtures of Choline Chloride and Dihydric Alcohols. J. Chem. Eng. Data 2014, 59, 1247–1253. [Google Scholar] [CrossRef]
- Lu, M.; Han, G.; Jiang, Y.; Zhang, X.; Deng, D.; Ai, N. Solubilities of carbon dioxide in the eutectic mixture of levulinic acid (or furfuryl alcohol) and choline chloride. J. Chem. Thermodyn. 2015, 88, 72–77. [Google Scholar] [CrossRef]
- Li, G.; Deng, D.; Chen, Y.; Shan, H.; Ai, N. Solubilities and thermodynamic properties of CO2 in choline-chloride based deep eutectic solvents. J. Chem. Thermodyn. 2014, 75, 58–62. [Google Scholar] [CrossRef]
- Leron, R.B.; Caparanga, A.; Li, M.-H. Carbon dioxide solubility in a deep eutectic solvent based on choline chloride and urea at T=303.15–343.15K and moderate pressures. J. Taiwan Inst. Chem. Eng. 2013, 44, 879–885. [Google Scholar] [CrossRef]
- Ali, E.; Hadj-Kali, M.K.; Mulyono, S.; Alnashef, I.; Fakeeha, A.; Mjalli, F.; Hayyan, A. Solubility of CO2 in deep eutectic solvents: Experiments and modelling using the Peng–Robinson equation of state. Chem. Eng. Res. Des. 2014, 92, 1898–1906. [Google Scholar] [CrossRef]
- Zubeir, L.F.; Lacroix, M.H.M.; Kroon, M.C. Low Transition Temperature Mixtures as Innovative and Sustainable CO2 Capture Solvents. J. Phys. Chem. B 2014, 118, 14429–14441. [Google Scholar] [CrossRef]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep eutectic solvents: Physicochemical properties and gas separation applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Yang, X.; Zou, Q.; Zhao, T.; Chen, P.; Liu, Z.; Liu, F.; Lin, Q. Deep Eutectic Solvents as Efficient Catalysts for Fixation of CO2 to Cyclic Carbonates at Ambient Temperature and Pressure through Synergetic Catalysis. ACS Sustain. Chem. Eng. 2021, 9, 10437–10443. [Google Scholar] [CrossRef]
- Komarova, A.O.; Dick, G.R.; Luterbacher, J.S. Diformylxylose as a new polar aprotic solvent produced from renewable biomass. Green Chem. 2021, 23, 4790–4799. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, H.; Wang, Y.; Zhao, J.; Ren, W.; Wang, B.; Qin, P.; Chen, F.; Wang, Y.; Cai, D. Non-isocyanate polyurethane from sweet potato residual and the application in food preservation. Ind. Crops Prod. 2022, 186, 115224. [Google Scholar] [CrossRef]
- Peters, R.; Decker, M.; Eggemann, L.; Schemme, S.; Schorn, F.; Breuer, J.L.; Weiske, S.; Pasel, J.; Samsun, R.C.; Stolten, D. Thermodynamic and ecological preselection of synthetic fuel intermediates from biogas at farm sites. Energy Sustain. Soc. 2020, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Yu, B.; Zhang, H.; Zhao, Y.; Chen, Y.; Ma, Z.; Ji, G.; Gao, X.; Han, B.; Liu, Z. Metalated Mesoporous Poly(triphenylphosphine) with Azo Functionality: Efficient Catalysts for CO2 Conversion. ACS Catal. 2016, 6, 1268–1273. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Villeneuve, P. Deep eutectic solvents: Synthesis, application, and focus on lipase-catalyzed reactions. Eur. J. Lipid Sci. Technol. 2013, 115, 379–385. [Google Scholar] [CrossRef]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep Eutectic Solvents: The Organic Reaction Medium of the Century. Eur. J. Org. Chem. 2016, 2016, 612–632. [Google Scholar] [CrossRef]
- Cheng, W.; Fu, Z.; Wang, J.; Sun, J.; Zhang, S. ZnBr2-Based Choline Chloride Ionic Liquid for Efficient Fixation of CO2 to Cyclic Carbonate. Synth. Commun. 2012, 42, 2564–2573. [Google Scholar] [CrossRef]
- Liu, F.; Gu, Y.; Xin, H.; Zhao, P.; Gao, J.; Liu, M. Multifunctional Phosphonium-Based Deep Eutectic Ionic Liquids: Insights into Simultaneous Activation of CO2 and Epoxide and Their Subsequent Cycloaddition. ACS Sustain. Chem. Eng. 2019, 7, 16674–16681. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, H.; Lai, S.L.; Gao, J.; Gao, L. Lignin modified by deep eutectic solvents as green, reusable, and bio-based catalysts for efficient chemical fixation of CO2. React. Funct. Polym. 2020, 149, 104502. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Nie, Y.; Zhang, X.; Ji, X. Natural Deep Eutectic Solvents Enhanced Electro-Enzymatic Conversion of CO2 to Methanol. Front. Chem. 2022, 10, 894106. [Google Scholar] [CrossRef]
- Jiang, Z.; Xiao, T.; Kuznetsov, V.Á.; Edwards, P.Á. Turning carbon dioxide into fuel. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3343–3364. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lin, Z.; Peng, S.H.; Sage, V.; Sun, Z. A critical perspective on CO2 conversions into chemicals and fuels. J. Nanosci. Nanotechnol. 2019, 19, 3097–3109. [Google Scholar] [CrossRef] [PubMed]
- Jeffry, L.; Ong, M.Y.; Nomanbhay, S.; Mofijur, M.; Mubashir, M.; Show, P.L. Greenhouse gases utilization: A review. Fuel 2021, 301, 121017. [Google Scholar] [CrossRef]
- Mondal, B.; Song, J.; Neese, F.; Ye, S. Bio-inspired mechanistic insights into CO2 reduction. Curr. Opin. Chem. Biol. 2015, 25, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, J.; Wang, J.; Chang, A. Valence-induced distortion controls the resistivity and thermal stability of Co2.77Mn1.71Fe1.10Zn0.42O8 ceramics. Mater. Des. 2020, 192, 108736. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product selectivity of photocatalytic CO2 reduction reactions. Mater. Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, Y.; Jiang, Z.; Wang, X.; Wang, X.; Zhang, S.; Han, P.; Yang, C. Enzymatic conversion of carbon dioxide. Chem. Soc. Rev. 2015, 44, 5981–6000. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Shi, J.; Wu, H.; Jiang, Z.; Zhang, W.; Song, X.; Ai, Q. Bioinspired Approach to Multienzyme Cascade System Construction for Efficient Carbon Dioxide Reduction. ACS Catal. 2014, 4, 962–972. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, F.; Nie, Y.; Zhang, X.; Zhang, S.; Ji, X. Zinc-based deep eutectic solvent—An efficient carbonic anhydrase mimic for CO2 hydration and conversion. Sep. Purif. Technol. 2021, 276, 119446. [Google Scholar] [CrossRef]
- Gautam, R.K.; Seth, D. Thermal conductivity of deep eutectic solvents. J. Therm. Anal. Calorim. 2020, 140, 2633–2640. [Google Scholar] [CrossRef]
- Shahbaz, K.; Mjalli, F.S.; Vakili-Nezhaad, G.; AlNashef, I.M.; Asadov, A.; Farid, M.M. Thermogravimetric measurement of deep eutectic solvents vapor pressure. J. Mol. Liq. 2016, 222, 61–66. [Google Scholar] [CrossRef]
- Wu, J.; Liang, Q.; Yu, X.; Lv, Q.-F.; Ma, L.; Qin, X.; Chen, G.; Li, B. Deep Eutectic Solvents for Boosting Electrochemical Energy Storage and Conversion: A Review and Perspective. Adv. Funct. Mater. 2021, 31, 2011102. [Google Scholar] [CrossRef]
- Pätzold, M.; Siebenhaller, S.; Kara, S.; Liese, A.; Syldatk, C.; Holtmann, D. Deep Eutectic Solvents as Efficient Solvents in Biocatalysis. Trends Biotechnol. 2019, 37, 943–959. [Google Scholar] [CrossRef]
- Liao, H.-G.; Jiang, Y.-X.; Zhou, Z.-Y.; Chen, S.-P.; Sun, S.-G. Shape-Controlled Synthesis of Gold Nanoparticles in Deep Eutectic Solvents for Studies of Structure–Functionality Relationships in Electrocatalysis. Angew. Chem. Int. Ed. 2008, 47, 9100–9103. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Zhu, Q.; Chen, C.; Liu, H.; Liu, Z.; Zhao, Z.; Zhang, X.; Liu, S.; Han, B. Selective electroreduction of carbon dioxide to methanol on copper selenide nanocatalysts. Nat. Commun. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.-X.; Zhao, L.-Q.; Wang, J.; Song, G.-L.; Liu, H.-M.; Cheng, H.; Yang, Z. Improving Whole-Cell Biocatalysis by Addition of Deep Eutectic Solvents and Natural Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 5713–5722. [Google Scholar] [CrossRef]
- Rahman, M.S.; Roy, R.; Montoya, C.; Halim, M.A.; Raynie, D.E. Acidic and basic amino acid-based novel deep eutectic solvents and their role in depolymerization of lignin. J. Mol. Liq. 2022, 362, 119751. [Google Scholar] [CrossRef]
- Yang, S.-L.; Duan, Z.-Q. Insight into enzymatic synthesis of phosphatidylserine in deep eutectic solvents. Catal. Commun. 2016, 82, 16–19. [Google Scholar] [CrossRef]
- Chemat, F.; You, H.J.; Muthukumar, K.; Murugesan, T. Effect of l-arginine on the physical properties of choline chloride and glycerol based deep eutectic solvents. J. Mol. Liq. 2015, 212, 605–611. [Google Scholar] [CrossRef]
- Toledo, M.L.; Pereira, M.M.; Freire, M.G.; Silva, J.P.A.; Coutinho, J.A.P.; Tavares, A.P.M. Laccase Activation in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 11806–11814. [Google Scholar] [CrossRef]
- Słupek, E.; Makoś, P.; Gębicki, J. Theoretical and economic evaluation of low-cost deep eutectic solvents for effective biogas upgrading to bio-methane. Energies 2020, 13, 3379. [Google Scholar] [CrossRef]
- Deng, D.; Deng, X.; Duan, X.; Gong, L. Protic guanidine isothiocyanate plus acetamide deep eutectic solvents with low viscosity for efficient NH3 capture and NH3/CO2 separation. J. Mol. Liq. 2021, 324, 114719. [Google Scholar] [CrossRef]
- Mulia, K.; Putri, S.; Krisanti, E.; Nasruddin. Natural deep eutectic solvents (NADES) as green solvents for carbon dioxide capture. AIP Conf. Proc. 2017, 1823, 020022. [Google Scholar]
- Siani, G.; Tiecco, M.; Di Profio, P.; Guernelli, S.; Fontana, A.; Ciulla, M.; Canale, V. Physical absorption of CO2 in betaine/carboxylic acid-based Natural Deep Eutectic Solvents. J. Mol. Liq. 2020, 315, 113708. [Google Scholar] [CrossRef]
- Alkhatib, I., II; Ferreira, M.L.; Alba, C.G.; Bahamon, D.; Llovell, F.l.; Pereiro, A.B.; Araújo, J.M.M.; Abu-Zahra, M.R.M.; Vega, L.F. Screening of ionic liquids and deep eutectic solvents for physical CO2 absorption by Soft-SAFT using key performance indicators. J. Chem. Eng. Data 2020, 65, 5844–5861. [Google Scholar] [CrossRef]
- Brennecke, J.F.; Gurkan, B.E. Ionic Liquids for CO2 Capture and Emission Reduction. J. Phys. Chem. Lett. 2010, 1, 3459–3464. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Scaccia, S.; Tizzani, C.; Alessandrini, F.; Passerini, S. Synthesis of hydrophobic ionic liquids for electrochemical applications. J. Electrochem. Soc. 2006, 153, A1685. [Google Scholar] [CrossRef]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible deep eutectic solvents based on choline chloride: Characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Development of deep eutectic solvents applied in extraction and separation. J. Sep. Sci. 2016, 39, 3505–3520. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Tomé, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–2824. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Yu, J.; Yu, H.; Liu, Y.; Hussain, A. Carbon dioxide capture using liquid absorption methods: A review. Environ. Chem. Lett. 2021, 19, 77–109. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, J.; Wang, D.; Li, X.; Meng, Q.; Zhang, Z.; An, J.; Liu, X.; Li, M. Study on the Desulfurization and Regeneration Performance of Functional Deep Eutectic Solvents. ACS Omega 2020, 5, 15353–15361. [Google Scholar] [CrossRef]
- Ahmad, N.; Lin, X.; Wang, X.; Xu, J.; Xu, X. Understanding the CO2 capture performance by MDEA-based deep eutectics solvents with excellent cyclic capacity. Fuel 2021, 293, 120466. [Google Scholar] [CrossRef]
- Li, A.; Ren, S.; Teng, C.; Liu, H.; Zhang, Q. The role of deep eutectic solvents in chiral capillary electrokinetic chromatography: A comparative study based on α-cyclodextrin chiral selector. J. Mol. Liq. 2022, 359, 119281. [Google Scholar] [CrossRef]
- Bagherzadeh, A.; Shahini, N.; Saber, D.; Yousefi, P.; Alizadeh, S.M.S.; Ahmadi, S.; Shahdost, F.T. Developing a global approach for determining the molar heat capacity of deep eutectic solvents. Measurement 2022, 188, 110630. [Google Scholar] [CrossRef]
- Abdollahzadeh, M.; Khosravi, M.; Hajipour Khire Masjidi, B.; Samimi Behbahan, A.; Bagherzadeh, A.; Shahkar, A.; Tat Shahdost, F. Estimating the density of deep eutectic solvents applying supervised machine learning techniques. Sci. Rep. 2022, 12, 4954. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Thakur, R.; Das, A.B. Effect of natural deep eutectic solvents on thermal stability, syneresis, and viscoelastic properties of high amylose starch. Int. J. Biol. Macromol. 2021, 187, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sequeira, R.A.; Pereira, M.M.; Maity, T.K.; Chudasama, N.A.; Prasad, K. Are ionic liquids and deep eutectic solvents the same?: Fundamental investigation from DNA dissolution point of view. J. Mol. Liq. 2021, 328, 115386. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, K.-q.; Chen, Q.; He, M.-y. Facile and environmentally friendly halogenation of BODIPYs in deep eutectic solvent. Dye. Pigment. 2015, 112, 274–279. [Google Scholar] [CrossRef]
- Krishnan, A.; Gopinath, K.P.; Vo, D.-V.N.; Malolan, R.; Nagarajan, V.M.; Arun, J. Ionic liquids, deep eutectic solvents and liquid polymers as green solvents in carbon capture technologies: A review. Environ. Chem. Lett. 2020, 18, 2031–2054. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Maugeri, Z.; de María, P.D.J.R.A. Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: Levulinic acid and sugar-based polyols. R. Soc. Chem. 2012, 2, 421–425. [Google Scholar] [CrossRef]
- Bernasconi, R.; Panzeri, G.; Accogli, A.; Liberale, F.; Nobili, L.; Magagnin, L. Electrodeposition from deep eutectic solvents. In Progress and Developments in Ionic Liquids; Intech Open: Rijeka, Croatia, 2017; pp. 235–261. [Google Scholar]
- Su, W.C.; Wong, D.S.H.; Li, M.H. Effect of water on solubility of carbon dioxide in (aminomethanamide + 2-hydroxy-N, N, N-trimethylethanaminium chloride). J. Chem. Eng. Data 2009, 54, 1951–1955. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Leron, R.B.; Li, M.-H. Solubility of carbon dioxide in aqueous mixtures of (reline+monoethanolamine) at T=(313.2 to 353.2)K. J. Chem. Thermodyn. 2014, 72, 94–99. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Zubeir, L.F.; Peters, C.J.; Kroon, M.C. A new low transition temperature mixture (LTTM) formed by choline chloride+lactic acid: Characterization as solvent for CO2 capture. Fluid Phase Equilibria 2013, 340, 77–84. [Google Scholar] [CrossRef]

| Absorbents | Advantages | Disadvantages | References |
|---|---|---|---|
| Biphasic solvents | Low energy consumption and low viscosity | Complex equipment | [23] |
| MEA | Low price | High volatility, corrosion, and high energy consumption | [24,25,26,27,28] |
| ILs | Nonvolatile, low corrosion, and high solubility | Biotoxicity, high price, and high viscosity | [29] |
| DESs | Nonvolatile, low corrosion, high solubility, nontoxic, low price, and biodegradable nature | High viscosity | [30] |
| Cation | Structure | Anion | Structure |
|---|---|---|---|
| Pyridinium | 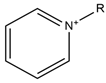 | Acetate | 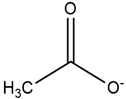 |
| Imidazolium | 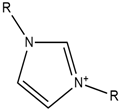 | Nitrate | 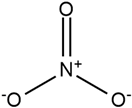 |
| Pyrrolidinium | 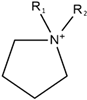 | Bis(trifluorophosphate)imide | 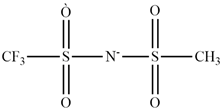 |
|
Quaternary ammonium | 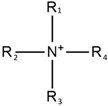 | Hexafluoro borate | 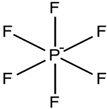 |
|
Tetra alkyl phosphonium | 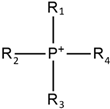 | Tetrafluoroborate | 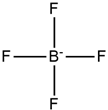 |
| Piperidinium | 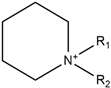 | Dicyanamide | 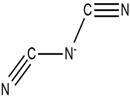 |
| Morpholinium | 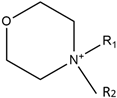 | Triflate | 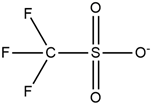 |
| Guanidinium | 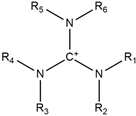 | Alkylsulfate | 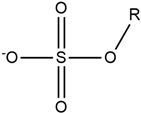 |
| Halide Salt | Structure | Hydrogen Bond | Structure |
|---|---|---|---|
| Choline chloride | 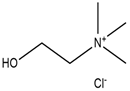 | Lactic acid | 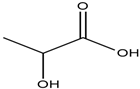 |
| Choline nitrate | 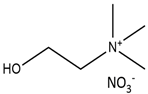 | Acetamide |  |
| Choline acetate |  | Glycerol |  |
| Ethyl ammonium chloride |  | Melic acid |  |
| N-Ethyl-2-hydroxy-N,N-dimethylethanaminium chloride | 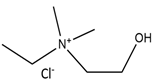 | Oxalic acid | 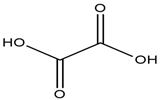 |
| 2-Chloro-N,N,N-trimethylethanaminium chloride | 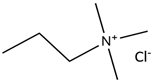 | Phenol | 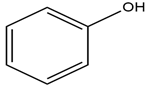 |
| Tetrabutylammonium bromide | 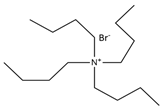 | Malonic acid | 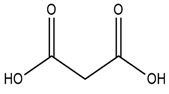 |
| N,N,N-Trimethyl(phenyl)methenamine chloride | 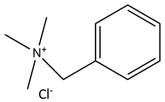 | D-Glucose |  |
| Lithium bis[(trifluoromethyl)sulfonyl] imide | 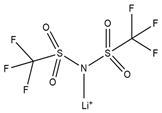 | D-Fructose |  |
| 1-Butyl-3 methyl-imidazolium chloride | 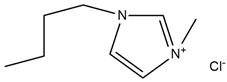 | D-Isosorbide | 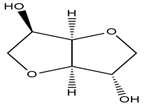 |
| Methyltriphenylphosphonium bromide | 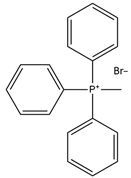 | Sorbitol |  |
| 2-(Acetyloxy)-N,N,N-trimethylethanaminium chloride | 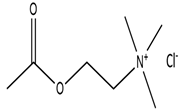 | Xylitol |  |
| Benzyltriphenylphosphonium chloride |  | Xylenol |  |
| 1-Ethyl-3-butylbenzotriazolium hexafluorophosphate |  | Trifluoro acetamide |  |
| 1-Butyl-3-methylimidazolium chloride |  | Glutaric acid | 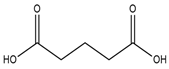 |
| Tetra propylammonium bromide | 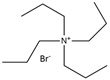 | O-Cresol | 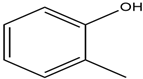 |
| 2-Fluoro-N,N,N-trimethylethanaminium bromide | 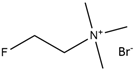 | Levulinic acid | 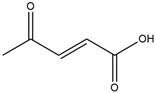 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.A.; Mulk, W.U.; Ullah, Z.; Khan, H.; Zahid, A.; Shah, M.U.H.; Shah, S.N. Recent Advances in the Synthesis, Application and Economic Feasibility of Ionic Liquids and Deep Eutectic Solvents for CO2 Capture: A Review. Energies 2022, 15, 9098. https://doi.org/10.3390/en15239098
Ali SA, Mulk WU, Ullah Z, Khan H, Zahid A, Shah MUH, Shah SN. Recent Advances in the Synthesis, Application and Economic Feasibility of Ionic Liquids and Deep Eutectic Solvents for CO2 Capture: A Review. Energies. 2022; 15(23):9098. https://doi.org/10.3390/en15239098
Chicago/Turabian StyleAli, Syed Awais, Waqad Ul Mulk, Zahoor Ullah, Haris Khan, Afrah Zahid, Mansoor Ul Hassan Shah, and Syed Nasir Shah. 2022. "Recent Advances in the Synthesis, Application and Economic Feasibility of Ionic Liquids and Deep Eutectic Solvents for CO2 Capture: A Review" Energies 15, no. 23: 9098. https://doi.org/10.3390/en15239098








