Rapid Prediction of Retired Ni-MH Batteries Capacity Based on Reliable Multi-Parameter Driven Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Capacity Testing
2.2. Parameter Acquisition Test
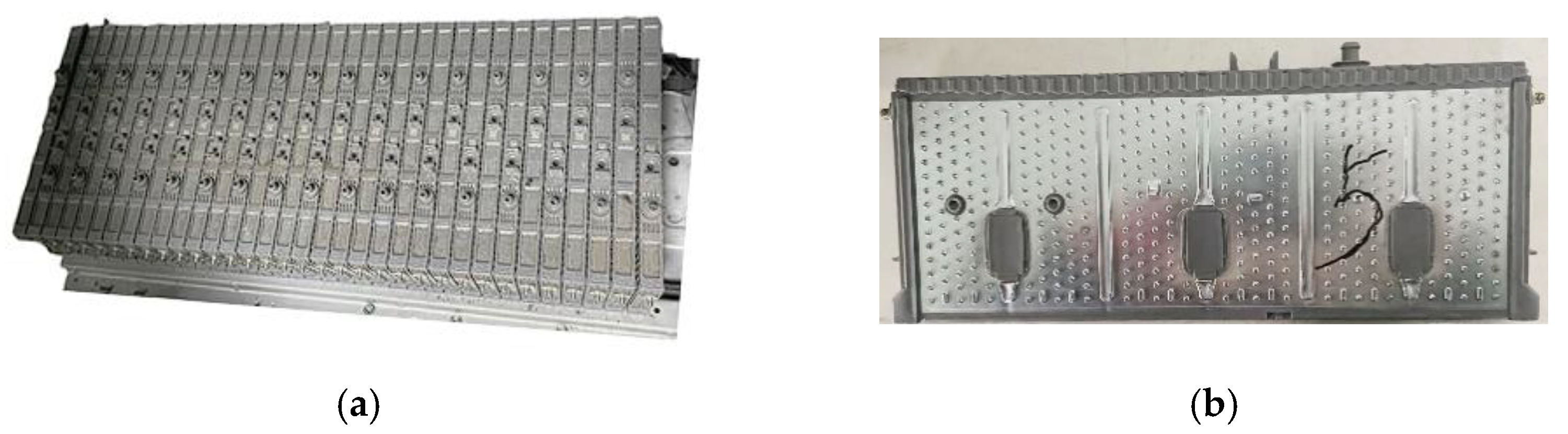
2.2.1. Difference in Volume Expansion Thickness
2.2.2. AC Internal Resistance and Terminal Voltage
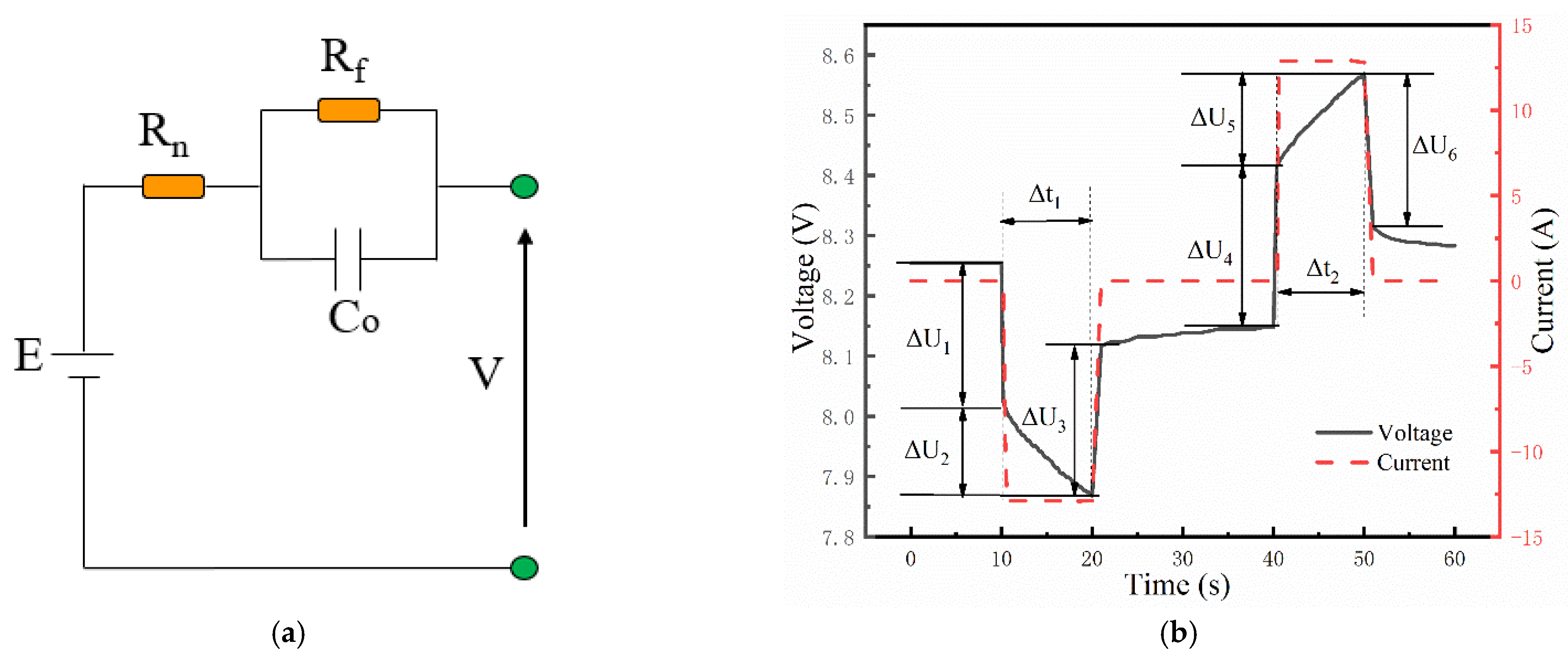
2.2.3. Pulse Test
3. Results and Discussion
3.1. Analysis of Charging and Discharging Results
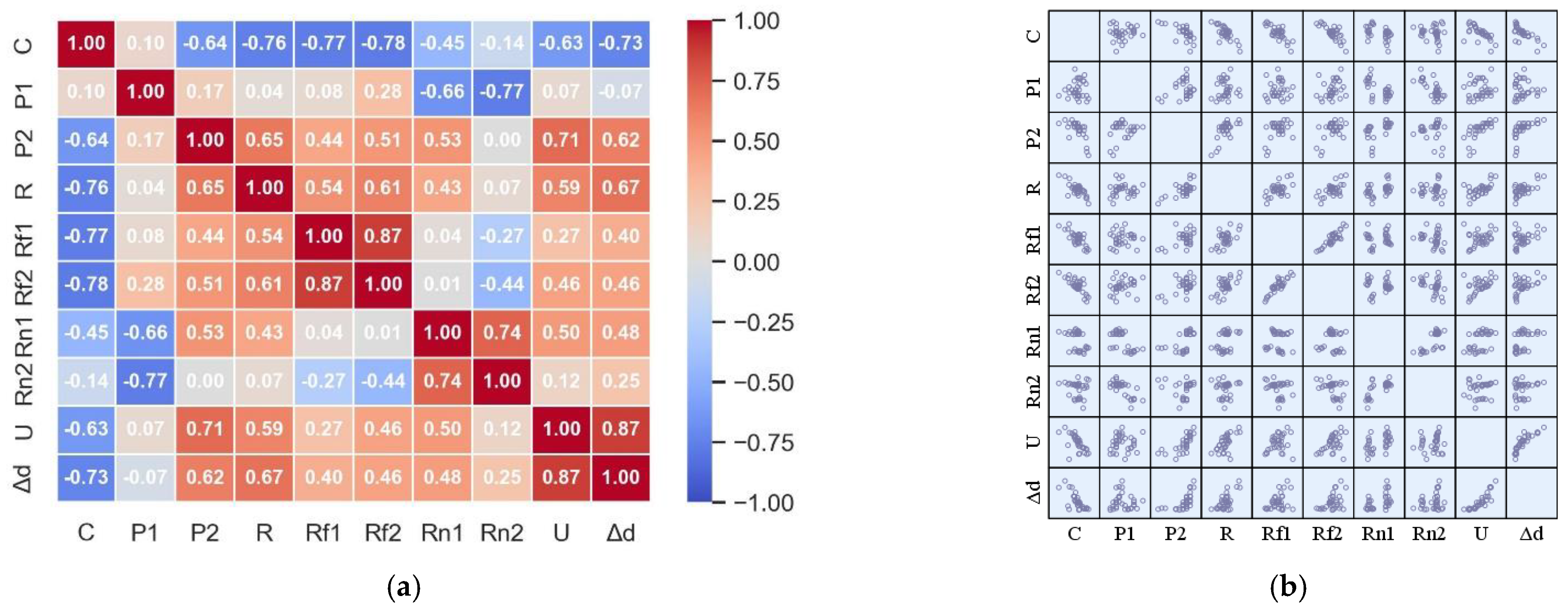
3.2. Multiparameter Correlation Extraction Results
3.3. Same Distribution Verification
4. Method Validation
5. Conclusions
- (1)
- The R-N6 batteries have no obvious voltage plateau during charging and discharging. Their discharge capacity is distributed below 4 Ah, and battery attenuation is more serious.
- (2)
- By using Pearson correlation coefficient on the parameters, it is found that the discharge power P1 is positively correlated with the discharge capacity. Other parameters are negatively correlated with the discharge capacity. Among them, Δd, R, U, Rf1, Rf2 and P2 show strong correlation with the discharge capacity of R-N6 batteries. These characteristic parameters obtained by rapid testing can effectively and reliably reflect the internal structure characteristics of the battery.
- (3)
- The KS-test was used to verify the same distribution of the multi-feature parameters, and then support vector regression (SVR) was used to establish the discharge capacity prediction model. The average relative error of capacity prediction is 5.87% and the lowest error is 1.32%. It shows that the prediction for Ni-MH batteries by the method presented in this paper is acceptable and reliable.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ouyang, L.; Huang, J.; Wang, H.; Liu, J.; Zhu, M. Progress of hydrogen storage alloys for Ni-MH rechargeable power batteries in electric vehicles: A review. Mater. Chem. Phys. 2017, 200, 164–178. [Google Scholar] [CrossRef]
- Zhu, W.H.; Zhu, Y.; Davis, Z.; Tatarchuk, B.J. Energy efficiency and capacity retention of Ni–MH batteries for storage applications. Appl. Energy 2013, 106, 307–313. [Google Scholar] [CrossRef]
- Miao, Y.; Hynan, P.; von Jouanne, A.; Yokochi, A. Current Li-Ion Battery Technologies in Electric Vehicles and Opportunities for Advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Chu, A.; Li, D.; Yuan, Y.; Fan, X.; Deng, Y. Development of the cycling life model of Ni-MH power batteries for hybrid electric vehicles based on real-world operating conditions. J. Energy Storage 2021, 34, 101999. [Google Scholar] [CrossRef]
- Chu, H.L.; Qiu, S.J.; Sun, L.X.; Zhang, Y.; Xu, F.; Jiang, T.; Hu, W.Y. The improved electrochemical properties of novel La–Mg–Ni-based hydrogen storage composites. Electrochim. Acta 2007, 52, 6700–6706. [Google Scholar] [CrossRef]
- Kang, J.; Yan, F.; Zhang, P.; Du, C. Comparison of comprehensive properties of Ni-MH (nickel-metal hydride) and Li-ion (lithium-ion) batteries in terms of energy efficiency. Energy 2014, 70, 618–625. [Google Scholar] [CrossRef]
- Piao, C.-H.; Fu, W.-L.; Lei, G.-H.; Cho, C.-D. Online Parameter Estimation of the Ni-MH Batteries Based on Statistical Methods. Energies 2010, 3, 206–215. [Google Scholar] [CrossRef]
- Assefi, M.; Maroufi, S.; Yamauchi, Y.; Sahajwalla, V. Pyrometallurgical recycling of Li-ion, Ni–Cd and Ni–MH batteries: A minireview. Curr. Opin. Green Sustain. Chem. 2020, 24, 26–31. [Google Scholar] [CrossRef]
- Wu, J.B.; Tu, J.P.; Han, T.A.; Yang, Y.Z.; Zhang, W.K.; Zhao, X.B. High-rate discharge ability enhancement of Ni/MH rechargeable batteries by addition of nanoscale CoO to positive electrodes. J. Power Sources 2006, 156, 667–672. [Google Scholar] [CrossRef]
- He, H.; Xiong, R.; Fan, J. Evaluation of lithium-ion battery equivalent circuit models for state of charge estimation by an experimental approach. Energies 2011, 4, 582–598. [Google Scholar] [CrossRef]
- Barai, A.; Uddin, K.; Widanage, W.D.; McGordon, A.; Jennings, P.A. study of the influence of measurement timescale on internal resistance characterisation methodologies for lithium-ion cells. Sci. Rep. 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strange, C.; dos Reis, G. Prediction of future capacity and internal resistance of Li-ion cells from one cycle of input data. Energy AI 2021, 5, 100097. [Google Scholar] [CrossRef]
- Hoque, M.A.; Nurmi, P.; Kumar, A.; Varjonen, S.; Song, J.; Pecht, M.G.; Tarkoma, S. Data driven analysis of lithium-ion battery internal resistance towards reliable state of health prediction. J. Power Sources 2021, 513, 230519. [Google Scholar] [CrossRef]
- Mao, W.J.; Lou, Y.; Zhang, H. Internal gas pressure applied in charging control and SOC estimation of MH/Ni battery. Battery Bimon. 2011, 41, 254–257. [Google Scholar]
- Wang, Z.; Song, C.; Yuan, C.; Li, X. A flexible method for state-of-health estimation of lithium battery energy storage system. Energy Rep. 2021, 7, 6375–6383. [Google Scholar] [CrossRef]
- Ahmeid, M.; Muhammad, M.; Lambert, S.; Attidekou, P.S.; Milojevic, Z. A rapid capacity evaluation of retired electric vehicle battery modules using partial discharge test. J. Energy Storage 2022, 50, 104562. [Google Scholar] [CrossRef]
- Wang, L.J.; Li, M. Research on state of charge estimation method with Ni-MH battery for electric vehicle. Mod. Electron. Technol. 2015, 155, 149–151. [Google Scholar]
- Jiao, H.M.; Yu, Q.M. Research on capacity predication of battery based on BP network and genetic algorithm. Comput. Simul. 2006, 23, 218–220. [Google Scholar]
- Fei, Z.; Yang, F.; Tsui, K.-L.; Li, L.; Zhang, Z. Early prediction of battery lifetime via a machine learning based framework. Energy 2021, 225, 120205. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zhao, S.; Zhang, B. A fast classification method based on factor analysis and K-means clustering for retired electric vehicle batteries. Power Syst. Prot. Control 2021, 49, 41. [Google Scholar]
- He, Z.L.; Zhou, P. Research on Sorting of Retired Lithium Battery Based on Radial Basis Function Neural Network. Electron. Sci. Technol. 2021, 34, 38. [Google Scholar]
- Cha, C.; Yu, J.; Zhang, J. Comparative experimental study of gas evolution and gas consumption reactions in sealed Ni–Cd and Ni–MH cells. J. Power Sources 2004, 129, 347–357. [Google Scholar] [CrossRef]
- Uddin, K.; Picarelli, A.; Lyness, C.; Taylor, N.; Marco, J. An Acausal Li-Ion Battery Pack Model for Automotive Applications. Energies 2014, 7, 5675–5700. [Google Scholar] [CrossRef]
- Su, Q.; Wang, Y.; Jiang, X.; Chen, F.; Lu, W.-C. A Cancer Gene Selection Algorithm Based on the K-S Test and CFS. BioMed Res. Int. 2017, 2017, 1645619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Xu, P.; Chu, Y.; Li, W.; Wu, Y.; Ni, L.; Bao, Y.; Wang, K. Short-term electrical load forecasting using the Support Vector Regression (SVR) model to calculate the demand response baseline for office buildings. Appl. Energy 2017, 195, 659–670. [Google Scholar] [CrossRef]


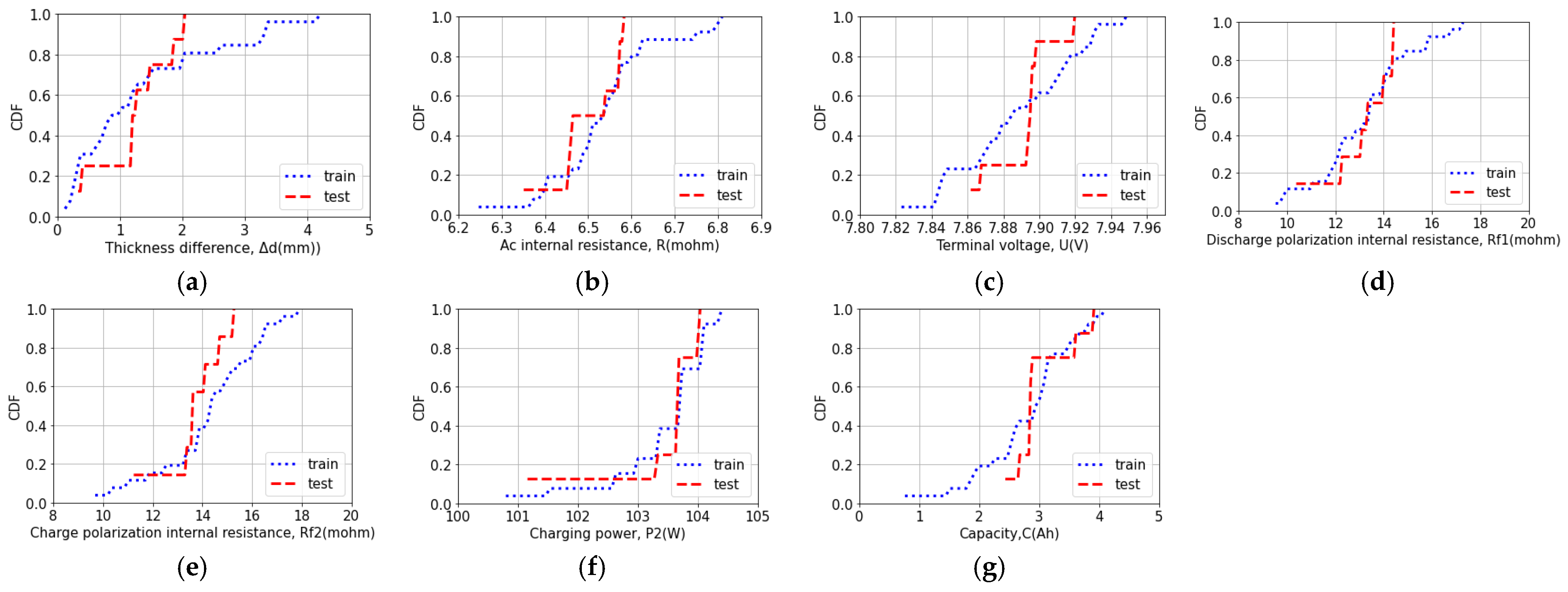

| Feature | Statistic | p-Value |
|---|---|---|
| Thickness difference | 0.365 | 0.308 |
| AC internal resistance | 0.269 | 0.671 |
| Terminal voltage | 0.327 | 0.438 |
| Discharge polarization internal resistance | 0.192 | 0.943 |
| Charge polarization internal resistance | 0.356 | 0.330 |
| Charging power | 0.135 | 0.999 |
| Capacity | 0.327 | 0.438 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Bie, C.; Luo, F.; Kang, J.; Zhang, Y. Rapid Prediction of Retired Ni-MH Batteries Capacity Based on Reliable Multi-Parameter Driven Analysis. Energies 2022, 15, 9156. https://doi.org/10.3390/en15239156
Liu H, Bie C, Luo F, Kang J, Zhang Y. Rapid Prediction of Retired Ni-MH Batteries Capacity Based on Reliable Multi-Parameter Driven Analysis. Energies. 2022; 15(23):9156. https://doi.org/10.3390/en15239156
Chicago/Turabian StyleLiu, Hongling, Chuanyu Bie, Fan Luo, Jianqiang Kang, and Yuping Zhang. 2022. "Rapid Prediction of Retired Ni-MH Batteries Capacity Based on Reliable Multi-Parameter Driven Analysis" Energies 15, no. 23: 9156. https://doi.org/10.3390/en15239156




