Production, Activation and CO2 Uptake Capacity of a Carbonaceous Microporous Material from Palm Oil Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
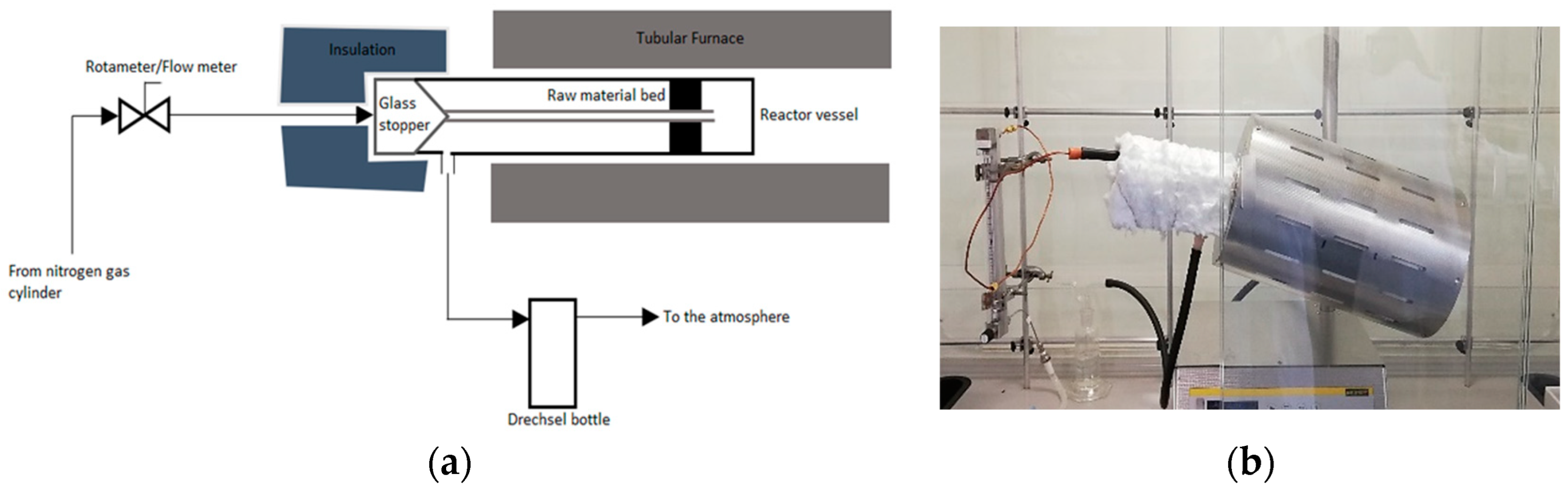
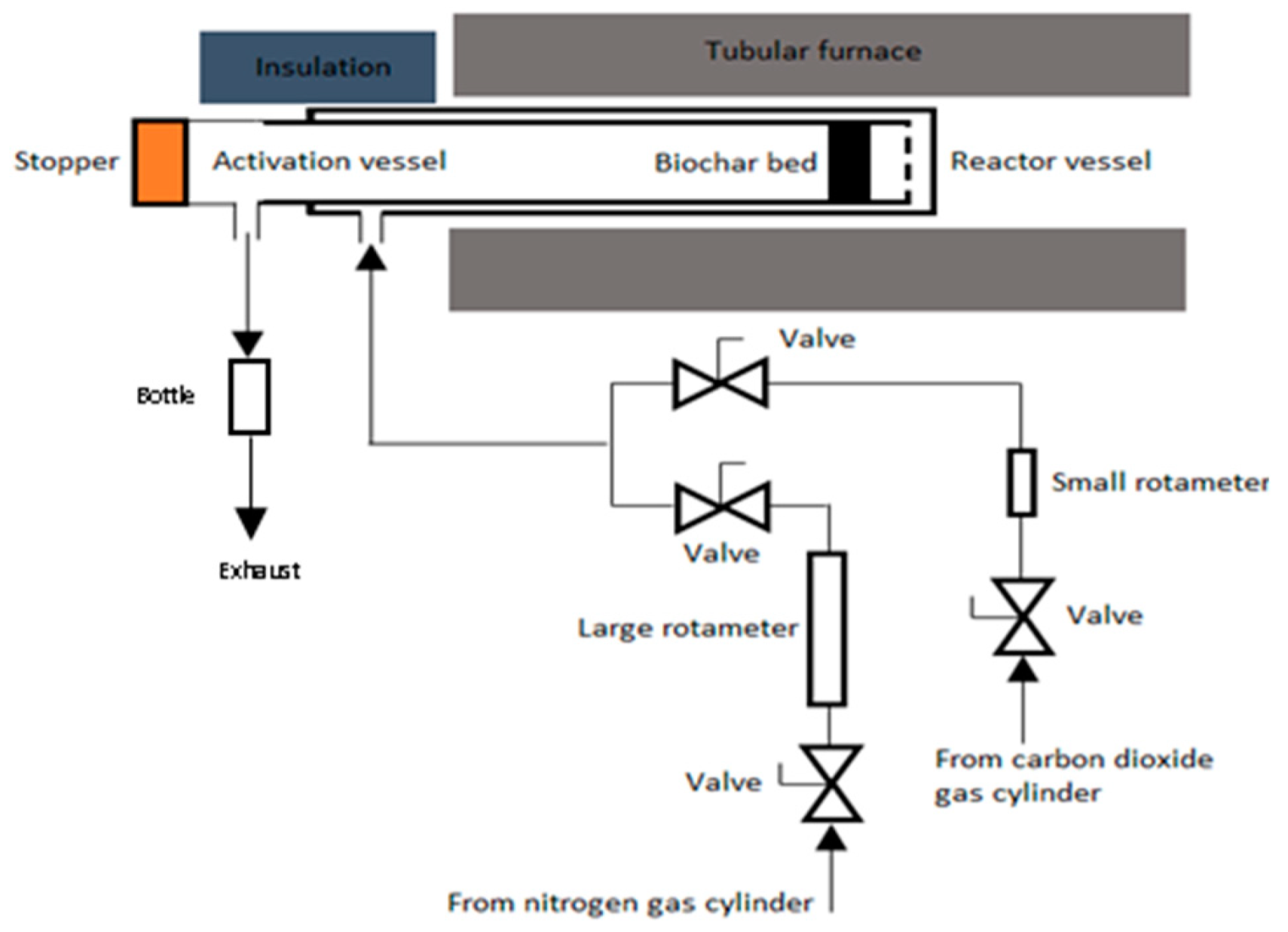
2.2. Preparation of Porous Carbons
2.3. Carbon Activation Procedure
2.4. CO2 Uptake
3. Results and Discussion
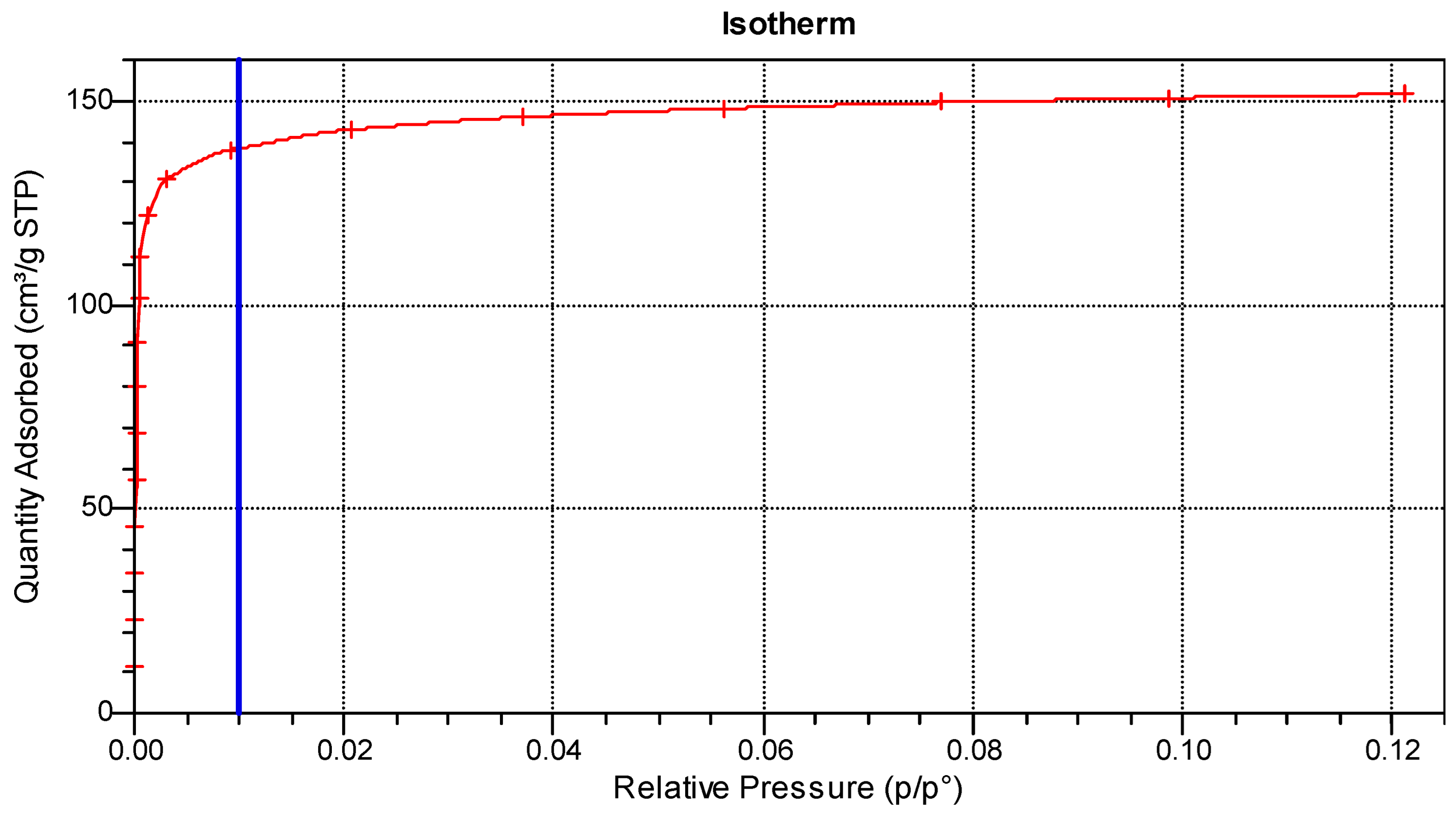
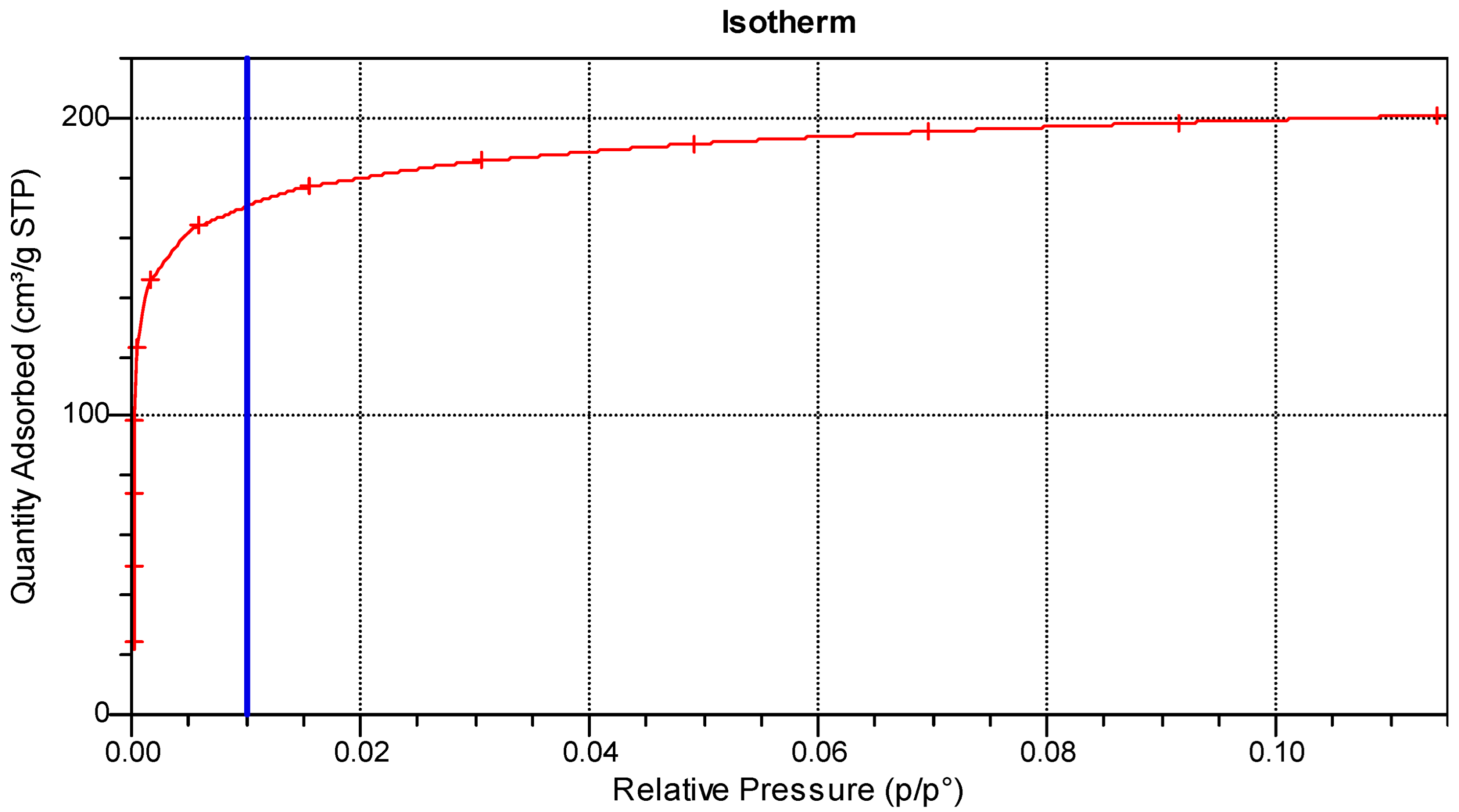
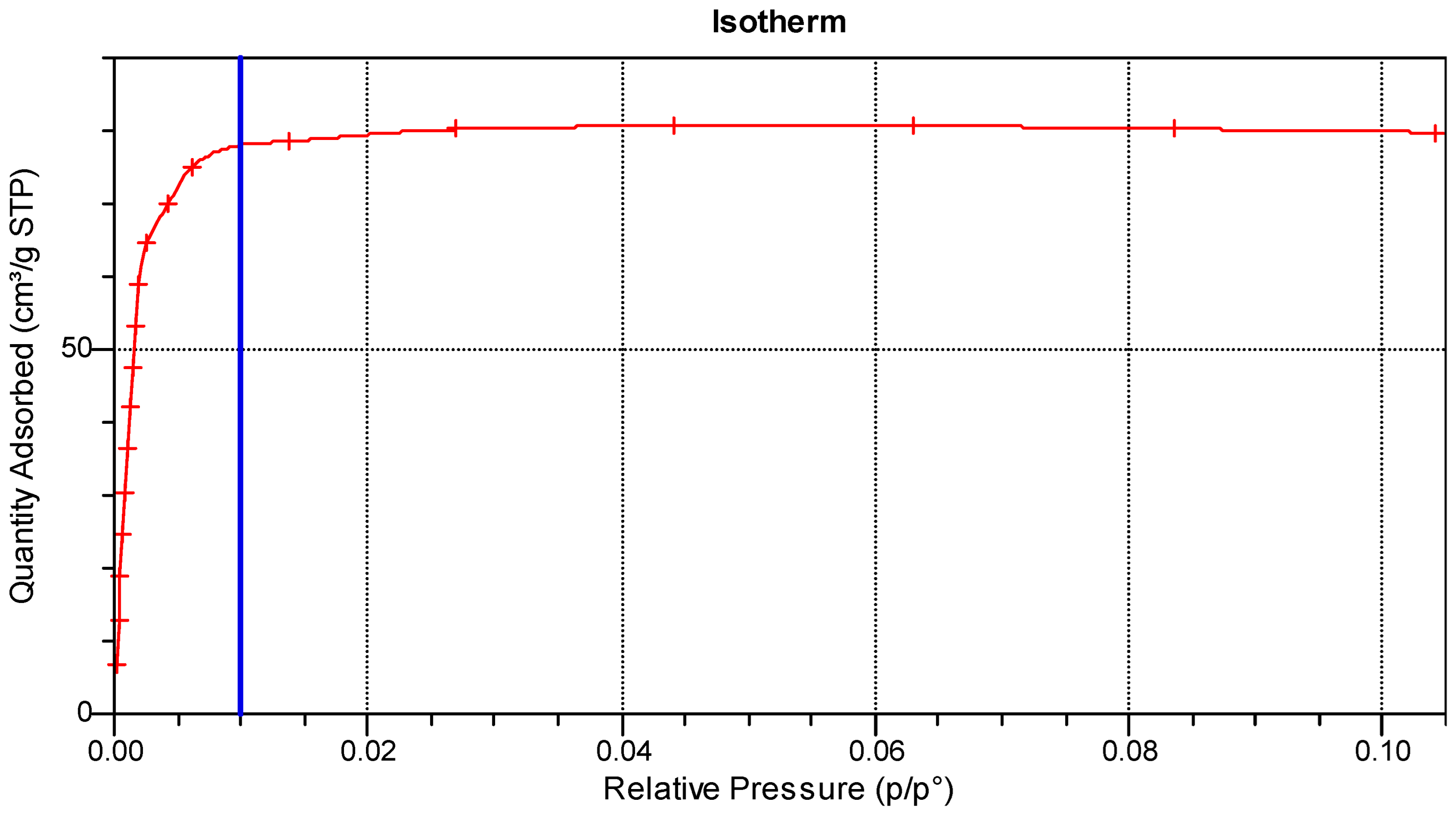
3.1. Production and Characterisation of Activated Carbons
3.1.1. Physical Activation
3.1.2. Chemical Activation
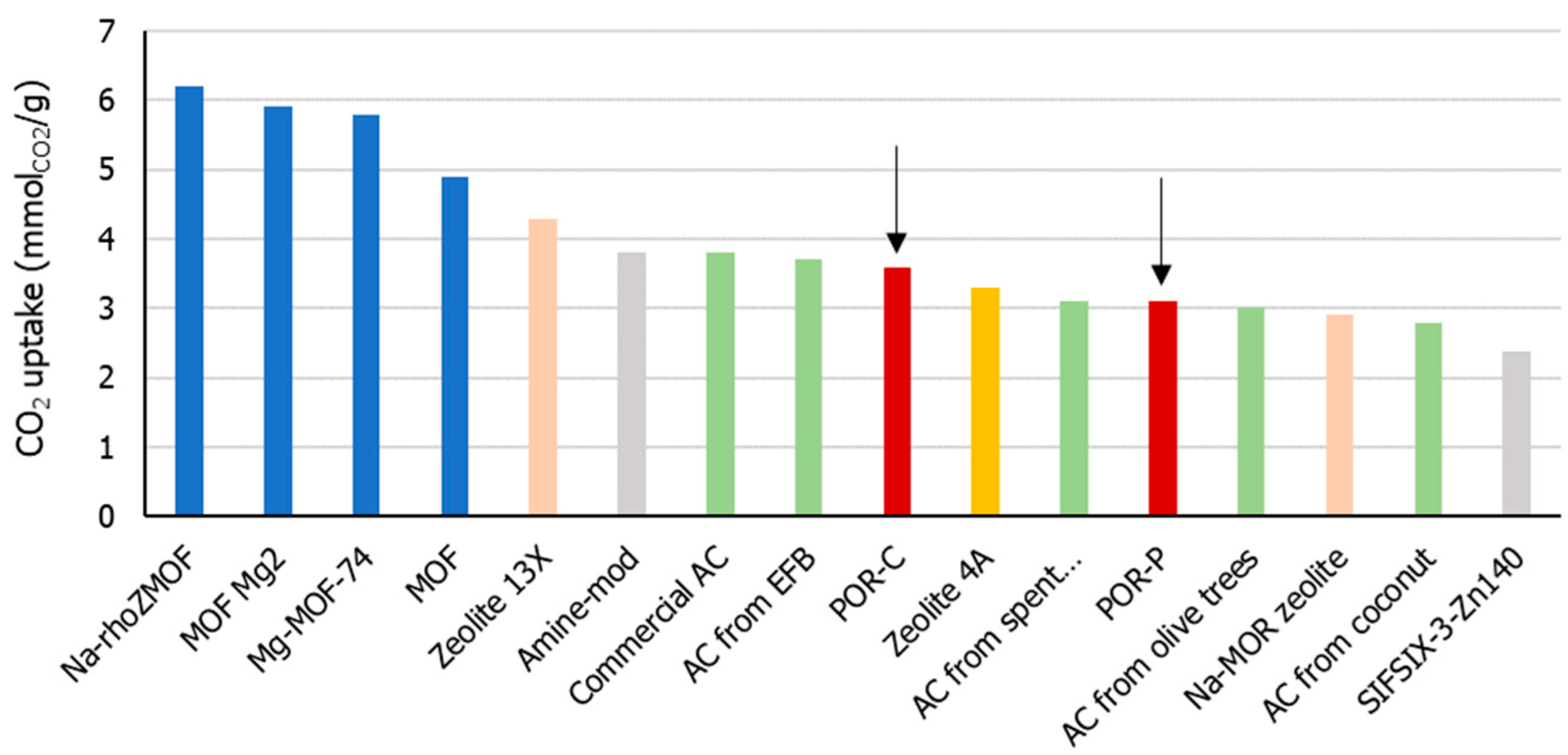
3.2. Influence of Temperature on CO2 Uptake
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- FAOSTAT. Food and Agriculture Organisation of the United Nations. Available online: http://www.fao.org/home/en (accessed on 18 January 2022).
- Zakaria, Z.; Rahim, A.R.A.; Aman, Z. Issues and Challenges of Oil Palm Cooperatives towards Greater Sustainability: A Proposal of Conceptual Framework. Int. J. Acad. Res. Bus. Soc. Sci. 2020, 10, 46–69. [Google Scholar] [CrossRef] [PubMed]
- Zaki, H.H.M.; Rahim, M.A. Palm Oil: Malaysia—EU Trade Issue; Khazanah Research Institute: Kuala Lumpur, Malaysia, 2019. [Google Scholar]
- Abdullah, N.; Sulaim, F. The Oil Palm Wastes in Malaysia. In Biomass Now—Sustainable Growth and Use; InTech: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Kaniapan, S.; Hassan, S.; Ya, H.; Nesan, K.P.; Azeem, M. The Utilisation of Palm Oil and Oil Palm Residues and the Related Challenges as a Sustainable Alternative in Biofuel, Bioenergy, and Transportation Sector: A Review. Sustainability 2021, 13, 3110. [Google Scholar] [CrossRef]
- Pardakhti, M.; Jafari, T.; Tobin, Z.; Dutta, B.; Moharreri, E.; Shemshaki, N.S.; Suib, S.; Srivastava, R. Trends in Solid Adsorbent Materials Development for CO2 Capture. ACS Appl. Mater. Interfaces 2019, 11, 34533–34559. [Google Scholar] [CrossRef]
- Yahya, M.A.; Mansor, M.H.; Zolkarnaini, W.A.A.W.; Rusli, N.S.; Aminuddin, A.; Mohamad, K.; Sabhan, F.A.M.; Atik, A.A.A.; Ozair, L.N. A Brief Review on Activated Carbon Derived from Agriculture By-Product; AIP Publishing LLC: Melville, NY, USA, 2018; p. 030023. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Deng, S.; Wei, H.; Chen, T.; Wang, B.; Huang, J.; Yu, G. Superior CO2 adsorption on pine nut shell-derived activated carbons and the effective micropores at different temperatures. Chem. Eng. J. 2014, 253, 46–54. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production-A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.-Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Ukanwa, K.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A Review of Chemicals to Produce Activated Carbon from Agricultural Waste Biomass. Sustainability 2019, 11, 6204. [Google Scholar] [CrossRef]
- Hinkov, I.P.I.; Lamari, F.; Langlois, P.; Dicko, M.; Chilev, C. Carbon Dioxide Capture by Adsorption (review). J. Chem. Technol. Metall. (JCTM) 2016, 51, 609–626. [Google Scholar]
- Ben-Mansour, R.; Habib, M.A.; Bamidele, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; Laoui, T.; Ali, M.J.A.E. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations—A review. Appl. Energy 2016, 161, 225–255. [Google Scholar] [CrossRef]
- Promraksa, A.; Rakmak, N. Biochar production from palm oil mill residues and application of the biochar to adsorb carbon dioxide. Heliyon 2020, 6, e04019. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Tsubota, T.; Hassan, M.A.; Andou, Y. Surface Functionalization of Biochar from Oil Palm Empty Fruit Bunch through Hydrothermal Process. Processes 2021, 9, 149. [Google Scholar] [CrossRef]
- Lawal, A.A.; Hassan, M.A.; Farid, M.A.A.; Yasim-Anuar, T.A.T.; Yusoff, M.Z.M.; Zakaria, M.R.; Roslan, A.M.; Mokhtar, M.N.; Shirai, Y. Production of biochar from oil palm frond by steam pyrolysis for removal of residual contaminants in palm oil mill effluent final discharge. J. Clean. Prod. 2020, 265, 121643. [Google Scholar] [CrossRef]
- Cristina, A.M.; Antonucci, B.; Focacci, S.; Heap, J.M.; Martel, A.M.; Hamzah, F.; Martín, C.F.; Martinez-Felipe, A. Molecular dynamics simulation of the interactions between carbon dioxide and a natural-based carbonaceous microporous material. Chem. Eng. Trans. 2021, 86, 1111–1116. [Google Scholar] [CrossRef]
- Arami-Niya, A.; Daud, W.M.A.W.; Mjalli, F.S. Comparative study of the textural characteristics of oil palm shell activated carbon produced by chemical and physical activation for methane adsorption. Chem. Eng. Res. Des. 2011, 89, 657–664. [Google Scholar] [CrossRef]
- Nuilerd, J.C.T.; Pongyeela, P. Pellet activated carbon production using parawood charcoal from gasifier by KOH activation for adsorption of iron in water. Songklanakarin J. Sci. Technol. 2018, 40, 264–270. [Google Scholar] [CrossRef]
- Evans, M.J.B.; Halliop, E.; MacDonald, J.A.F. The production of chemically-activated carbon. Carbon N. Y. 1999, 37, 269–274. [Google Scholar] [CrossRef]
- Correa, C.R.; Ngamying, C.; Klank, D.; Kruse, A. Investigation of the textural and adsorption properties of activated carbon from HTC and pyrolysis carbonizates. Biomass Convers. Biorefinery 2018, 8, 317–328. [Google Scholar] [CrossRef]
- Li, W.; Yang, K.; Peng, J.; Zhang, L.; Guo, S.; Xia, H. Effects of carbonization temperatures on characteristics of porosity in coconut shell chars and activated carbons derived from carbonized coconut shell chars. Ind. Crops Prod. 2008, 28, 190–198. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Biomass derived low-cost microporous adsorbents for efficient CO2 capture. Fuel 2015, 148, 246–254. [Google Scholar] [CrossRef]
- Ello, A.S.; de Souza, L.K.C.; Trokourey, A.; Jaroniec, M. Development of microporous carbons for CO2 capture by KOH activation of African palm shells. J. CO2 Util. 2013, 2, 35–38. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. Production of palm kernel shell-based activated carbon by direct physical activation for carbon dioxide adsorption. Environ. Sci. Pollut. Res. 2019, 26, 33732–33746. [Google Scholar] [CrossRef] [PubMed]
- Sulistianti, I.M.I.; Krisnandi, Y.K. Study of CO2 adsorption capacity of mesoporous carbon and activated carbon modified by triethylenetetramine (TETA). IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 012041. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Xing, W.; Xue, Q.; Yan, Z.; Zhuo, S.; Qiao, S.Z. Critical role of small micropores in high CO2 uptake. Phys. Chem. Chem. Phys. 2013, 15, 2523. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Shah, B.B.; Zhao, D. CO2 Capture in Metal-Organic Framework Adsorbents: An Engineering Perspective. Adv. Sustain. Syst. 2019, 3, 1800080. [Google Scholar] [CrossRef] [Green Version]
- Xiang, S.; He, Y.; Zhang, Z.; Wu, H.; Zhou, W.; Krishna, R.; Chen, B. Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions. Nat. Commun. 2012, 3, 954. [Google Scholar] [CrossRef] [Green Version]
- Qasem, N.A.A.; Ben-Mansour, R. Adsorption breakthrough and cycling stability of carbon dioxide separation from CO2/N2/H2O mixture under ambient conditions using 13X and Mg-MOF-74. Appl. Energy 2018, 230, 1093–1107. [Google Scholar] [CrossRef]
- Liao, P.Q.; Chen, X.W.; Liu, S.Y.; Li, X.Y.; Xu, Y.T.; Tang, M.; Rui, Z.; Ji, H.; Zhang, J.P.; Chen, X.M. Putting an ultrahigh concentration of amine groups into a metal–organic framework for CO2 capture at low pressures. Chem. Sci. 2016, 7, 6528–6533. [Google Scholar] [CrossRef] [Green Version]
- Villarreal, A.; Garbarino, G.; Riani, P.; Finocchio, E.; Bosio, B.; Ramirez, J.; Busca, G. Adsorption and separation of CO2 from N2 -rich gas on zeolites: Na-X faujasite vs. Na-mordenite. J. CO2 Util. 2017, 19, 266–275. [Google Scholar] [CrossRef]
- Vaidhyanathan, R.; Iremonger, S.S.; Dawson, K.W.; Shimizu, G.K.H. An amine-functionalized metal organic framework for preferential CO2 adsorption at low pressures. Chem. Commun. 2009, 5230. [Google Scholar] [CrossRef]
- Yang, G.; Ye, J.; Yan, Y.; Tang, Z.; Yu, D.; Yang, J. Preparation and CO2 adsorption properties of porous carbon from camphor leaves by hydrothermal carbonization and sequential potassium hydroxide activation. RSC Adv. 2017, 7, 4152–4160. [Google Scholar] [CrossRef] [Green Version]
- Dreisbach, F.; Staudt, R.; Keller, J.U. High Pressure Adsorption Data of Methane, Nitrogen, Carbon Dioxide and their Binary and Ternary Mixtures on Activated Carbon. Adsorption 1999, 5, 215–227. [Google Scholar] [CrossRef]
- González, A.S.; Plaza, M.G.; Rubiera, F.; Pevida, C. Sustainable biomass-based carbon adsorbents for post-combustion CO2 capture. Chem. Eng. J. 2013, 230, 456–465. [Google Scholar] [CrossRef] [Green Version]
- Hauchhum, L.; Mahanta, P. Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed. Int. J. Energy Environ. Eng. 2014, 5, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Forrest, K.A.; Pham, T.; Elsaidi, S.K.; Mohamed, M.H.; Thallapally, P.K.; Zaworotko, M.J.; Space, B. Investigating CO2 Sorption in SIFSIX-3-M (M = Fe, Co, Ni, Cu, Zn) through Computational Studies. Cryst. Growth Des. 2019, 19, 3732–3743. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef]



| Sample | Activation Time (min) | Burn-Off (%) |
|---|---|---|
| POR-P20 | 390 | 23.13 |
| POR-P40 | 1130 | 39.57 |
| Sample | C:NaOH Mass Ratio | Burn-off (-) |
|---|---|---|
| POR-C11 | 1:1 | 4.34 |
| POR-C12 | 1:2 | 4.90 |
| POR-C13 | 1:3 | 3.39 |
| Sample | Average Pore Diameter, dp (nm) * | BET Surface Area, SBET (m2·g−1) ** | Total Pore Volume, VTOTAL (cm3·g−1) *** |
|---|---|---|---|
| POR-P20 | 0.67 | 653.5 | 0.22 |
| POR-P40 | 0.68 | 881.5 | 0.30 |
| POR-C11 | 0.80 | 319.0 | 0.11 |
| POR-C12 | 0.77 | 356.0 | 0.22 |
| Sample | CO2 Uptake (mmol/g) |
|---|---|
| POR-P20 | 2.41 |
| POR-P40 | 3.58 |
| POR-C11 | 3.36 |
| POR-C12 | 3.75 |
| POR-C13 | 3.81 |
| Commercial AC [27] | 3.84 |
| Sample | CO2 Uptake (mmol/g) | |
|---|---|---|
| 125 °C | 225 °C | |
| POR-C12 | 1.20 | 0.32 |
| POR-C13 | 0.78 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moliner, C.; Focacci, S.; Antonucci, B.; Moreno, A.; Biti, S.; Hamzah, F.; Martinez-Felipe, A.; Arato, E.; Fernández Martín, C. Production, Activation and CO2 Uptake Capacity of a Carbonaceous Microporous Material from Palm Oil Residues. Energies 2022, 15, 9160. https://doi.org/10.3390/en15239160
Moliner C, Focacci S, Antonucci B, Moreno A, Biti S, Hamzah F, Martinez-Felipe A, Arato E, Fernández Martín C. Production, Activation and CO2 Uptake Capacity of a Carbonaceous Microporous Material from Palm Oil Residues. Energies. 2022; 15(23):9160. https://doi.org/10.3390/en15239160
Chicago/Turabian StyleMoliner, Cristina, Simona Focacci, Beatrice Antonucci, Aldo Moreno, Simba Biti, Fazlena Hamzah, Alfonso Martinez-Felipe, Elisabetta Arato, and Claudia Fernández Martín. 2022. "Production, Activation and CO2 Uptake Capacity of a Carbonaceous Microporous Material from Palm Oil Residues" Energies 15, no. 23: 9160. https://doi.org/10.3390/en15239160








