Electrochemical Failure Results Inevitable Capacity Degradation in Li-Ion Batteries—A Review
Abstract
:1. Introduction
2. Battery Failure Mechanism
Failure of Cathode Material
3. Anode Materials and Their Interface Failure Problems
4. Lithium Plating
5. Battery Failure Detection
6. Destructive Detection
7. Nondestructive Detection
8. Methods to Prevent Battery Failure
8.1. Modification of Electrode Structure
8.2. Modification of Cathode Materials

9. SEI Design and Modification of Anode Materials
10. Optimization of Charging Strategy
11. Battery Failure Prediction
12. Summary and Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, X.; Manthiram, A. Sustainable Battery Materials for Next-Generation Electrical Energy Storage. Adv. Energy Sustain. Res. 2021, 2, 2000102. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Y. A retrospective on lithium-ion batteries. Nat. Commun. 2020, 11, 2499. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kong, X.; Liu, C.; Zhao, J. Study on thermal stability of nickel-rich/silicon-graphite large capacity lithium ion battery. Appl. Therm. Eng. 2019, 161, 114144. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.-J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-Rich Layered Lithium Transition-Metal Oxide for High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Hynan, P.; von Jouanne, A.; Yokochi, A. Current Li-Ion Battery Technologies in Electric Vehicles and Opportunities for Advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Bae, C.; Denlinger, A.; Miller, T. Electric Vehicles Batteries: Requirements and Challenges. Joule 2020, 4, 511–515. [Google Scholar] [CrossRef]
- Hannan, M.A.; Hoque, M.M.; Mohamed, A.; Ayob, A. Review of energy storage systems for electric vehicle applications: Issues and challenges. Renew. Sustain. Energy Rev. 2017, 69, 771–789. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Krupp, A.; Beckmann, R.; Diekmann, T.; Ferg, E.; Schuldt, F.; Agert, C. Calendar aging model for lithium-ion batteries considering the influence of cell characterization. J. Energy Storage 2022, 45, 103506. [Google Scholar] [CrossRef]
- Eduardo, R.; Pascal, V.; Pelissier, S. Calendar and cycling ageing combination of batteries in electric vehicles. Microelectron. Reliab. 2018, 88–90, 1212–1215. [Google Scholar]
- Maik, N.; Michael, S.; Peter, K.; Holger, C.H.; Andreas, J. Analysis and modeling of calendar aging of a commercial LiFePO4/graphite cell. J. Energy Storage 2018, 17, 153–169. [Google Scholar]
- Ren, D.; Hsu, H.; Li, R.; Feng, X.; Guo, D.; Han, X.; Lu, L.; He, X.; Gao, S.; Hou, J.; et al. A comparative investigation of aging effects on thermal runaway behavior of lithium-ion batteries. eTransportation 2019, 2, 100034. [Google Scholar] [CrossRef]
- Han, X.; Lu, L.; Zheng, Y.; Feng, X.; Li, Z.; Li, J.; Ouyang, M. A review on the key issues of the lithium ion battery degradation among the whole life cycle. eTransportation 2019, 1, 100005. [Google Scholar] [CrossRef]
- Fleischhammer, M.; Waldmann, T.; Bisle, G.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Interaction of cyclic ageing at high-rate and low temperatures and safety in lithium-ion batteries. J. Power Sources 2015, 274, 432–439. [Google Scholar] [CrossRef]
- Kim, K.M.; Ly, N.V.; Won, J.H.; Lee, Y.-G.; Cho, W.I.; Ko, J.M.; Kaner, R.B. Improvement of lithium-ion battery performance at low temperature by adopting polydimethylsiloxane-based electrolyte additives. Electrochim. Acta 2014, 136, 182–188. [Google Scholar] [CrossRef]
- Mussa, A.S.; Liivat, A.; Marzano, F.; Klett, M.; Philippe, B.; Tengstedt, C.; Lindbergh, G.; Edström, K.; Lindström, R.W.; Svens, P. Fast-charging effects on ageing for energy-optimized automotive LiNi1/3Mn1/3Co1/3O2/graphite prismatic lithium-ion cells. J. Power Sources 2019, 422, 175–184. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Käbitz, S.; Gerschler, J.B.; Ecker, M.; Yurdagel, Y.; Emmermacher, B.; André, D.; Mitsch, T.; Sauer, D.U. Cycle and calendar life study of a graphite|LiNi1/3Mn1/3Co1/3O2 Li-ion high energy system. Part A: Full cell characterization. J. Power Sources 2013, 239, 572–583. [Google Scholar] [CrossRef]
- Zheng, J.; Gu, M.; Xiao, J.; Zuo, P.; Wang, C.; Zhang, J.-G. Corrosion/Fragmentation of Layered Composite Cathode and Related Capacity/Voltage Fading during Cycling Process. Nano Lett. 2013, 13, 3824–3830. [Google Scholar] [CrossRef]
- He, Y.; Jiang, L.; Chen, T.; Xu, Y.; Jia, H.; Yi, R.; Xue, D.; Song, M.; Genc, A.; Bouchet-Marquis, C.; et al. Progressive growth of the solid–electrolyte interphase towards the Si anode interior causes capacity fading. Nat. Nanotechnol. 2012, 16, 10. [Google Scholar] [CrossRef]
- Atalay, S.; Sheikh, M.; Mariani, A.; Merla, Y.; Bower, E.; Widanage, W.D. Theory of battery ageing in a lithium-ion battery: Capacity fade, nonlinear ageing and lifetime prediction. J. Power Sources 2020, 478, 229026. [Google Scholar] [CrossRef]
- Han, X.; Ouyang, M.; Lu, L.; Li, J.; Zheng, Y.; Li, Z. A comparative study of commercial lithium ion battery cycle life in electrical vehicle: Aging mechanism identification. J. Power Sources 2014, 251, 38–54. [Google Scholar] [CrossRef]
- He, W.; Guo, W.; Wu, H.; Lin, L.; Liu, Q.; Han, X.; Xie, Q.; Liu, P.; Zheng, H.; Wang, L.; et al. Challenges and Recent Advances in High Capacity Li-Rich Cathode Materials for High Energy Density Lithium-Ion Batteries. Adv. Mater. 2021, 33, 2005937. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Wu, X.; Wang, K.; Feng, Z.; Cheng, T.; Liu, Y.; Wang, M.; Chen, R.; Xu, L.; Zhou, J.; et al. An Overview on the Advances of LiCoO2 Cathodes for Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2000982. [Google Scholar] [CrossRef]
- Zhang, J.-C.; Liu, Z.-D.; Zeng, C.-H.; Luo, J.-W.; Deng, Y.-D.; Cui, X.-Y.; Chen, Y.-N. High-voltage LiCoO2 cathodes for high-energy-density lithium-ion battery. Rare Met. 2022, 41, 3946–3956. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P.; Bai, P.; Wan, H.; Liu, S.; Hou, S.; Pu, X.; Xia, J.; Zhang, W.; Wang, Z.; et al. Interfacial Design for a 4.6 V High-Voltage Single-Crystalline LiCoO2 Cathode. Adv. Mater. 2022, 34, 2108353. [Google Scholar] [CrossRef]
- Li, L.; Wu, L.; Wu, F.; Song, S.; Zhang, X.; Fu, C.; Yuan, D.; Xiang, Y. Review—Recent Research Progress in Surface Modification of LiFePO4 Cathode Materials. J. Electrochem. Soc. 2017, 164, A2138. [Google Scholar] [CrossRef]
- Chen, S.-P.; Lv, D.; Chen, J.; Zhang, Y.-H.; Shi, F.-N. Review on Defects and Modification Methods of LiFePO4 Cathode Material for Lithium-Ion Batteries. Energy Fuels 2022, 36, 1232–1251. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Guang, T.; Fan, B.; Zhu, K.; Wang, X. Controlled Hydrothermal/Solvothermal Synthesis of High-Performance LiFePO4 for Li-Ion Batteries. Small Methods 2021, 5, 2100193. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Dai, P.; Kong, X.; Yang, H.; Li, J.; Zeng, J.; Zhao, J. Single-Crystal Ni-Rich Layered LiNi0.9Mn0.1O2 Enables Superior Performance of Co-Free Cathodes for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 4381–4390. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Kong, X.; Yang, H.; Zeng, J.; Zhao, J. Insight into the Kinetic Degradation of Stored Nickel-Rich Layered Cathode Materials for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2021, 9, 10547–10556. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Hu, Y.; Chai, Y.; Li, D.; Zuo, W.; Zhang, Z.; Yang, Y. Failure mechanism of Li1+x(NCM)1−xO2 layered oxide cathode material during capacity degradation. Energy Storage Sci. Technol. 2019, 8, 1003. [Google Scholar] [CrossRef]
- Xia, Y.; Zheng, J.; Wang, C.; Gu, M. Designing principle for Ni-rich cathode materials with high energy density for practical applications. Nano Energy 2018, 49, 434–452. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Lv, D.; Wei, Y.; Zheng, J.; Wang, Z.; Kuppan, S.; Yu, J.; Luo, L.; Edwards, D.; et al. Atomic-Resolution Visualization of Distinctive Chemical Mixing Behavior of Ni, Co, and Mn with Li in Layered Lithium Transition-Metal Oxide Cathode Materials. Chem. Mater. 2015, 27, 5393–5401. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, K. Electronic Structure and Comparative Properties of LiNixMnyCozO2 Cathode Materials. J. Phys. Chem. C 2017, 121, 6002–6010. [Google Scholar] [CrossRef]
- Lin, Q.; Guan, W.; Meng, J.; Huang, W.; Wei, X.; Zeng, Y.; Li, J.; Zhang, Z. A new insight into continuous performance decay mechanism of Ni-rich layered oxide cathode for high energy lithium ion batteries. Nano Energy 2018, 54, 313–321. [Google Scholar] [CrossRef]
- Jung, S.-K.; Gwon, H.; Hong, J.; Park, K.-Y.; Seo, D.-H.; Kim, H.; Hyun, J.; Yang, W.; Kang, K. Understanding the Degradation Mechanisms of LiNi0.5Co0.2Mn0.3O2 Cathode Material in Lithium Ion Batteries. Adv. Energy Mater. 2014, 4, 1300787. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Yang, H.; Kong, X.; Li, J.; Dai, P.; Zeng, J.; Yang, Y.; Zhao, J. In-situ construction of a thermodynamically stabilized interface on the surface of single crystalline Ni-rich cathode materials via a one-step molten-salt route. Nano Res. 2022, 6, 1–9. [Google Scholar] [CrossRef]
- Li, D.; Li, H.; Danilov, D.L.; Gao, L.; Chen, X.; Zhang, Z.; Zhou, J.; Eichel, R.-A.; Yang, Y.; Notten, P.H. Degradation mechanisms of C6/LiNi0.5Mn0.3Co0.2O2 Li-ion batteries unraveled by non-destructive and post-mortem methods. J. Power Sources 2019, 416, 163–174. [Google Scholar] [CrossRef]
- Liu, X.; Ren, D.; Hsu, H.; Feng, X.; Xu, G.-L.; Zhuang, M.; Gao, H.; Lu, L.; Han, X.; Chu, Z.; et al. Thermal Runaway of Lithium-Ion Batteries without Internal Short Circuit. Joule 2018, 2, 2047–2064. [Google Scholar] [CrossRef]
- Hou, J.; Feng, X.; Wang, L.; Liu, X.; Ohma, A.; Lu, L.; Ren, D.; Huang, W.; Li, Y.; Yi, M.; et al. Unlocking the self-supported thermal runaway of high-energy lithium-ion batteries. Energy Storage Mater. 2021, 39, 395–402. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Andersen, H.L.; Djuandhi, L.; Mittal, U.; Sharma, N. Strategies for the Analysis of Graphite Electrode Function. Adv. Energy Mater. 2021, 11, 2102693. [Google Scholar] [CrossRef]
- Cui, Q.; Zhong, Y.; Pan, L.; Zhang, H.; Yang, Y.; Liu, D.; Teng, F.; Bando, Y.; Yao, J.; Wang, X. Recent Advances in Designing High-Capacity Anode Nanomaterials for Li-Ion Batteries and Their Atomic-Scale Storage Mechanism Studies. Adv. Sci. 2018, 5, 1700902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Russell, J.A.; Fang, Z.; Barnes, P.; Li, L.; Efaw, C.; Muenzer, A.; May, J.; Hamal, K.; Cheng, I.F.; et al. A Comparison of Solid Electrolyte Interphase Formation and Evolution on Highly Oriented Pyrolytic and Disordered Graphite Negative Electrodes in Lithium-Ion Batteries. Small 2021, 17, 2105292. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef] [Green Version]
- Peled, E.; Menkin, S. Review—SEI: Past, Present and Future. J. Electrochem. Soc. 2017, 164, A1703. [Google Scholar] [CrossRef]
- Yoshio, M.; Tsumura, T.; Dimov, N. Electrochemical behaviors of silicon based anode material. J. Power Sources 2005, 146, 10–14. [Google Scholar] [CrossRef]
- Pidaparthy, S.; Luo, M.; Rodrigues, M.-T.F.; Zuo, J.-M.; Abraham, D.P. Physicochemical Heterogeneity in Silicon Anodes from Cycled Lithium-Ion Cells. ACS Appl. Mater. Interfaces 2022, 14, 38660–38668. [Google Scholar] [CrossRef]
- Xiao, Q.; Gu, M.; Yang, H.; Li, B.; Zhang, C.; Liu, Y.; Liu, F.; Dai, F.; Yang, L.; Liu, Z.; et al. Inward lithium-ion breathing of hierarchically porous silicon anodes. Nat. Commun. 2015, 6, 8844. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Wu, M.; Xiao, X.; Vukmirovic, N.; Xun, S.; Das, P.K.; Song, X.; Olalde-Velasco, P.; Wang, D.; Weber, A.Z.; Wang, L.-W.; et al. Toward an Ideal Polymer Binder Design for High-Capacity Battery Anodes. J. Am. Chem. Soc. 2013, 135, 12048–12056. [Google Scholar] [CrossRef] [Green Version]
- Ko, M.; Chae, S.; Cho, J. Challenges in Accommodating Volume Change of Si Anodes for Li-Ion Batteries. ChemElectroChem 2015, 2, 1645–1651. [Google Scholar] [CrossRef] [Green Version]
- Wetjen, M.; Solchenbach, S.; Pritzl, D.; Hou, J.; Tileli, V.; Gasteiger, H.A. Morphological Changes of Silicon Nanoparticles and the Influence of Cutoff Potentials in Silicon-Graphite Electrodes. J. Electrochem. Soc. 2018, 165, A1503. [Google Scholar] [CrossRef] [Green Version]
- McDowell, M.T.; Ryu, I.; Lee, S.W.; Wang, C.; Nix, W.D.; Cui, Y. Studying the Kinetics of Crystalline Silicon Nanoparticle Lithiation with In Situ Transmission Electron Microscopy. Adv. Mater. 2012, 24, 6034–6041. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Xu, H.; Wang, L.; Lu, X.; He, X. Li4Ti5O12 spinel anode: Fundamentals and advances in rechargeable batteries. InfoMat 2022, 4, e12228. [Google Scholar] [CrossRef]
- Yuan, T.; Tan, Z.; Ma, C.; Yang, J.; Ma, Z.-F.; Zheng, S. Challenges of Spinel Li4Ti5O12 for Lithium-Ion Battery Industrial Applications. Adv. Energy Mater. 2017, 7, 1601625. [Google Scholar] [CrossRef]
- Chen, Z.; Belharouak, I.; Sun, Y.-K.; Amine, K. Li4Ti5O12 for High-Power, Long-Life, and Safe Lithium-Ion Batteries. In Lithium Batteries; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 277–290. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghamouss, F. Role of Electrolytes in the Stability and Safety of Lithium Titanate-Based Batteries. Front. Mater. 2020, 7, 186. [Google Scholar] [CrossRef]
- He, Y.-B.; Li, B.; Liu, M.; Zhang, C.; Lv, W.; Yang, C.; Li, J.; Du, H.; Zhang, B.; Yang, Q.-H.; et al. Gassing in Li4Ti5O12-based batteries and its remedy. Sci. Rep. 2012, 2, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Aubin, M.; Mehta, A.; Tian, H.; Chang, J.; Kushima, A.; Sohn, Y.; Yang, Y. Stabilization of Sn Anode through Structural Reconstruction of a Cu–Sn Intermetallic Coating Layer. Adv. Mater. 2020, 32, 2003684. [Google Scholar] [CrossRef] [PubMed]
- Derrien, G.; Hassoun, J.; Panero, S.; Scrosati, B. Nanostructured Sn–C Composite as an Advanced Anode Material in High-Performance Lithium-Ion Batteries. Adv. Mater. 2007, 19, 2336–2340. [Google Scholar] [CrossRef]
- Ying, H.; Han, W.-Q. Metallic Sn-Based Anode Materials: Application in High-Performance Lithium-Ion and Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1700298. [Google Scholar] [CrossRef] [PubMed]
- Zinth, V.; von Lüders, C.; Hofmann, M.; Hattendorff, J.; Buchberger, I.; Erhard, S.; Rebelo-Kornmeier, J.; Jossen, A.; Gilles, R. Lithium plating in lithium-ion batteries at sub-ambient temperatures investigated by in situ neutron diffraction. J. Power Sources 2014, 271, 152–159. [Google Scholar] [CrossRef]
- Gao, T.; Han, Y.; Fraggedakis, D.; Das, S.; Zhou, T.; Yeh, C.-N.; Xu, S.; Chueh, W.C.; Li, J.; Bazant, M.Z. Interplay of Lithium Intercalation and Plating on a Single Graphite Particle. Joule 2021, 5, 393–414. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Liaw, B.Y.; Metzler, V.; Zhang, J. A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J. Power Sources 2014, 254, 168–182. [Google Scholar] [CrossRef]
- Mei, W.; Jiang, L.; Liang, C.; Sun, J.; Wang, Q. Understanding of Li-plating on graphite electrode: Detection, quantification and mechanism revelation. Energy Storage Mater. 2021, 41, 209–221. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. Study of the charging process of a LiCoO2-based Li-ion battery. J. Power Sources 2006, 160, 1349–1354. [Google Scholar] [CrossRef]
- Li, H.; Ji, W.; Zhang, P.; Zhao, J. Safety boundary of power battery based on quantitative lithium deposition. J. Energy Storage 2022, 52, 104789. [Google Scholar] [CrossRef]
- Sarkar, A.; Shrotriya, P.; Nlebedim, I.C. Anodic Interfacial Evolution in Extremely Fast Charged Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 3179–3188. [Google Scholar] [CrossRef]
- Ho, A.S.; Parkinson, D.Y.; Finegan, D.P.; Trask, S.E.; Jansen, A.N.; Tong, W.; Balsara, N.P. 3D Detection of Lithiation and Lithium Plating in Graphite Anodes during Fast Charging. ACS Nano 2021, 15, 10480–10487. [Google Scholar] [CrossRef]
- Petzl, M.; Kasper, M.; Danzer, M.A. Lithium plating in a commercial lithium-ion battery—A low-temperature aging study. J. Power Sources 2015, 275, 799–807. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.; Ren, D.; Ouyang, M.; Lu, L.; Han, X. Thermal Runaway Triggered by Plated Lithium on the Anode after Fast Charging. ACS Appl. Mater. Interfaces 2019, 11, 46839–46850. [Google Scholar] [CrossRef]
- Sreenarayanan, B.; Tan, D.H.S.; Bai, S.; Li, W.; Bao, W.; Meng, Y.S. Quantification of lithium inventory loss in micro silicon anode via titration-gas chromatography. J. Power Sources 2022, 531, 231327. [Google Scholar] [CrossRef]
- Li, H.; Wen, Z.; Wu, D.; Ji, W.; He, Z.; Wang, F.; Yang, Y.; Zhang, P.; Zhao, J. Achieving a Stable Solid Electrolyte Interphase and Enhanced Thermal Stability by a Dual-Functional Electrolyte Additive toward a High-Loading LiNi0.8Mn0.1Co0.1O2/Lithium Pouch Battery. ACS Appl. Mater. Interfaces 2021, 13, 57142–57152. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, Y.; Li, J.; Yang, H.; Dai, P.; Zeng, J.; Zhao, J. Single-crystal structure helps enhance the thermal performance of Ni-rich layered cathode materials for lithium-ion batteries. Chem. Eng. J. 2022, 434, 134638. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Guo, B.; Zhu, C.; Zhou, X.; Wang, L.; Cao, J. A novel quantitative electrochemical aging model considering side reactions for lithium-ion batteries. Electrochim. Acta 2020, 343, 136070. [Google Scholar] [CrossRef]
- Yang, X.-G.; Liu, T.; Gao, Y.; Ge, S.; Leng, Y.; Wang, D.; Wang, C.-Y. Asymmetric Temperature Modulation for Extreme Fast Charging of Lithium-Ion Batteries. Joule 2019, 3, 3002–3019. [Google Scholar] [CrossRef]
- Wang, H.; Dai, L.; Mao, L.; Liu, Y.; Jin, Y.; Wu, Q. In Situ Detection of Lithium-Ion Battery Pack Capacity Inconsistency Using Magnetic Field Scanning Imaging. Small Methods 2019, 6, 2101358. [Google Scholar] [CrossRef]
- Deng, Z.; Huang, Z.; Shen, Y.; Huang, Y.; Ding, H.; Luscombe, A.; Johnson, M.; Harlow, J.E.; Gauthier, R.; Dahn, J.R. Ultrasonic Scanning to Observe Wetting and “Unwetting” in Li-Ion Pouch Cells. Joule 2020, 4, 2017–2029. [Google Scholar] [CrossRef]
- Zhang, S.S. Problems and their origins of Ni-rich layered oxide cathode materials. Energy Storage Mater. 2020, 24, 247–254. [Google Scholar] [CrossRef]
- Ryu, H.H.; Park, K.J.; Yoon, C.S.; Sun, Y.K. Capacity Fading of Ni-Rich Li[NixCoyMn1–x–y]O2 (0.6 ≤ x ≤ 0.95) Cathodes for High-Energy-Density Lithium-Ion Batteries: Bulk or Surface Degradation? Chem. Mater. 2018, 3, 1155–1163. [Google Scholar] [CrossRef]
- Li, H.; Ji, W.; He, Z.; Zhang, Y.; Zhao, J. Distinct capacity fade modes of Nickel-rich/Graphite-SiOx power lithium ion battery. J. Energy Storage 2022, 47, 103830. [Google Scholar] [CrossRef]
- Mendoza-Hernandez, O.S.; Hosono, E.; Asakura, D.; Matsuda, H.; Shironita, S.; Umeda, M.; Sone, Y. Impact of Calendar Degradation on the Performance of LiFePO4—Graphite Li-Ion Cells during Charge-Discharge Cycling at −5 °C. J. Electrochem. Soc. 2019, 166, A3525. [Google Scholar] [CrossRef]
- Mouravieff, C. Unlocking cell chemistry evolution with operando fibre optic infrared spectroscopy in commercial Na(Li)-ion batteries. Nature Energy 2022. [Google Scholar] [CrossRef]
- Peng, J.; Zhou, X.; Jia, S.; Jin, Y.; Xu, S.; Chen, J. High precision strain monitoring for lithium ion batteries based on fiber Bragg grating sensors. J. Power Sources 2019, 433, 226692. [Google Scholar] [CrossRef]
- Scharf, J.; Chouchane, M.; Finegan, D.P.; Lu, B.; Redquest, C.; Kim, M.-C.; Yao, W.; Franco, A.A.; Gostovic, D.; Liu, Z.; et al. Bridging nano- and microscale X-ray tomography for battery research by leveraging artificial intelligence. Nat. Nanotechnol. 2022, 17, 446–459. [Google Scholar] [CrossRef]
- Gayon-Lombardo, A.; Mosser, L.; Brandon, N.P.; Cooper, S.J. Pores for thought: Generative adversarial networks for stochastic reconstruction of 3D multi-phase electrode microstructures with periodic boundaries. NPJ Comput. Mater. 2020, 6, 82. [Google Scholar] [CrossRef]
- Taiwo, O.O.; Paz-García, J.M.; Hall, S.A.; Heenan, T.M.; Finegan, D.P.; Mokso, R.; Villanueva-Pérez, P.; Patera, A.; Brett, D.J.; Shearing, P.R. Microstructural degradation of silicon electrodes during lithiation observed via operando X-ray tomographic imaging. J. Power Sources 2017, 342, 904–912. [Google Scholar] [CrossRef]
- Pastor-Fernández, C.; Uddin, K.; Chouchelamane, G.H.; Widanage, W.D.; Marco, J. A Comparison between Electrochemical Impedance Spectroscopy and Incremental Capacity-Differential Voltage as Li-ion Diagnostic Techniques to Identify and Quantify the Effects of Degradation Modes within Battery Management Systems. J. Power Sources 2017, 360, 301–318. [Google Scholar] [CrossRef]
- Gordon, I.J.; Genies, S.; Larbi, G.S.; Boulineau, A.; Daniel, L.; Alias, M. Original implementation of Electrochemical Impedance Spectroscopy (EIS) in symmetric cells: Evaluation of post-mortem protocols applied to characterize electrode materials for Li-ion batteries. J. Power Sources 2016, 307, 788–795. [Google Scholar] [CrossRef]
- Gaberšček, M. Understanding Li-Based Battery Materials Via Electrochemical Impedance Spectroscopy. Nat. Commun. 2021, 12, 6513. [Google Scholar] [CrossRef]
- Teliz, E.; Zinola, C.F.; Díaz, V. Identification and quantification of ageing mechanisms in Li-ion batteries by Electrochemical impedance spectroscopy. Electrochim. Acta 2022, 426, 140801. [Google Scholar] [CrossRef]
- Jiang, B.; Zhu, J.; Wang, X.; Wei, X.; Shang, W.; Dai, H. A comparative study of different features extracted from electrochemical impedance spectroscopy in state of health estimation for lithium-ion batteries. Appl. Energy 2022, 322, 119502. [Google Scholar] [CrossRef]
- R-Smith, N.A.-Z.; Leitner, M.; Alic, I.; Toth, D.; Kasper, M.; Romio, M.; Surace, Y.; Jahn, M.; Kienberger, F.; Ebner, A.; et al. Assessment of lithium ion battery ageing by combined impedance spectroscopy, functional microscopy and finite element modelling. J. Power Sources 2021, 512, 230459. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J. An overview of modification strategies to improve LiNi0.8Co0.1Mn0.1O2 (NCM811) cathode performance for automotive lithium-ion batteries. eTransportation 2021, 7, 100105. [Google Scholar] [CrossRef]
- Sun, H.H.; Kim, U.-H.; Park, J.-H.; Park, S.-W.; Seo, D.-H.; Heller, A.; Mullins, C.B.; Yoon, C.S.; Sun, Y.-K. Transition metal-doped Ni-rich layered cathode materials for durable Li-ion batteries. Nat. Commun. 2021, 12, 6552. [Google Scholar] [CrossRef]
- Kim, U.-H.; Kuo, L.-Y.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.-K. Quaternary Layered Ni-Rich NCMA Cathode for Lithium-Ion Batteries. ACS Energy Lett. 2019, 4, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.-R.; Zhang, R.; Deng, L.; Yi, T.-F.; Ye, M.-F.; Yao, J.-H.; Dai, C.-S. Lithium-Ion Insertion Kinetics of Na-Doped LiFePO4 as Cathode Materials for Lithium-Ion Batteries. Metall. Mater. Trans. E 2015, 2, 33–38. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, Y.-J.; Deng, J.-L.; Luo, G.-Y.; Wu, F.-Z.; Mai, Y.; Dai, X.-Y.; Li, J.-Q. Effect of doped Mn on improving the electrochemical performance of LiFePO4. J. Mater. Sci. Mater. Electron. 2020, 31, 2887–2894. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Hu, X.; Tian, H.; Zhang, Y.; Yang, X. Realization of Ti Doping by Electrostatic Assembly to Improve the Stability of LiCoO2 Cycled to 4.5 V. J. Electrochem. Soc. 2019, 166, A1793. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, Y.; Amine, K.; Sun, Y.-K. Role of surface coating on cathode materials for lithium-ion batteries. J. Mater. Chem. 2010, 20, 7606–7612. [Google Scholar] [CrossRef]
- Nisar, U.; Muralidharan, N.; Essehli, R.; Amin, R.; Belharouak, I. Valuation of Surface Coatings in High-Energy Density Lithium-ion Battery Cathode Materials. Energy Storage Mater. 2021, 38, 309–328. [Google Scholar] [CrossRef]
- Hall, D.S.; Gauthier, R.B.; Eldesoky, A.; Murray, V.S.; Dahn, J.R. New Chemical Insights into the Beneficial Role of Al2O3 Cathode Coatings in Lithium-ion Cells. ACS Appl. Mater. Interfaces 2019, 11, 14095–14100. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Ren, D.; Hsu, H.; Xu, G.-L.; Hou, J.; Wang, L.; Feng, X.; Lu, L.; Xu, W.; et al. Toward a high-voltage fast-charging pouch cell with TiO2 cathode coating and enhanced battery safety. Nano Energy 2020, 71, 104643. [Google Scholar] [CrossRef]
- Yi, D.; Cui, X.; Li, N.; Zhang, L.; Yang, D. Enhancement of Electrochemical Performance of LiFePO4@C by Ga Coating. ACS Omega 2020, 5, 9752–9758. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Zhang, Y.; Peng, S.; Zeng, J.; Zhao, J. Superiority of Single-Crystal to Polycrystalline LiNixCoyMn1–x–yO2 Cathode Materials in Storage Behaviors for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 14938–14948. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, J.; Liang, Z.; Potapenko, H.; Zhou, M.; Tang, X.; Zhong, S. The use of a single-crystal nickel-rich layered NCM cathode for excellent cycle performance of lithium-ion batteries. New J. Chem. 2021, 45, 3652–3659. [Google Scholar] [CrossRef]
- Li, F.; Kong, L.; Sun, Y.; Jin, Y.; Hou, P. Micron-sized monocrystalline LiNi1/3Co1/3Mn1/3O2 as high-volumetric-energy-density cathode for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 12344–12352. [Google Scholar] [CrossRef]
- Guo, J.; Li, W. Synthesis of Single-Crystal LiNi0.7Co0.15Mn0.15O2 Materials for Li-Ion Batteries by a Sol–Gel Method. ACS Appl. Energy Mater. 2022, 5, 397–406. [Google Scholar] [CrossRef]
- Klein, S.; Bärmann, P.; Fromm, O.; Borzutzki, K.; Reiter, J.; Fan, Q.; Winter, M.; Placke, T.; Kasnatscheew, J. Prospects and limitations of single-crystal cathode materials to overcome cross-talk phenomena in high-voltage lithium ion cells. J. Mater. Chem. A 2021, 9, 7546–7555. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Y.; Ou, X.; Zhang, J.; Zhang, B.; Wang, D.; Hu, G. Unravelling the influence of quasi single-crystalline architecture on high-voltage and thermal stability of LiNi0.5Co0.2Mn0.3O2 cathode for lithium-ion batteries. Chem. Eng. J. 2020, 393, 124709. [Google Scholar] [CrossRef]
- Huang, S.; Huang, X.; Huang, Y.; He, X.; Zhuo, H.; Chen, S. Rational Design of Effective Binders for LiFePO4 Cathodes. Polymers 2021, 13, 3146. [Google Scholar] [CrossRef]
- Cholewinski, A.; Si, P.; Uceda, M.; Pope, M.; Zhao, B. Polymer Binders: Characterization and Development toward Aqueous Electrode Fabrication for Sustainability. Polymers 2021, 13, 631. [Google Scholar] [CrossRef]
- Huang, S.; Shao, Z.; Wang, D.; Wang, W.; Wang, F.; Wang, J. Enhanced Electrochemical Properties of LiFePO4 Cathode Using Waterborne Lithiated Ionomer Binder in Li-Ion Batteries with Low Amount. ACS Sustain. Chem. Eng. 2018, 6, 12650–12657. [Google Scholar] [CrossRef]
- Qiu, L.; Shao, Z.; Wang, D.; Wang, F.; Wang, W.; Wang, J. Carboxymethyl cellulose lithium (CMC-Li) as a novel binder and its electrochemical performance in lithium-ion batteries. Cellulose 2014, 21, 2789–2796. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, X.; Yu, G. Material and Structural Design of Novel Binder Systems for High-Energy, High-Power Lithium-Ion Batteries. Acc. Chem. Res. 2017, 50, 2642–2652. [Google Scholar] [CrossRef] [Green Version]
- Xin, C.; Gao, J.; Luo, R.; Zhou, W. Prelithiation Reagents and Strategies on High Energy Lithium-Ion Batteries. Chem. Eur. J. 2022, 28, e202104282. [Google Scholar] [CrossRef]
- Heiskanen, S.K.; Kim, J.; Lucht, B.L. Generation and Evolution of the Solid Electrolyte Interphase of Lithium-Ion Batteries. Joule 2019, 3, 2322–2333. [Google Scholar] [CrossRef]
- Prado, A.Y.R.; Rodrigues, M.-T.F.; Trask, S.E.; Shaw, L.; Abraham, D.P. Electrochemical Dilatometry of Si-Bearing Electrodes: Dimensional Changes and Experiment Design. J. Electrochem. Soc. 2020, 167, 160551. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Z.; Liu, Y.; Xie, J. Achieving SEI preformed graphite in flow cell to mitigate initial lithium loss. Carbon 2022, 196, 589–595. [Google Scholar] [CrossRef]
- Choi, N.-S.; Yew, K.H.; Lee, K.Y.; Sung, M.; Kim, H.; Kim, S.-S. Effect of fluoroethylene carbonate additive on interfacial properties of silicon thin-film electrode. J. Power Sources 2006, 161, 1254–1259. [Google Scholar] [CrossRef]
- Choi, S.; Kwon, T.-W.; Coskun, A.; Choi, J.W. Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries. Science 2017, 357, 279–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yang, Z.; Mao, B.; Wang, Y.; Jiang, Y.; Cao, M. Transgenic Engineering on Silicon Surfaces Enables Robust Interface Chemistry. ACS Energy Lett. 2022, 7, 2781–2791. [Google Scholar] [CrossRef]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.-W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Lai, Y.; Yang, Z.; Yang, Q.; Liu, Y.; Zheng, Z.; Liu, Y.; Sun, Y.; Zhong, B.; et al. Revisiting the Preparation Progress of Nano-Structured Si Anodes toward Industrial Application from the Perspective of Cost and Scalability. Adv. Energy Mater. 2022, 12, 2102181. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Zhou, J.; Lin, C.; Lv, F.; Wang, K.; Feng, J.; Xu, Z.; Li, J.; Guo, S. Hollow Si/SiOx nanosphere/nitrogen-doped carbon superstructure with a double shell and void for high-rate and long-life lithium-ion storage. J. Mater. Chem. A 2018, 6, 8039–8046. [Google Scholar] [CrossRef]
- Hwang, T.H.; Lee, Y.M.; Kong, B.-S.; Seo, J.-S.; Choi, J.W. Electrospun Core–Shell Fibers for Robust Silicon Nanoparticle-Based Lithium Ion Battery Anodes. Nano Lett. 2012, 12, 802–807. [Google Scholar] [CrossRef]
- An, W.; Gao, B.; Mei, S.; Xiang, B.; Fu, J.; Wang, L.; Zhang, Q.; Chu, P.K.; Huo, K. Scalable synthesis of ant-nest-like bulk porous silicon for high-performance lithium-ion battery anodes. Nat. Commun. 2019, 10, 1447. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Wang, C. A polymer scaffold binder structure for high capacity silicon anode of lithium-ion battery. Chem. Commun. 2010, 46, 1428–1430. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A Major Constituent of Brown Algae for Use in High-Capacity Li-Ion Batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef]
- Su, Y.; Feng, X.; Zheng, R.; Lv, Y.; Wang, Z.; Zhao, Y.; Shi, L.; Yuan, S. Binary Network of Conductive Elastic Polymer Constraining Nanosilicon for a High-Performance Lithium-Ion Battery. ACS Nano 2021, 15, 14570–14579. [Google Scholar] [CrossRef]
- Liu, G.; Xun, S.; Vukmirovic, N.; Song, X.; Olalde-Velasco, P.; Zheng, H.; Battaglia, V.S.; Wang, L.; Yang, W. Polymers with Tailored Electronic Structure for High Capacity Lithium Battery Electrodes. Adv. Mater. 2011, 23, 4679–4683. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Yu, G.; Zhai, D.; Lee, H.R.; Zhao, W.; Liu, N.; Wang, H.; Tee, B.C.K.; Shi, Y.; Cui, Y.; et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef] [Green Version]
- Ling, M.; Qiu, J.; Li, S.; Yan, C.; Kiefel, M.J.; Liu, G.; Zhang, S. Multifunctional SA-PProDOT Binder for Lithium Ion Batteries. Nano Lett. 2015, 15, 4440–4447. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’Kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L.; et al. Lithium-ion battery fast charging: A review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Trentadue, G.; Lucas, A.; Otura, M.; Pliakostathis, K.; Zanni, M.; Scholz, H. Evaluation of Fast Charging Efficiency under Extreme Temperatures. Energies 2018, 11, 1937. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Shang, F.; Salameh, M.; Krishnamurthy, M. Challenges and Advancements in Fast Charging Solutions for EVs: A Technological Review. In Proceedings of the 2018 IEEE Transportation Electrification Conference and Expo (ITEC), Long Beach, CA, USA, 13–14 June 2018; pp. 695–701. [Google Scholar] [CrossRef]
- Waldmann, T.; Kasper, M.; Wohlfahrt-Mehrens, M. Optimization of Charging Strategy by Prevention of Lithium Deposition on Anodes in high-energy Lithium-ion Batteries—Electrochemical Experiments. Electrochim. Acta 2015, 178, 525–532. [Google Scholar] [CrossRef]
- Anseán, D.; Dubarry, M.; Devie, A.; Liaw, B.; García, V.; Viera, J.; González, M. Fast charging technique for high power LiFePO4 batteries: A mechanistic analysis of aging. J. Power Sources 2016, 321, 201–209. [Google Scholar] [CrossRef]
- Abdel-Monem, M.; Trad, K.; Omar, N.; Hegazy, O.; van den Bossche, P.; van Mierlo, J. Influence analysis of static and dynamic fast-charging current profiles on ageing performance of commercial lithium-ion batteries. Energy 2017, 120, 179–191. [Google Scholar] [CrossRef]
- Zhang, S.S. The effect of the charging protocol on the cycle life of a Li-ion battery. J. Power Sources 2006, 161, 1385–1391. [Google Scholar] [CrossRef]
- Yang, X.-G.; Vishnugopi, B.S.; Mukherjee, P.P.; Wang, W.; Sun, F.; Wang, C.-Y. Advancements in extreme fast charging to foster sustainable electrification. One Earth 2022, 5, 216–219. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, J.; Amine, K.; Pan, F. Depolarization effect to enhance the performance of lithium ions batteries. Nano Energy 2017, 33, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Murphy, E.; Winnick, J.; Kohl, P.A. The effects of pulse charging on cycling characteristics of commercial lithium-ion batteries. J. Power Sources 2001, 102, 302–309. [Google Scholar] [CrossRef]
- Qin, Y.; Zuo, P.; Chen, X.; Yuan, W.; Huang, R.; Yang, X.; Du, J.; Lu, L.; Han, X.; Ouyang, M. An ultra-fast charging strategy for lithium-ion battery at low temperature without lithium plating. J. Energy Chem. 2022, 72, 442–452. [Google Scholar] [CrossRef]
- Reis, M.S.; Jiang, B. Predicting the lifetime of Lithium–Ion batteries: Integrated feature extraction and modeling through sequential Unsupervised-Supervised Projections (USP). Chem. Eng. Sci. 2022, 252, 117510. [Google Scholar] [CrossRef]
- Zhang, J.; Lee, J. A review on prognostics and health monitoring of Li-ion battery. J. Power Sources 2011, 196, 6007–6014. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, R.; He, H.; Qu, X.; Pecht, M. Aging characteristics-based health diagnosis and remaining useful life prognostics for lithium-ion batteries. eTransportation 2019, 1, 100004. [Google Scholar] [CrossRef]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of Galvanostatic Charge and Discharge of the Lithium/Polymer/Insertion Cell. J. Electrochem. Soc. 1993, 140, 1526. [Google Scholar] [CrossRef]
- Xiong, R.; Li, L.; Li, Z.; Yu, Q.; Mu, H. An electrochemical model based degradation state identification method of Lithium-ion battery for all-climate electric vehicles application. Appl. Energy 2018, 219, 264–275. [Google Scholar] [CrossRef]
- Lin, C.; Mu, H.; Xiong, R.; Cao, J. Multi-Model Probabilities Based State Fusion Estimation Method of Lithium-Ion Battery for Electric Vehicles: State-of-energy—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0306261916306626 (accessed on 24 November 2022).
- Jin, X.; Vora, A.; Hoshing, V.; Saha, T.; Shaver, G.; García, R.E.; Wasynczuk, O.; Varigonda, S. Physically-based reduced-order capacity loss model for graphite anodes in Li-ion battery cells. J. Power Sources 2017, 342, 750–761. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Wang, S.; Pecht, M.; Zhao, L.; Ye, Z. Interacting multiple model particle filter for prognostics of lithium-ion batteries. Microelectron. Reliab. 2017, 70, 59–69. [Google Scholar] [CrossRef]
- Sulzer, V.; Mohtat, P.; Aitio, A.; Lee, S.; Yeh, Y.T.; Steinbacher, F.; Khan, M.U.; Lee, J.W.; Siegel, J.B.; Stefanopoulou, A.G.; et al. The challenge and opportunity of battery lifetime prediction from field data. Joule 2021, 5, 1934–1955. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, X.; Pan, R.; Wang, Y.; Chen, Z. A novel Gaussian process regression model for state-of-health estimation of lithium-ion battery using charging curve. J. Power Sources 2018, 384, 387–395. [Google Scholar] [CrossRef]
- Wang, J.; Liu, P.; Hicks-Garner, J.; Sherman, E.; Soukiazian, S.; Verbrugge, M.; Tataria, H.; Musser, J.; Finamore, P. Cycle-life model for graphite-LiFePO4 cells. J. Power Sources 2011, 196, 3942–3948. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, C.; Chen, Z. An online method for lithium-ion battery remaining useful life estimation using importance sampling and neural networks. Appl. Energy 2016, 173, 134–140. [Google Scholar] [CrossRef]
- Su, L.; Zhang, J.; Huang, J.; Ge, H.; Li, Z.; Xie, F.; Liaw, B.Y. Path dependence of lithium ion cells aging under storage conditions. J. Power Sources 2016, 315, 35–46. [Google Scholar] [CrossRef]
- Song, L.; Zhang, K.; Liang, T.; Han, X.; Zhang, Y. Intelligent state of health estimation for lithium-ion battery pack based on big data analysis. J. Energy Storage 2020, 32, 101836. [Google Scholar] [CrossRef]
- Finegan, D.P.; Cooper, S.J. Battery Safety: Data-Driven Prediction of Failure. Joule 2019, 3, 2599–2601. [Google Scholar] [CrossRef]

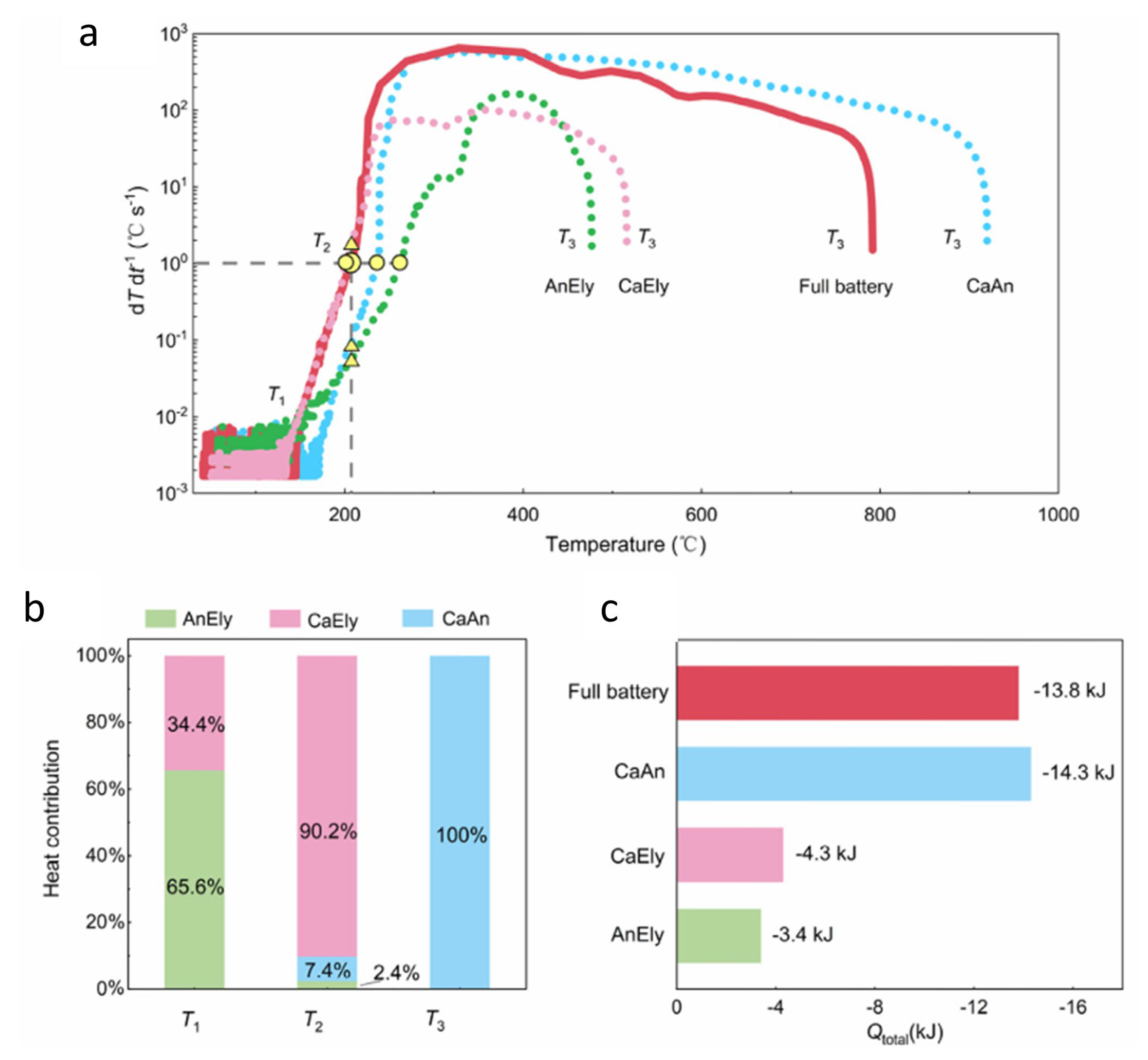

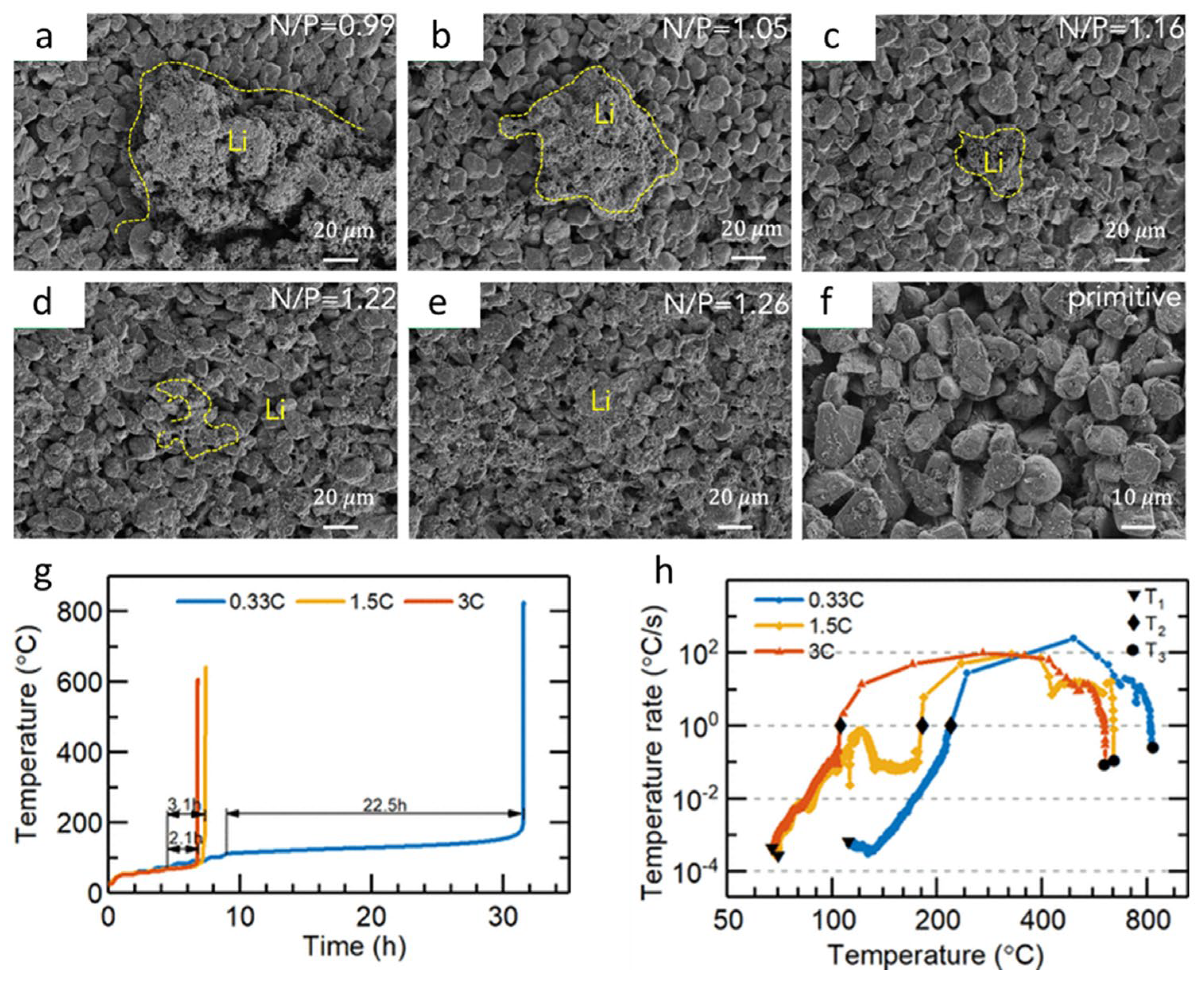

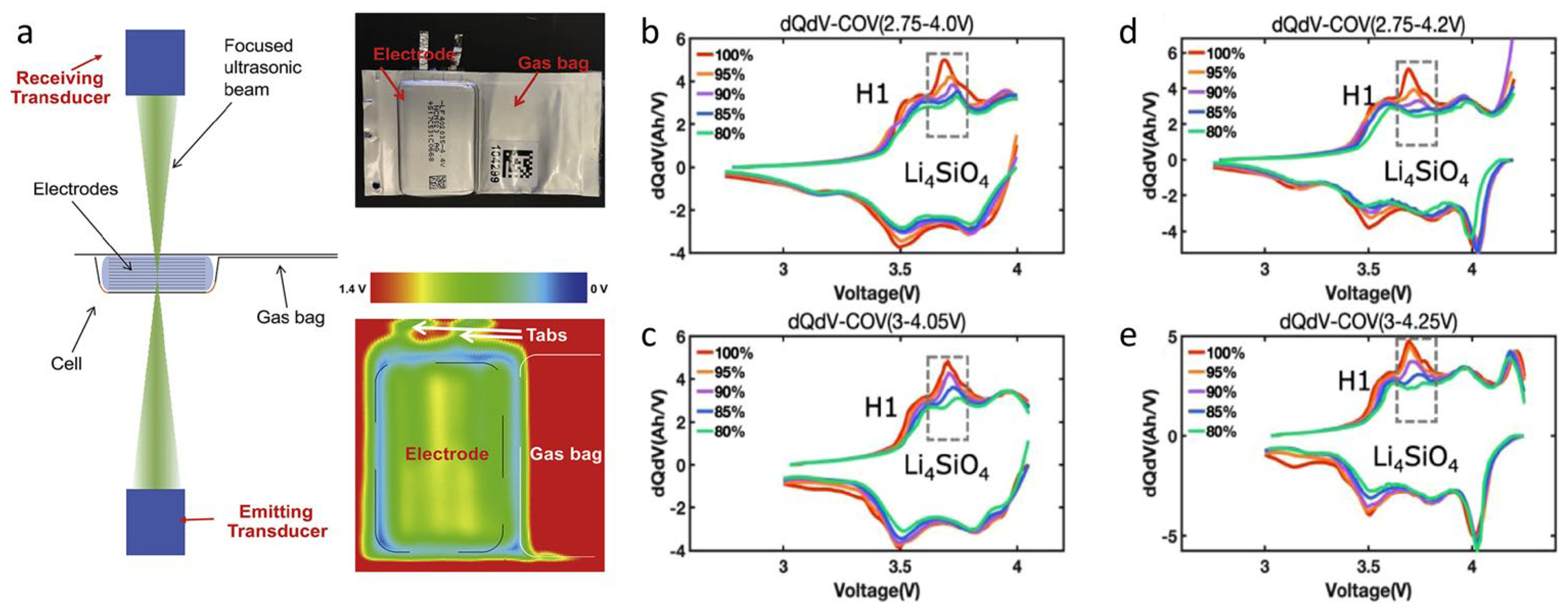

| Cathode Materials | Theoretical Capacity (mAh·g−1) | Potential (V vs. Li/Li+) | Crystal Structure |
|---|---|---|---|
| LMO | 148 | 3.0–4.3 | Spinel structure |
| LFP | 170 | 3.2–3.7 | Olivine structure |
| LCO | 274 | 3.0–4.5 | layered structure |
| NCM | 273–285 | 2.5–4.6 | layered structure |
| NCA | 279 | 3.0–4.2 | layered structure |
| V2O5 | 294 | 2.0–4.0 | layered structure |
| Anode Materials | Theoretical Capacity (mAh·g−1) | Potential (V vs Li/Li+) | Volume Expansion Rate (%) |
|---|---|---|---|
| Graphite | 372 | 0.01–0.2 | 12 |
| Li4Ti5O12 | 175 | 1.4–1.6 | 1 |
| Si | 4200 | 0.2 | 300 |
| Sn | 994 | 0.6–0.1 | 260 |
| Electrode Material | Method of Modification | Performance | Reference |
|---|---|---|---|
| LCO | All-fluorinated electrolyte improving CEI | The fabricated 4.5 V high loading (4 mAh cm−2) LiCoO2||graphite full cell delivered an energy density of 430 Wh kg−1, with an excellent capacity retention of 80% after 500 cycles. | [26] |
| NCM811 | Facile molten-salt route | NCM811 material realizes high capacity of 92% after 200 cycles at 0.5 C. | [40] |
| NC90 | Doping with various dopants (Mg2+, Al3+, Ti4+, Ta5+, Mo6+) | After doping Ta5+ and Mo6+ for 3000 cycles at 200 mA g−1, NC90 can still maintain about 81.5% of its initial specific capacity. | [99] |
| NCM | Doping Al to NCM materials | The capacity of NCMA cathode is 228 mAh g−1, 85% of the initial capacity after 1000 cycles. | [100] |
| LFP | Doping Na to LFP materials | Na doping increased the diffusion coefficient of LFP (Li1−xNaxFePO4 (x = 0, 0.05, 0.1, 0.2) 1.21 × 10−16, 1.06 × 10−14, 7.95 × 10−15, and 9.17 × 10−15 cm2 s−1, respectively). | [101] |
| LFP | Doping Mn to LFP materials | The LiFe0.98Mn0.02PO4/C specific discharge capacity reached 156.0 mAh g−1 at a rate of 0.1 C, even at a high rate of 5 C, it can still reach 110.0 mAh g−1. | [102] |
| LCO | Doping Ti to LCO materials | Ti-doped LiCoO2 exhibits a capacity of 205 mAh g−1 at a cut-off voltage of 4.5 V. After 200 cycles, the capacity retention rate is 97%. | [103] |
| NCM523 | TiO2-coated NCM cathode | The trigger temperature of thermal runaway for the battery using TiO2-coated NCM523 as cathode material was 257 °C, which was higher than NCM523 cathode (251 °C). | [107] |
| LFP | Ga-coated LFP cathode | The discharge capacity of 152.6 mAh g−1 at 1 C after 100 cycles and a discharge capacity retention rate of 98.77%. | [108] |
| Modified Binder | Electrochemical Performance | Reference |
|---|---|---|
| Incorporation 5% polyrotaxane to conventional polyacrylic acid binder | Average Coulombic efficiency of 99.92% in the cycle numbers of 2 to 50. | [125] |
| Sodium carboxymethyl cellulose porous scaffold | At 250 mA g−1 charge/discharge rate, the battery can retain capacity of 930 mAh g−1 after 85 cycles. | [132] |
| Mixing Si nanopowder with alginate | At a current density of 4200 mA/g, the reversible Li extraction specific capacity of an alginate-based Si anode is in the range of 1700 to 2000 mAh g−1. | [133] |
| A multifunctional polymeric binder synthesized by the cross-linking of conducting polymer and poly (ether thioureas). | 2081 mAh g−1 after 300 cycles and 908 mAh g−1 at 8 A g−1. | [134] |
| Novel conducting polymer based on polyfluorene polymers and introduced carbonyl and methylbenzoic ester | Composite anodes based on this polymer and commercial Si particles exhibit 2100 mAh g−1 in Si after 650 cycles without any conductive additive. | [135] |
| Multifunctional polyaniline hydrogel | 83% capacitance retention after 10,000 cycles | [136] |
| Modified sodium alginate | Without the conductive additives, the resultant batteries have achieved the theoretical specific capacity of LiFePO4 (C/10) and 120 mAh g−1 at 1 C for more than 400 cycles. | [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Li, H.; He, Z.; Ji, W.; Zeng, J.; Li, X.; Zhang, Y.; Zhang, P.; Zhao, J. Electrochemical Failure Results Inevitable Capacity Degradation in Li-Ion Batteries—A Review. Energies 2022, 15, 9165. https://doi.org/10.3390/en15239165
Li W, Li H, He Z, Ji W, Zeng J, Li X, Zhang Y, Zhang P, Zhao J. Electrochemical Failure Results Inevitable Capacity Degradation in Li-Ion Batteries—A Review. Energies. 2022; 15(23):9165. https://doi.org/10.3390/en15239165
Chicago/Turabian StyleLi, Wei, Hang Li, Zheng He, Weijie Ji, Jing Zeng, Xue Li, Yiyong Zhang, Peng Zhang, and Jinbao Zhao. 2022. "Electrochemical Failure Results Inevitable Capacity Degradation in Li-Ion Batteries—A Review" Energies 15, no. 23: 9165. https://doi.org/10.3390/en15239165





