Research Progress and Prospect of Carbon Dioxide Utilization and Storage Based on Unconventional Oil and Gas Development
Abstract
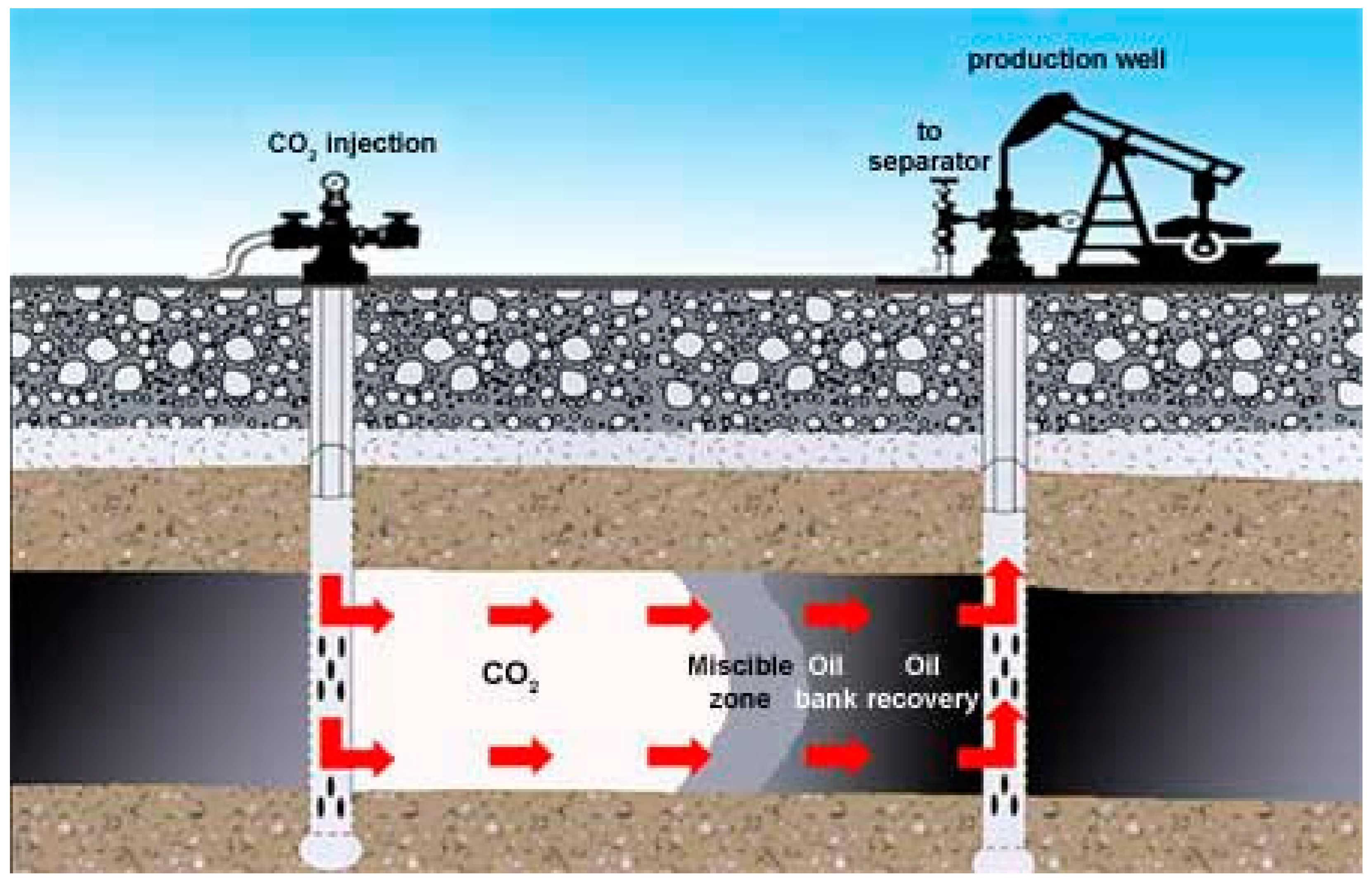
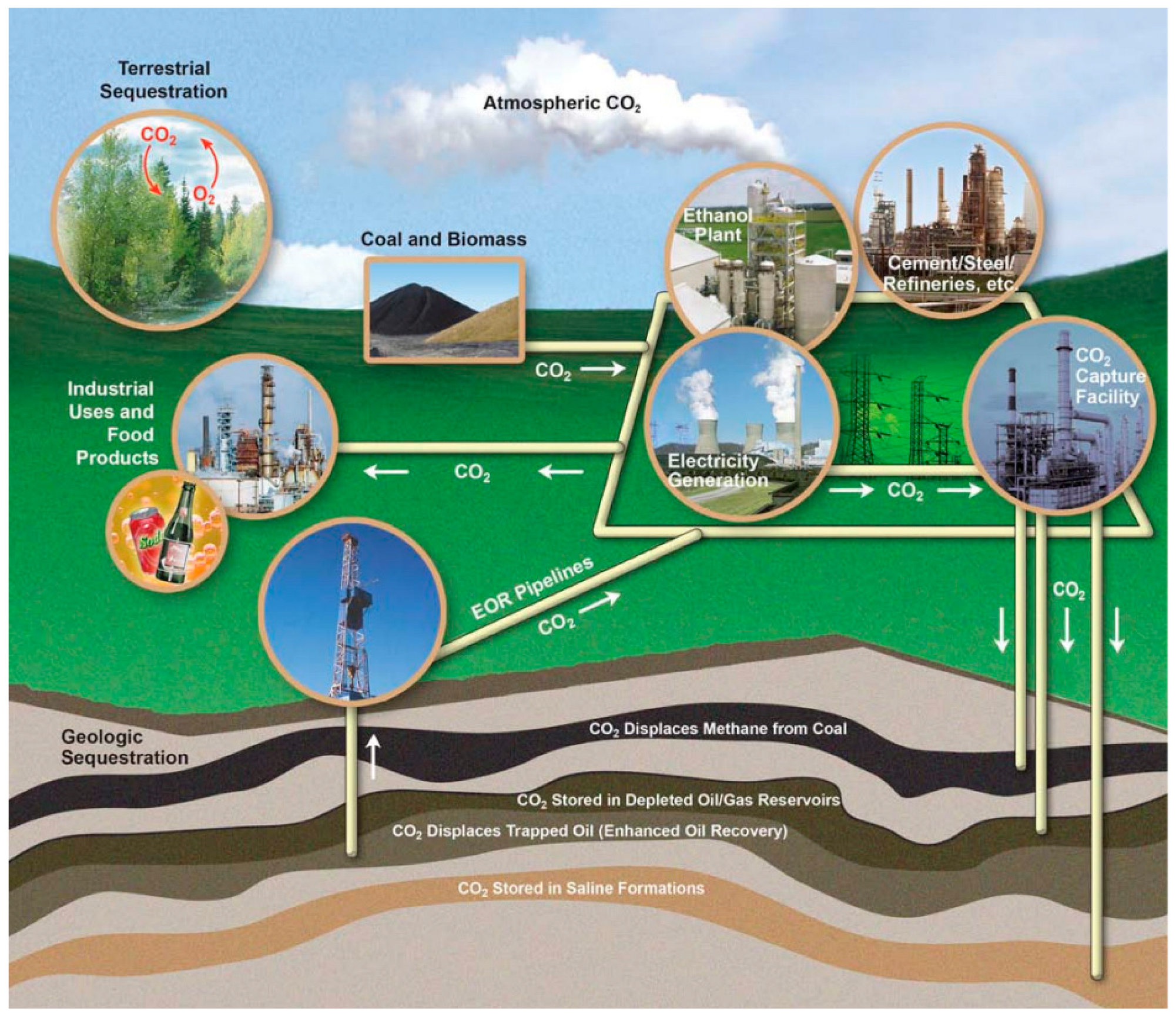
:1. Introduction to CO2-EOR-CCUS Technology in Unconventional Reservoirs
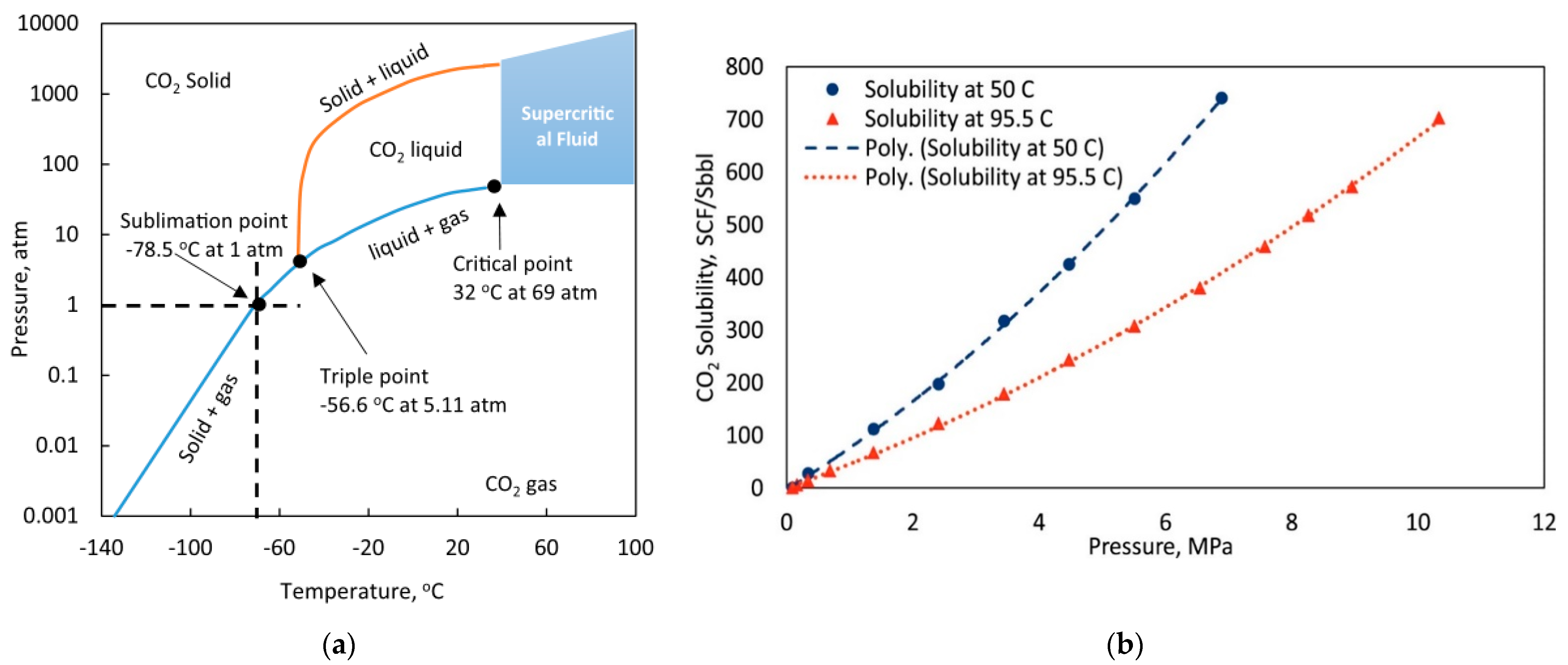
2. Study on the Influence of CO2 Storage on Formation Fluid
2.1. Dissolution, Diffusion and Storage of CO2 in Crude Oil
2.2. Dissolution, Diffusion and Storage of CO2 and Brine
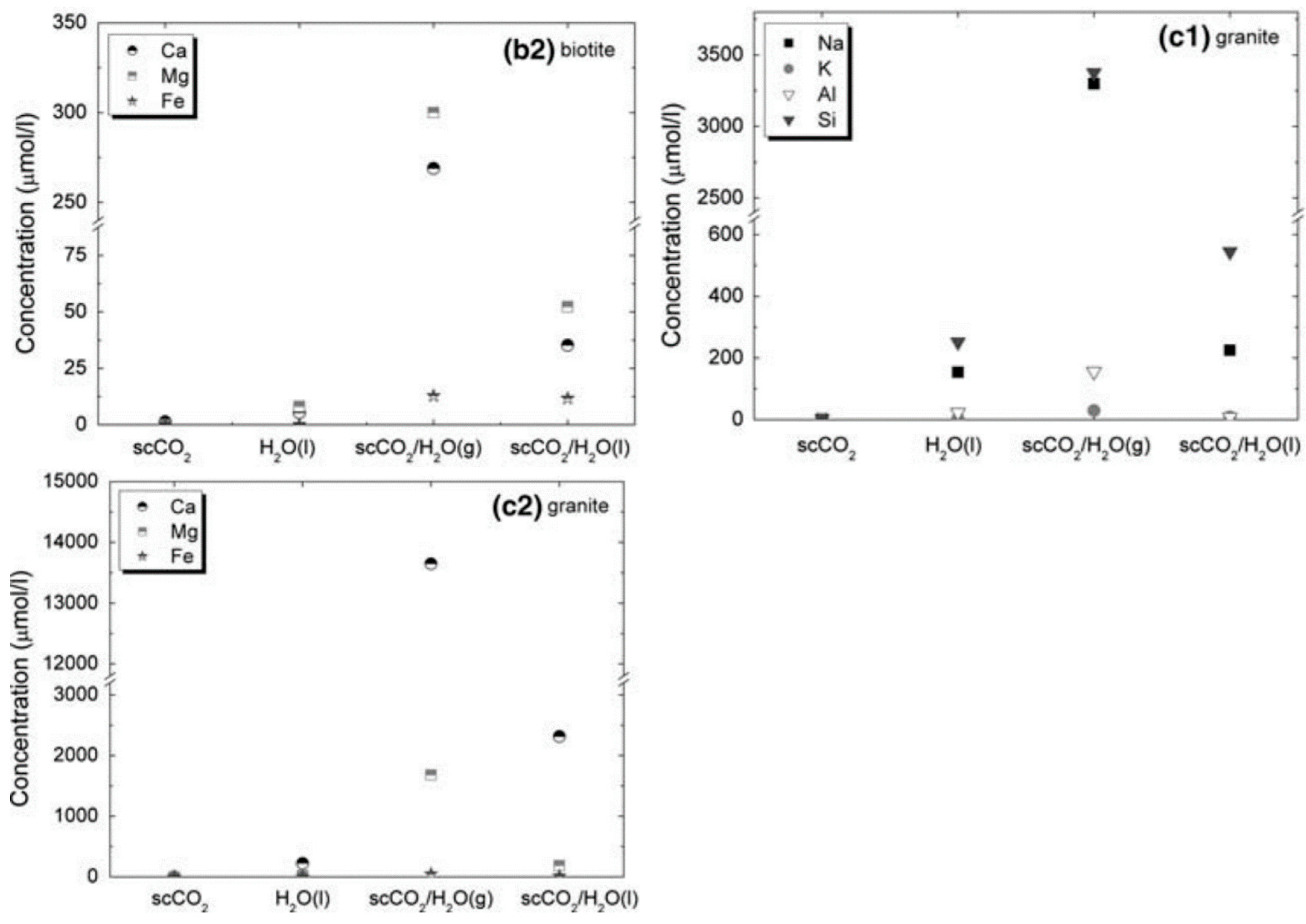
3. The Effect on Reservoir Property with the Reaction between CO2 and Rock for Unconventional Reservoir
4. Study on Leakage and Gas Detection Mechanism and Chemical Control Method in CO2 Flooding Process
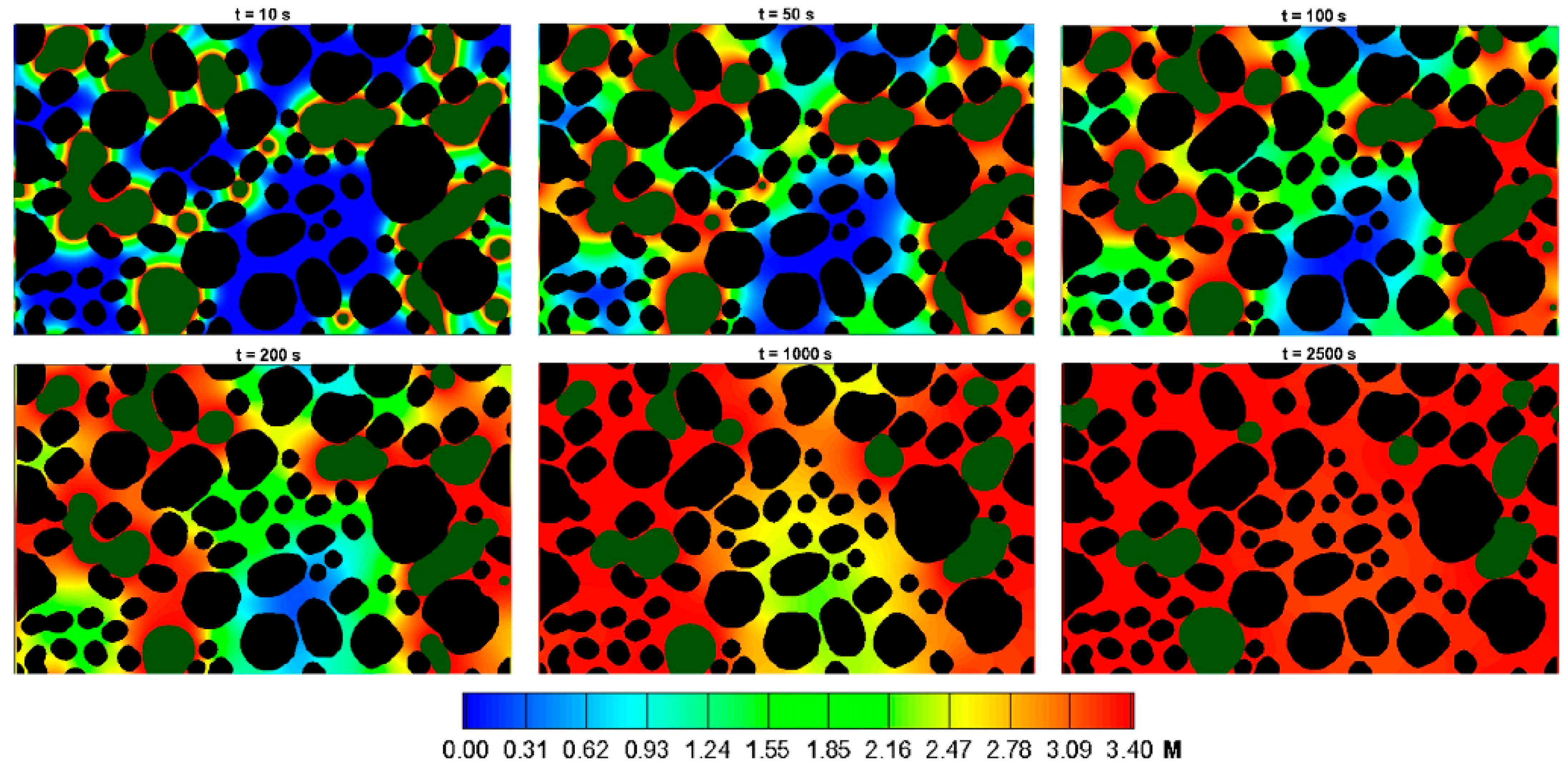
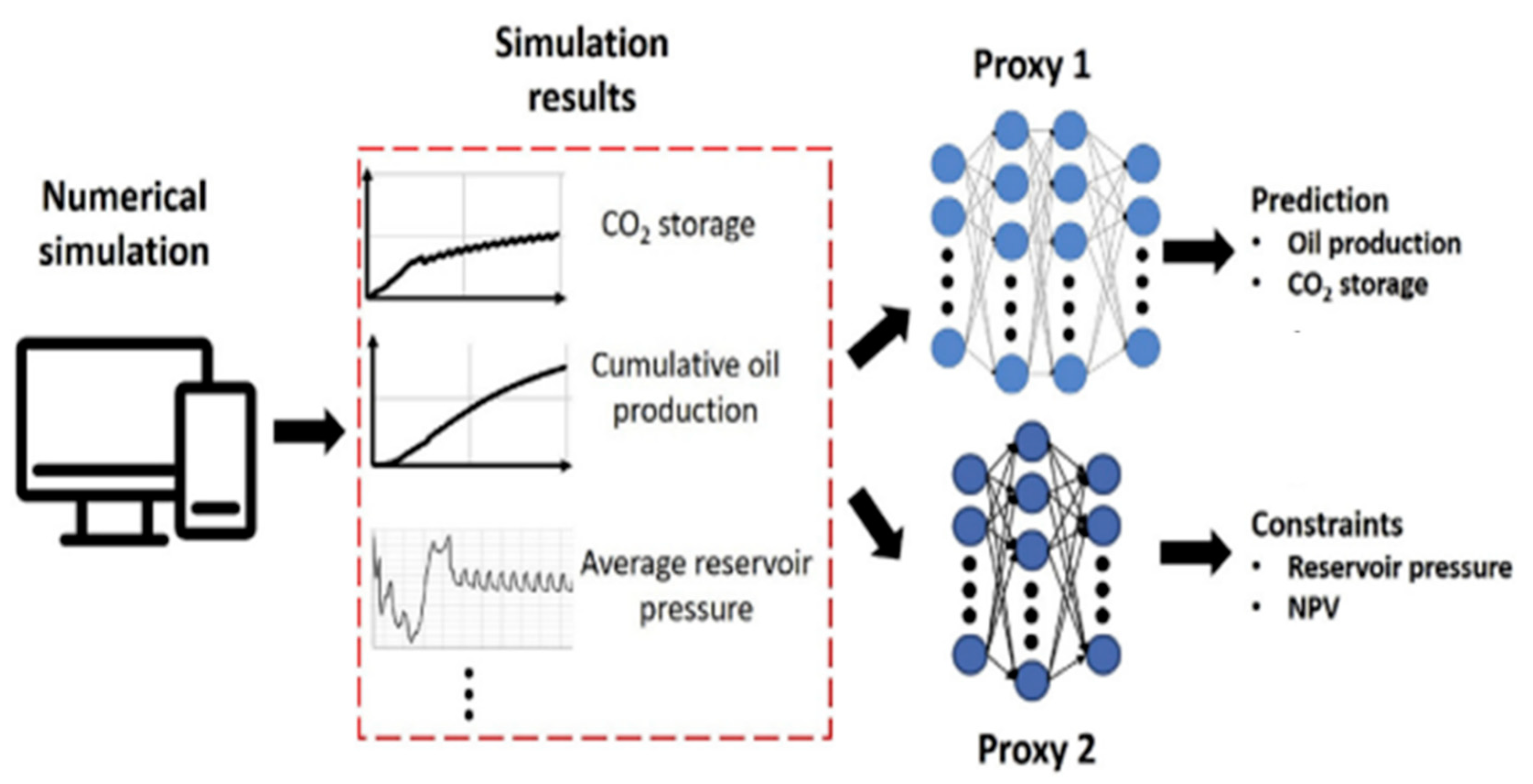
4.1. Numerical Simulation and Uncertainty Quantitative Analysis of CO2 Storage Problems
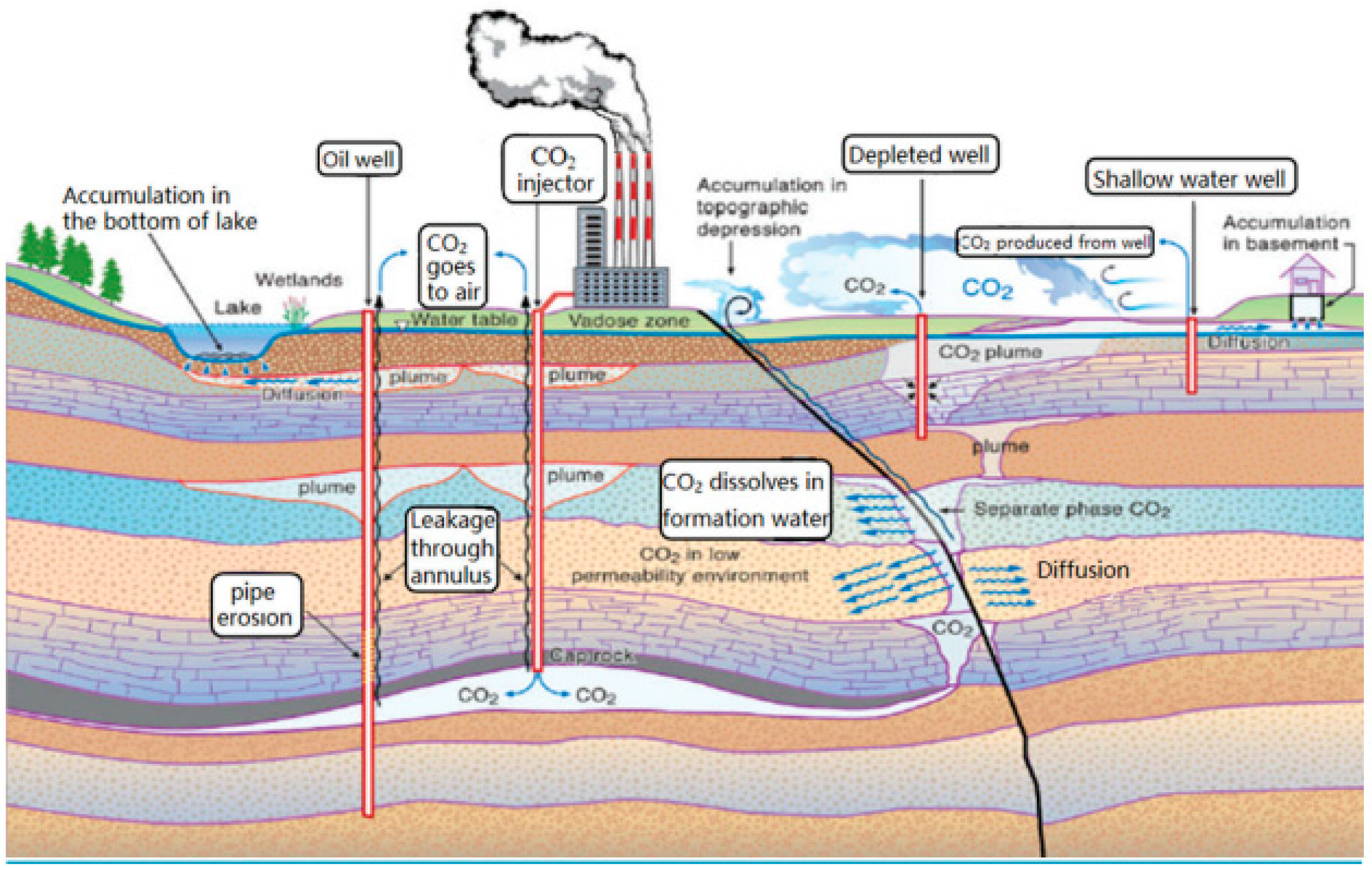
4.2. Leakage Paths and Risk Analysis of CO2 Geological Storage
- (1)
- Geological storage in oil and gas reservoirs
- (2)
- Geological storage in deep saline aquifers
- (3)
- Geological storage in coal beds
4.3. Sealing Method for CO2 Leakage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Busch, A.; Alles, S.; Gensterblum, Y.; Prinz, D.; Dewhurst, D.N.; Raven, M.D.; Stanjek, H.; Krooss, B.M. Carbon dioxide storage potential of shales. Int. J. Greenh. Gas Control 2008, 2, 297–308. [Google Scholar] [CrossRef]
- Zhao, J.; Li, C.; Yuan, X. Discussion on the ways and countermeasures of carbon dioxide reduction in China. Environ. Sci. Guide 2010, 29, 3–6. [Google Scholar]
- Qu, J.; Zeng, J. Technology, Practice and law of CO2 capture and sequestration—Analysis of legal issues in international promotion of carbon dioxide capture and sequestration. Worldw. Sci. Technol. Res. Dev. 2007, 29, 78–83. [Google Scholar]
- Ansarizadeh, M.; Dodds, K.; Gurpinar, D.; Kalfa, U.; Ramakrishnan, T.S.; Sacuta, N.; Whittaker, S. Carbon dioxide—Challenges and opportunities. Oilfield Rev. 2015, 27, 36–50. [Google Scholar]
- Blondes, M.S.; Brennan, S.T.; Merrill, M.D.; Buursink, M.L.; Warwick, P.D.; Cahan, S.M.; Corum, M.D.; Cook, T.A.; Craddock, W.H.; Devera, C.A.; et al. National Assessment of Geologic Carbon Dioxide Storage Resources Methodology Implementation; USGS Open-File Report; U.S. Geological Survey: Reston, VA, USA, 2013; p. 26. [CrossRef]
- Bradshaw, J.; Bachu, S.; Bonijoly, D.; Burruss, R.; Holloway, S.; Christensen, N.P.; Matthiessen, O.M. CO2 storage capacity estimation: Issues and development of standards. Int. J. Greenh. Gas Control 2007, 1, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, G. Carbon dioxide sequestration technology and research status. Energy Environ. 2007, 2, 33–35. [Google Scholar]
- Shen, P.; Jiang, H. Resource utilization and underground storage of enhanced recovery of greenhouse gases. Eng. Sci. 2009, 11, 54–59. [Google Scholar]
- Sun, L.; Chen, W. CO2 sequestration potential assessment of Onshore reservoirs in China. China Popul. Resour. Environ. 2012, 22, 76–81. [Google Scholar]
- Shen, P.; Liao, W. CO2 Geological Storage and Enhanced Oil Recovery Technology; Petroleum Industry Press: Beijing, China, 2009. [Google Scholar]
- Advanced Resources International (ARI). World Shale Gas Resources: An Initial Assessment of 14 Regions Outside the United States; U.S. Energy Information Administration (EIA): Washington, DC, USA, 2011.
- Advanced Resources International. Geologic, Engineering, and Economic Evaluation of the CO2 Sequestration Capacity of New York’s Gas Shales: Final Report; New York State Energy Research and Development Authority: Albany, NY, USA, 2011.
- Ahlbrandt, T.S.; Blaise, J.R.; Blystad, P.; Kelter, D.; Gabrielyants, G.; Heiberg, S.; Martinez, A.; Ross, J.G.; Slavov, S.; Subelj, A.; et al. Updated United Nations framework classification for reserves and resources of extractive industries. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 26–29 September 2004; p. 90839. [Google Scholar]
- Bachu, S. Drainage and imbibition CO2/brine relative permeability curves at in situ conditions for sandstone formations in western Canada. Energy Procedia 2013, 37, 4428–4436. [Google Scholar] [CrossRef] [Green Version]
- Bachu, S.; Bennion, D.B. Interfacial tension between CO2, freshwater and brine in the range of pressure from (2 to 27) MPa, temperature from (20 to 125) °C, and water salinity from (0 to 334,000) mg/L. J. Chem. Eng. Data 2009, 54, 765–775. [Google Scholar] [CrossRef]
- Bachu, S.; Haug, K.; Michael, K.; Buschkuehle, B.E.; Adams, J.J. Deep injection of acid–gas in western Canada. Dev. Water Sci. 2005, 52, 623–635. [Google Scholar]
- Bachu, S.; Bonijoly, D.; Bradshaw, J.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Methodology andgaps. Int. J. Greenh. Gas Control 2007, 1, 430–443. [Google Scholar] [CrossRef] [Green Version]
- Bachu, S.; Melnyk, A.; Bistran, R. Approach to evaluating the CO2 storage capacity in Devonian deep saline aquifers for emissions from oil sands operations in the Athabasca area. Energy Procedia 2014, 63, 5093–5102. [Google Scholar] [CrossRef] [Green Version]
- Bader, A.; Thibeau, S.; Vincké, O.; Jannaud, F.D.; Saysset, S.; Joffre, G.; Giger, F.; David, M.; Gimenez, M.; Dieulin, A.; et al. CO2 storage capacity evaluation in deep saline aquifers for an industrial pilot selection. Methodology and results of the France Nord project. Energy Procedia 2014, 63, 2779–2788. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.; Zhang, L.; Zhang, L. CO2 geological storage: Foreign demonstration projects and their implications for China. J. China Univ. Pet. (Ed. Nat. Sci.) 2010, 34, 94–98. [Google Scholar]
- Li, Z.; Yao, J.; Kou, J. Mixture composition effect on hydrocarbon-water transport in shale organic nanochannels. J. Phys. Chem. Lett. 2019, 10, 4291–4296. [Google Scholar] [CrossRef]
- Mehmani, A.; Kelly, S.; Torres-Verdín, C.; Balhoff, M. Residual oil saturation following gas injection in sandstones: Microfluidic quantification of the impact of pore-scale surface roughness. Fuel 2019, 251, 147–161. [Google Scholar] [CrossRef]
- Enhanced Oil Recovery Utilizing CO2 Flood Technology Overview. Available online: https://ehrsolutions.ca/CO2-enhanced-oil-recovery (accessed on 1 June 2022).
- Nguyen, P.; Carey, J.W.; Viswanathan, H.S.; Porter, M. Effectiveness of supercritical-CO2 and N2 huff-and-puff methods of enhanced oil recovery in shale fracture networks using microfluidic experiments. Appl. Energy 2018, 230, 160–174. [Google Scholar] [CrossRef]
- de Almeida, J.M.; Miranda, C.R. Improved oil recovery in nanopores: Nano IOR. Sci. Rep. 2016, 6, 28128. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.; Hou, J.; Wu, K.; Chen, Z. Phase equilibria of confined fluids in nanopores of tight and shale rocks considering the effect of capillary pressure and adsorption film. Ind. Eng. Chem. Res. 2016, 55, 798–811. [Google Scholar] [CrossRef]
- Nojabaei, B.; Johns, R.T.; Chu, L. Effect of capillary pressure on phase behavior in tight rocks and shales. SPE Reserv. Eval. Eng. 2013, 16, 281–289. [Google Scholar] [CrossRef]
- Teklu, T.W.; Alharthy, N.; Kazemi, H.; Yin, X.; Graves, R.M.; AlSumaiti, A.M. Phase behavior and minimum miscibility pressure in nanopores. SPE Reserv. Eval. Eng. 2014, 17, 396–403. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, Y.; Fu, Y.; Wu, G.; Liu, P. Optimization of CO2 flooding injection method in low permeability reservoir. Spec. Oil Gas Reserv. 2012, 19, 8284. [Google Scholar]
- Rezk, M.G.; Foroozesh, J. Phase behavior and fluid interactions of a CO2-Light oil system at high pressures and temperatures. Heliyon 2019, 5, e02057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawez, H.; Ahmed, Z. Enhanced Oil Recovery by CO2 Injection in Carbonate Reservoirs. Energy Sustain. 2014, 186, 547–558. [Google Scholar]
- Al-Dhahli, A.; Geiger, S.; Dijke, M.I. Accurate modelling of pore-scale film and layer flow for three-phase EOR in carbonate rocks with arbitrary wettability. In Proceedings of the Improved Oil Recovery Symposium, SPE-154019-MS, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Andrew, M.; Bijeljic, B.; Blunt, M.J. Pore-scale contact angle measurements at reservoir conditions using X-ray microtomography. Adv. Water Resour. 2014, 68, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.R.; Azadi, R.; Salimi, E. Capturing of interface topological changes in two-phase gasliquid flows using a coupled volume-of-fluid and level-set method. Comput. Fluids 2016, 125, 82–100. [Google Scholar] [CrossRef]
- Falk, K.; Coasne, B.A.; Pellenq, R.J.; Ulm, F.-J.; Bocquet, L. Subcontinuum mass transport of condensed hydrocarbons in nanoporous media. Nat. Commun. 2015, 6, 6949. [Google Scholar] [CrossRef] [Green Version]
- Oldenburg, C. Migration mechanisms and potential impacts of CO2 leakage and seepage. In Carbon Capture and Sequestration: Integrating Technology, Monitoring, Regulation; Wilson, E.J., Gerard, D., Eds.; Wiley-Blackwell: New York, NY, USA, 2007. [Google Scholar]
- Waseem Arshad, M.; Fosbøl, P.L.; von Solms, N.; Thomsen, K. CO2 capture with liquid-liquid phase change solvents: A thermodynamic study. Energy Procedia 2017, 114, 1671–1681. [Google Scholar] [CrossRef]
- U.S. Department of Energy’s Office of Fossil Energy. Carbon Utilization and Storage Atlas, 5th ed.; U.S. Department of Energy, Office of Fossil Energy: Peachtree Corners, GA, USA, 2012; p. 4.
- Golparvar, A.; Zhou, Y.; Wu, K.; Ma, J.; Yu, Z. A comprehensive review of pore scale modeling methodologies for multiphase flow in porous media. Adv. Geo-Energy Res. 2018, 2, 418–440. [Google Scholar] [CrossRef] [Green Version]
- Adler, P.M. Fractal Porous Media. In Transport Processes in Porous Media; Springer: Dordrecht, The Netherlands, 1991; pp. 723–743. [Google Scholar]
- Ahrenholz, B.; Tölke, J.; Lehmann, P.; Peters, A.; Kaestner, A.; Krafczyk, M.; Durner, W. Prediction of capillary hysteresis in a porous material using Lattice-Boltzmann methods and comparison to experimental data and a morphological pore network model. Adv. Water Resour. 2008, 31, 1151–1173. [Google Scholar] [CrossRef]
- Bachu, S.; Bennion, D.B. Experimental assessment of brine and or CO2 leakage through well cements at reservoir conditions. Int. J. Greenh. Gas Control 2009, 3, 494–501. [Google Scholar] [CrossRef]
- Benge, G. Improving wellbore seal integrity in CO2 injection wells. In Proceedings of the SPE/IADC Drilling Conference and Exhibition, Amsterdam, The Netherlands, 17–19 March 2009. [Google Scholar]
- Benson, S.M.; Cole, D.R. CO2 sequestration in deep sedimentary formations. Elements 2008, 4, 325–331. [Google Scholar] [CrossRef]
- Brandl, A.; Cutler, J.; Seholm, A.; Sansil, M.; Braun, G. Cementing solutions for corrosive well environments. SPE Drill. Complet. 2011, 26, 208–219. [Google Scholar] [CrossRef]
- Gaurina-Međimurec, N.; Pašić, B.; Simon, K. CO2 underground storage and wellbore integrity. Int. J. Trans-Port Logist. 2010, 8, 11–17. [Google Scholar]
- Chang, K.W.; Hesse, M.A.; Nicot, J.-P. Dissipation of overpressure into ambient mudrocks during geological carbon dioxide storage. Energy Procedia 2013, 37, 4457–4464. [Google Scholar] [CrossRef] [Green Version]
- Cihan, A.; Birkholzer, J.T.; Zhou, Q. Pressure buildup and brine migration during CO2 storage in multilayered aquifers. Groundwater 2013, 51, 252–267. [Google Scholar]
- Cihan, A.; Birkholzer, J.T.; Bianchi, M. Targeted pressure management during CO2 sequestration: Optimization of well placement and brine extraction. Energy Procedia 2014, 63, 5325–5332. [Google Scholar] [CrossRef] [Green Version]
- Craig, J.; Gorecki, C.D.; Ayash, S.C.; Liu, G.; Braunberger, J. A comparison of volumetric and dynamic storage efficiency in deep saline reservoirs: An overview of IEAGHG study. Energy Procedia 2014, 63, 5185–5191. [Google Scholar] [CrossRef] [Green Version]
- Domenico, P.A.; Schwartz, F.W. Sequestering carbon dioxide in a closed underground volume. J. Pet. Sci. Eng. 1990, 70, 123–130. [Google Scholar]
- Ehlig-Economides, C.A.; Economides, M.J. Reply to: Open or closed? A discussion of the mistaken assumptions in the Economides analysis of carbon sequestration. J. Petr. Sci. Eng. 2010, 74, 111–112. [Google Scholar] [CrossRef]
- Emami-Meybodi, H.; Hasssanzadeh, H.; Green, C.P.; Ennis-King, J. Convective dissolution of CO2 in saline aquifers: Progress in modelling and experiments. Int. J. Greenh. Gas Control 1990, 90, 23–33. [Google Scholar] [CrossRef]
- Enick, R.M.; Klara, S.M. CO2 solubility in water and brine under reservoir conditions. Chem. Eng. Commun. 1990, 90, 23–33. [Google Scholar] [CrossRef]
- Ennis-King, J.; Paterson, L. Coupling of geochemical reactions and convective mixing in the long-term geological storage of carbon dioxide. Int. J. Greenh. Gas Control 2007, 1, 86–93. [Google Scholar] [CrossRef]
- Doughty, C.; Freifeld, B.M.; Trautz, R.C. Site characterization for CO2 geologic storage and vice versa: The Frio Brine Pilot, Texas, USA as a case study. Environ. Geol. 2008, 54, 1635–1656. [Google Scholar] [CrossRef]
- Zhang, D.P. Study on Integrity of CO2 Buried Formation under Chemical Seepage Stress. Ph.D. Thesis, Northeast Petroleum University, Daqing, China, 2020. [Google Scholar]
- Hou, Z. CO2 Geological Storage Technology and Potential; Klausthal University of Technology: Clausthal-Zellerfeld, Germany, 2010. [Google Scholar]
- Ren, S.; Ren, B.; Li, Y.; Zhang, L.; Kang, W.; Liu, Y.; Chen, G.; Zhang, H. Analysis of CO2 geological storage monitoring technology and its application. J. China Univ. Pet. (Ed. Nat. Sci.) 2012, 36, 106–111. [Google Scholar]
- Zhang, H.T.; Wen, D.G.; Li, Y.L.; Zhang, J.; Lu, J. Geological conditions of CO2 in China and some suggestions. Geol. Bull. China 2005, 24, 29–32. [Google Scholar]
- Xu, Z.; Chen, D.; Zeng, R.; Guo, L.; Wang, X. Principle and conditions of CO2 underground geological storage. J. Southwest Pet. Univ. (Sci. Technol. Ed.) 2009, 31, 91–97. [Google Scholar]
- Han, B.; Bian, X. A hybrid PSO-SVM-based model for determination of oil recovery factor in the low-permeability reservoir. Petroleum 2018, 4, 43–49. [Google Scholar] [CrossRef]
- Hu, Y.; Hao, M.; Chen, G.; Sun, R.; Li, S. CO2 flooding and storage Technology in China and its practice. Pet. Explor. Dev. 2019, 46, 716–727. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Gorecki, C.D.; Sorensen, J.A.; Steadman, E.N.; Harju, J.A.; Melzer, S. Hydrocarbon mobilization mechanisms from upper, middle, and lower Bakken reservoir rocks exposed to CO2. In Proceedings of the SPE Unconventional Resources Conference, OnePetro, Calgary, AB, Canada, 5 November 2013. [Google Scholar]
- Zhao, C.L.; Guo, P.; Long, F. Thermo-hydraulic coupled simulation of immiscible CO2 flooding. Appl. Ecol. Environ. Res. 2018, 17, 409–425. [Google Scholar] [CrossRef]
- Lee, H.-S.; Cho, J.; Lee, Y.-W.; Lee, K.-S. Compositional Modeling of Impure CO2Injection for Enhanced Oil Recovery and CO2 Storage. Appl. Sci. 2021, 11, 7907. [Google Scholar] [CrossRef]
- Alfarge, D.; Wei, M.; Bai, B. CO2-EOR mechanisms in huff-n-puff operations in shale oil reservoirs based on history matching results. Fuel 2018, 226, 112–120. [Google Scholar] [CrossRef]
- Knapik, E.; Chruszcz-Lipska, K. Chemistry of Reservoir Fluids in the Aspect of CO2 Injection for Selected Oil Reservoirs in Poland. Energies 2020, 13, 6456. [Google Scholar] [CrossRef]
- Lee, E.; Hornafius, J.S.; Dean, E.; Kazemi, H. Potential of Denver Basin oil fields to store CO2 and produce Bio-CO2-EOR oil. Int. J. Greenh. Gas Control 2019, 81, 137–156. [Google Scholar] [CrossRef]
- Pranesh, V. Subsurface CO2 storage estimation in Bakken tight oil and Eagle Ford shale gas condensate reservoirs by retention mechanism. Fuel 2018, 215, 580–591. [Google Scholar] [CrossRef]
- Li, S.; Qiao, C.; Zhang, C.; Li, Z. Determination of diffusion coefficients of supercritical CO2 under tight oil reservoir conditions with pressure-decay method. J. CO2 Util. 2018, 24, 430–443. [Google Scholar] [CrossRef]
- Hosseininoosheri, P.; Hosseini, S.; Lopez, V.N.; Lake, L. Impact of field development strategies on CO2 trapping mechanisms in a CO2-EOR field: A case study in the Permian basin (SACROC unit). Int. J. Greenh. Gas Control 2018, 72, 92–104. [Google Scholar] [CrossRef] [Green Version]
- Ringrose, P.S. The CCS hub in Norway: Some insights from 22 years of saline aquifer storage. Energy Procedia 2018, 146, 166–172. [Google Scholar] [CrossRef]
- Liu, W.; Du, L.; Luo, X.; Liu, W.; Sun, Q.; Zhang, N. Experimental and microscopic simulation study on CO2 diffusion in an oil-water liquid system. Chem. Eng. Sci. 2021, 245, 116950. [Google Scholar] [CrossRef]
- Li, D.; Saraji, S.; Jiao, Z.; Zhang, Y. CO2 injection strategies for enhanced oil recovery and geological sequestration in a tight reservoir: An experimental study. Fuel 2021, 284, 119013. [Google Scholar] [CrossRef]
- Khather, M.; Saeedi, A.; Myers, M.B.; Verrall, M. An experimental study for carbonate reservoirs on the impact of CO2-EOR on petrophysics and oil recovery. Fuel 2019, 235, 1019–1038. [Google Scholar] [CrossRef]
- Zhao, D.; Yin, D. Effect of Fluid/Rock Interactions on Physical Character of Tight Sandstone Reservoirs during CO2 Flooding. Geofluids 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Valle, L.M.; Grima, C.; Rodríguez, R.; Llopis, C. Effect of scCO2-brine mixture on injectivity and storage capacity in rock samples of naturally fractured carbonate formations. J. Nat. Gas Sci. Eng. 2020, 81, 103452. [Google Scholar] [CrossRef]
- Emberley, S.; Hutcheon, I.; Shevalier, M.; Durocher, K.; Mayer, B.; Gunter, W.D.; Perkins, E.H. Monitoring of fluid–rock interaction and CO2 storage through produced fluid sampling at the Weyburn CO2-injection enhanced oil recovery site, Saskatchewan, Canada. Appl. Geochem. 2005, 20, 1131–1157. [Google Scholar] [CrossRef]
- Cui, G.; Zhu, L.; Zhou, Q.; Ren, S.; Wang, J. Geochemical reactions and their effect on CO2 storage efficiency during the whole process of CO2 EOR and subsequent storage. Int. J. Greenh. Gas Control 2021, 108, 103335. [Google Scholar] [CrossRef]
- Zandvakili, A.; Shahrouzi, J.R.; Tabatabaei-Nejad, S.A.; Khodapanah, E. Experimental investigation of CO2–rock–brine interaction for injection of CO2 in an Iranian oil reservoir as an EOR method. Environ. Earth Sci. 2020, 79, 480. [Google Scholar] [CrossRef]
- Cui, G.; Wang, Y.; Rui, Z.; Chen, B.; Ren, S.; Zhang, L. Assessing the combined influence of fluid-rock interactions on reservoir properties and injectivity during CO2 storage in saline aquifers. Energy 2018, 155, 281–296. [Google Scholar] [CrossRef]
- Utomo, G.P.; Güleç, N. Preliminary geochemical investigation of a possible CO2 injection in the Ungaran geothermal field, Indonesia: Equilibrium and kinetic modeling. Greenh. Gases Sci. Technol. 2021, 11, 3–18. [Google Scholar] [CrossRef]
- Mason, H.E.; Smith, M.M.; Carroll, S.A. Calibration of NMR porosity to estimate permeability in carbonate reservoirs. Int. J. Greenh. Gas Control 2019, 87, 19–26. [Google Scholar] [CrossRef]
- Raza, A.; Gholami, R.; Sarmadivaleh, M. Feasibility of limestone reservoirs as a carbon dioxide storage site: An experimental study. AAPG Bull. 2020, 104, 83–96. [Google Scholar] [CrossRef]
- Kim, K.Y.; Oh, J.; Han, W.S.; Park, K.G.; Shinn, Y.J.; Park, E. Two-phase flow visualization under reservoir conditions for highly heterogeneous conglomerate rock: A core-scale study for geologic carbon storage. Sci. Rep. 2018, 8, 4869. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Yu, C.; Hu, X.; Yu, Z.; Jiang, X. Characterization of mineral and pore evolution under CO2-brine-rock interaction at in-situ conditions. Adv. Geo-Energy Res. 2022, 6, 177–178. [Google Scholar] [CrossRef]
- Liu, B.; Fu, X.; Li, Z. Impacts of CO2-brine-rock interaction on sealing efficiency of sand caprock: A case study of Shihezi formation in Ordos basin. Adv. Geo-Energy Res. 2018, 2, 380–392. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Hu, R.; Cai, J. Subsurface multiphase reactive flow in geologic CO2 storage: Key impact factors and characterization approaches. Adv. Geo-Energy Res. 2022, 6, 179–180. [Google Scholar] [CrossRef]
- Fakher, S.; El-Tonbary, A.; Abdelaal, H.; Elgahawy, Y.; Imqam, A. Increasing oil recovery from unconventional shale reservoirs using cyclic carbon dioxide injection. In Proceedings of the SPE Europec, OnePetro, Virtual, 1–3 December 2020. [Google Scholar]
- Oomole, O.; Osoba, J.S. Carbon dioxide-dolomite rock interaction during CO2 flooding process. In Proceedings of the Annual Technical Meeting, OnePetro, Banff, AB, Canada, 9 May 1983. [Google Scholar]
- Matter, J.M.; Takahashi, T.; Goldberg, D. Experimental evaluation of in situ CO2-water-rock reactions during CO2 injection in basaltic rocks: Implications for geological CO2 sequestration. Geochem. Geophys. Geosystems 2007, 8, 2. [Google Scholar] [CrossRef]
- Liu, L.; Suto, Y.; Bignall, G.; Yamasaki, N.; Hashida, T. CO2 injection to granite and sandstone in experimental rock/hot water systems. Energy Convers. Manag. 2003, 44, 1399–1410. [Google Scholar] [CrossRef]
- Lin, H.; Fujii, T.; Takisawa, R.; Takahashi, T.; Hashida, T. Experimental evaluation of interactions in supercritical CO2/water/rock minerals system under geologic CO2 sequestration conditions. J. Mater. Sci. 2008, 43, 2307–2315. [Google Scholar] [CrossRef]
- Ueda, A.; Kato, K.; Ohsumi, T.; Yajima, T.; Ito, H.; Kaieda, H.; Metcalfe, R.; Takase, H. Experimental studies of CO2-rock interaction at elevated temperatures under hydrothermal conditions. Geochem. J. 2005, 39, 417–425. [Google Scholar] [CrossRef]
- Lu, J.; Nicot, J.P.; Mickler, P.J.; Ribeiro, L.H.; Darvari, R. Alteration of Bakken reservoir rock during CO2-based fracturing-An autoclave reaction experiment. J. Unconv. Oil Gas Resour. 2016, 14, 72–85. [Google Scholar] [CrossRef]
- Zhao, D.F.; Liao, X.W.; Yin, D.D. An experimental study for the effect of CO2-brine-rock interaction on reservoir physical properties. J. Energy Inst. 2015, 88, 27–35. [Google Scholar] [CrossRef]
- Ma, B.; Cao, Y.; Zhang, Y.; Eriksson, K.A. Role of CO2-water-rock interactions and implications for CO2 sequestration in Eocene deeply buried sandstones in the Bonan Sag, eastern Bohai Bay Basin, China. Chem. Geol. 2020, 541, 119585. [Google Scholar] [CrossRef]
- Pearce, J.K.; Dawson GK, W.; Golab, A.; Knuefing, L.; Sommacal, S.; Rudolph, V.; Golding, S.D. A combined geochemical and μCT study on the CO2 reactivity of Surat Basin reservoir and cap-rock cores: Porosity changes, mineral dissolution and fines migration. Int. J. Greenh. Gas Control 2019, 80, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Seyyedi, M.; Giwelli, A.; White, C.; Esteban, L.; Verrall, M.; Clennell, B. Effects of geochemical reactions on multi-phase flow in porous media during CO2 injection. Fuel 2020, 269, 117421. [Google Scholar] [CrossRef]
- Zou, Y.; Li, S.; Ma, X.; Zhang, S.; Li, N.; Chen, M. Effects of CO2-brine-rock interaction on porosity/permeability and mechanical properties during supercritical-CO2 fracturing in shale reservoirs. J. Nat. Gas Sci. Eng. 2018, 49, 157–168. [Google Scholar] [CrossRef]
- Iglauer, S.; Pentland, C.H.; Busch, A. CO2 wettability of seal and reservoir rocks and the implications for carbon geo-sequestration. Water Resour. Res. 2015, 51, 729–774. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Ranjith, P.G. Review of application of molecular dynamics simulations in geological sequestration of carbon dioxide. Fuel 2019, 255, 115644. [Google Scholar] [CrossRef]
- Chen, C.; Wan, J.; Li, W.; Song, Y. Water contact angles on quartz surfaces under supercritical CO2 sequestration conditions: Experimental and molecular dynamics simulation studies. Int. J. Greenh. Gas Control 2015, 42, 655–665. [Google Scholar] [CrossRef] [Green Version]
- Javanbakht, G.; Sedghi, M.; Welch, W.; Goual, L. Molecular dynamics simulations of CO2/water/quartz interfacial properties: Impact of CO2 dissolution in water. Langmuir 2015, 31, 5812–5819. [Google Scholar] [CrossRef]
- McCaughan, J.; Iglauer, S.; Bresme, F. Molecular dynamics simulation of water/CO2-quartz interfacial properties: Application to subsurface gas injection. Energy Procedia 2013, 37, 5387–5402. [Google Scholar] [CrossRef] [Green Version]
- Myshakin, E.M.; Saidi, W.A.; Romanov, V.N.; Cygan, R.T.; Jordan, K.D. Molecular dynamics simulations of carbon dioxide intercalation in hydrated Na-montmorillonite. J. Phys. Chem. C 2013, 117, 11028–11039. [Google Scholar] [CrossRef]
- Tian, Z.; Xing, H.; Tan, Y.; Gao, J. A coupled lattice Boltzmann model for simulating reactive transport in CO2 injection. Phys. A Stat. Mech. Its Appl. 2014, 403, 155–164. [Google Scholar] [CrossRef]
- An, S.; Erfani, H.; Hellevang, H.; Niasar, V. Lattice-Boltzmann simulation of dissolution of carbonate rock during CO2-saturated brine injection. Chem. Eng. J. 2021, 408, 127235. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Barati, R. Pore-scale hydrodynamic evolution within carbonate rock during CO2 injection and sequestration. In Proceedings of the EGU General Assembly Conference Abstracts, Virtual, 19–30 April 2021; p. EGU21-9578. [Google Scholar]
- Gao, J.; Xing, H.; Tian, Z.; Pearce, J.K.; Sedek, M.; Golding, S.D.; Rudolph, V. Reactive transport in porous media for CO2 sequestration: Pore scale modeling using the lattice Boltzmann method. Comput. Geosci. 2017, 98, 9–20. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, J. Lattice Boltzmann simulation of dissolution-induced changes in permeability and porosity in 3D CO2 reactive transport. J. Hydrol. 2018, 557, 276–290. [Google Scholar] [CrossRef]
- Fazeli, H.; Patel, R.A.; Ellis, B.R.; Hellevang, H. Three-dimensional pore-scale modeling of fracture evolution in heterogeneous carbonate caprock subjected to CO2-enriched brine. Environ. Sci. Technol. 2019, 53, 4630–4639. [Google Scholar] [CrossRef]
- Chen, L.; Wang, M.; Kang, Q.; Tao, W. Pore scale study of multiphase multicomponent reactive transport during CO2 dissolution trapping. Adv. Water Resour. 2018, 116, 208–218. [Google Scholar] [CrossRef]
- Dashtian, H.; Bakhshian, S.; Hajirezaie, S.; Nicot, J.-P.; Hosseini, S.A. Convection-diffusion-reaction of CO2-enriched brine in porous media: A pore-scale study. Comput. Geosci. 2019, 125, 19–29. [Google Scholar] [CrossRef]
- Nghiem, L.; Sammon, P.; Grabenstetter, J.; Ohkuma, H. Modeling CO2 storage in aquifers with a fully-coupled geochemical EOS compositional simulator. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, OnePetro, Tulsa, OK, USA, 17–20 April 2004. [Google Scholar]
- Nghiem, L.; Shrivastava, V.; Kohse, B.F.; Hassam, M.; Yang, C. Simulation and optimization of trapping processes for CO2 storage in saline aquifers. J. Can. Pet. Technol. 2010, 49, 15–22. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Agarwal, R.K. Numerical simulation of long-term storage of CO2 in Yanchang shale reservoir of the Ordos basin in China. Chem. Geol. 2016, 440, 288–305. [Google Scholar] [CrossRef]
- Graupner, B.J.; Li, D.; Bauer, S. The coupled simulator ECLIPSE–Open Geo Sys for the simulation of CO2 storage in saline formations. Energy Procedia 2011, 4, 3794–3800. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Hou, M.Z.; Were, P.; Gou, Y.; Xiong, L.; Sun, X. Modelling CO2-brine-rock interactions in the Upper Paleozoic formations of Ordos Basin used for CO2 sequestration. Environ. Earth Sci. 2015, 73, 2205–2222. [Google Scholar] [CrossRef]
- Bachu, S. Review of CO2 storage efficiency in deep saline aquifers. Int. J. Greenh. Gas Control 2015, 40, 188–202. [Google Scholar] [CrossRef]
- Senel, O.; Will, R.; Butsch, R.J. Integrated reservoir modeling at the Illinois Basin—Decatur Project. Greenh. Gases Sci. Technol. 2015, 4, 662–684. [Google Scholar] [CrossRef]
- Bao, J.; Xu, Z.; Fang, Y. A coupled thermal-hydro-mechanical simulation for carbon dioxide sequestration. Environ. Geotech. 2014, 3, 312–324. [Google Scholar] [CrossRef]
- Yin, Z.; Siahkoohi, A.; Louboutin, M.; Herrmann, F.J. Learned coupled inversion for carbon sequestration monitoring and forecasting with Fourier neural operators. In Proceedings of the SEG/AAPG International Meeting for Applied Geoscience & Energy, Houston, TX, USA, 28 August–1 September 2022. [Google Scholar]
- Wei, X.; Li, Q.; Li, X.; Niu, Z. Modeling the hydromechanical responses of sandwich structure faults during underground fluid injection. Environ. Earth Sci. 2016, 75, 1155. [Google Scholar] [CrossRef]
- Bao, J.; Chu, Y.; Xu, Z.; Tartakovsky, A.M.; Fang, Y. Uncertainty quantification for the impact of injection rate fluctuation on the geomechanically response of geological carbon sequestration. Int. J. Greenh. Gas Control 2014, 20, 160–167. [Google Scholar] [CrossRef]
- Dai, Z.; Xu, L.; Xiao, T.; McPherson, B.; Zhang, X.; Zheng, L.; Dong, S.; Yang, Z.; Soltanian, M.R.; Yang, C.; et al. Reactive chemical transport simulations of geologic carbon sequestration: Methods and applications. Earth-Sci. Rev. 2020, 208, 103265. [Google Scholar] [CrossRef]
- Han, W.S.; McPherson, B.J.; Lichtner, P.C.; Wang, F.P. Evaluation of trapping mechanisms in geologic CO2 sequestration: Case study of SACROC northern platform, a 35-year CO2 injection site. Am. J. Sci. 2010, 310, 282–324. [Google Scholar] [CrossRef]
- Shabani, B.; Vilcáez, J. TOUGHREACT-CO2Bio—A new module to simulate geological carbon storage under biotic conditions (Part 1): The multiphase flow of CO2-CH4-H2-H2S gas mixtures. J. Nat. Gas Sci. Eng. 2019, 63, 85–94. [Google Scholar] [CrossRef]
- Moodie, N.; Ampomah, W.; Heath, J.; Jia, W.; McPherson, B. Quantitative analysis of the influence of capillary pressure on geologic carbon storage forecasts case study: CO2-EOR in the Anadarko basin, Texas. Int. J. Greenh. Gas Control 2021, 109, 103373. [Google Scholar] [CrossRef]
- Bao, J.; Hou, Z.; Fang, Y.; Ren, H.; Lin, G. Uncertainty quantification for evaluating impacts of caprock and reservoir properties on pressure buildup and ground surface displacement during geological CO2 sequestration. Greenh. Gases Sci. Technol. 2013, 3, 338–358. [Google Scholar] [CrossRef]
- Akber Hassan, W.A.; Jiang, X. Upscaling and its application in numerical simulation of long-term CO2 storage. Greenh. Gases Sci. Technol. 2012, 2, 408–418. [Google Scholar] [CrossRef]
- Ngoc, T.D.T.; Doughty, C.; Lefebvre, R.; Malo, M. Injectivity of carbon dioxide in the St. Lawrence Platform, Quebec (Canada): A sensitivity study. Greenh. Gases Sci. Technol. 2013, 3, 516–540. [Google Scholar] [CrossRef]
- Yan, B.; Chen, B.; Harp, D.R.; Jia, W.; Pawar, R.J. A Robust Deep Learning Workflow to Predict Multiphase Flow Behavior during Geological CO2 Sequestration Injection and Post-Injection Periods. J. Hydrol. 2021, 607, 127542. [Google Scholar] [CrossRef]
- Zheng, T.; Guo, B.; Shao, H. A hybrid multiscale framework coupling multilayer dynamic reconstruction and full-dimensional models for CO2 storage in deep saline aquifers. J. Hydrol. 2021, 600, 126649. [Google Scholar] [CrossRef]
- Chen, Q.; Lai, Z. Hydromechanical modelling of CO2 sequestration using a component-based multiphysics code. J. Environ. Geotech. 2018, 8, 38–54. [Google Scholar] [CrossRef]
- You, J.; Ampomah, W.; Sun, Q.; Kutsienyo, E.J.; Balch, R.S.; Dai, Z.; Cather, M.; Zhang, X. Machine learning based co-optimization of carbon dioxide sequestration and oil recovery in CO2-EOR project. J. Clean. Prod. 2020, 260, 120866. [Google Scholar] [CrossRef]
- Biondi, B.; Jennings, J.; Jun Park, M.; Farris, S.; Clapp, B. Integration of deep neural networks into seismic workflows for low-carbon energy. In Proceedings of the SEG/AAPG International Meeting for Applied Geoscience & Energy, Houston, TX, USA, 28 August–1 September 2022. [Google Scholar]
- Chen, B.; Harp, D.R.; Lin, Y.; Keating, E.H.; Pawar, R.J. Geologic CO2 sequestration monitoring design: A machine learning and uncertainty quantification based approach. Appl. Energy 2018, 225, 332–345. [Google Scholar] [CrossRef]
- Mo, S.; Shi, X.; Lu, D.; Ye, M.; Wu, J. An adaptive Kriging surrogate method for efficient uncertainty quantification with an application to geological carbon sequestration modeling. Comput. Geosci. 2019, 125, 69–77. [Google Scholar] [CrossRef]
- Mathias, S.A.; Gluyas, J.G.; de Miguel, G.J.; Bryant, S.L.; Wilson, D. On relative permeability data uncertainty and CO2 injectivity estimation for brine aquifers. Int. J. Greenh. Gas Control 2013, 12, 200–212. [Google Scholar] [CrossRef] [Green Version]
- White, M.; McPherson, B.; Grigg, R.; Ampomah, W.; Appold, M. Numerical Simulation of Carbon Dioxide Injection in the Western Section of the Farnsworth Unit. Energy Procedia 2014, 63, 7891–7912. [Google Scholar] [CrossRef] [Green Version]
- Kalantari-Dahaghi, A.; Mohaghegh, S.; Esmaili, S. Data-driven Proxy at Hydraulic Fracture Cluster Level: A Technique for Efficient CO2- Enhanced Gas Recovery and Storage Assessment in Shale Reservoir. J. Nat. Gas Sci. Eng. 2015, 27, 515–530. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Ahmadinia, M.; Shariatipour, S.M.; Lawton, D.; Osadetz, K.; Saeedfar, A. Impact of Reservoir Permeability, Permeability Anisotropy and Designed Injection Rate on CO2 Gas Behavior in the Shallow Saline Aquifer at the CaMI Field Research Station, Brooks, Alberta. Nat. Resour. Res. 2020, 29, 2735–2752. [Google Scholar] [CrossRef]
- Onishi, T.; Nguyen, M.C.; Carey, J.W.; Will, B.; Zaluski, W.; Bowen, D.W.; Devault, B.C.; Duguid, A.; Zhou, Q.; Fairweather, S.H.; et al. Potential CO2 and brine leakage through wellbore pathways for geologic CO2 sequestration using the National Risk Assessment Partnership tools: Application to the Big Sky Regional Partnership. Int. J. Greenh. Gas Control 2019, 81, 44–65. [Google Scholar] [CrossRef]
- Ampomah, W.; Balch, R.S.; Grigg, R.B.; McPherson, B.; Will, R.A.; Lee, S.Y.; Dai, Z.; Pan, F. Co-optimization of CO2-EOR and storage processes in mature oil reservoirs. Greenh. Gases Sci. Technol. 2017, 7, 128–142. [Google Scholar] [CrossRef]
- Cheng, C.-L.; Gragg, M.; Perfect, E.; White, M.; Lemiszki, P.; McKay, L. Sensitivity of injection costs to input petrophysical parameters in numerical geologic carbon sequestration models. Int. J. Greenh. Gas Control 2013, 18, 277–284. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2005. [Google Scholar]
- Godec, M.; Koperna, G.; Petrusak, R.; Oudinot, A. Enhanced gas recovery and CO2 storage in gas shales: A summary review of its status and potential. Energy Procedia 2014, 63, 5849–5857. [Google Scholar] [CrossRef] [Green Version]
- Takase, K.; Barhate, Y.; Hashimoto, H.; Lunkad, S.F. Cement-sheath wellbore integrity for CO2 injection and storage wells. In Proceedings of the SPE Oil and Gas India Conference and Exhibition, Mumbai, India, 20–22 January 2010. [Google Scholar]
- Nogues, J.P.; Nordbotten, J.M.; Celia, M.A. Detecting leakage of brine or CO2 through abandoned wells in a geological sequestration operation using pressure monitoring wells. Energy Procedia 2011, 4, 3620–3627. [Google Scholar] [CrossRef] [Green Version]
- Carroll, S.A.; Iyer, J.; Walsh, S.D.C. Influence of chemical, mechanical, and transport processes on wellbore leakage from geologic CO2 storage reservoirs. Acc. Chem. Res. 2017, 50, 1829–1837. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, A.B.; Lauchnor, E.; Eldring, J.; Esposito, R.; Mitchell, A.C.; Gerlach, R.; Phillips, A.J.; Ebigbo, A.; Spangler, L.H. Abandoned well CO2 leakage mitigation using biologically induced mineralization: Current progress and future directions. Greenh. Gases Sci. Technol. 2013, 3, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Saripalli, K.P.; Mahasenan, N.M.; Cook, E.M. Risk and hazard assessment for projects involving the geological sequestration of CO2. In Proceedings of the Greenhouse Gas Control Technologies-6th International Conference, Pergamon, Turkey, 1 January 2003; pp. 511–516. [Google Scholar]
- Dejam, M.; Hassanzadeh, H. Diffusive leakage of brine from aquifers during CO2 geological storage. Adv. Water Resour. 2018, 111, 36–57. [Google Scholar] [CrossRef]
- Hou, Z.; Rockhold, M.L.; Murray, C.J. Evaluating the impact of caprock and reservoir properties on potential risk of CO2 leakage after injection. Environ. Earth Sci. 2012, 66, 2403–2415. [Google Scholar] [CrossRef]
- Bouchard, R.; Delaytermoz, A. Integrated path towards geological storage. Energy 2004, 29, 1339–1346. [Google Scholar] [CrossRef]
- Dexiang, L.; Shaoran, R.; Hongxing, R. CO2 Leakage Behaviors in Typical Caprock–Aquifer System during Geological Storage Process. ACS Omega 2019, 4, 17874–17879. [Google Scholar]
- Yujia, M.; Doyoon, K.; Young-Shin, J. Effects of Na+ and K+ Exchange in Interlayers on Biotite Dissolution under High-Temperature and High-CO2-Pressure Conditions. Environ. Sci. Technol. 2018, 52, 13638–13646. [Google Scholar]
- Wang, Y.; Zhang, L.; Ren, S.; Ren, B.; Chen, B.; Lu, J. Identification of potential CO2 leakage pathways and mechanisms in oil reservoirs using fault tree analysis. Greenh. Gases Sci. Technol. 2020, 10, 331–346. [Google Scholar] [CrossRef]
- Li, Z.; Dong, M.; Li, S.; Huang, S. CO2 sequestration in depleted oil and gas reservoirs-caprock characterization and storage capacity. Energy Convers. Manag. 2006, 47, 1372–1382. [Google Scholar] [CrossRef]
- Li, S.; Dong, M.; Li, Z.; Huang, S.; Qing, H.; Nickel, E. Gas breakthrough pressure for hydrocarbon reservoir seal rocks: Implications for the security of long-term CO2 storage in the Weyburn field. Geofluids 2005, 5, 326–334. [Google Scholar] [CrossRef]
- De Silva, G.P.D.; Ranjith, P.G.; Perera, M.S.A. Geochemical aspects of CO2 sequestration in deep saline aquifers: A review. Fuel 2015, 155, 128–143. [Google Scholar] [CrossRef]
- Ren, J.; Wang, Y.; Feng, D.; Gong, J. CO2 migration and distribution in multiscale-heterogeneous deep saline aquifers. Adv. Geo-Energy Res. 2021, 5, 333–346. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, H.; Kim, J.; Lee, J. Prediction of storage efficiency on CO2 sequestration in deep saline aquifers using artificial neural network. Appl. Energy 2017, 185, 916–928. [Google Scholar] [CrossRef]
- Bachu, S.; Adams, J.J. Sequestration of CO2 in geological media in response to climate change: Capacity of deep saline aquifers to sequester CO2 in solution. Energy Convers. Manag. 2003, 44, 3151–3175. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, Y.; Jin, K.; Guo, H.; Liu, Q.; Dong, J.; Li, W. Effects of supercritical CO2 fluids on pore morphology of coal: Implications for CO2 geological sequestration. Energy Fuels 2017, 31, 4731–4741. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Su, E.; Jiang, Z.; Wang, C.; Chu, Y.; Ye, C. Pore structure and diffusion characteristics of intact and tectonic coals: Implications for selection of CO2 geological sequestration site. J. Nat. Gas Sci. Eng. 2020, 81, 103388. [Google Scholar] [CrossRef]
- Zhu, D.; Peng, S.; Zhao, S.; Wei, M.; Bai, B. Comprehensive review of sealant materials for leakage remediation technology in geological CO2 capture and storage process. Energy Fuels 2021, 35, 4711–4742. [Google Scholar] [CrossRef]
- Li, Q.; Lim, Y.M.; Flores, K.M.; Kranjc, K.; Jun, Y.-S. Chemical reactions of Portland cement with aqueous CO2 and their impacts on cement’s mechanical properties under geologic CO2 sequestration conditions. Environ. Sci. Technol. 2015, 49, 6335–6343. [Google Scholar] [CrossRef]
- Carey, J.W. Geochemistry of wellbore integrity in CO 2 sequestration: Portland cement-steel-brine-CO 2 interactions. In Proceedings of the AGU Fall Meeting Abstracts, San Fransico, CA, USA, 9–13 December 2013; Volume 2013, p. V31D-05. [Google Scholar]
- Nasvi, M.; Ranjith, P.; Sanjayan, J. The permeability of geopolymer at down-hole stress conditions: Application for carbon dioxide sequestration wells. Appl. Energy 2013, 102, 1391–1398. [Google Scholar] [CrossRef]
- Nasvi, M.C.M.; Ranjith, P.G.; Sanjayan, J. Effect of different mix compositions on apparent carbon dioxide (CO2) permeability of geopolymer: Suitability as well cement for CO2 sequestration wells. Appl. Energy 2014, 114, 939–948. [Google Scholar] [CrossRef]
- Khalifeh, M.; Saasen, A.; Vrålstad, T. Potential Utilization of Geopolymers in Plug and Abandonment Operations. In Proceedings of the SPE Bergen One Day Seminar, Bergen, Norway, 2 April 2014; p. SPE-169231-MS. [Google Scholar]
- Abid, K.; Gholami, R.; Choate, P.; Nagaratnam, B.H. A review on cement degradation under CO2-rich environment of sequestration projects. J. Nat. Gas Sci. Eng. 2015, 27, 1149–1157. [Google Scholar] [CrossRef] [Green Version]
- Tiong, M.; Gholami, R.; Rahman, M.E. Cement degradation in CO2 storage sites: A review on potential applications of nanomaterials. J. Pet. Explor. Prod. Technol. 2019, 9, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Mosleh, M.H.; Govindan, R.; Shi, J.-Q.; Durucan, S.; Korre, A. The use of polymer-gel remediation for CO2 leakage through faults and fractures in the caprock. Energy Procedia 2017, 114, 4164–4171. [Google Scholar] [CrossRef]
- Durucan, S.; Korre, A.; Shi, J.-Q.; Govindan, R.; Mosleh, M.H.; Syed, A. The use of polymer-gel solutions for CO2 flow diversion and mobility control within storage sites. Energy Procedia 2016, 86, 450–459. [Google Scholar] [CrossRef]
- Pizzocolo, F.; Hewson, C.; Ter Heege, J. Polymer-Gel Remediation of CO2 Migration through Faults and Caprock: Numerical Simulations Addressing Feasibility of Novel Approaches. In Proceedings of the 50th U.S. Rock Mechanics/Geomechanics Symposium, Houston, TX, USA, 26–29 June 2016; p. ARMA-2016-100. [Google Scholar]
- Phillips, A.J.; Lauchnor, E.; Eldring, J.; Esposito, R.; Mitchell, A.C.; Gerlach, R.; Cunningham, A.B.; Spangler, L.H. Potential CO2 leakage reduction through biofilm-induced calcium carbonate precipitation. Environ. Sci. Technol. 2013, 47, 142–149. [Google Scholar] [CrossRef]
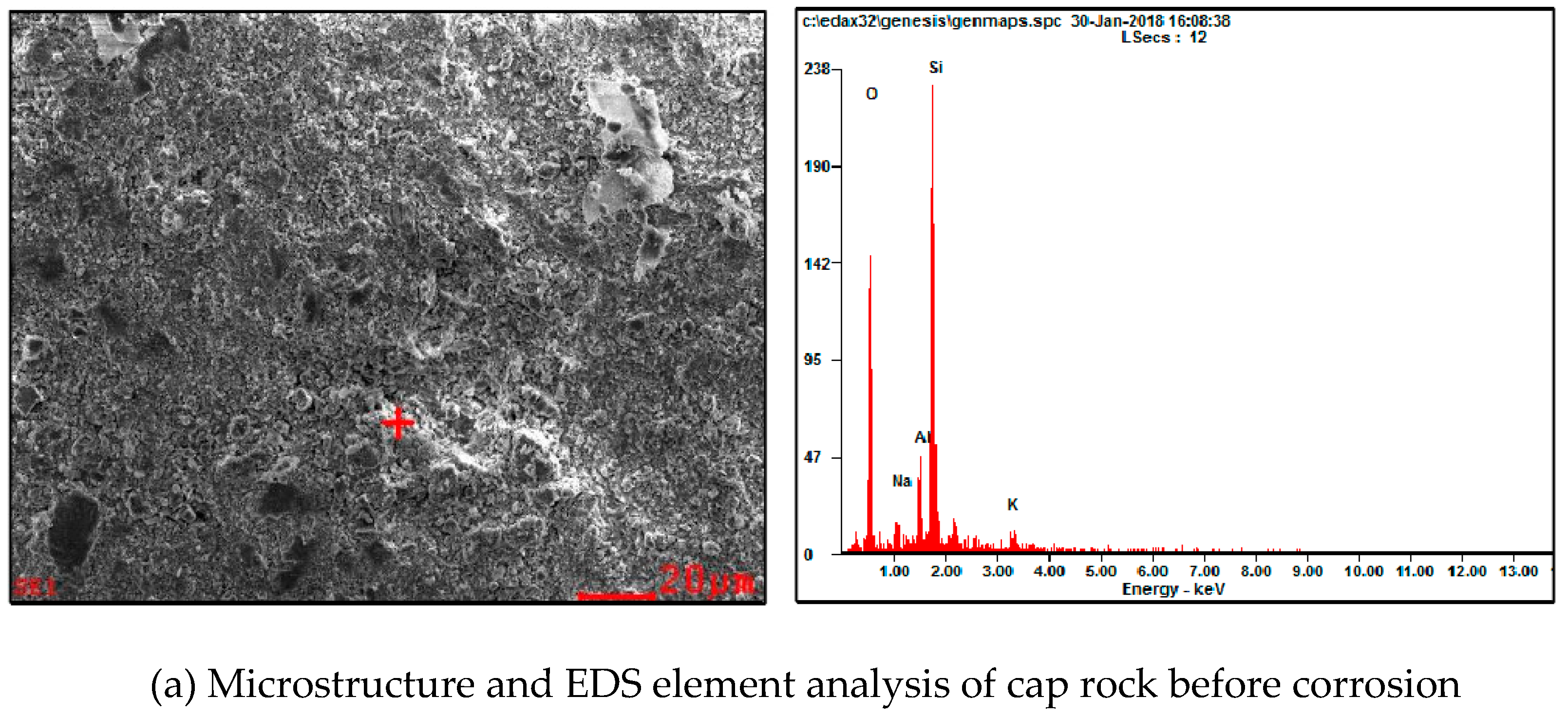
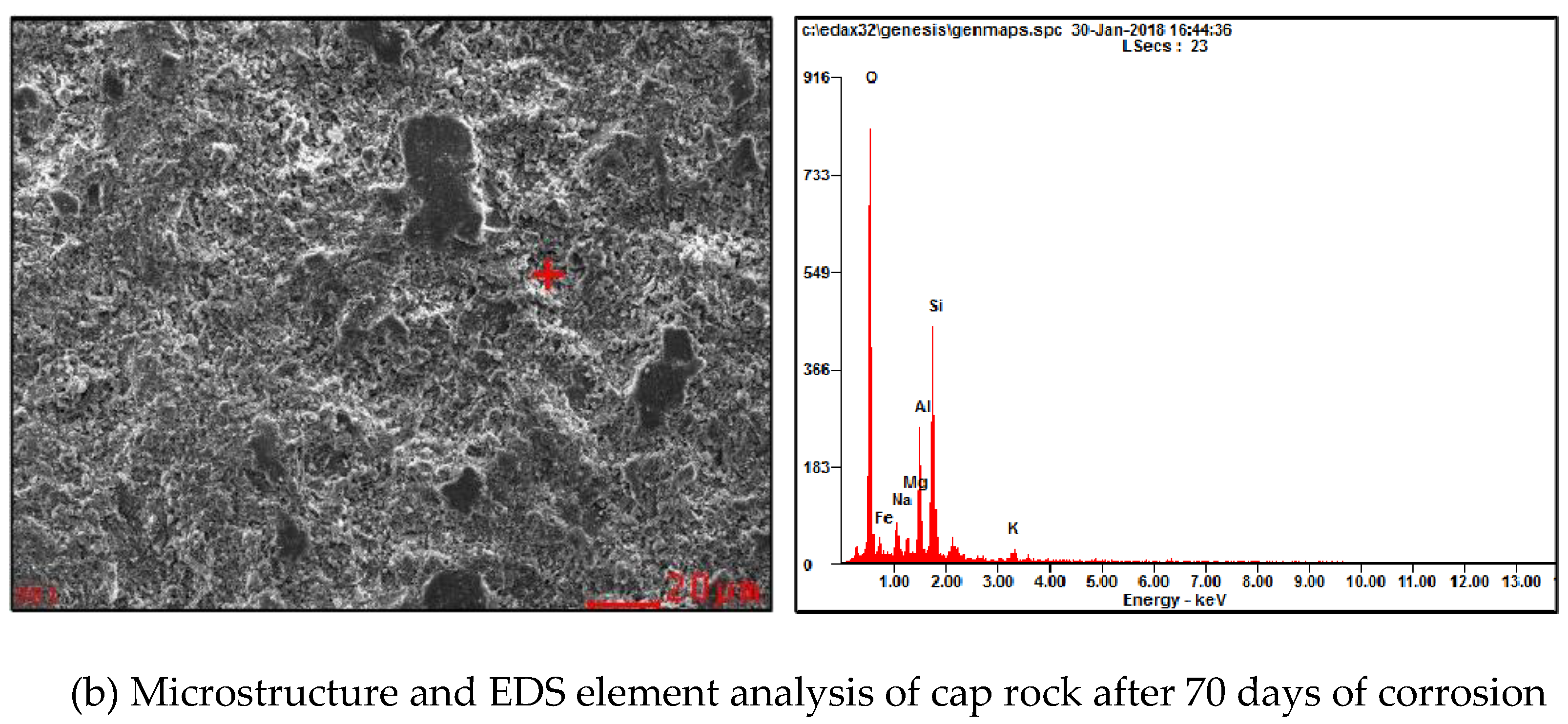







| Project Site | Location | Source of CO2 | Buried Body Type | The Total Storage Q/Mt | Storage Costs Dollar/t |
|---|---|---|---|---|---|
| Weyburn | Williston Basin, Canada | Fuel plant pipeline transportation | Injection into the field to enhance oil recovery | 20.0 | 20 |
| Recopol | Silesian coalfield, Poland | Chemical plant truck canning | Strengthen the exploitation of coal seam methane gas | 1 × 10−5 | - |
| Sleipner | Norway’s north sea | CO2 associated with gas fields (9.5%) | Injected into the Utsira brine formation above the gas reservoir | 20.0 | 17 |
| K12-B | Netherlands offshore North Sea | CO2 associated with gas fields (13%) | Injection of waste gas reservoir to bury and inject gas field to enhance gas recovery | 8.0 | 7–14 |
| InSalah | Central Algeria oil and gas field | CO2 associated with gas fields (10%) | The reservoir pressure is maintained by injecting water into the reservoir | 17.0 | 8 |
| Gorgon | 130 km off the west coast of Australia | CO2 associated with gas fields (14%) | Injected into the LDupuy salt water layer below Barrow Island | - | 6–10 |
| Snohvit | Barents Sea, Norway | CO2 associated with gas fields (5%~8%) | Injected into the Tubasen brine formation below the gas reservoir | - | 8 |
| QinShui basin | China | - | Strengthen the exploitation of coal seam methane gas | 1.5 × 10−4 | - |
| Storage Form | Storage Mechanism | Main Control Factors |
|---|---|---|
| Construct storage | Part of CO2 enters the micro-pore and is permanently sealed | Capillary pressure |
| Dissolve sequestration | CO2 partially dissolves in brine and crude oil, increasing its viscosity, volume, and storage capacity | Brine salinity, Crude oil, Brine composition, Temperature, Pressure |
| The free storage | After CO2 supersaturation, part of CO2 exists free. Cap layer is the key to sealing | Temperature, Pressure, Rock compressibility, Cap sealing |
| Mineral sequestration | CO2-formation water-rock interaction eventually consolidates as minerals | Mineral composition, Reaction time, CO2 content, Temperature, Pressure |
| Formation | Methods | CO2-Crude Oil Interaction Mechanism | CO2-Crude Oil Interaction Efficiency | CO2 Storage Efficiency | Authors, Year |
|---|---|---|---|---|---|
| The fields in Malaysia | Laboratory environment | CO2 dissolution, oil viscosity reduction, oil volume expansion, interfacial tension reduction | Effective | Effective | Mohamed Gamal Rezk, 2019 [29] |
| Oil Reservoirs in Poland | Laboratory environment | CO2 dissolution | High potential | Effective | Ewa Knapik, 2020 [68] |
| Denver Basin oil fields | Mathematical model and numerical simulation | CO2 dissolution | Effective (106 million barrels could be produced in 22 years.) | Effective | Erik Lee, 2019 [69] |
| Bakken tight oil | Numerical simulation and laboratory environment | CO2 dissolution, CO2-crude oil miscible | Effective | Effective (>90%) | Venkat Pranesh, 2018 [70] |
| Eagle Ford shale gas | Numerical simulation and laboratory environment | CO2 dissolution, CO2-crude oil miscible | Effective | Effective (>90%) | Venkat Pranesh, 2018 [70] |
| Changji Oilfield, Xinjing | Mathematical model and laboratory environment | CO2 diffusion | Effective | / | Songyan Li, 2018 [71] |
| Permian Basin | Mathematical model and laboratory environment | CO2 diffusion | Effective | Effective (WAG achieves a good balance between maximizing oil production and carbon monoxide) | P. Hosseininoosheri, 2018 [72] |
| Formation | Methods | CO2-Brine Interaction Mechanism | CO2 Storage Efficiency | Authors, Year |
|---|---|---|---|---|
| Permian Basin | Mathematical model and laboratory environment | CO2 diffusion | Effective | P. Hosseininoosheri, 2018 [72] |
| Block X of Ordos Basin | Numerical simulation and laboratory environment | CO2 diffusion, CO2-brine scaling | Effective | Dongfeng Zhao, 2021 [77] |
| Weyburn Oil Field | Laboratory environment | CO2 dissolution | Effective (CO2 to be precipitated as calcite) | S. Emberley, 2005 [79] |
| - | Numerical simulation | CO2 diffusion, CO2-brine scaling | Effective (Salt precipitation is a detrimental influence) | Guodong Cui, 2018 [80] |
| Iranian hydrocarbon reservoir | Laboratory environment | CO2 dissolution and deposition of minerals | Effective | Amin Zandvakili, 2020 [81] |
| - | Numerical simulation | CO2 diffusion, CO2 dissolution, CO2-water-rock geochemical reactions | Effective (WAG) | Guodong Cui, 2021 [82] |
| The hot water in the Ungaran geothermal field, Java, Indonesia. | Numerical simulation | CO2 diffusion, CO2-brine scaling | Effective (Achieve CO2 brine rock balance within 10 years) | Gagas Pambudi Utomo, 2021 [83] |
| Author | Simulation Methods | Research Content of Reacton |
|---|---|---|
| Iglauer S et al. [102] | MD | Review of CO2 reaction |
| Ma Z et al. [103] | MD | Wettability estimation of different rock types |
| Chen C et al. [104] | MD | Factors on capillary capture amount |
| Javanbakht G et al. [105] | MD | The effect of IFT |
| McCaughan J et al. [106] | MD | Different liquid-solid system impact |
| Myshakin E et al. [107] | MD | Effect of clay swelling |
| Tian Z et al. [108] | LBM | Effect of fluid velocity |
| An S et al. [109] | LBM | Dissolution patterns under different factors |
| Zhang C et al. [110] | LBM | Different rock property impacting on reaction |
| Gao J et al. [111] | LBM | Impact on hydraulic property |
| Tian Z et al. [112] | LBM | Connectivity of pore channels and properties of matrix |
| Fazeli H et al. [113] | LBM | Fractural formation |
| Chen L et al. [114] | LBM | Non-equilibrium process with CO2 dissolution |
| Dashtian H et al. [115] | Pore network | Effect of two dimensionless numbers |
| Nghiem L et al. [117] | CMG | Combination of CMG-GHG |
| Liu D et al. [118] | CMG | Effect of CO2 absorption |
| Cui G et al. [80] | CMG | Factors on EOR and CCS |
| Graupner B J et al. [119] | ECLIPSE | CO2 storage in deep saline aquifers |
| Liu H et al. [120] | TOUGHREACT | The dissolution and precipitation on minerals |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhang, X.; Liu, J.; Xie, Q.; Zhou, X.; Zheng, J.; Su, Y. Research Progress and Prospect of Carbon Dioxide Utilization and Storage Based on Unconventional Oil and Gas Development. Energies 2022, 15, 9383. https://doi.org/10.3390/en15249383
Li L, Zhang X, Liu J, Xie Q, Zhou X, Zheng J, Su Y. Research Progress and Prospect of Carbon Dioxide Utilization and Storage Based on Unconventional Oil and Gas Development. Energies. 2022; 15(24):9383. https://doi.org/10.3390/en15249383
Chicago/Turabian StyleLi, Lei, Xue Zhang, Jiahui Liu, Qiuheng Xie, Xiaomei Zhou, Jianyang Zheng, and Yuliang Su. 2022. "Research Progress and Prospect of Carbon Dioxide Utilization and Storage Based on Unconventional Oil and Gas Development" Energies 15, no. 24: 9383. https://doi.org/10.3390/en15249383
APA StyleLi, L., Zhang, X., Liu, J., Xie, Q., Zhou, X., Zheng, J., & Su, Y. (2022). Research Progress and Prospect of Carbon Dioxide Utilization and Storage Based on Unconventional Oil and Gas Development. Energies, 15(24), 9383. https://doi.org/10.3390/en15249383







