Emerging Lignin-Based Materials in Electrochemical Energy Systems
Abstract
:1. Introduction
2. Lignin and Industrial Lignin
2.1. Fundamental Structures of Lignin
2.2. Industrial Lignin from the Pulping Process
3. Lignin in Lithium Batteries (LIBs)
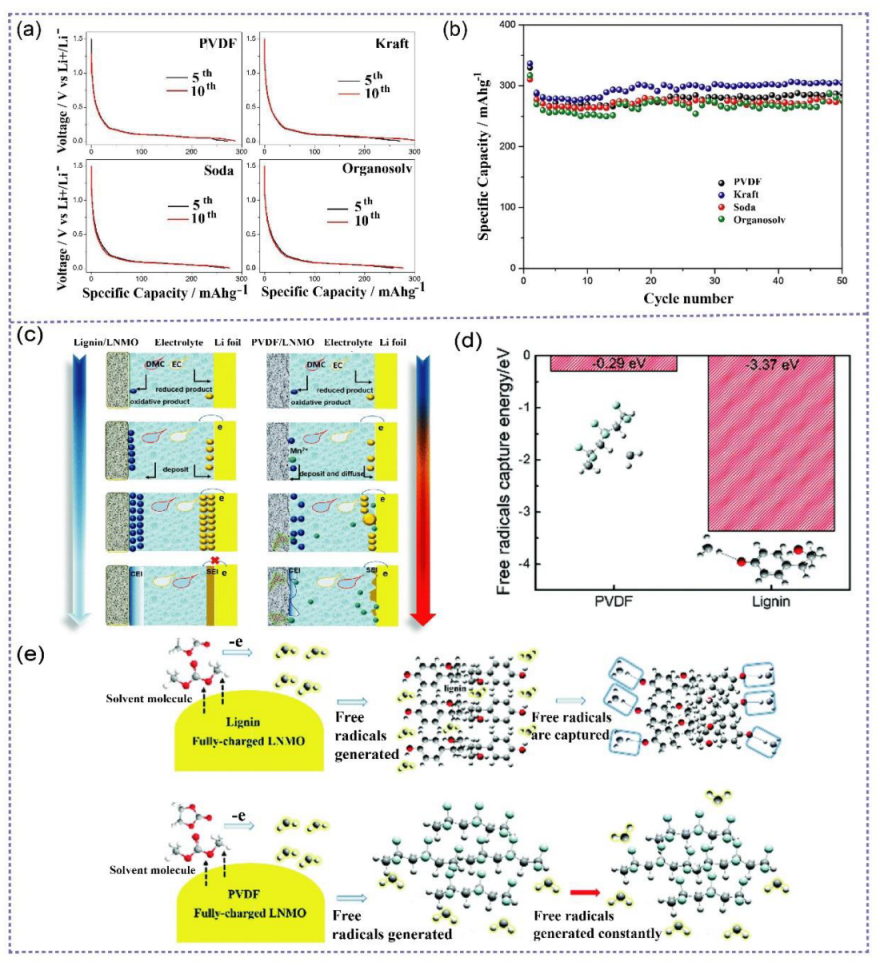
3.1. Lignin-Based Electrodes
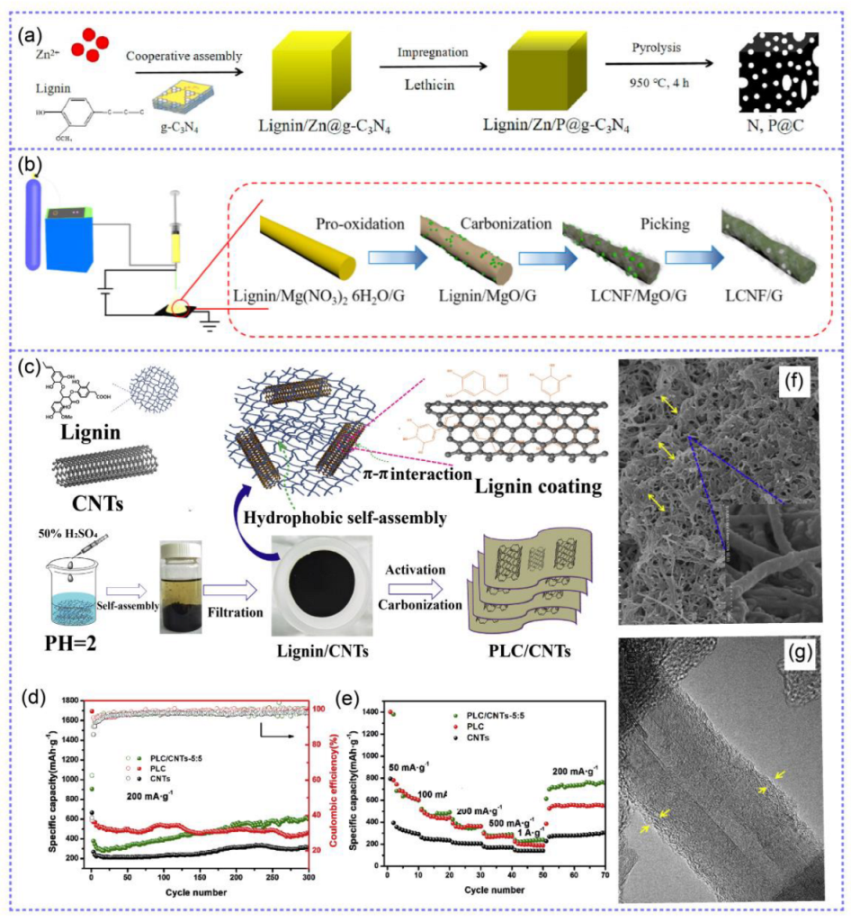
3.2. Lignin-Based Gel Electrolytes
3.3. Lignin as Separators for LIBs
3.4. Lignin-Based Binders for LIBs
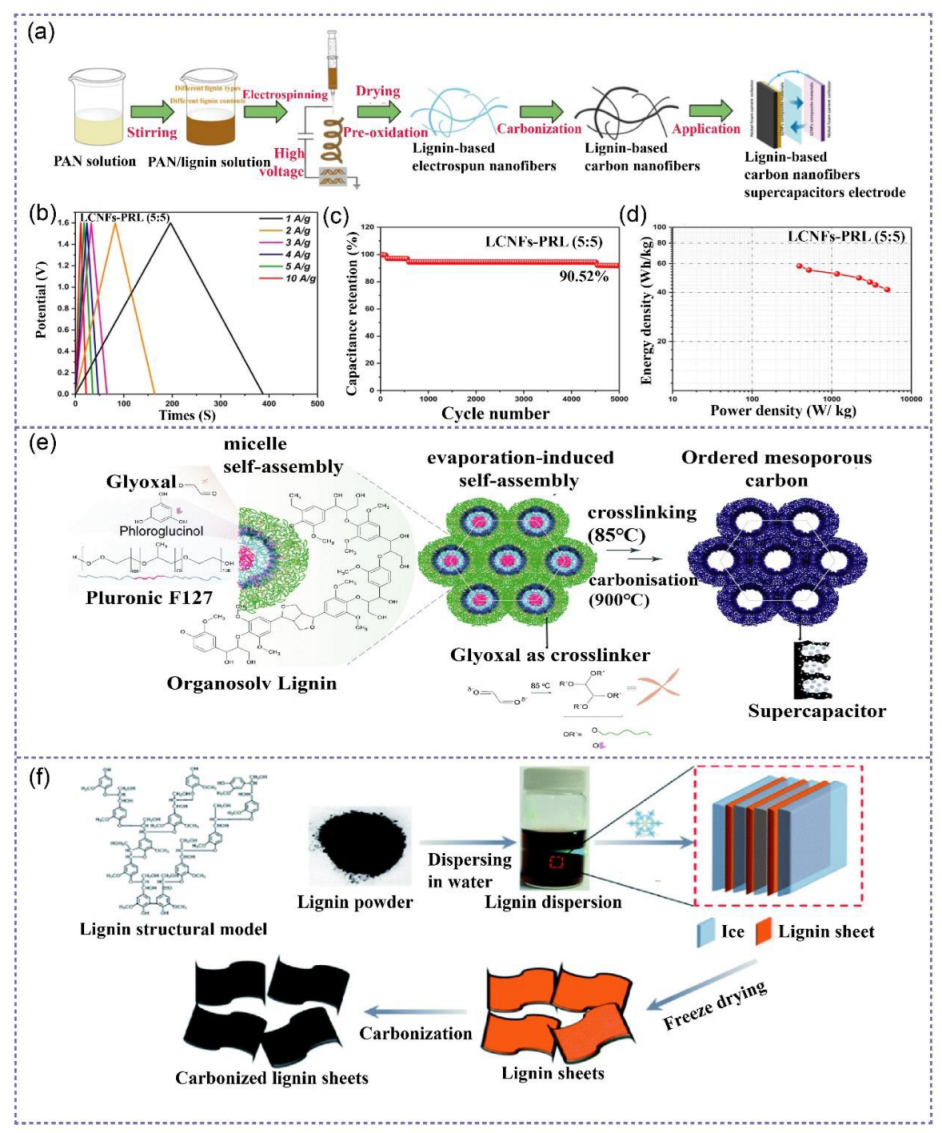
4. Lignin in Supercapacitors
4.1. Lignin-Based Electrodes for Supercapacitors
4.1.1. Lignin-Derived Electrodes for Double-Layer Capacitors
4.1.2. Lignin-Based Electrodes for Pseudocapacitors (PCs)
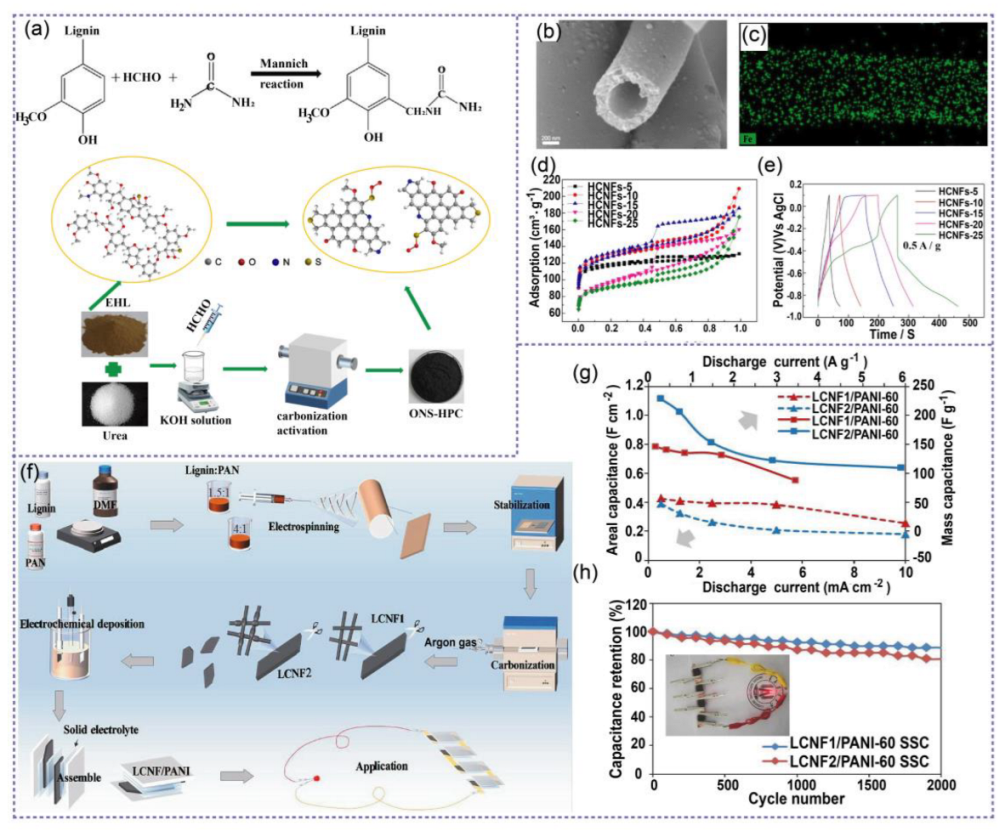
4.2. Lignin-Based Hydrogel Electrolytes
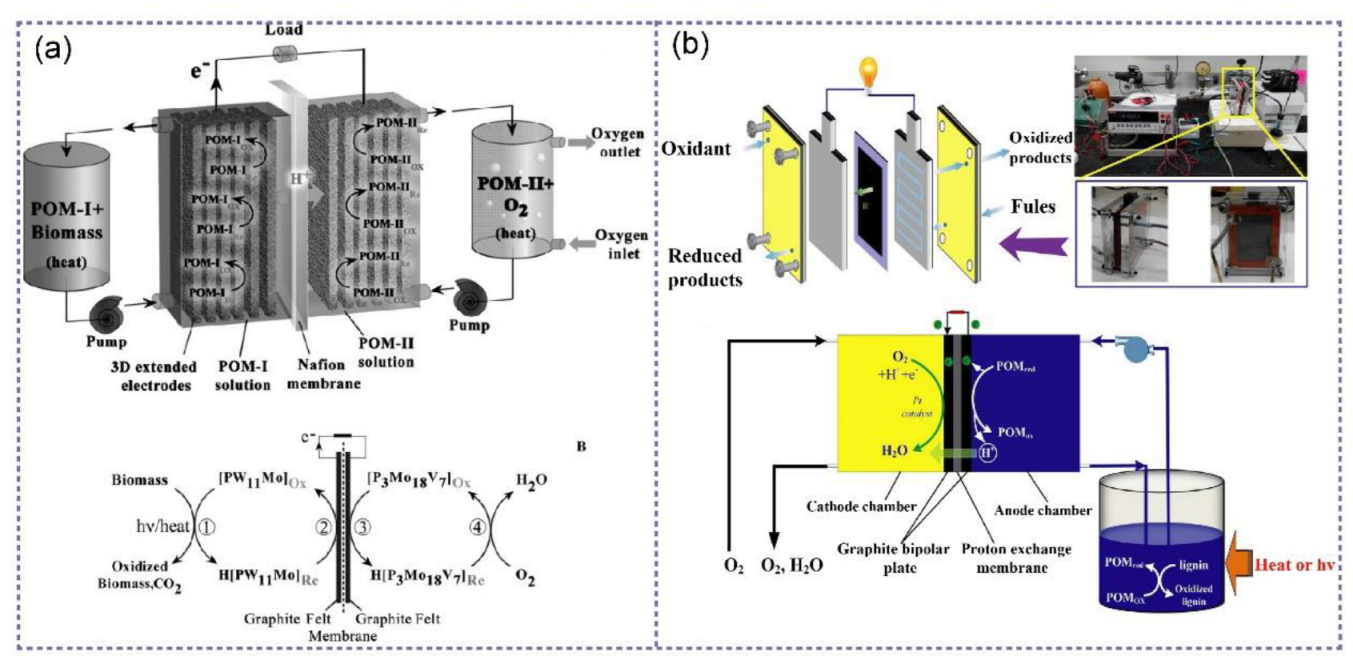
5. Lignin in Fuel Cells
6. Lignin in Solar Cells

7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Fadzillah, D.M.; Kamarudin, S.K.; Zainoodin, M.A.; Masdar, M.S. Critical challenges in the system development of direct alcohol fuel cells as portable power supplies: An overview. Int. J. Hydrogen Energy 2019, 44, 3031–3054. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hou, Y.; Li, Y.M.; Xiao, H.I. Heteroatom-doped porous carbon microspheres derived from ionic liquid-lignin solution for high performance supercapacitors. J. Colloid Interface Sci. 2022, 614, 566–573. [Google Scholar] [CrossRef]
- Wu, X.Y.; Jiang, J.H.; Wang, C.M.; Liu, J.; Pu, Y.Q.; Ragauskas, A.; Li, S.M.; Yang, B. Lignin-derived electrochemical energy materials and systems. Biofuel. Bioprod. Bior. 2020, 14, 650–672. [Google Scholar] [CrossRef]
- Zhu, J.D.; Yan, C.Y.; Zhang, X.; Yang, C.; Jiang, M.J.; Zhang, X.W. A sustainable platform of lignin: From bioresources to materials and their applications in rechargeable batteries and supercapacitors. Prog. Energy Combust. Sci. 2020, 76, 100788. [Google Scholar] [CrossRef]
- Cai, C.; Bao, Y.; Zhan, X.J.; Lin, X.L.; Lou, H.M.; Pang, Y.X.; Qian, Y.; Qiu, X. Recovering cellulase and increasing glucose yield during lignocellulosic hydrolysis using lignin-MPEG with a sensitive pH response. Green Chem. 2019, 21, 1141–1151. [Google Scholar] [CrossRef]
- Choi, J.H.; Jang, S.K.; Kim, J.H.; Park, S.Y.; Kim, J.C.; Jeong, H.; Kim, H.Y.; Choi, I.G. Simultaneous production of glucose, furfural, and ethanol organosolv lignin for total utilization of high recalcitrant biomass by organosolv pretreatment. Renew. Energy 2019, 130, 952–960. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Rippert, P.; Puyaubert, J.; Grisollet, D.; Derrier, L.; Matringe, M. Tyrosine and Phenylalanine Are Synthesized within the Plastids in Arabidopsis. Plant Physiol. 2009, 149, 1251–1260. [Google Scholar] [CrossRef]
- Ten, E.; Vermerris, W. Recent developments in polymers derived from industrial lignin. J. Appl. Polym. Sci. 2015, 132, 42069. [Google Scholar] [CrossRef] [Green Version]
- Upton, B.M.; Kasko, A.M. Strategies for the Conversion of Lignin to High-Value Polymeric Materials: Review and Perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kelley, S.S.; Venditti, R.A. Lignin-Based Thermoplastic Materials. ChemSusChem 2016, 9, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.Q.; Cao, S.L.; Ragauskas, A.J. Application of quantitative P-31 NMR in biomass lignin and biofuel precursors characterization. Energy Environ. Sci. 2011, 4, 3154–3166. [Google Scholar] [CrossRef]
- Espinoza-Acosta, J.L.; Torres-Chavez, P.I.; Olmedo-Martinez, J.L.; Vega-Rios, A.; Flores-Gallardo, S.; Zaragoza-Contreras, E.A. Lignin in storage and renewable energy applications: A review. J. Energy Chem. 2018, 27, 1422–1438. [Google Scholar] [CrossRef]
- Beckham, G.T.; Johnson, C.W.; Karp, E.M.; Salvachua, D.; Vardon, D.R. Opportunities and challenges in biological lignin valorization. Curr. Opin. Biotechnol. 2016, 42, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Z.; Zhao, X.C.; Wang, A.Q.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Buranov, A.U.; Mazza, G. Lignin in straw of herbaceous crops. Ind. Crops Prod. 2008, 28, 237–259. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef] [PubMed]
- Vishtal, A.; Kraslawski, A. Challenges in Industrial Applications of Technical Lignins. Bioresources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Delmas, G.H.; Benjelloun-Mlayah, B.; Le Bigot, Y.; Delmas, M. Functionality of Wheat Straw Lignin Extracted in Organic Acid Media. J. Appl. Polym. Sci. 2011, 121, 491–501. [Google Scholar] [CrossRef]
- Laurichesse, S.; Averous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Poveda-Giraldo, J.A.; Solarte-Toro, J.C.; Alzate, C.A.C. The potential use of lignin as a platform product in biorefineries: A review. Renew. Sustain. Energy Rev. 2021, 138, 110688. [Google Scholar] [CrossRef]
- Gharehkhani, S.; Zhang, Y.Q.; Fatehi, P. Lignin-derived platform molecules through TEMPO catalytic oxidation strategies. Prog. Energy Combust. Sci. 2019, 72, 59–89. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef] [Green Version]
- Tribot, A.; Amer, G.; Alio, M.A.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.D.; Callois, J.M.; Vial, C.; Michaud, P.; et al. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Zhou, W.P.; Zhang, H.; Chen, F.G. Modified lignin: Preparation and use in reversible gel via Diels-Alder reaction. Int. J. Biol. Macromol. 2018, 107, 790–795. [Google Scholar] [CrossRef]
- Bernardini, J.; Cinelli, P.; Anguillesi, I.; Coltelli, M.B.; Lazzeri, A. Flexible polyurethane foams green production employing lignin or oxypropylated lignin. Eur. Polym. J. 2015, 64, 147–156. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H.; Pizzi, A.; Honarbakhsh-Raouf, A.; Nemati, F. The effect of soda bagasse lignin modified by ionic liquids on properties of the urea-formaldehyde resin as a wood adhesive. J. Adhes 2017, 93, 914–925. [Google Scholar] [CrossRef]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crops Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafan-Vidales, H.I.; Longoria, A.; Sebastian, P.J.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- Deng, B.B.; Shen, L.; Liu, Y.A.; Yang, T.; Zhang, M.S.; Liu, R.J.; Huang, Z.H.; Fang, M.H.; Wu, X.W. Porous Si/C composite as anode materials for high-performance rechargeable lithium-ion battery. Chin. Chem. Lett. 2017, 28, 2281–2284. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Lu, Z.; Liu, J.; Bai, L.; Dong, J.; Nan, D. Constructing porous lignin-based carbon nanofiber anodes with flexibility for high-performance lithium/sodium-ion batteries. Mater. Today Sustain. 2022, 20, 100234. [Google Scholar] [CrossRef]
- Jo, G.; Jeon, H.; Park, M.J. Synthesis of Polymer Electrolytes Based on Poly(ethylene oxide) and an Anion-Stabilizing Hard Polymer for Enhancing Conductivity and Cation Transport. ACS Macro Lett. 2015, 4, 225–230. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, J.; Chong, C.B.; Yu, X.W.; Wanga, L.L.; Shi, Z.Q. An electrospun lignin/polyacrylonitrile nonwoven composite separator with high porosity and thermal stability for lithium-ion batteries. RSC Adv. 2015, 5, 101115–101120. [Google Scholar] [CrossRef]
- Yi, X.L.; He, W.; Zhang, X.D.; Yang, G.H.; Wang, Y.Y. Hollow mesoporous MnO/MnS/SiC/S-CN composites prepared from soda pulping black liquor for lithium-ion batteries. J. Alloys Compd. 2018, 735, 1306–1313. [Google Scholar] [CrossRef]
- Nowak, A.P.; Hagberg, J.; Leijonmarck, S.; Schweinebarth, H.; Baker, D.; Uhlin, A.; Tomani, P.; Lindbergh, G. Lignin-based carbon fibers for renewable and multifunctional lithium-ion battery electrodes. Holzforschung 2018, 72, 81–90. [Google Scholar] [CrossRef]
- Song, S.J.; Ma, F.W.; Wu, G.; Ma, D.; Geng, W.D.; Wan, J.F. Facile self-templating large scale preparation of biomass-derived 3D hierarchical porous carbon for advanced supercapacitors. J. Mater. Chem. A 2015, 3, 18154–18162. [Google Scholar] [CrossRef]
- Zhao, H.J.; Wang, Q.J.; Deng, Y.H.; Shi, Q.; Qian, Y.; Wang, B.B.; Lu, L.; Qiu, X.Q. Preparation of renewable lignin-derived nitrogen-doped carbon nanospheres as anodes for lithium-ion batteries. RSC Adv. 2016, 6, 77143–77150. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Song, K.X.; Wang, X.F.; Yu, K.F.; Liang, C. Rice Husk Lignin-Derived Porous Carbon Anode Material for Lithium-Ion Batteries. Chemistryselect 2019, 4, 4178–4184. [Google Scholar] [CrossRef]
- Zhang, W.L.; Yin, J.; Lin, Z.Q.; Lin, H.B.; Lu, H.Y.; Wang, Y.; Huang, W.M. Facile preparation of 3D hierarchical porous carbon from lignin for the anode material in lithium ion battery with high rate performance. Electrochim. Acta 2015, 176, 1136–1142. [Google Scholar] [CrossRef]
- Yun, L.; Jia, M.Q.; Yang, H.H.; Zheng, H.Y.; Ao, H. Structural Characteristics and Electrochemical Performance of N,P- Codoped Porous Carbon as a Lithium-Ion Battery Anode Electrode. ACS Omega 2022, 38, 34109–34116. [Google Scholar]
- Chen, F.; Wu, L.; Zhou, Z.P.; Ju, J.J.; Zhao, Z.P.; Zhong, M.Q.; Kuang, T.R. MoS2 decorated lignin-derived hierarchical mesoporous carbon hybrid nanospheres with exceptional Li-ion battery cycle stability. Chin. Chem. Lett. 2019, 30, 197–202. [Google Scholar] [CrossRef]
- Wang, S.X.; Yang, L.P.; Stubbs, L.P.; Li, X.; He, C.B. Lignin-Derived Fused Electrospun Carbon Fibrous Mats as High Performance Anode Materials for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 12275–12282. [Google Scholar] [CrossRef]
- Tenhaeff, W.E.; Rios, O.; More, K.; McGuire, M.A. Highly Robust Lithium Ion Battery Anodes from Lignin: An Abundant, Renewable, and Low-Cost Material. Adv. Funct. Mater. 2014, 24, 86–94. [Google Scholar] [CrossRef]
- Yi, X.L.; He, W.; Zhang, X.D.; Yue, Y.Z.; Yang, G.H.; Wang, Z.Y.; Zhou, M.J.; Wang, L.Z. Graphene-like carbon sheet/Fe3O4 nanocomposites derived from soda papermaking black liquor for high performance lithium ion batteries. Electrochim. Acta 2017, 232, 550–560. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.Y.; Zhou, J.Q.; Qian, T.; Wang, M.F.; Yan, C.L. Confined silicon nanospheres by biomass lignin for stable lithium ion battery. Nanotechnology 2017, 28, 405410. [Google Scholar] [CrossRef]
- Chou, C.Y.; Kuo, J.R.; Yen, S.C. Silicon-Based Composite Negative Electrode Prepared from Recycled Silicon-Slicing Slurries and Lignin/Lignocellulose for Li-Ion Cells. ACS Sustain. Chem. Eng. 2018, 6, 4759–4766. [Google Scholar] [CrossRef]
- Zhou, Z.P.; Chen, F.; Kuang, T.R.; Chang, L.Q.; Yang, J.T.; Fan, P.; Zhao, Z.P.; Zhong, M.Q. Lignin-derived hierarchical mesoporous carbon and NiO hybrid nanospheres with exceptional Li-ion battery and pseudocapacitive properties. Electrochim. Acta 2018, 274, 288–297. [Google Scholar] [CrossRef]
- Xi, Y.B.; Yang, D.J.; Liu, W.F.; Qin, Y.L.; Qiu, X.Q. Preparation of porous lignin-derived carbon/carbon nanotube composites by hydrophobic self-assembly and carbonization to enhance lithium storage capacity. Electrochim. Acta 2019, 303, 1–8. [Google Scholar] [CrossRef]
- Cheng, J.J.; Pan, Y.; Pan, J.A.; Song, H.J.; Ma, Z.S. Sulfur/bamboo charcoal composites cathode for lithium–sulfur batteries. RSC Adv. 2014, 5, 68–74. [Google Scholar] [CrossRef]
- Yuan, Y.; Haibo, L. In situ growth of CoO nanosheets on a carbon fiber derived from corn cellulose as an advanced hybrid anode for lithium-ion batteries. N. J. Chem. 2022, 46, 18664–18670. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.; Chen, X.; Zhang, M.; Huang, Q.; Du, X.; Liu, Y.; Li, X. Microtubular carbon fibers derived from bamboo and wood as sustainable anodes for lithium and sodium ion batteries. J. Porous Mater. 2019, 26, 1821–1830. [Google Scholar] [CrossRef]
- Chen, T.; Hu, J.Z.; Zhang, L.; Pan, J.; Liu, Y.Y.; Cheng, Y.T. High performance binder-free SiOx/C composite LIB electrode made of SiOx and lignin. J. Power Sources 2017, 362, 236–242. [Google Scholar] [CrossRef]
- Gong, S.D.; Huang, Y.; Cao, H.J.; Lin, Y.H.; Li, Y.; Tang, S.H.; Wang, M.S.; Li, X. A green and environment-friendly gel polymer electrolyte with higher performances based on the natural matrix of lignin. J. Power Sources 2016, 307, 624–633. [Google Scholar] [CrossRef]
- Wang, Q.J.; Song, W.L.; Fan, L.Z.; Song, Y. Facile fabrication of polyacrylonitrile/alumina composite membranes based on triethylene glycol diacetate-2-propenoic acid butyl ester gel polymer electrolytes for high-voltage lithium-ion batteries. J. Membr. Sci. 2015, 486, 21–28. [Google Scholar] [CrossRef]
- Yang, P.T.; Zhang, P.; Shi, C.; Chen, L.X.; Dai, J.H.; Zhao, J.B. The functional separator coated with core-shell structured silica-poly (methyl methacrylate) sub-microspheres for lithium-ion batteries. J. Membr. Sci. 2015, 474, 148–155. [Google Scholar] [CrossRef]
- Li, L.Y.; Chen, Y.X.; Guo, X.D.; Zhong, B.H. Preparation of sodium trimetaphosphate and its application as an additive agent in a novel polyvinylidene fluoride based gel polymer electrolyte in lithium sulfur batteries. Polym. Chem. 2015, 6, 1619–1626. [Google Scholar] [CrossRef]
- Che, Q.T.; Zhu, Z.F.; Chen, N.; Zhai, X. Methylimidazolium group-Modified polyvinyl chloride (PVC) doped with phosphoric acid for high temperature proton exchange membranes. Mater. Des. 2015, 87, 1047–1055. [Google Scholar] [CrossRef]
- Rajeswari, N.; Selvasekarapandian, S.; Karthikeyan, S.; Prabu, M.; Hirankumar, G.; Nithya, H.; Sanjeeviraja, C. Conductivity and dielectric properties of polyvinyl alcohol-polyvinylpyrrolidone poly blend film using non-aqueous medium. J. Non-Cryst. Solids 2011, 357, 3751–3756. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Wang, A.L.; Liu, X.; Chan, J.; Wang, Z.N.; Zeng, Q.H.; Zhou, H.H.; Jiang, X.R.; Zhang, L.Y. Polymer-Laden Composite Lignin-Based Electrolyte Membrane for High-Performance Lithium Batteries. ACS Sustain. Chem. Eng. 2018, 6, 14460–14469. [Google Scholar] [CrossRef]
- Uddin, M.J.; Alaboina, P.K.; Zhang, L.F.; Cho, S.J. A low-cost, environment-friendly lignin-polyvinyl alcohol nanofiber separator using a water-based method for safer and faster lithium-ion batteries. Mater. Sci. Eng. B Solid State Mater Adv. Technol. 2017, 223, 84–90. [Google Scholar] [CrossRef]
- Dominguez-Robles, J.; Sanchez, R.; Diaz-Carrasco, P.; Espinosa, E.; Garcia-Dominguez, M.T.; Rodriguez, A. Isolation and characterization of lignins from wheat straw: Application as binder in lithium batteries. Int. J. Biol. Macromol. 2017, 104, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, K.; Ma, J.; Xu, G.J.; Dong, S.M.; Chen, B.B.; Li, J.D.; Chen, Z.; Zhou, X.H.; Cui, G.L. A biomass based free radical scavenger binder endowing a compatible cathode interface for 5 V lithium-ion batteries. Energy Environ. Sci. 2019, 12, 273–280. [Google Scholar] [CrossRef]
- Wang, D.; Lee, S.H.; Kim, J.; Park, C.B. “Waste to Wealth”: Lignin as a Renewable Building Block for Energy Harvesting/Storage and Environmental Remediation. ChemSusChem 2020, 13, 2807–2827. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Xu, T.; Liu, K.; Zhang, M.; Liu, W.; Li, H.; Du, H.S.; Si, C.L. Lignin-based electrodes for energy storage application. Ind. Crops Prod. 2021, 165, 113425. [Google Scholar] [CrossRef]
- Long, C.; Zhuang, J.L.; Xiao, Y.; Zheng, M.T.; Hu, H.; Dong, H.W.; Lei, B.F.; Zhang, H.R.; Liu, Y.L. Nitrogen-doped porous carbon with an ultrahigh specific surface area for superior performance supercapacitors. J. Power Sources 2016, 310, 145–153. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, N.; Lei, S.W.; Yan, R.; Tian, X.D.; Wang, J.Z.; Song, Y.; Xu, D.F.; Guo, Q.G.; Liu, L. Promising biomass-based activated carbons derived from willow catkins for high performance supercapacitors. Electrochim. Acta 2015, 166, 1–11. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Lu, M.; Tao, P.Y.; Zhang, Y.J.; Gong, X.T.; Yang, Z.; Zhang, G.Q.; Li, H.L. Hierarchically porous and heteroatom doped carbon derived from tobacco rods for supercapacitors. J. Power Sources 2016, 307, 391–400. [Google Scholar] [CrossRef]
- Cai, Y.J.; Luo, Y.; Xiao, Y.; Zhao, X.; Liang, Y.; Hu, H.; Dong, H.W.; Sun, L.Y.; Liu, Y.L.; Zheng, M.T. Facile Synthesis of Three-Dimensional Heteroatom-Doped and Hierarchical Egg-Box-Like Carbons Derived from Moringa oleifera Branches for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 33060–33071. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Benabithe, Z.; Castro, C.D.; Quintana, G. Kraft lignin as a raw material of activated carbon for supercapacitor electrodes. J. Mater. Sci. Mater. Electron. 2022, 33, 7031–7047. [Google Scholar] [CrossRef]
- Zhang, W.L.; Zhao, M.Z.; Liu, R.Y.; Wang, X.F.; Lin, H.B. Hierarchical porous carbon derived from lignin for high performance supercapacitor. Colloids Surf. A 2015, 484, 518–527. [Google Scholar] [CrossRef]
- Song, Y.G.; Liu, J.L.; Sun, K.; Xu, W. Synthesis of sustainable lignin-derived mesoporous carbon for supercapacitors using a nano-sized MgO template coupled with Pluronic F127. RSC Adv. 2017, 7, 48324–48332. [Google Scholar] [CrossRef] [Green Version]
- Lim, G.H.; Lee, J.W.; Choi, J.H.; Kang, Y.C.; Roh, K.C. Efficient utilization of lignin residue for activated carbon in supercapacitor applications. Mater. Chem. Phys. 2022, 284, 126073. [Google Scholar] [CrossRef]
- Yi, Y.J.; Hou, Y.; Li, Y.M. Preparation of High Conductivity Hierarchical Porous Carbon Based on Sodium Lignosulfonate with Pre-crosslinking. Bioresources 2020, 15, 8995–9012. [Google Scholar] [CrossRef]
- Liu, W.S.; Yao, Y.M.; Fu, O.L.; Jiang, S.H.; Fang, Y.C.; Wei, Y.; Lu, X.H. Lignin-derived carbon nanosheets for high-capacitance supercapacitors. RSC Adv. 2017, 7, 48537–48543. [Google Scholar] [CrossRef]
- Herou, S.; Ribadeneyra, M.C.; Madhu, R.; Araullo-Peters, V.; Jensen, A.; Schlee, P.; Titirici, M. Ordered mesoporous carbons from lignin: A new class of biobased electrodes for supercapacitors. Green Chem. 2019, 21, 550–559. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, J.M.; Lei, E.; Ma, C.H.; Luo, S.; Wu, Z.W.; Li, W.; Liu, S.X. Effects of the Pore Structure of Commercial Activated Carbon on the Electrochemical Performance of Supercapacitors. J. Energy Storage 2022, 45, 103457. [Google Scholar] [CrossRef]
- Du, B.Y.; Zhu, H.W.; Chai, L.F.; Cheng, J.L.; Wang, X.; Chen, X.H.; Zhou, J.H.; Sun, R.C. Effect of lignin structure in different biomass resources on the performance of lignin-based carbon nanofibers as supercapacitor electrode. Ind. Crops Prod. 2021, 170, 113745. [Google Scholar] [CrossRef]
- Zhou, B.J.; Li, J.; Liu, W.; Jiang, H.M.; Li, S.B.; Tan, L.X.; Dong, L.C.; She, L.; Wei, Z.D. Functional-Group Modification of Kraft Lignin for Enhanced Supercapacitors. ChemSusChem 2020, 13, 2628–2633. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Cao, Y.H.; Wang, X.M.; Castro, M.A.; Luo, B.; Gu, Z.R.; Liu, J.; Hoefelmeyer, J.D.; Fan, Q.H. Rod-shape porous carbon derived from aniline modified lignin for symmetric supercapacitors. J. Power Sources 2016, 307, 462–467. [Google Scholar] [CrossRef] [Green Version]
- Milczarek, G.; Nowicki, M. Carbon nanotubes/kraft lignin composite: Characterization and charge storage properties. Mater. Res. Bull. 2013, 48, 4032–4038. [Google Scholar] [CrossRef]
- Leguizamon, S.; Diaz-Orellana, K.P.; Velez, J.; Thies, M.C.; Roberts, M.E. High charge-capacity polymer electrodes comprising alkali lignin from the Kraft process. J. Mater. Chem. A 2015, 3, 11330–11339. [Google Scholar] [CrossRef]
- Xu, H.L.; Jiang, H.; Li, X.W.; Wang, G.C. Synthesis and electrochemical capacitance performance of polyaniline doped with lignosulfonate. RSC Adv. 2015, 5, 76116–76121. [Google Scholar] [CrossRef]
- Ajjan, F.N.; Casado, N.; Rebis, T.; Elfwing, A.; Solin, N.; Mecerreyes, D.; Inganas, O. High performance PEDOT/lignin biopolymer composites for electrochemical supercapacitors. J. Mater. Chem. A 2016, 4, 1838–1847. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.T.; Wang, W.D.; Lu, Q.F.; Zhao, H.B.; Zhang, X.Q.; Lin, Q.L. Graphene-wrapped nitrogen-containing carbon spheres for electrochemical supercapacitor application. J. Anal. Appl. Pyrolysis. 2015, 113, 545–550. [Google Scholar] [CrossRef]
- Yu, B.M.; Gele, A.; Wang, L.P. Iron oxide/lignin-based hollow carbon nanofibers nanocomposite as an application electrode materials for supercapacitors. Int. J. Biol. Macromol. 2018, 118, 478–484. [Google Scholar] [CrossRef]
- Shi, F.Y.; Tong, Y.; Li, H.S.; Li, J.J.; Cong, Z.Y.; Zhai, S.R.; An, Q.D.; Wang, K. Synthesis of oxygen/nitrogen/sulfur codoped hierarchical porous carbon from enzymatically hydrolyzed lignin for high-performance supercapacitors. J. Energy Storage 2022, 52, 104992. [Google Scholar] [CrossRef]
- Hu, P.Y.; Jin, H.; Wang, K.L.; Zhao, Z.Z.; Qu, W.D. Lignin-based carbon nanofibe rs: Morphologies, properties, and features as substrates for pseudocapacitor electrodes. Int. J. Biol. Macromol. 2021, 193, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Gao, F.X.; Wang, X.Q.; Zhang, N.; Ma, M.M. Strong and Robust Polyaniline-Based Supramolecular Hydrogels for Flexible Supercapacitors. Angew. Chem. Int. Ed. 2016, 55, 9196–9201. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, X.; Li, C.; Sun, X.Z.; Meng, Q.H.; Ma, Y.W.; Wei, Z.X. Chemically Crosslinked Hydrogel Film Leads to Integrated Flexible Supercapacitors with Superior Performance. Adv. Mater. 2015, 27, 7451. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhong, M.; Shi, F.K.; Liu, X.Y.; Tang, Z.J.; Wang, Y.K.; Huang, Y.; Hou, H.Q.; Xie, X.M.; Zhi, C.Y. An Intrinsically Stretchable and Compressible Supercapacitor Containing a Polyacrylamide Hydrogel Electrolyte. Angew. Chem. Int. Ed. 2017, 56, 9141–9145. [Google Scholar] [CrossRef]
- Park, J.H.; Rana, H.H.; Lee, J.Y.; Park, H.S. Renewable flexible supercapacitors based on all-lignin-based hydrogel electrolytes and nanofiber electrodes. J. Mater. Chem. A 2019, 7, 16962–16968. [Google Scholar] [CrossRef]
- Liu, T.; Ren, X.L.; Zhang, J.M.; Liu, J.; Ou, R.X.; Guo, C.G.; Yu, X.Y.; Wang, Q.W.; Liu, Z.Z. Highly compressible lignin hydrogel electrolytes via double-crosslinked strategy for superior foldable supercapacitors. J. Power Sources 2020, 449, 227532. [Google Scholar] [CrossRef]
- Lima, R.B.; Raza, R.; Qin, H.Y.; Li, J.B.; Lindstrom, M.E.; Zhu, B. Direct lignin fuel cell for power generation. RSC Adv. 2013, 3, 5083–5089. [Google Scholar] [CrossRef]
- Shewa, W.A.; Lalman, J.A.; Chaganti, S.R.; Heath, D.D. Electricity production from lignin photocatalytic degradation byproducts. Energy 2016, 111, 774–784. [Google Scholar] [CrossRef]
- Liu, W.; Mu, W.; Deng, Y.L. High-Performance Liquid-Catalyst Fuel Cell for Direct Biomass-into-Electricity Conversion. Angew. Chem. Int. Ed. 2014, 53, 13558–13562. [Google Scholar] [CrossRef]
- Zhao, X.B.; Zhu, J.Y. Efficient Conversion of Lignin to Electricity Using a Novel Direct Biomass Fuel Cell Mediated by Polyoxometalates at Low Temperatures. ChemSusChem 2016, 9, 197–207. [Google Scholar] [CrossRef]
- Li, Y.D.; Liu, T.F.; Qiu, X.Q.; Zhou, Y.H.; Li, Y. Enhancing Efficiency and Durability of Inverted Perovskite Solar Cells with Phenol/Unsaturated Carbon-Carbon Double Bond Dual-Functionalized Poly(3,4-ethylenedioxythiophene) Hole Extraction Layer. ACS Sustain. Chem. Eng. 2019, 7, 961–968. [Google Scholar] [CrossRef]
- Thomas, J.P.; Leung, K.T. Mixed co-solvent engineering of PEDOT:PSS to enhance its conductivity and hybrid solar cell properties. J. Mater. Chem. A 2016, 4, 17537–17542. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.Y.; Li, Y.; Yuan, J.Y.; Hong, Q.M.; Shi, G.Z.; Yuan, D.X.; Wei, J.; Huang, C.C.; Tang, J.X.; Fung, M.K. Improved performance of inverted planar perovskite solar cells with F4-TCNQ doped PEDOT:PSS hole transport layers. J. Mater. Chem. A 2017, 5, 5701–5708. [Google Scholar] [CrossRef]
- Xie, B.S.; Hou, Y.; Liu, C.; Li, Y.M. Hydrophobic magnetic bilayer micro-particles from OA@Lignin@Fe3O4 for high-efficient oil adsorption. Eur. Polym. J. 2022, 168, 111090. [Google Scholar] [CrossRef]
- Hu, H.C.; Xu, H.M.; Wu, J.Y.; Li, L.J.; Yue, F.X.; Huang, L.L.; Chen, L.H.; Zhang, X.Y.; Ouyang, X.H. Secondary Bonds Modifying Conjugate-Blocked Linkages of Biomass-Derived Lignin to Form Electron Transfer 3D Networks for Efficiency Exceeding 16% Nonfullerene Organic Solar Cells. Adv. Funct. Mater. 2020, 30, 2001494. [Google Scholar] [CrossRef]
- Ma, X.J.; Elbohy, H.; Sigdel, S.; Lai, C.L.; Qiao, Q.Q.; Fong, H. Electrospun carbon nano-felt derived from alkali lignin for cost-effective counter electrodes of dye-sensitized solar cells. RSC Adv. 2016, 6, 11481–11487. [Google Scholar] [CrossRef]
| Kraft Lignin | Lignosulfonate | Soda Lignin | Organosolv Lignin | |
|---|---|---|---|---|
| Extraction methods | NaOH, Na2S, 150–170 °C, 1–2 h | Sulfur dioxide (Na, Ca or Mg as counter ion), 125–150 °C, 1–5 h | NaOH or NaOH- anthraquinone, 140–170 °C, 1–2 h | Acetic acid/formic acid/water, 80–130 °C, 1–4 h |
| Isolation methods | Acid precipitation | Ultrafiltration | Acid precipitation | Antisolvent precipitation |
| Molecular weight (×103 g mol−1) | 1.0–6.0 | 15–50 | 0.8–3.0 | 0.5–5.0 |
| Polydispersity | 2.5–3.5 | 6.0–8.0 | 2.5–3.5 | 1.5–4.0 |
| Impurities (%) | Sulfur 1–3 | Sulfur 3–8 | Sulfur < 0.1% | ash < 10 |
| Tg (°C) | 140–150 | 130 | 140 | 90–110 |
| Solubility | Organic solvents and alkali | Water | Alkali | Organic solvents |
| Lignin Type | Lignin-Based Materials | SSA (m2 g−1) | Rate (A g−1) | Specific Capacitance (mA h g−1) | Ref. |
|---|---|---|---|---|---|
| KL | Carbon fibers | - | 0.1 C | 335 | [40] |
| SL | Hollow mesoporous spheres | - | 1 | 756 | [39] |
| AL | 3D hierarchical porous carbon | - | 0.2 | 470 | [44] |
| AL | N-doped carbon nanospheres | 419.2 | 0.06 | 225 | [42] |
| AL | 3D porous carbon | 167.5 | 0.2 | 469 | [43] |
| AL | N, P- codoped porous carbon | 675.4 | 1 | 1463.8 | [45] |
| LS | Hierarchical mesoporous carbon nanospheres | 462.8 | 0.1 | 520 | [46] |
| OSL | Carbon nanofibers | 381 | 2 | 200 | [47] |
| OSL | Carbon nanofibers | - | 0.015 | 193 | [48] |
| Lignin-Based Materials | Porogen | SSA (m2 g−1) | Rate (A g−1) | Specific Capacitance (mA h g−1) | Ref. |
|---|---|---|---|---|---|
| AL/MgO/G | Mg (NO3)2·6H2O | 628.09 | 2 C | 1064.7 | [36] |
| SL/Fe3O4 | FeCl3·6H2O and Fe (NO3)3·9H2O | - | 1 | 750 | [49] |
| AL/SiNPs | Self-assembly | - | 9 | 800 | [50] |
| AL/Si | Mixing mixture | - | 0.3 | 880 | [51] |
| LS/NiO | Ni (OH)2 | 851.8 | 0.1 | 863 | [52] |
| OSL/PEO | urea | 381 | 2 | 200 | [47] |
| EHL/CNTs | K2CO3 | 740 | 1 | 240 | [53] |
| Materials | Porogen | Electrolyte | Rate (A g−1) | Specific Capacitance (F g−1) | Ref. |
|---|---|---|---|---|---|
| AL | Freeze drying | 1 M H2SO4 | 0.5 | 281 | [74] |
| AL | KOH | 6 M KOH | 0.2 | 286.7 | [75] |
| AL | F127, MgO | 6 M KOH | 0.2 | 186.3 | [76] |
| KL | KOH | 1 M H2SO4 | 0.63 | 196.5 | [77] |
| LS | KOH | 6 M KOH | 0.5 | 305 | [78] |
| OL | KOH | 1 M TEABF4 | 1 mA cm−2 | 131 | [79] |
| OL | Self-assembly | 6 M KOH | - | 90 F cm−3 | [80] |
| Commercial AC | - | 6 M KOH | 1 | 139.35 | [81] |
| Materials | Porogen | Electrolyte | Rate (A g−1) | Specific Capacitance (F g−1) | Ref. |
|---|---|---|---|---|---|
| KL/PANI | 6 M KOH | 0.5 | 141.3 | [82] | |
| KL/Fe2O3 | - | 1 M H2SO4 | 0.5 | 390 | [83] |
| KL/aniline | KOH | 6 M KOH | 1 | 333 | [84] |
| KL/CNT | - | 1 M H2SO4 | 2.5 | 177 | [85] |
| AL/PPy | - | 0.5 M H2SO4 | 0.5 mA g−1 | 444 | [86] |
| LS/PANI | - | 1 M H2SO4 | 10 | 377.2 | [87] |
| LS/PEDOT | - | 0.1 M HClO4/acetonitrile | 1 | 170.4 | [88] |
| LS/PANI/GO | - | 6 M KOH | 0.5 | 266.7 | [89] |
| OL/Fe(acac)3 | - | 1 M Na2SO3 | 0.5 | 121 | [90] |
| EHL/urea | KOH | 6 M KOH | 0.5 | 318 | [91] |
| EHL/PANI | - | 0.5 M H2SO4 | 0.29 | 229 | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Y.; Zhuang, J.; Liu, C.; Lei, L.; He, S.; Hou, Y. Emerging Lignin-Based Materials in Electrochemical Energy Systems. Energies 2022, 15, 9450. https://doi.org/10.3390/en15249450
Yi Y, Zhuang J, Liu C, Lei L, He S, Hou Y. Emerging Lignin-Based Materials in Electrochemical Energy Systems. Energies. 2022; 15(24):9450. https://doi.org/10.3390/en15249450
Chicago/Turabian StyleYi, Yanjie, Jingshun Zhuang, Chao Liu, Lirong Lei, Shuaiming He, and Yi Hou. 2022. "Emerging Lignin-Based Materials in Electrochemical Energy Systems" Energies 15, no. 24: 9450. https://doi.org/10.3390/en15249450








