The P2A system was placed on shore for the system. An offshore P2A system that transports ammonia by ship or by pipe to the land can also be a viable consideration. Despite the advantages such as low ammonia shipping costs, low electrical losses, not taking up land space onshore, and potential refuel locations for ammonia-powered marine ships [
15], offshore P2A systems on an offshore platform have significant economic complexities for an accurate analysis. For example, the cost of building these offshore platforms are usually confidential but were estimated to be around 100–500 million USD [
17] which is a significantly large range. The logistics and costs of operating and maintenance of offshore P2A systems are difficult to estimated. In addition, repurposing existing oil rigs could have a variable cost depending on the types of processing equipment on the rigs. Therefore, an onshore plant was chosen to have a more accurate capital expenditure (CAPEX) estimation.
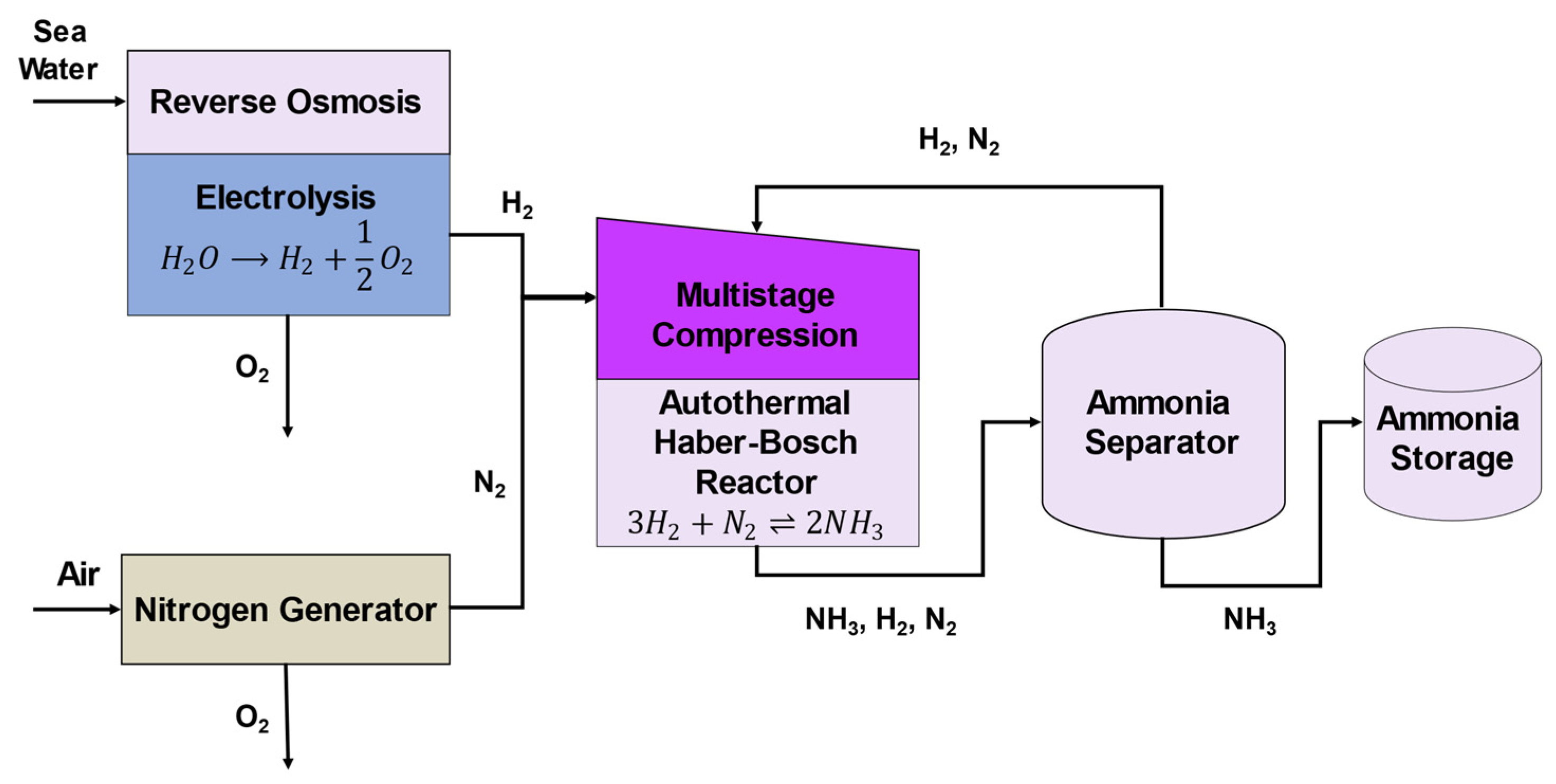
3.2.1. RO and Electrolyzer
The RO and electrolyser subsystem have inputs of seawater and electricity and outputs oxygen and hydrogen. Seawater will be inputted into the RO system which will remove salt and impurities so that it is suitable for use in the electrolysis. The PEM electrolyser uses electrical power to convert water into hydrogen and oxygen.
The sizing of the subsystem was based on 15,000 kg of ammonia per day. The electrolyser was sized first and the RO system second. Using overall mass balances and assuming an 97% overall conversion in the ammonia reactor (details in
Section 3.2.4), the amount of hydrogen required for that basis would be about 2743 kg day
−1 from an electrolyser that can output around 1271.5 Nm
3 h
−1 of hydrogen. A company by the name of Nel with the electrolyser model M2000 met this requirement with a possible volumetric hydrogen output of 2000 Nm
3 h
−1 [
33]. Nel was chosen over other vendors as that had the most available specifications. According to the product specifications, the electrolyser unit would result in average power consumption of around 4.5 kWh/Nm
3 of hydrogen [
33]. This means that about 5.7 MW of power would be required for each ton of ammonia. With a safety factor of about 20%, the power that the electrolyser was sized to handle was 6.86 MW. The capital cost of the electrolyser was estimated using an article that stated the cost of an electrolyser stack as around 400 USD/kW [
34] and using a power of 7 MW. With the assumption that 50% of the total cost of the electrolyser goes to the stack, the calculated capital cost of the electrolyser was estimated to be around 7.2 million CAD.
With the electrolyser information above, it was calculated that about 27,417 kg day
−1 of processed water would be required in the RO system which is about 7128 gallons per day. A 10,000 gallon per day capacity RO system by ForeverPure [
35] was selected for this report. With a 26% recovery rate, the amount of seawater inputted into the reverse osmosis system was about 105,000 kg of water per day [
35]. The power consumed on the RO system was based off a consumption of around 3 kWh m
−3 of water [
36] which is typical for many seawater RO systems. The cost of the RO system was taken to be 23,056 CAD which was an average of three RO systems that have similar capacities [
37,
38,
39].
A hydrogen storage tank will be placed at the end of the subsystem. The hydrogen storage will have a holdup time of about 15 min. The size of the storage tank will have a volume of around 12 m
3 and will hold the hydrogen at 25 °C and 30 bar. Using Ulrich and Vasudevan methods [
40], the cost of a stainless steel cylindrical hydrogen tank with a length of 6 m and a diameter of 2 m would be about 500,000 CAD.
3.2.4. Ammonia Reactor
The ammonia reactor synthesizes ammonia by taking hydrogen and nitrogen as inputs. Modern Haber catalysts such as ruthenium and iron oxide operate at moderate conditions (380 °C and 86 bar), compared with the traditional Haber magnetite catalysts at 450 °C and 300 bar [
44,
45,
46]. Iron oxide was selected as the catalyst due to its lower cost compared to ruthenium. A typical radial flow bed reactor was chosen, because it reduces pressure drop across the catalyst bed [
3,
46,
47,
48]. The reactor was designed according to main design specifications required, i.e., recycle rate and the size of the catalyst beds. Typical large-scale systems achieve up to 26% single-pass conversion and between 97–99% overall conversion with the recycle stream [
44,
45]. Yet, the small modular scale of our design is uncommon in the literature, so more conservative specifications, i.e., 20% single-pass conversion and 97% overall conversion with recycling were chosen. The conversion for the first bed is calculated by dividing the amount reacted in the first bed by the amount inputted into the first bed. The overall conversion is calculated by dividing the total amount that was reacted in both beds by the total amount of input to the two reactor beds. This is significant because there is an interstage quench stream which is inputted into the process in between the two beds.
As the offshore wind power is intermittent [
3], it is important to understand the operating flexibility of the process to function under different production rates. Once the reactor is sized and built, the bed size will not change but the production rates could change. The inlet flow rate of reactants can vary depending on the power available to produce hydrogen and nitrogen. The chosen configuration was based on the work by Iqbal Cheema et al. [
3] where they designed a multistage ammonia synthesis reactor that uses the heat generated in the exothermic reaction to heat the feed to the reactor and make the reaction self-sustaining. Following their work, the assumption was made that heat losses were negligible.
Figure 2 is a process diagram of the ammonia reactor depicting the multiple catalyst beds and the interstage quench stream.
The designed output of the reactor is 15 tonnes ammonia per day. The maximum reactor temperature is 480 °C. The feed is a stoichiometric mixture at 325 °C and 86 bar. The reactor has two beds: the first one is 0.40 m
3 and the second bed is 0.74 m
3. The catalyst properties which were used in the sizing the reactor (i.e., rate constant, activation energy, etc.) were based on the industrial catalyst ZA-5 described by Liu et al. [
46]. These parameters are summarized in
Table 1. These parameters were used in Equations (5) and (6) which describe the reaction kinetics.
The reactor packed beds were modelled as ideal packed bed reactors with the following reaction: [
3,
46,
49].
The mass and energy balances are:
where
is the molar flow rate of species
in mol s
−1,
is the catalyst weight in kg,
is the rate of reaction mol kg
−1 s
−1,
is the stoichiometric coefficient,
is the temperature in ℃ or K,
is the specific heat capacity of species
in kJ mol
−1 K
−1 and
is the heat of reaction in kJ mol
−1.
The reaction kinetics are modelled using the Temkin equation described by Equations (4)–(6) [
3,
46,
49,
50]; where
is the rate constant in atm
0.5 s
−1;
is the catalyst bulk density in kg m
−3,
is the equilibrium constant in atm
−1,
is a parameter specific to the catalyst, dimensionless,
is the pre-exponential factor in atm
0.5 s
−1,
is the activation energy in kJ mol
−1, and
is the ideal gas constant. The fugacity,
, of each component is calculated using Equation (7), where
and
are the mole fraction and fugacity coefficient of component
respectively and
is the pressure in atm.
is not meant to be confused with the molar flowrates of a species but is a simplifying term to represent the large kinetic term in (5). For the selected catalyst size (2–3 mm), the rate of reaction can be taken without corrective factor and the pressure drop can be neglected [
3].
Liu et al. [
46] and Gillespie et al. [
51] provide correlations for calculating the heat capacity of each reactive component, the Arrhenius parameters and the equilibrium constant. Panahandeh et al. [
47] provides a correlation for computing the heat of reaction. Dyson et al. [
50] provide correlations for calculating the fugacity coefficients. A summary of the correlations utilized by the modelling of the reactor can be found in
Table 2 below:
The modeling Equations (2) and (3) were solved using MATLAB’s solver ode45.
Figure 3 shows the nominal profile of temperature and conversion along the reactor beds. The nominal profile is based on a constant output of 15 tonnes of ammonia per day.
The ammonia reactor is an autothermal reactor. The heat generated by the reaction in both beds are used to preheat the feed through heat exchangers before bed 1 to sustain the overall reaction, and the system exhibits multiple steady states.
Figure 4 shows the dependence of conversion in the reactor on the inlet flow rate to the reactor, using the method by Iqbal Cheema et al. [
3] for this autothermal reactor set-up. Firstly, the operating line of the beds was found by solving the ordinary differential Equations (2) and (3) at a given inlet
T while flowrate is held constant. This will solve for the temperature at the reactor outlet at a given temperature at the inlet. Secondly, operating lines of the heat exchanger is obtained by using the effectiveness equation from Iqbal [
3]. Then, one can find where the operating lines intersect, and each intersection represents one steady state. The upper and lower ones are considered stable. This process is repeated with different flowrates until a graph like
Figure 4 is made where all the steady states are plotted at a range of flowrates.
When the flow rates are higher than the nominal design flow rate, there is not enough catalyst available to achieve high conversions. As a result, less heat is generated in the reactor, which results in a further decrease in conversion because there is not enough heat available to sustain the reaction. Thus,
Figure 4 shows that the system drops to a lower steady state where conversion is very low and considered not viable for production. This means that it would not be possible to ramp-up production when there is excess wind power beyond the nominal operating point. The reactor is quite flexible for inlet flow rates below the nominal point. The system can remain at an upper steady state without causing a shutdown.
The lower production bound is then limited by other components in the P2A process. According to Nel, the M2000 PEM electrolysis systems can operate down to 10% of their maximum production capacity [
52]. The lower operating bound of the compressors is usually limited by the surge boundary which is the minimum operable flow for a compressor. This depends on parameters such as the type of compressor, the shape of the impeller, the impeller speed, etc. [
53]. This boundary is usually located 45 to 65% of the nominal flow rate, but this could also be varied by using variable speed compressors or compressor trains in parallel [
53]. In this study, 55% was chosen as the lower production rate.
The ammonia reactor’s cost was analyzed by estimating the cost of two stainless steel process vessels and the catalyst required. The two stainless steel process vessels would hold the two beds. The first vessel would have a bed length of 1.519 m, a vessel radius of around 0.47 m, and a length of 1.719 m. The second vessel would have a bed length of 1.783 m, a vessel radius of around 0.56 m, and a length of 1.98 m. The cost of the vessels was estimated using the costing charts from Ulrich and Vasudevan [
40] be around 340,000 CAD and 680,000 CAD each. The catalyst bed’s cost was estimated to be around 1.8 euros per kilogram of catalyst [
45] and there was in total about 3700 kg of catalyst within the two beds combined. This would mean that it would cost about 9500 CAD. Summing it all up, the ammonia reactor is expected to cost around 1 million CAD.