Towards Integration of Two-Dimensional Hexagonal Boron Nitride (2D h-BN) in Energy Conversion and Storage Devices
Abstract
:1. Introduction
2. Application of 2D h-BN
2.1. Batteries
2.1.1. Li-Ion Batteries (LIBs)
2.1.2. Mg Metal-Based Batteries (MMBs)
2.1.3. Zinc Flow Batteries (ZFBs)
2.1.4. Other
2.2. Supercapacitors
2.2.1. Electrodes
2.2.2. Electrolyte
2.3. Thermoelectrics
2.4. Fuel Cells
2.4.1. Membrane
2.4.2. Cathode Catalyst
2.4.3. Hydrogen Environmental Barrier Coating
2.4.4. Seal
2.5. Solar Cell
3. Future Challenges and Remarks
Funding
Conflicts of Interest
References
- Gong, Y.; Xu, Z.-Q.; Li, D.; Zhang, J.; Aharonovich, I.; Zhang, Y. Two-Dimensional Hexagonal Boron Nitride for Building Next-Generation Energy-Efficient Devices. ACS Energy Lett. 2021, 6, 985–996. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, S.; Yapici, N.; Oakley, R.; Sharma, S.; Parashar, V.; Yap, Y.K. Emerging Applications of Boron Nitride Nanotubes in Energy Harvesting, Electronics, and Biomedicine. ACS Omega 2021, 6, 20722–20728. [Google Scholar] [CrossRef]
- Glavin, N.R.; Rao, R.; Varshney, V.; Bianco, E.; Apte, A.; Roy, A.; Ringe, E.; Ajayan, P.M. Emerging Applications of Elemental 2D Materials. Adv. Mater. 2020, 32, e1904302. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Angizi, S.; Akbar, M.A.; Darestani-Farahani, M.; Kruse, P. Review—Two-Dimensional Boron Carbon Nitride: A Comprehensive Review. ECS J. Solid State Sci. Technol. 2020, 9, 83004. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Benu, D.P.; Xu, X.; Yuliarto, B.; Yamauchi, Y.; Golberg, D. Borophene: Two-dimensional Boron Monolayer: Synthesis, Properties, and Potential Applications. Chem. Rev. 2022, 122, 1000–1051. [Google Scholar] [CrossRef] [PubMed]
- Angizi, S.; Alem, S.A.A.; Hasanzadeh Azar, M.; Shayeganfar, F.; Manning, M.I.; Hatamie, A.; Pakdel, A.; Simchi, A. A comprehensive review on planar boron nitride nanomaterials: From 2D nanosheets towards 0D quantum dots. Prog. Mater. Sci. 2022, 124, 100884. [Google Scholar] [CrossRef]
- Han, R.; Liu, F.; Wang, X.; Huang, M.; Li, W.; Yamauchi, Y.; Sun, X.; Huang, Z. Functionalised hexagonal boron nitride for energy conversion and storage. J. Mater. Chem. A 2020, 8, 14384–14399. [Google Scholar] [CrossRef]
- Khalaj, M.; Golkhatmi, S.Z.; Alem, S.A.A.; Baghchesaraee, K.; Azar, M.H.; Angizi, S. Recent Progress in the Study of Thermal Properties and Tribological Behaviors of Hexagonal Boron Nitride-Reinforced Composites. J. Compos. Sci. 2020, 4, 116. [Google Scholar] [CrossRef]
- Pakdel, A.; Zhi, C.; Bando, Y.; Nakayama, T.; Golberg, D. Boron Nitride Nanosheet Coatings with Controllable Water Repellency. ACS Nano 2011, 5, 6507–6515. [Google Scholar] [CrossRef]
- Zeng, H.; Zhi, C.; Zhang, Z.; Wei, X.; Wang, X.; Guo, W.; Bando, Y.; Golberg, D. “White Graphenes”: Boron Nitride Nanoribbons via Boron Nitride Nanotube Unwrapping. Nano Lett. 2010, 10, 5049–5055. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, A.; Bando, Y.; Golberg, D. Plasma-Assisted Interface Engineering of Boron Nitride Nanostructure Films. ACS Nano 2014, 8, 10631–10639. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, A.; Bando, Y.; Golberg, D. Nano boron nitride flatland. Chem. Soc. Rev. 2014, 43, 934–959. [Google Scholar] [CrossRef]
- Angizi, S.; Khalaj, M.; Alem, S.A.A.; Pakdel, A.; Willander, M.; Hatamie, A.; Simchi, A. Review—Towards the Two-Dimensional Hexagonal Boron Nitride (2D h-BN) Electrochemical Sensing Platforms. J. Electrochem. Soc. 2020, 167, 126513. [Google Scholar] [CrossRef]
- Falin, A.; Cai, Q.; Santos, E.J.G.; Scullion, D.; Qian, D.; Zhang, R.; Yang, Z.; Huang, S.; Watanabe, K.; Taniguchi, T.; et al. Mechanical properties of atomically thin boron nitride and the role of interlayer interactions. Nat. Commun. 2017, 8, 15815. [Google Scholar] [CrossRef] [Green Version]
- Angizi, S.; Yu, E.Y.C.; Dalmieda, J.; Saha, D.; Selvaganapathy, P.R.; Kruse, P. Defect Engineering of Graphene to Modulate pH Response of Graphene Devices. Langmuir 2021, 37, 12163–12178. [Google Scholar] [CrossRef] [PubMed]
- Angizi, S.; Hatamie, A.; Ghanbari, H.; Simchi, A.A. Mechanochemical Green Synthesis of Exfoliated Edge-Functionalized Boron Nitride Quantum Dots: Application to Vitamin C Sensing through Hybridization with Gold Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 28819–28827. [Google Scholar] [CrossRef]
- Angizi, S.; Shayeganfar, F.; Azar, M.H.; Simchi, A. Surface/edge functionalized boron nitride quantum dots: Spectroscopic fingerprint of bandgap modification by chemical functionalization. Ceram. Int. 2020, 46, 978–985. [Google Scholar] [CrossRef]
- Pakdel, A.; Zhi, C.; Bando, Y.; Golberg, D. Low-dimensional boron nitride nanomaterials. Mater. Today 2012, 15, 256–265. [Google Scholar] [CrossRef]
- Lin, Y.; Williams, T.V.; Cao, W.; Elsayed-Ali, H.E.; Connell, J.W. Defect Functionalization of Hexagonal Boron Nitride Nanosheets. J. Phys. Chem. C 2010, 114, 17434–17439. [Google Scholar] [CrossRef]
- Ma, P.; Spencer, J.T. Non-covalent stabilization and functionalization of boron nitride nanosheets (BNNSs) by organic polymers: Formation of complex BNNSs-containing structures. J. Mater. Sci. 2015, 50, 313–323. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, X.; Wang, X.; Bando, Y.; Golberg, D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, J.; Stagi, L.; Innocenzi, P. Hydroxylated boron nitride materials: From structures to functional applications. J. Mater. Sci. 2021, 56, 4053–4079. [Google Scholar] [CrossRef]
- Pakdel, A.; Bando, Y.; Shtansky, D.; Golberg, D. Nonwetting and optical properties of BN nanosheet films. Surf. Innov. 2013, 1, 32–39. [Google Scholar] [CrossRef]
- Wang, X.; Pakdel, A.; Zhang, J.; Weng, Q.; Zhai, T.; Zhi, C.; Golberg, D.; Bando, Y. Large-surface-area BN nanosheets and their utilization in polymeric composites with improved thermal and dielectric properties. Nanoscale Res. Lett. 2012, 7, 662. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Das, D.; Samanta, A.; de los Reyes, C.A.; Deng, L.; Alemany, L.B.; Weldeghiorghis, T.K.; Khabashesku, V.N.; Kochat, V.; Jin, Z.; et al. Fluorinated h-BN as a magnetic semiconductor. Sci. Adv. 2017, 3, e1700842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wu, X.; Zeng, X.C.; Yang, J. Band-Gap Engineering via Tailored Line Defects in Boron-Nitride Nanoribbons, Sheets, and Nanotubes. ACS Nano 2012, 6, 4104–4112. [Google Scholar] [CrossRef] [PubMed]
- Mballo, A.; Srivastava, A.; Sundaram, S.; Vuong, P.; Karrakchou, S.; Halfaya, Y.; Gautier, S.; Voss, P.L.; Ahaitouf, A.; Salvestrini, J.P.; et al. Towards P-Type Conduction in Hexagonal Boron Nitride: Doping Study and Electrical Measurements Analysis of hBN/AlGaN Heterojunctions. Nanomaterials 2021, 11, 211. [Google Scholar] [CrossRef]
- Singh, R.S.; Tay, R.Y.; Chow, W.L.; Tsang, S.H.; Mallick, G.; Teo, H.T.E. Band gap effects of hexagonal boron nitride using oxygen plasma. Appl. Phys. Lett. 2014, 104, 163101. [Google Scholar] [CrossRef]
- Herrera-Reinoza, N.; dos Santos, A.C.; de Lima, L.H.; Landers, R.; de Siervo, A. Atomically Precise Bottom-Up Synthesis of h-BNC: Graphene Doped with h-BN Nanoclusters. Chem. Mater. 2021, 33, 2871–2882. [Google Scholar] [CrossRef]
- Emanet, M.; Şen, O.; Taşkin, I.; Çulha, M. Synthesis, Functionalization, and Bioapplications of Two-Dimensional Boron Nitride Nanomaterials. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef]
- Krečmarová, M.; Canet-Albiach, R.; Pashaei-Adl, H.; Gorji, S.; Muñoz-Matutano, G.; Nesládek, M.; Martínez-Pastor, J.P.; Sánchez-Royo, J.F. Extrinsic Effects on the Optical Properties of Surface Color Defects Generated in Hexagonal Boron Nitride Nanosheets. ACS Appl. Mater. Interfaces 2021, 13, 46105–46116. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Kawahara, K.; Fukamachi, S.; Ago, H. Chemical Vapor Deposition Growth of Uniform Multilayer Hexagonal Boron Nitride Driven by Structural Transformation of a Metal Thin Film. ACS Appl. Electron. Mater. 2020, 2, 3270–3278. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Q.; Yu, H.; Bulin, C.; Sun, H.; Li, R.; Ge, X.; Xing, R. High-Efficient Liquid Exfoliation of Boron Nitride Nanosheets Using Aqueous Solution of Alkanolamine. Nanoscale Res. Lett. 2017, 12, 1–7. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, G.; Huang, F.; Liu, H.; Gong, C.; Wen, S.; Hu, Y.; Zheng, G.; Chen, D. Liquid Phase Exfoliated Hexagonal Boron Nitride/Graphene Heterostructure Based Electrode Toward Asymmetric Supercapacitor Application. Front. Chem. 2019, 7, 544. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kwon, S.; Cho, D.-H.; Kang, B.; Kwon, H.; Kim, Y.; Park, S.O.; Jung, G.Y.; Shin, E.; Kim, W.-G.; et al. Direct exfoliation and dispersion of two-dimensional materials in pure water via temperature control. Nat. Commun. 2015, 6, 8294. [Google Scholar] [CrossRef]
- Cao, L.; Emami, S.; Lafdi, K. Large-scale exfoliation of hexagonal boron nitride nanosheets in liquid phase. Mater. Express 2014, 4, 165–171. [Google Scholar] [CrossRef]
- Deepika, D.; Li, L.H.; Glushenkov, A.M.; Hait, S.K.; Hodgson, P.; Chen, Y. High-Efficient Production of Boron Nitride Nanosheets via an Optimized Ball Milling Process for Lubrication in Oil. Sci. Rep. 2014, 4, 7288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Z.; Chen, K.; Sun, S.; Zhang, J.; Cui, W.; Xie, Z.; Liu, G. Crystalline boron nitride nanosheets by sonication-assisted hydrothermal exfoliation. J. Adv. Ceram. 2019, 8, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Hu, C.; Wang, X. One-pot solvothermal synthesis of water-soluble boron nitride nanosheets and fluorescent boron nitride quantum dots. Mater. Lett. 2018, 234, 306–310. [Google Scholar] [CrossRef]
- Mahdizadeh, A.; Farhadi, S.; Zabardasti, A. Microwave-assisted rapid synthesis of graphene-analogue hexagonal boron nitride (h-BN) nanosheets and their application for the ultrafast and selective adsorption of cationic dyes from aqueous solutions. RSC Adv. 2017, 7, 53984–53995. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, J.; Zhu, L.; Wang, H.; Han, S.; Jin, Y.; Zhao, G.; Zhu, Y.; Guo, X.; Hou, J.; Yin, H.; et al. Hexagonal Boron Nitride/Blue Phosphorene Heterostructure as a Promising Anode Material for Li/Na-Ion Batteries. J. Phys. Chem. C 2018, 122, 23329–23335. [Google Scholar] [CrossRef]
- Shi, X.; Wang, K.; Tian, J.; Yin, X.; Guo, B.; Xi, G.; Wang, W.; Wu, W. Few-Layer Hydroxyl-Functionalized Boron Nitride Nanosheets for Nanoscale Thermal Management. ACS Appl. Nano Mater. 2020, 3, 2310–2321. [Google Scholar] [CrossRef]
- Huang, K.; Liang, L.; Chai, S.; Tumuluri, U.; Li, M.; Wu, Z.; Sumpter, B.G.; Dai, S. Aminopolymer functionalization of boron nitride nanosheets for highly efficient capture of carbon dioxide. J. Mater. Chem. A 2017, 5, 16241–16248. [Google Scholar] [CrossRef]
- Muhabie, A.A.; Cheng, C.-C.; Huang, J.-J.; Liao, Z.-S.; Huang, S.-Y.; Chiu, C.-W.; Lee, D.-J. Non-Covalently Functionalized Boron Nitride Mediated by a Highly Self-Assembled Supramolecular Polymer. Chem. Mater. 2017, 29, 8513–8520. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, D.; Guo, H.; Zhao, J.; Wang, Z.; Hu, H.; Xu, J.; Fu, C. Functionalized Boron Nitride Nanosheets/Poly(l-lactide) Nanocomposites and Their Crystallization Behavior. Polymers 2019, 11, 440. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Gao, D.; Zhao, S.; Xiao, Y.; Guo, Z.; Fang, Y.; Lin, J.; Liu, Z.; Huang, Y.; Guo, K.; et al. Carbon doped hexagonal boron nitride nanoribbon as efficient metal-free electrochemical nitrogen reduction catalyst. Chem. Eng. J. 2021, 410, 128419. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Li, Q.; Lin, J.; Guo, Z.; Zhang, X.; Lu, Z.; Ma, Y.; Huang, Y.; Tang, C. Fluorine doped porous boron nitride for efficient CO2 capture and separation: A DFT study. Appl. Surf. Sci. 2021, 556, 149775. [Google Scholar] [CrossRef]
- Lei, W.; Zhang, H.; Wu, Y.; Zhang, B.; Liu, D.; Qin, S.; Liu, Z.; Liu, L.; Ma, Y.; Chen, Y. Oxygen-doped boron nitride nanosheets with excellent performance in hydrogen storage. Nano Energy 2014, 6, 219–224. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, X.; Li, C.; Bendavid, A.; Westerhausen, M.T.; Bradac, C.; Toth, M.; Aharonovich, I.; Tran, T.T. Bottom-Up Synthesis of Hexagonal Boron Nitride Nanoparticles with Intensity-Stabilized Quantum Emitters. Small 2021, 17, 2008062. [Google Scholar] [CrossRef] [PubMed]
- Kainthola, A.; Bijalwan, K.; Negi, S.; Sharma, H.; Dwivedi, C. Hydrothermal synthesis of highly stable boron nitride nanoparticles. Mater. Today Proc. 2020, 28, 138–140. [Google Scholar] [CrossRef]
- Jing, X.; Puglisi, F.M.; Akinwande, D.; Lanza, M. Chemical vapor deposition of hexagonal boron nitride on metal-coated wafers and transfer-free fabrication of resistive switching devices. 2D Mater. 2019, 6, 35021. [Google Scholar] [CrossRef]
- McLean, B.D.; Webber, G.B.; Page, A.J. Boron Nitride Nucleation Mechanism during Chemical Vapor Deposition. J. Phys. Chem. C 2018, 122, 24341–24349. [Google Scholar] [CrossRef]
- Ren, X.; Dong, J.; Yang, P.; Li, J.; Lu, G.; Wu, T.; Wang, H.; Guo, W.; Zhang, Z.; Ding, F.; et al. Grain boundaries in chemical-vapor-deposited atomically thin hexagonal boron nitride. Phys. Rev. Mater. 2019, 3, 14004. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Zhang, J.; Weng, Z.; Kong, D.; Tao, Y.; Ding, F.; Ruan, D.; Yang, Q.-H. Two-dimensional materials for lithium/sodium-ion capacitors. Mater. Today Energy 2019, 11, 30–45. [Google Scholar] [CrossRef]
- Chen, K.-S.; Balla, I.; Luu, N.S.; Hersam, M.C. Emerging Opportunities for Two-Dimensional Materials in Lithium-Ion Batteries. ACS Energy Lett. 2017, 2, 2026–2034. [Google Scholar] [CrossRef]
- Rojaee, R.; Shahbazian-Yassar, R. Two-Dimensional Materials to Address the Lithium Battery Challenges. ACS Nano 2020, 14, 2628–2658. [Google Scholar] [CrossRef]
- Peng, L.; Zhu, Y.; Chen, D.; Ruoff, R.S.; Yu, G. Two-Dimensional Materials for Beyond-Lithium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600025. [Google Scholar] [CrossRef]
- Jeong, J.-M.; Choi, B.G.; Lee, S.C.; Lee, K.G.; Chang, S.-J.; Han, Y.-K.; Lee, Y.B.; Lee, H.U.; Kwon, S.; Lee, G.; et al. Hierarchical Hollow Spheres of Fe2O3@Polyaniline for Lithium Ion Battery Anodes. Adv. Mater. 2013, 25, 6250–6255. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lai, C.; Xiao, C.; Gao, X. Enhanced high rate capability of dual-phase Li4Ti5O12–TiO2 induced by pseudocapacitive effect. Electrochim. Acta 2011, 56, 9152–9158. [Google Scholar] [CrossRef]
- Li, H.; Tay, R.Y.; Tsang, S.H.; Liu, W.; Teo, E.H.T. Reduced Graphene Oxide/Boron Nitride Composite Film as a Novel Binder-Free Anode for Lithium Ion Batteries with Enhanced Performances. Electrochim. Acta 2015, 166, 197–205. [Google Scholar] [CrossRef]
- Zhang, F.; Németh, K.; Bareño, J.; Dogan, F.; Bloom, I.D.; Shaw, L.L. Experimental and theoretical investigations of functionalized boron nitride as electrode materials for Li-ion batteries. RSC Adv. 2016, 6, 27901–27914. [Google Scholar] [CrossRef]
- Ma, T.; Wang, R.; Jin, S.; Zheng, S.; Li, L.; Shi, J.; Cai, Y.; Liang, J.; Tao, Z. Functionalized Boron Nitride-Based Modification Layer as Ion Regulator Toward Stable Lithium Anode at High Current Densities. ACS Appl. Mater. Interfaces 2021, 13, 391–399. [Google Scholar] [CrossRef]
- de Moraes, A.C.M.; Hyun, W.J.; Seo, J.T.; Downing, J.R.; Lim, J.; Hersam, M.C. Ion-Conductive, Viscosity-Tunable Hexagonal Boron Nitride Nanosheet Inks. Adv. Funct. Mater. 2019, 29, 1902245. [Google Scholar] [CrossRef]
- Rodrigues, M.-T.F.; Kalaga, K.; Gullapalli, H.; Babu, G.; Reddy, A.L.M.; Ajayan, P.M. Hexagonal Boron Nitride-Based Electrolyte Composite for Li-Ion Battery Operation from Room Temperature to 150 °C. Adv. Energy Mater. 2016, 6, 1600218. [Google Scholar] [CrossRef]
- Waqas, M.; Ali, S.; Lv, W.; Chen, D.; Boateng, B.; He, W. High-Performance PE-BN/PVDF-HFP Bilayer Separator for Lithium-Ion Batteries. Adv. Mater. Interfaces 2019, 6, 1801330. [Google Scholar] [CrossRef] [Green Version]
- De Moraes, A.C.M.; Hyun, W.J.; Luu, N.S.; Lim, J.-M.; Park, K.-Y.; Hersam, M.C. Phase-Inversion Polymer Composite Separators Based on Hexagonal Boron Nitride Nanosheets for High-Temperature Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 8107–8114. [Google Scholar] [CrossRef]
- Orendorff, C.J.; Lambert, T.N.; Chavez, C.A.; Bencomo, M.; Fenton, K.R. Polyester Separators for Lithium-Ion Cells: Improving Thermal Stability and Abuse Tolerance. Adv. Energy Mater. 2013, 3, 314–320. [Google Scholar] [CrossRef]
- Yu, L.; Miao, J.; Lin, J.Y.S.; Jin, Y. A comparative study on polypropylene separators coated with different inorganic materials for lithium-ion batteries. Front. Chem. Sci. Eng. 2017, 11, 346–352. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mateti, S.; Cai, Q.; Sultana, I.; Fan, Y.; Wang, X.; Hou, C.; Chen, Y. High temperature and high rate lithium-ion batteries with boron nitride nanotubes coated polypropylene separators. Energy Storage Mater. 2019, 19, 352–359. [Google Scholar] [CrossRef]
- Le, H.T.T.; Ngo, D.T.; Kalubarme, R.S.; Cao, G.; Park, C.-N.; Park, C.-J. Composite Gel Polymer Electrolyte Based on Poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) with Modified Aluminum-Doped Lithium Lanthanum Titanate (A-LLTO) for High-Performance Lithium Rechargeable Batteries. ACS Appl. Mater. Interfaces 2016, 8, 20710–20719. [Google Scholar] [CrossRef]
- Shim, J.; Kim, H.J.; Kim, B.G.; Kim, Y.S.; Kim, D.-G.; Lee, J.-C. 2D boron nitride nanoflakes as a multifunctional additive in gel polymer electrolytes for safe, long cycle life and high rate lithium metal batteries. Energy Environ. Sci. 2017, 10, 1911–1916. [Google Scholar] [CrossRef]
- Monroe, C.; Newman, J. The Impact of Elastic Deformation on Deposition Kinetics at Lithium/Polymer Interfaces. J. Electrochem. Soc. 2005, 152, A396–A404. [Google Scholar] [CrossRef]
- Varzi, A.; Raccichini, R.; Passerini, S.; Scrosati, B. Challenges and prospects of the role of solid electrolytes in the revitalization of lithium metal batteries. J. Mater. Chem. A 2016, 4, 17251–17259. [Google Scholar] [CrossRef] [Green Version]
- Dukovic, J.O.; Tobias, C.W. Simulation of Leveling in Electrodeposition. J. Electrochem. Soc. 1990, 137, 3748–3755. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Rao, Z.; Xu, X.; Cheng, Z.; Liao, Y.; Yuan, L.; Xie, X.; Li, Z.; Huang, Y. Electrolyte with boron nitride nanosheets as leveling agent towards dendrite-free lithium metal anodes. Nano Energy 2020, 72, 104725. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Graff, G.L.; Zhang, J.; Sushko, M.L.; Chen, X.; Shao, Y.; Engelhard, M.H.; Nie, Z.; Xiao, J.; et al. Dendrite-Free Lithium Deposition via Self-Healing Electrostatic Shield Mechanism. J. Am. Chem. Soc. 2013, 135, 4450–4456. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Liu, X.; Yu, B.; Mateti, S.; O’Dell, L.A.; Rong, Q.; Chen, Y. Amine-Functionalized Boron Nitride Nanosheets: A New Functional Additive for Robust, Flexible Ion Gel Electrolyte with High Lithium-Ion Transference Number. Adv. Funct. Mater. 2020, 30, 1910813. [Google Scholar] [CrossRef]
- Aydın, H.; Çelik, S.; Bozkurt, A. Electrolyte loaded hexagonal boron nitride/polyacrylonitrile nanofibers for lithium ion battery application. Solid State Ionics 2017, 309, 71–76. [Google Scholar] [CrossRef]
- Venkateswarlu, G.; Madhu, D.; Rani, J.V. Graphene Supported Boron Nitride Nanosheets as Advanced Electroanalytical Performance for Rechargeable Magnesium Storage System. ChemistrySelect 2020, 5, 2247–2254. [Google Scholar] [CrossRef]
- Zhang, R.; Ling, C. Status and challenge of Mg battery cathode. MRS Energy Sustain. 2016, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Venkateswarlu, G.; Madhu, D.; Rani, J.V. An effective performance of F-Doped hexagonal boron nitride nanosheets as cathode material in magnesium battery. Mater. Chem. Phys. 2019, 226, 356–361. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, R.; Yang, S.; Ma, H.; Liang, J.; Chen, J. Rechargeable Mg Batteries with Graphene-like MoS2 Cathode and Ultrasmall Mg Nanoparticle Anode. Adv. Mater. 2010, 23, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Fan, H.J. From aqueous Zn-ion battery to Zn-MnO2 flow battery: A brief story. J. Energy Chem. 2021, 54, 194–201. [Google Scholar] [CrossRef]
- Chen, D.; Kang, C.; Duan, W.; Yuan, Z.; Li, X. A non-ionic membrane with high performance for alkaline zinc-iron flow battery. J. Membr. Sci. 2020, 618, 118585. [Google Scholar] [CrossRef]
- Lu, W.; Xu, P.; Shao, S.; Li, T.; Zhang, H.; Li, X. Multifunctional Carbon Felt Electrode with N-Rich Defects Enables a Long-Cycle Zinc-Bromine Flow Battery with Ultrahigh Power Density. Adv. Funct. Mater. 2021, 2102913. [Google Scholar] [CrossRef]
- Jian, Q.P.; Wu, M.C.; Jiang, H.R.; Lin, Y.K.; Zhao, T.S. A trifunctional electrolyte for high-performance zinc-iodine flow batteries. J. Power Sources 2020, 484, 229238. [Google Scholar] [CrossRef]
- Hu, J.; Yue, M.; Zhang, H.; Yuan, Z.; Li, X. A Boron Nitride Nanosheets Composite Membrane for a Long-Life Zinc-Based Flow Battery. Angew. Chem. Int. Ed. 2020, 59, 6715–6719. [Google Scholar] [CrossRef]
- Gong, K.; Xu, F.; Grunewald, J.B.; Ma, X.; Zhao, Y.; Gu, S.; Yan, Y. All-Soluble All-Iron Aqueous Redox-Flow Battery. ACS Energy Lett. 2016, 1, 89–93. [Google Scholar] [CrossRef]
- Khor, A.; Leung, P.; Mohamed, M.R.; Flox, C.; Xu, Q.; An, L.; Wills, R.G.A.; Morante, J.R.; Shah, A.A. Review of zinc-based hybrid flow batteries: From fundamentals to applications. Mater. Today Energy J. 2018, 8, 80–108. [Google Scholar] [CrossRef]
- Nejati, K.; Hosseinian, A.; Bekhradnia, A.; Vessally, E.; Edjlali, L. Na-ion batteries based on the inorganic BN nanocluster anodes: DFT studies. J. Mol. Graph. Model. 2017, 74, 1–7. [Google Scholar] [CrossRef]
- Song, L.; Ci, L.; Lu, H.; Sorokin, P.B.; Jin, C.; Ni, J.; Kvashnin, A.G.; Kvashnin, D.G.; Lou, J.; Yakobson, B.I.; et al. Large Scale Growth and Characterization of Atomic Hexagonal Boron Nitride Layers. Nano Lett. 2010, 10, 3209–3215. [Google Scholar] [CrossRef]
- Kansara, S.; Gupta, S.K.; Sonvane, Y.; Pajtler, M.V.; Ahuja, R. Inquisitive Geometric Sites in h-BN Monolayer for Alkali Earth Metal Ion Batteries. J. Phys. Chem. C 2019, 123, 19340–19346. [Google Scholar] [CrossRef]
- Nejati, K.; Hosseinian, A.; Edjlali, L.; Vessally, E. The effect of structural curvature on the cell voltage of BN nanotube based Na-ion batteries. J. Mol. Liq. 2017, 229, 167–171. [Google Scholar] [CrossRef]
- Ergen, O. Hexagonal boron nitride incorporation to achieve high performance Li4Ti5O12 electrodes. AIP Adv. 2020, 10, 45040. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, S.; Yin, L.; Li, C.; Xia, C.; An, Y.; Wei, S.Y. Silicene/boron nitride heterostructure for the design of highly efficient anode materials in lithium-ion battery. J. Phys. Condens. Matter 2020, 32, 355502. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, D.; Rahman, M.; Tao, T.; Lei, W.; Mateti, S.; Yu, B.; Wang, J.; Yang, C.; Chen, Y. Repelling Polysulfide Ions by Boron Nitride Nanosheet Coated Separators in Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2019, 2, 2620–2628. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Q.; Jiang, P.; Huang, X. Rapid, high-efficient and scalable exfoliation of high-quality boron nitride nanosheets and their application in lithium-sulfur batteries. Nano Res. 2020, 14, 2424–2431. [Google Scholar] [CrossRef]
- Hyun, W.J.; De Moraes, A.C.M.; Lim, J.-M.; Downing, J.R.; Park, K.-Y.; Tan, M.T.Z.; Hersam, M.C. High-Modulus Hexagonal Boron Nitride Nanoplatelet Gel Electrolytes for Solid-State Rechargeable Lithium-Ion Batteries. ACS Nano 2019, 13, 9664–9672. [Google Scholar] [CrossRef]
- Qui, M.; Jia, H.; Lan, C.; Liu, H.; Fu, S. An enhanced kinetics and ultra-stable zinc electrode by functionalized boron nitride intermediate layer engineering. Energy Storage Mater. 2022, 45, 1175–1182. [Google Scholar] [CrossRef]
- Gao, T.; Gong, L.-J.; Wang, Z.; Yang, Z.-K.; Pan, W.; He, L.; Zhang, J.; Ou, E.-C.; Xiong, Y.; Xu, W. Boron nitride/reduced graphene oxide nanocomposites as supercapacitors electrodes. Mater. Lett. 2015, 159, 54–57. [Google Scholar] [CrossRef]
- Maity, C.K.; Goswami, N.; Verma, K.; Sahoo, S.; Nayak, G.C. A facile synthesis of boron nitride supported zinc cobalt sulfide nano hybrid as high-performance pseudocapacitive electrode material for asymmetric supercapacitors. J. Energy Storage 2020, 32, 101993. [Google Scholar] [CrossRef]
- Saha, S.; Jana, M.; Samanta, P.; Murmu, N.C.; Kim, N.H.; Kuila, T.; Lee, J.H. Investigation of band structure and electrochemical properties of h-BN/rGO composites for asymmetric supercapacitor applications. Mater. Chem. Phys. 2017, 190, 153–165. [Google Scholar] [CrossRef]
- Maity, C.K.; Santra, D.K.; Verma, K.; Sahoo, S.; Cotts, S.; Akinwande, D.; Berry, V.; Nayak, G.C. Induced conducting energy-levels in a boron nitride nano-framework for asymmetric supercapacitors in high charge-mobility ionic electrolytes. Compos. Part B Eng. 2021, 212, 108728. [Google Scholar] [CrossRef]
- Rajendran, J.; Reshetilov, A.N.; Sundramoorthy, A.K. Preparation of hybrid paper electrode based on hexagonal boron nitride integrated graphene nanocomposite for free-standing flexible supercapacitors. RSC Adv. 2021, 11, 3445–3451. [Google Scholar] [CrossRef]
- Maity, C.K.; Sahoo, S.; Verma, K.; Behera, A.K.; Nayak, G.C. Facile functionalization of boron nitride (BN) for the development of high-performance asymmetric supercapacitors. New J. Chem. 2020, 44, 8106–8119. [Google Scholar] [CrossRef]
- Gunday, S.T.; Cevik, E.; Yusuf, A.; Bozkurt, A. Nanocomposites composed of sulfonated polysulfone/hexagonal boron nitride/ionic liquid for supercapacitor applications. J. Energy Storage 2019, 21, 672–679. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Wang, C.; Hu, K.; Liu, Y.; Zhang, M.-R.; Wang, Z.; Li, Z. Flexible Supercapacitors Based on Graphene/Boron Nitride Nanosheets Electrodes and PVA/PEI Gel Electrolytes. Materials 2021, 14, 1955. [Google Scholar] [CrossRef]
- Li, T.; Jiao, X.; You, T.; Dai, F.; Zhang, P.; Yu, F.; Hu, L.; Ding, L.; Zhang, L.; Wen, Z.; et al. Hexagonal boron nitride nanosheet/carbon nanocomposite as a high-performance cathode material towards aqueous asymmetric supercapacitors. Ceram. Int. 2019, 45, 4283–4289. [Google Scholar] [CrossRef]
- Song, F.; Chen, Q.; Li, Y.; Li, Y.; Zhang, L. High energy density supercapacitors based on porous mSiO2@Ni3S2/NiS2 promoted with boron nitride and carbon. Chem. Eng. J. 2020, 390, 124561. [Google Scholar] [CrossRef]
- Byun, S.; Kim, J.H.; Song, S.H.; Lee, M.; Park, J.-J.; Lee, G.; Hong, S.H.; Lee, D. Ordered, Scalable Heterostructure Comprising Boron Nitride and Graphene for High-Performance Flexible Supercapacitors. Chem. Mater. 2016, 28, 7750–7756. [Google Scholar] [CrossRef]
- Hassan, M.; Gondal, M.A.; Cevik, E.; Qahtan, T.F.; Bozkurt, A.; Dastageer, M.A. High performance pliable supercapacitor fabricated using activated carbon nanospheres intercalated into boron nitride nanoplates by pulsed laser ablation technique. Arab. J. Chem. 2020, 13, 6696–6707. [Google Scholar] [CrossRef]
- Saha, S.; Jana, M.; Khanra, P.; Samanta, P.; Koo, H.; Murmu, N.C.; Kuila, T. Band Gap Engineering of Boron Nitride by Graphene and Its Application as Positive Electrode Material in Asymmetric Supercapacitor Device. ACS Appl. Mater. Interfaces 2015, 7, 14211–14222. [Google Scholar] [CrossRef]
- Khan, A.F.; Down, M.P.; Smith, G.C.; Foster, C.W.; Banks, C.E. Surfactant-exfoliated 2D hexagonal boron nitride (2D-hBN): Role of surfactant upon the electrochemical reduction of oxygen and capacitance applications. J. Mater. Chem. A 2017, 5, 4103–4113. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Xie, K.; Liu, X.; Guo, S.; Shen, C.; Liu, X.; Li, X.; Wang, J.-G.; Wei, B. Dramatically Enhanced Ion Conductivity of Gel Polymer Electrolyte for Supercapacitor via h-BN Nanosheets Doping. Electrochim. Acta 2017, 227, 455–461. [Google Scholar] [CrossRef]
- Zheng, S.; Lei, W.; Qin, J.; Wu, Z.-S.; Zhou, F.; Wang, S.; Shi, X.; Sun, C.; Chen, Y.; Bao, X. All-solid-state high-energy planar asymmetric supercapacitors based on all-in-one monolithic film using boron nitride nanosheets as separator. Energy Storage Mater. 2018, 10, 24–31. [Google Scholar] [CrossRef]
- Mohammed, M.H. Electronic and thermoelectric properties of zigzag and armchair boron nitride nanotubes in the presence of C island. Chin. J. Phys. 2018, 56, 1622–1632. [Google Scholar] [CrossRef]
- Pakdel, A.; Guo, Q.; Nicolosi, V.; Mori, T. Enhanced thermoelectric performance of Bi–Sb–Te/Sb2O3 nanocomposites by energy filtering effect. J. Mater. Chem. A 2018, 6, 21341–21349. [Google Scholar] [CrossRef]
- Vishkayi, S.I.; Tagani, M.B.; Soleimani, H.R. Enhancement of thermoelectric efficiency by embedding hexagonal boron-nitride cells in zigzag graphene nanoribbons. J. Phys. D Appl. Phys. 2015, 48. [Google Scholar] [CrossRef]
- Khan, A.U.; Orabi, R.A.R.A.; Pakdel, A.; Vaney, J.-B.; Fontaine, B.; Gautier, R.; Halet, J.-F.; Mitani, S.; Mori, T. Sb Doping of Metallic CuCr2S4 as a Route to Highly Improved Thermoelectric Properties. Chem. Mater. 2017, 29, 2988–2996. [Google Scholar] [CrossRef]
- Pan, C.; Long, M.; He, J. Enhanced thermoelectric properties in boron nitride quantum-dot. Results Phys. 2017, 7, 1487–1491. [Google Scholar] [CrossRef]
- Mir, S.H. A computational study of physical, electronic, thermal and transport properties of one-dimensional boron and boron nitride systems. J. Solid State Chem. 2021, 297, 122037. [Google Scholar] [CrossRef]
- Jiang, X.; Ban, C.; Li, L.; Wang, C.; Chen, W.; Liu, X. Thermoelectric properties study on the BN nanoribbons via BoltzTrap first-principles. AIP Adv. 2021, 11, 55120. [Google Scholar] [CrossRef]
- Zberecki, K.; Swirkowicz, R.; Barnaś, J. Boron nitride zigzag nanoribbons: Optimal thermoelectric systems. Phys. Chem. Chem. Phys. 2015, 17, 22448–22454. [Google Scholar] [CrossRef] [PubMed]
- Kanahashi, K.; Pu, J.; Takenobu, T. 2D Materials for Large-Area Flexible Thermoelectric Devices. Adv. Energy Mater. 2020, 10, 1902842. [Google Scholar] [CrossRef]
- Xie, Z.-X.; Tang, L.-M.; Pan, C.-N.; Chen, Q.; Chen, K.-Q. Ballistic thermoelectric properties in boron nitride nanoribbons. J. Appl. Phys. 2013, 114, 144311. [Google Scholar] [CrossRef]
- Tran, V.-T.; Saint-Martin, J.; Dollfus, P. High thermoelectric performance in graphene nanoribbons by graphene/BN interface engineering. Nanotechnology 2015, 26, 495202. [Google Scholar] [CrossRef]
- Wang, J.; Mu, X.; Wang, X.; Wang, N.; Ma, F.; Liang, W.; Sun, M. The thermal and thermoelectric properties of in-plane C-BN hybrid structures and graphene/h-BN van der Waals heterostructures. Mater. Today Phys. 2018, 5, 29–57. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Liu, Y.; Cai, Y.; Zhao, Y.; Ng, H.K.; Watanabe, K.; Taniguchi, T.; Zhang, G.; Qiu, C.W.; et al. Large enhancement of thermoelectric performance in MoS2/h-BN heterostructure due to vacancy-induced band hybridization Proc. Natl. Acad. Sci. USA 2020, 117, 18127. [Google Scholar] [CrossRef]
- Ma, T.; Lin, C.-T.; Wang, Y. The dimensionality effect on phonon localization in graphene/hexagonal boron nitride superlattices. 2D Mater. 2020, 7, 35029. [Google Scholar] [CrossRef]
- Chen, C.-C.; Li, Z.; Shi, L.; Cronin, S.B. Thermoelectric transport across graphene/hexagonal boron nitride/graphene heterostructures. Nano Res. 2014, 8, 666–672. [Google Scholar] [CrossRef]
- Li, X.; Yin, J.; Zhou, J.; Wang, Q.; Guo, W. Exceptional high Seebeck coefficient and gas-flow-induced voltage in multilayer graphene. Appl. Phys. Lett. 2012, 100, 183108. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Kang, S.D.; Kim, W.; Lee, E.-S.; Woo, S.-J.; Kong, K.-J.; Kim, I.; Kim, H.-D.; Zhang, T.; Stroscio, J.A.; et al. Thermoelectric imaging of structural disorder in epitaxial graphene. Nat. Mater. 2013, 12, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Mahan, G.D.; Sofo, J.O.; Bartkowiak, M. Multilayer thermionic refrigerator and generator. J. Appl. Phys. 1998, 83, 4683–4689. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, R.; Mukherjee, S. Thermoelectric transport in graphene/h-BN/graphene heterostructures: A computational study. Phys. E Low-Dimens. Syst. Nanostructures 2016, 81, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, J.; Akaishi, A. Anomalous enhancement of Seebeck coefficients of the graphene/hexagonal boron nitride composites. Jpn. J. Appl. Phys. 2016, 55, 1102A9. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Chen, Y.; D'Agosta, R.; Xie, Y.; Zhong, J.; Rubio, A. Enhanced thermoelectric properties in hybrid graphene/boron nitride nanoribbons. Phys. Rev. B 2012, 86, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Algharagholy, L.; Al-Galiby, Q.; Marhoon, H.A.; Sadeghi, H.; Abduljalil, H.M.; Lambert, C.J. Tuning thermoelectric properties of graphene/boron nitride heterostructures. Nanotechnology 2015, 26, 475401. [Google Scholar] [CrossRef] [Green Version]
- Duan, S.; Cui, Y.; Yi, W.; Chen, X.; Yang, B.; Liu, X. Superior Conversion Efficiency Achieved in GeP3/h-BN Heterostructures as Novel Flexible and Ultralight Thermoelectrics. ACS Appl. Mater. Interfaces 2021, 13, 18800–18808. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.H.; Tamer, T.; Konsowa, A.H.; Farag, H.; Eldin, M.M. Organic-Inorganic Novel Green Cation Exchange Membranes for Direct Methanol Fuel Cells. Energies 2021, 14, 4686. [Google Scholar] [CrossRef]
- Velayutham, P.; Sahu, A.K.; Parthasarathy, S. A Nafion-Ceria Composite Membrane Electrolyte for Reduced Methanol Crossover in Direct Methanol Fuel Cells. Energies 2017, 10, 259. [Google Scholar] [CrossRef]
- Falcão, D.S.; Silva, R.A.; Rangel, C.M.; Pinto, A.M.F.R. Performance of an Active Micro Direct Methanol Fuel Cell Using Reduced Catalyst Loading MEAs. Energies 2017, 10, 1683. [Google Scholar] [CrossRef] [Green Version]
- Braz, B.A.; Oliveira, V.B.; Pinto, A.M.F.R. Experimental Evaluation of the Effect of the Anode Diffusion Layer Properties on the Performance of a Passive Direct Methanol Fuel Cell. Energies 2020, 13, 5198. [Google Scholar] [CrossRef]
- Zhou, Z.; Zholobko, O.; Wu, X.-F.; Aulich, T.; Thakare, J.; Hurley, J. Polybenzimidazole-Based Polymer Electrolyte Membranes for High-Temperature Fuel Cells: Current Status and Prospects. Energies 2021, 14, 135. [Google Scholar] [CrossRef]
- Song, H.-B.; Park, J.-H.; Park, J.-S.; Kang, M.-S. Pore-Filled Proton-Exchange Membranes with Fluorinated Moiety for Fuel Cell Application. Energies 2021, 14, 4433. [Google Scholar] [CrossRef]
- Kregar, A.; Frühwirt, P.; Ritzberger, D.; Jakubek, S.; Katrašnik, T.; Gescheidt, G. Sensitivity Based Order Reduction of a Chemical Membrane Degradation Model for Low-Temperature Proton Exchange Membrane Fuel Cells. Energies 2020, 13, 5611. [Google Scholar] [CrossRef]
- Parthiban, V.; Sahu, A.K. Performance enhancement of direct methanol fuel cells using a methanol barrier boron nitride–Nafion hybrid membrane. New J. Chem. 2020, 44, 7338–7349. [Google Scholar] [CrossRef]
- Yan, X.; Sun, J.; Gao, L.; Zheng, W.; Dai, Y.; Ruan, X.; He, G. A novel long-side-chain sulfonated poly(2,6-dimethyl-1,4-phenylene oxide) membrane for vanadium redox flow battery. Int. J. Hydrogen Energy 2018, 43, 301–310. [Google Scholar] [CrossRef]
- Guan, R.; Gong, C.; Lu, D.; Zou, H.; Lu, W. Development and characterization of homogeneous membranes prepared from sulfonated poly(phenylene oxide). J. Appl. Polym. Sci. 2005, 98, 1244–1250. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Recent advances in additive-enhanced polymer electrolyte membrane properties in fuel cell applications: An overview. Int. J. Energy Res. 2019, 43, 2756–2794. [Google Scholar] [CrossRef]
- Yadav, V.; Niluroutu, N.; Bhat, S.D.; Kulshrestha, V. Insight toward the Electrochemical Properties of Sulfonated Poly(2,6-dimethyl-1,4-phenylene oxide) via Impregnating Functionalized Boron Nitride: Alternate Composite Polymer Electrolyte for Direct Methanol Fuel Cell. ACS Appl. Energy Mater. 2020, 3, 7091–7102. [Google Scholar] [CrossRef]
- Yogarathinam, L.T.; Jaafar, J.; Ismail, A.F.; Goh, P.S.; Gangasalam, A.; Hanifah, M.F.R.; Wong, K.C.; Subramaniam, M.N.; Peter, J. Functionalized boron nitride embedded sulfonated poly (ether ether ketone) proton exchange membrane for direct methanol fuel cell applications. J. Environ. Chem. Eng. 2021, 9, 105876. [Google Scholar] [CrossRef]
- Mahalingam, S.; Ayyaru, S.; Ahn, Y.-H. Enhanced cathode performance of Fe2O3, boron nitride-doped rGO nanosheets for microbial fuel cell applications. Sustain. Energy Fuels 2020, 4, 1454–1468. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Checchetto, R.; Chayahara, A.; Horino, H.; Miotello, A.; Fujii, K. A study of deuterium permeation through thin BN films. Thin Solid Films 1997, 299, 5–9. [Google Scholar] [CrossRef]
- Checchetto, R.; Miotello, A. Deuterium diffusion through hexagonal boron nitride thin films. J. Appl. Phys. 2000, 87, 110–116. [Google Scholar] [CrossRef]
- He, L.; Wang, H.; Chen, L.; Wang, X.; Xie, H.; Jiang, C.; Lingxiu, C.; Elibol, K.; Meyer, J.; Watanabe, K.; et al. Isolating hydrogen in hexagonal boron nitride bubbles by a plasma treatment. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bull, S.K.; Champ, T.A.; Raj, S.V.; O'Brien, R.C.; Musgrave, C.B.; Weimer, A.W. Atomic Layer Deposited Boron Nitride Nanoscale Films Act as High Temperature Hydrogen Barriers. Appl. Surf. Sci. 2021, 565, 150428. [Google Scholar] [CrossRef]
- Weimer, A.W. Particle atomic layer deposition. J. Nanoparticle Res. 2019, 21, 1–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Wang, X.; Peng, L.; Jiang, B.; Yang, J.; Yan, D.; Pu, J.; Chi, B.; Li, J. Thermal cycling stability of novel hexagonal boron nitride (h-BN)/glass compressive seals for planar intermediate temperature solid oxide fuel cells. J. Alloys Compd. 2020, 843, 155620. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, D.-H.; So, J.-H.; Koo, H.-J. Toward Eco-Friendly Dye-Sensitized Solar Cells (DSSCs): Natural Dyes and Aqueous Electrolytes. Energies 2021, 15, 219. [Google Scholar] [CrossRef]
- Cao, V.M.H.; Bae, J.; Shim, J.; Hong, B.; Jee, H.; Lee, J. Fabrication of the Cu2ZnSnS4 Thin Film Solar Cell via a Photo-Sintering Technique. Appl. Sci. 2021, 12, 38. [Google Scholar] [CrossRef]
- Alhamada, T.F.; Hanim, M.A.A.; Jung, D.W.; Nuraini, A.A.; Hasan, W.Z.W. A Brief Review of the Role of 2D Mxene Nanosheets toward Solar Cells Efficiency Improvement. Nanomaterials 2021, 11, 2732. [Google Scholar] [CrossRef]
- Kalita, G.; Kobayashi, M.; Shaarin, M.D.; Mahyavanshi, R.D.; Tanemura, M. Schottky Barrier Diode Characteristics of Graphene-GaN Heterojunction with Hexagonal Boron Nitride Interfacial Layer. Phys. Status Solidi 2018, 215, 1800089. [Google Scholar] [CrossRef]
- Xu, S.J.; Luo, Y.F.; Zhong, W.; Xiao, Z.H.; Liu, X.Y. Investigation of Hexagonal Boron Nitride for Application as Counter Electrode in Dye-Sensitized Solar Cells. Adv. Mater. Res. 2012, 512, 242–245. [Google Scholar] [CrossRef]
- Lee, G.-H.; Cuong, T.-V.; Yeo, D.-K.; Cho, H.; Ryu, B.-D.; Kim, E.-M.; Nam, T.-S.; Suh, E.-K.; Seo, T.-H.; Hong, C.-H. Hexagonal Boron Nitride Passivation Layer for Improving the Performance and Reliability of InGaN/GaN Light-Emitting Diodes. Appl. Sci. 2021, 11, 9321. [Google Scholar] [CrossRef]
- Cho, A.-J.; Kwon, J.-Y. Hexagonal Boron Nitride for Surface Passivation of Two-Dimensional van der Waals Heterojunction Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 39765–39771. [Google Scholar] [CrossRef]
- Raj, V.; Chugh, D.; Black, L.E.; Shehata, M.M.; Li, L.; Kremer, F.; Macdonald, D.H.; Tan, H.H.; Jagadish, C. Passivation of InP solar cells using large area hexagonal-BN layers. NPJ 2D Mater. Appl. 2021, 5, 1–8. [Google Scholar] [CrossRef]
- Lee, W.-C.; Hu, C. Modeling gate and substrate currents due to conduction- and valence-band electron and hole tunneling [CMOS technology]. In Proceedings of the 2000 Symposium on VLSI Technology, Honolulu, HI, USA, 15 –17 June 2000; Digest of Technical Papers; IEEE: Piscataway, NJ, USA, 2002. [Google Scholar] [CrossRef]
- Rienacker, M.; Bossmeyer, M.; Merkle, A.; Romer, U.; Haase, F.; Krugener, J.; Brendel, R.; Peibst, R. Notice of Removal Junction resistivity of carrier selective polysilicon on oxide junctions and its impact on the solar cell performance. In Proceedings of the 2017 IEEE 44th Photovoltaic Specialist Conference (PVSC), Washington, DC, USA, 25–30 June 2017; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2018; pp. 1–7. [Google Scholar]
- Meng, J.-H.; Liu, X.; Zhang, X.-W.; Zhang, Y.; Wang, H.-L.; Yin, Z.-G.; Zhang, Y.-Z.; Liu, H.; You, J.-B.; Yan, H. Interface engineering for highly efficient graphene-on-silicon Schottky junction solar cells by introducing a hexagonal boron nitride interlayer. Nano Energy 2016, 28, 44–50. [Google Scholar] [CrossRef]
- Arifin, Z.; Suyitno, S.; Hadi, S.; Sutanto, B. Improved Performance of Dye-Sensitized Solar Cells with TiO2 Nanoparticles/Zn-Doped TiO2 Hollow Fiber Photoanodes. Energies 2018, 11, 2922. [Google Scholar] [CrossRef] [Green Version]
- Nien, Y.-H.; Chen, H.-H.C.; Hsu, H.-H.; Rangasamy, M.; Hu, G.-M.; Yong, Z.-R.; Kuo, P.-Y.; Chou, J.-C.; Lai, C.-H.; Ko, C.-C.; et al. Study of How Photoelectrodes Modified by TiO 2 / Ag Nanofibers in Various Structures Enhance the Low Illumination. Energies 2020, 13, 2248. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Wang, D.; Li, Y.; He, X.; Zhang, H.; Shen, J. ZnO@TiO2 Core/Shell Nanowire Arrays with Different Thickness of TiO2 Shell for Dye-Sensitized Solar Cells. Crystals 2020, 10, 325. [Google Scholar] [CrossRef] [Green Version]
- Tehare, K.K.; Navale, S.T.; Stadler, F.J.; He, Z.; Yang, H.; Xiong, X.; Liu, X.; Mane, R.S. Enhanced DSSCs performance of TiO2 nanostructure by surface passivation layers. Mater. Res. Bull. 2018, 99, 491–495. [Google Scholar] [CrossRef]
- Shanmugam, M.; Jacobs-Gedrim, R.; Durcan, C.; Yu, B. 2D layered insulator hexagonal boron nitride enabled surface passivation in dye sensitized solar cells. Nanoscale 2013, 5, 11275–11282. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Wang, F.; Kozawa, D.; Funahashi, K.; Mouri, S.; Miyauchi, Y.; Takenobu, T.; Matsuda, K. Enhanced photovoltaic performances of graphene/Si solar cells by insertion of a MoS2thin film. Nanoscale 2015, 7, 14476–14482. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Li, X.; Wang, P.; Xu, Z.; Zhang, S.; Zhong, H.; Wu, Z.; Xu, W.; Chen, H. Interface designed MoS2/GaAs heterostructure solar cell with sandwich stacked hexagonal boron nitride. Sci. Rep. 2015, 5, 15103. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lin, S.; Lin, X.; Xu, Z.; Wang, P.; Zhang, S.; Zhong, H.; Xu, W.; Wu, Z.; Fang, W. Graphene/h-BN/GaAs sandwich diode as solar cell and photodetector. Opt. Express 2016, 24, 134–145. [Google Scholar] [CrossRef]
- Shanmugam, M.; Jain, N.; Jacobs-Gedrim, R.; Xu, Y.; Yu, B. Layered insulator hexagonal boron nitride for surface passivation in quantum dot solar cell. Appl. Phys. Lett. 2013, 103, 243904. [Google Scholar] [CrossRef]
- Jabeen, M.; Haxha, S. 2D/3D graphene on h-BN interlayer-silicon solar cell with ZnO:Al buffer layer and enormous light captivation using Au/Ag NPs. Opt. Express 2020, 28, 12709–12728. [Google Scholar] [CrossRef] [PubMed]








| Process | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|
| Top-down | Sonication |
|
| [36,37] |
| Ball milling |
|
| [38] | |
| Hydrothermal/ Solvothermal |
|
| [39,40] | |
| Microwave |
|
| [41] | |
| Bottom-up | Hydrothermal/solvothermal |
|
| [51,52] |
| CVD (AP, PE, LP) |
|
| [53,54,55] |
| Application | Platform | Device Characteristic | Role of 2D h-BN | Ref. |
|---|---|---|---|---|
| LIB | h-BN/r-GO | Reversible capacity of 278 mAh/g Current density of 100 mA/g 200 cycle stability | Electrode | [62] |
| LIB | LTO/rGO/h-BN | Increased specific capacity to 200 mAh/g high capacity of 179 mAh.g−1 at a discharge rate of 20 C Ultrafast charge rate of >10 C. | Electrode | [96] |
| LIB | 2D h-BN/blue phosphorous | The low diffusion energy barrier of Li (0.08 eV) High theoretical specific capacity of 801 mAh/ g | Electrode | [63] |
| LIB | h-BN/silicene | Storage capacity of 1015 mAh/ g Low volume change (only 1.3%) | Electrode | [97] |
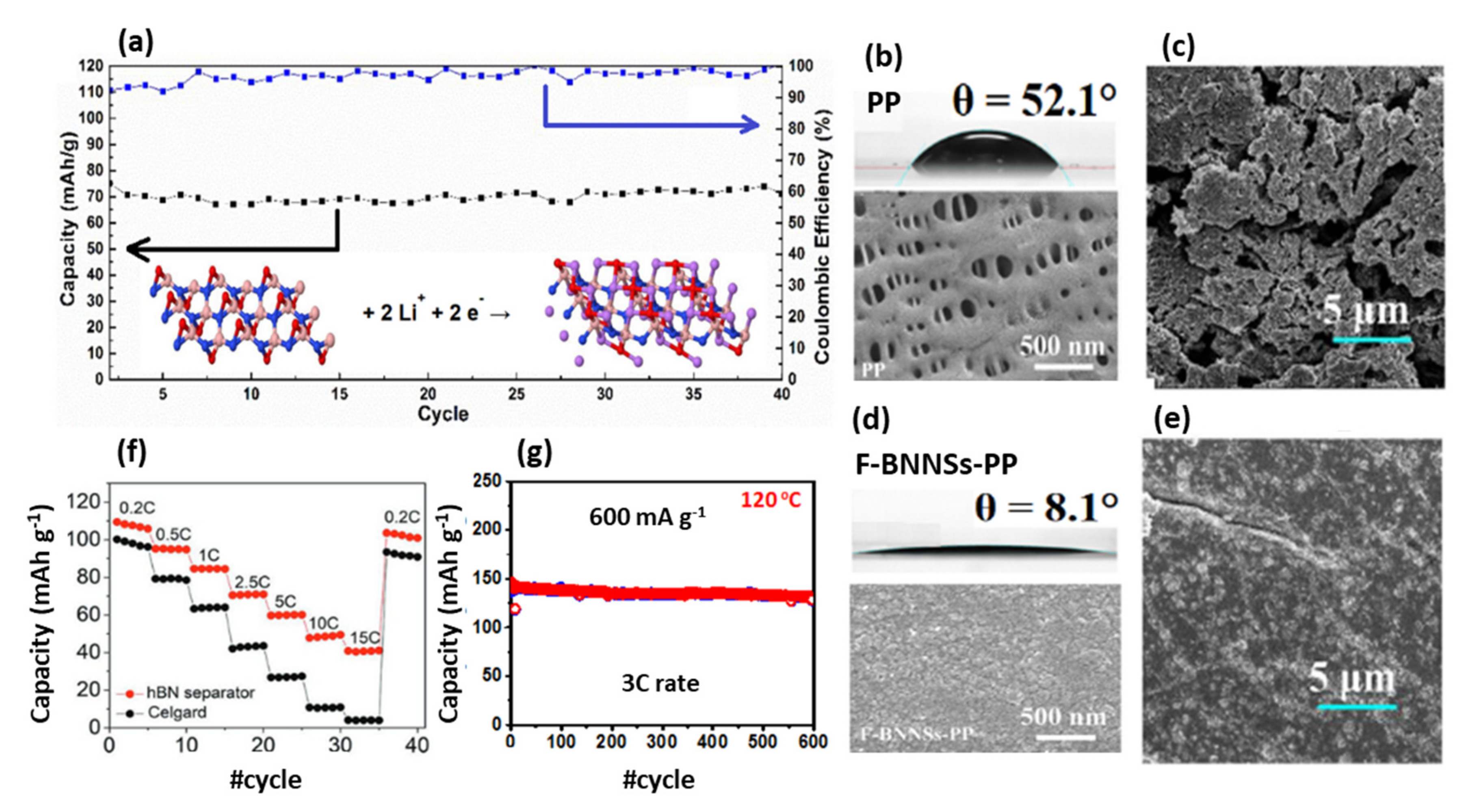
| LIB | BNNSs modified PP separator in a LiFePO4||Li full cell | Reducing separator’s contact angle from 52.1 to 8.1 Enhanced ionic conductivity to 0.255 mS/m Excellent long cycling performance (82% retention after 800 cycles) High current density (100 mA cm−2 in 800 h) | Separator | [64] |
| LIB | BNNTs modified PP separator in a LiFePO4||Li cell | Enhanced dimensional stability at 150 °C Li diffusion coefficient of 4.7 × 10−8 cm2/s High charge/discharge current rates of 5–10 C | Separator | [71] |
| LIB | h-BN flakes in PE-BN/PVDF-HFP bilayer separator in a LiFePO4||Li | Low thermal shrinkage (only 6.6 %) at 140 °C Rapid electrolyte uptake of 348% Low Rct of 6.67 Ω The superior capacity retention of 95% after 500 cycles | Separator | [67] |
| LIB | 2D h-BN ink separator in Li-manganese oxide (LMO)-graphene||Li (anode) | The capacity retention of 82% after 100 cycles Highly stable at 150 °C | Separator | [65] |
| Li-S | Celgard 2400 separator/functionalized h-BN flakes containing CO32- | The capacity retention of 91.5% at the high rate of 7 C Cyclic stability up to 1000 cycles | Separator | [98] |
| Li-S | BNNSs/PP | Improved ionic conductivity to 1.35 mS/cm Inhibiting dendrite nucleation The initial capacity of the PP-BNNSs (1405 mAh/g) Withstanding a high temperature of 80 °C for 1 min | Separator | [99] |
| LIB | (PFPE)-h-BN into P(VdF-co-HFP) (PVH) polymeric | High tLi+ = 0.62 Improved shear modulus (G) (5.2 times higher) Nearly 1940 h working hours | Electrolyte | [73] |
| Li-S | 2D h-BN in LiTFSI/1,3-dioxolane (DOL)—1,2-dimethoxymethane (DME) liquid electrolyte | High tLi+ = 0.55 Nearly 400 h constant working hours Deep plating up to 35 mAh/cm High mechanical stability of the electrolyte | Electrolyte | [77] |
| LIB | Amino-functionalized h-BN (AFBN)-based GPE in LiFePO4||Li | Enhanced ionic conductivity of 6.47 × 10−4 S/cm Enhanced tLi+ = 0.23 compared to h-BN free electrolyte (0.12) Cell retaining up to 92% | Electrolyte | [79] |
| LIB | Carbon/ h-BN in imidazolium ion liquids electrolyte | Excellent ionic conductivity (>1 mS/cm) Shear storage modulus (5 MPa) tLi+ value can be as high as 0.18 (200% improvement) Compatible with high-voltage cathodes (>5 V vs. Li/Li+) Working temperature up to 175 °C | Electrolyte | [100] |
| NIB | h-BN/Blue P | Theoretical specific capacity of 541 mAh/g Low diffusion barrier of 0.07 eV | Electrode | [43] |
| NIB | Monolayer h-BN | The theoretical capacity of 571.698 mAh/g The average electrode potential of 0.009 V | Electrode | [94] |
| MMBs | F-doped h-BN Celgrade as a separator with Mg (ClO4)2 [DBIm]Br | The good discharge capacity of 50 mAh/g Improved conductivity | Electrode | [83] |
| ZFBs | BNNSs | 500 cycles at 80 mA/cm2 Efficiency up to 80% at 200 mA/cm Working temperature up to 50 °C Columbic efficiency (CE) of 96.03% Voltage efficiency (VE) of 90.21% | Membrane | [89] |
| ZFBs | Sulfonate functionalized BNNSs | Long term stability (2500 h at 2 mAh/cm2) Small plating/stripping overpotential of 45 mV Cyclic stability up to 1200 cycles | Electrode | [101] |
| Supercapacitor | h-BN/rGO | The capacitance of 140 F/g (75% higher than bare r-GO electrode) Excellent cyclic stability (105.5% capacitance retention after 1000 cycles) | Electrode | [102] |
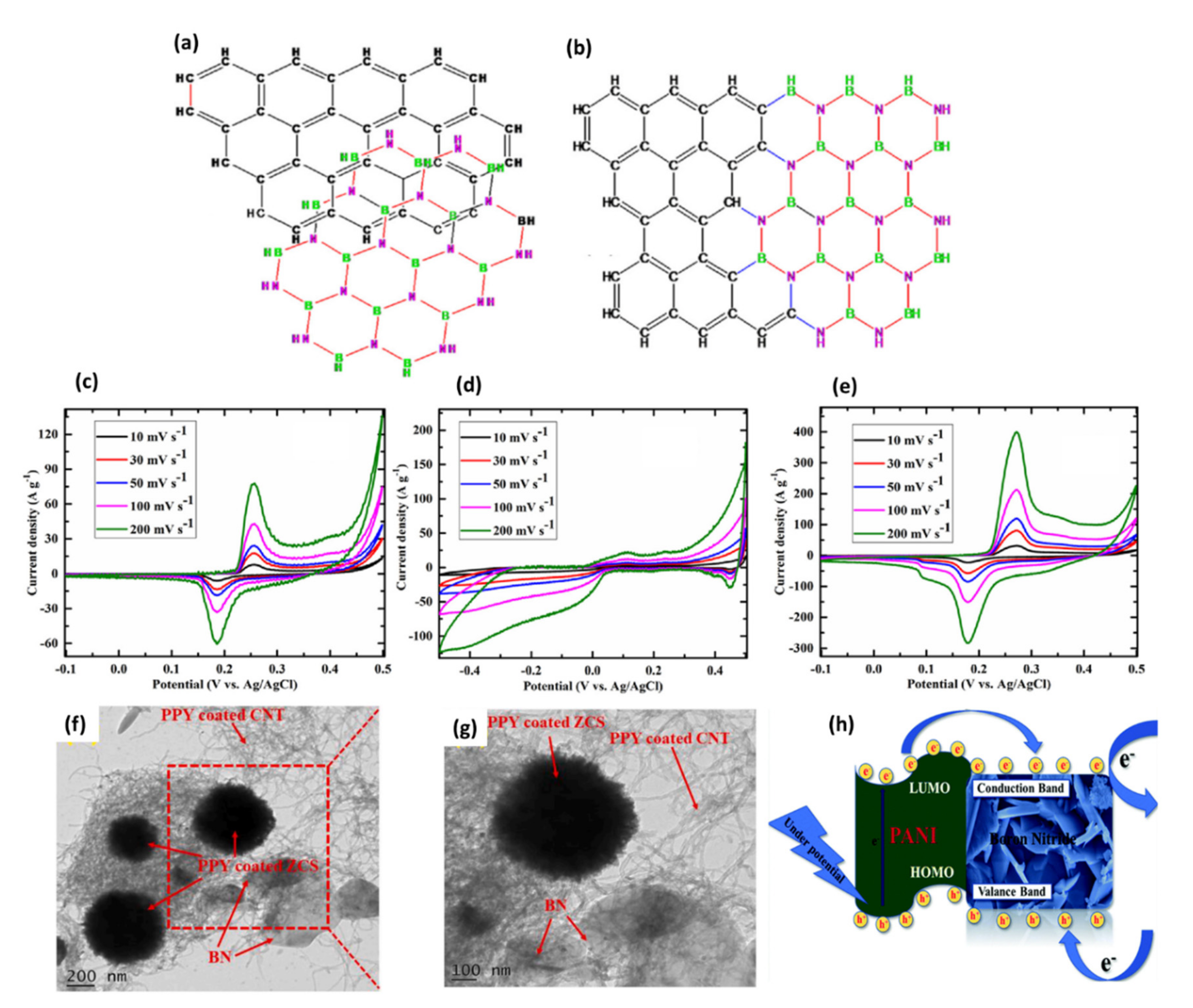
| Supercapacitor | ZnCo2S4 (ZCS)/h-BN/CNT/polypyrrole | The specific capacitance of 534 and 785 F/g in aqueous and organic media, respectivel Excellent cycling stability of 106% (after 10,000 charge/discharge cycles) in aqueous electrolyte | Electrode | [103] |
| Supercapacitor | h-BN/rGO | The specific capacitance of 960 F/g. Energy and power density of 73 Wh/kg and 14,000 W/kg, respectively. High stability (~80%) even after 10,000 cycles | Electrode | [104] |
| Supercapacitor | Zn-CdS/h-BN/CNT | The specific capacitance of 787 F/g The energy density of 78 Wh/kg High stability (99%) even after 10,000 cycles. | Electrode | [105] |
| Supercapacitor | h-BN/graphene | The specific capacitance of 322 F/g Energy and power density of 44.7 Wh/kg and 3588 W/kg, respectively. Good stability (96%) even after 6000 cycles. | Flexible electrode | [106] |
| Supercapacitor | h-BN/PANI/CNT | The specific capacitance of 515 F/g The energy density of 46 Wh/kg High stability (98%) even after 10,000 cycles. | Electrode | [107] |
| Supercapacitor | Sulfonated polysulfone/h-BN/ionic liquid | Proton conductivity of 1.3 × 10−3 S/cm at 100 The specific capacitance of 90.4 F/g Energy and power density of 43.8 Wh/kg and 1100 W/kg, respectively. High stability (98%) even after 10,000 cycles. | Electrolyte additive | [108] |
| Application | Platform | Role of 2D h-BN | Device Characteristics | Mechanisms | Ref. |
|---|---|---|---|---|---|
| Thermoelectrics | h-BN nanoribbons | Direct conversion of temperature difference to the electric voltage. | ZT = 0.2 at a chemical potential of 1.8 eV. | - | [128] |
| Thermoelectrics | Graphene/h-BN/graphene heterostructures | Heat dissipation across the graphene/h-BN junction. | Seebeck coefficient of −99.3 μV/K, power factor of 1.51 × 10–15 W/K2, and ZT of 1.05 × 10–6 | A large energy barrier at the interface caused a low ZT | [133] |
| Thermoelectrics | Graphene/h-BN hetero-nanoribbon doped with TTF | Acting as a tunnel barrier | Phonon thermal conductance of 0.2 nW/K, thermopower of −284 μv/K, and ZT of 0.9 at room temperature | Introducing additional phonon scattering by doping the structure and decreasing the phonon conductance. | [140] |
| Thermoelectrics | Graphene/h-BN heterostructures | Band gap opening in graphene | ZT = 1.48 | Increase in the phonon scattering andreduction the phonon thermal conductance | [129] |
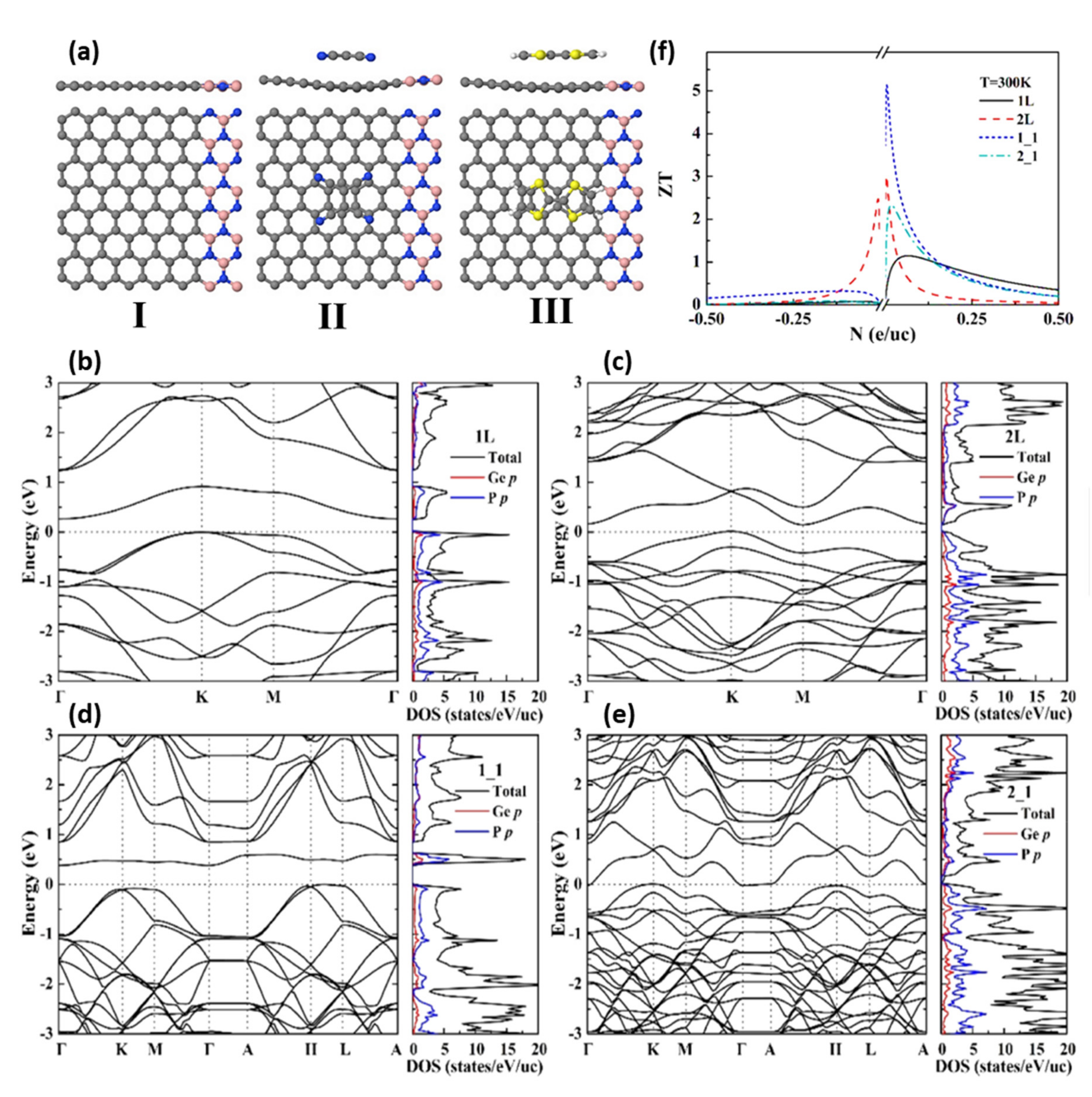
| Thermoelectrics | GeP3/h-BN heterostructures | Enlarging the bandgap of GeP3. | An ultrahigh ZT value of 5.13 at 300 K in p-type GeP3/h-BN. | Increase in the bandgap of GeP3 followed by the formation of an anisotropic electronic structure with a higher ZT. | [141] |
| Fuel cell | Nafion/h-BN | Reinforcement of the polymer electrolytes for direct methanol fuel cell | Proton conductivity of 214 × 10−3 S/cm (58% improvement). Water sorption of 36.5 (90% improvement). The ion exchange capacity of 1.13 meq/g (24% improvement). Methanol crossover density of 89 mA/cm2 (87% lower than pristine Nafion). Peak power density of 165 mW/cm2 (154% improvement). | The addition of hydrophilic sulfonated h-BN enhanced the membrane water uptake and provided a better proton-conducting pathway. The presence of –SO3H groups in the functionalized h-BN also improved the ion exchange capacity barrier effect in h-BN due to hindering the methanol crossover. | [149] |
| Fuel cell | sPPO/h-BN | Reinforcement of the polymer electrolytes used in direct methanol fuel cell | Proton conductivity of 4.64 × 10−2 S/cm (67% improvement). Water sorption of 50.61% (55% improvement). The ion Ion exchange capacity of 1.92 meq/g (23% improvement). Methanol permeability of 2.04 × 10−7 cm2/s (3 times lower than pristine sPPO). Peak power density of 122 mW/cm2 (103% improvement). | π-π interactions between sBN and sPPO and introducing long-range proton-conducting pathways owing to the presence of excess sulfonic acid groups and hydrogen bonding bridge. The barrier effect of h-BN due to impermeability to methanol. | [153] |
| Fuel cell | SPEEK/PANI/h-BN | Reinforcement of the polymer electrolyte for direct methanol fuel cell | Proton conductivity of 4.13 × 10−3 S/cm (141% improvement). Water sorption of 58.42 (38% improvement). The ion Ion exchange capacity of 1.76 meq/g (9.4% improvement). Methanol permeability of 3.08 × 10−7 cm2/s (46% lower than pristine SPEEK). Peak power density of 11.38 mW/cm2 (23% improvement). | The surface defects in acidified h-BN surface formed new pathways for water molecules transport inside the SPEEK. The barrier effect in h-BN hindered the methanol crossover. | [154] |
| Fuel cell | rGO/h-BN/Fe2O3 | The cathode catalyst of a microbial fuel cell | The maximum potential of 250 mVOpen circuit potential of 663 mV Power density of 1673 mW/m2 (81% of the conventional Pt/C electrode) | Enhancement of the surface area for facile transport of reactants and electrons into r-GO. | [155] |
| Fuel cell | h-BN/glass | Compressive seal in a solid oxide fuel cell | Having leakage rates lower than 0.012 sccm/cm under input gas pressure of 6.8 kPa (better than the leakage rates of Al2O3-based compressive seals, 0.02 sccm/cm) | Gradual formation of liquid B2O3 films on the h-BN surface, improving bondage for both interface and interior particles. | [162] |
| Fuel cell | h-BN coating on ZrO2 powders | Hydrogen environmental barrier coating | Effective against hydrogen diffusion up to 1713 K. | The high activation energy of 3.25 eV for atomic hydrogen diffusion into the (001) hexagonal BN surface. | [160] |
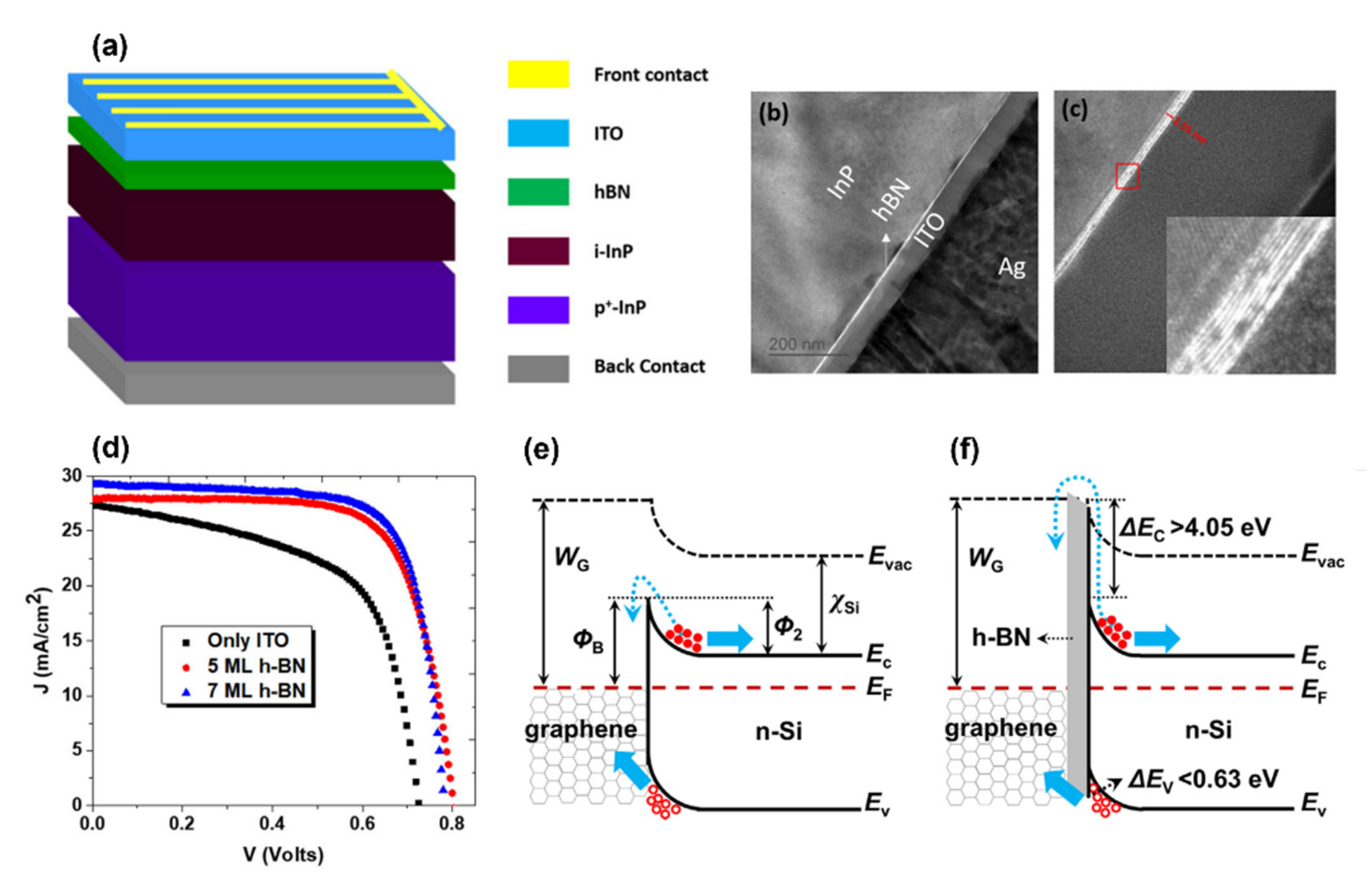
| Solar cell | p+-InP/i-InP/h-BN/ITO | Passivation of p+-InP/i-InP | FF of 75% (33% better than passivated device) Maximum efficiency of 17.2% (50% better than unpassivated device) The interface defect density of 2 × 1012 /eV cm2 | Surface passivation through the charge transfer from the surface defect states to 2D h-BN. | [170] |
| Solar cell | MoS2/WSe2/h-BN | Passivation of MoS2/WSe2 | JSC of 1.69 mA/cm2 (37.5% improvement) and VOC of 0.38 V (25.7% improvement) 74% improvement in PCE. Increase the average recombination lifetime from 112 ps to 131 ps before and after the addition of the h-BN layer. Long-term stability of the device. | Surface passivation through the charge transfer from the surface defect states to 2D h-BN. | [169] |
| Solar cell | TiO2/h-BN/CdSe/P3HT | Surface passivation of mesoporous TiO2 | JSC of 15.3 mA/cm2 (40% improvement), and VOC of 0.719 V (8.4% improvement). The maximum power output of 4.9 × 10−4 W (44% improvement), and PCE of 7% (46% improvement). Maximum EQE of 79% at 510 nm (23% improvement). | Surface passivation through the charge transfer from the surface defect states to 2D h-BN. | [182] |
| Solar cell | Dye-sensitized cell | Surface passivation of TiO2 | JSC of 15.7 mA/cm2 (23% improvement), and VOC of 0.782 V (1.6% improvement). Maximum power of 5.72 × 10−4 W (57% improvement). PCE of 8.2% (58% improvement). | Minimizing electron-hole recombination at the TiO2/dye/electrolyte interfaces. | [178] |
| Solar cell | Si /h-BN/graphene | The interface design of graphene/Si heterojunction | JSC of 33.49 mA/cm2 (28% improvement), and VOC of 0.547 V (33% improvement). FF of 60.8% (75% improvement. The series resistance of 1.9 Ω (21% less than the system without h-BN). reverse saturation current density of 2.56 × 10−4 mA/cm2 (one order of magnitude) | Appropriate band alignment and effective electron-blocking/hole-transporting mechanism | [173] |
| Solar cell | AuCl3:MoS2/h-BN/GaAs | The interface design of MoS2/GaAs heterojunction | JSC of 20.8 mA/cm2 (1% improvement), and VOC of 0.64 V (14% improvement). FF of 53.7% (15% improvement). PCE of 7.15% (33% improvement).Series resistance of 45.9 Ω (22% less than undoped system and without h-BN). | Appropriate band alignment and effective electron-blocking/hole-transporting mechanism | [180] |
| Solar cell | graphene/h-BN/GaAs | Interface design of graphene/GaAs heterojunction | Barrier height heterostructure of 1.02 eV (compare with 0.88 eV for the device without h-BN). PCE of 10.18% (18% improvement) | Appropriate band alignment and effective electron-blocking/hole-transporting mechanism | [181] |
| Solar cell | Si/h-BN interface | Interface of graphene/Si heterojunction | JSC of 30 mA/cm2 (19% improvement), and VOC of 0.42 V (2.4% improvement). FF of 71% (4.2% improvement). PCE of 8.94% (26.3% improvement). | Appropriate band alignment and effective electron-blocking/hole-transporting | [183] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angizi, S.; Alem, S.A.A.; Pakdel, A. Towards Integration of Two-Dimensional Hexagonal Boron Nitride (2D h-BN) in Energy Conversion and Storage Devices. Energies 2022, 15, 1162. https://doi.org/10.3390/en15031162
Angizi S, Alem SAA, Pakdel A. Towards Integration of Two-Dimensional Hexagonal Boron Nitride (2D h-BN) in Energy Conversion and Storage Devices. Energies. 2022; 15(3):1162. https://doi.org/10.3390/en15031162
Chicago/Turabian StyleAngizi, Shayan, Sayed Ali Ahmad Alem, and Amir Pakdel. 2022. "Towards Integration of Two-Dimensional Hexagonal Boron Nitride (2D h-BN) in Energy Conversion and Storage Devices" Energies 15, no. 3: 1162. https://doi.org/10.3390/en15031162








