Effect of Double-Sided 3D Patterned Cathode Catalyst Layers on Polymer Electrolyte Fuel Cell Performance
Abstract
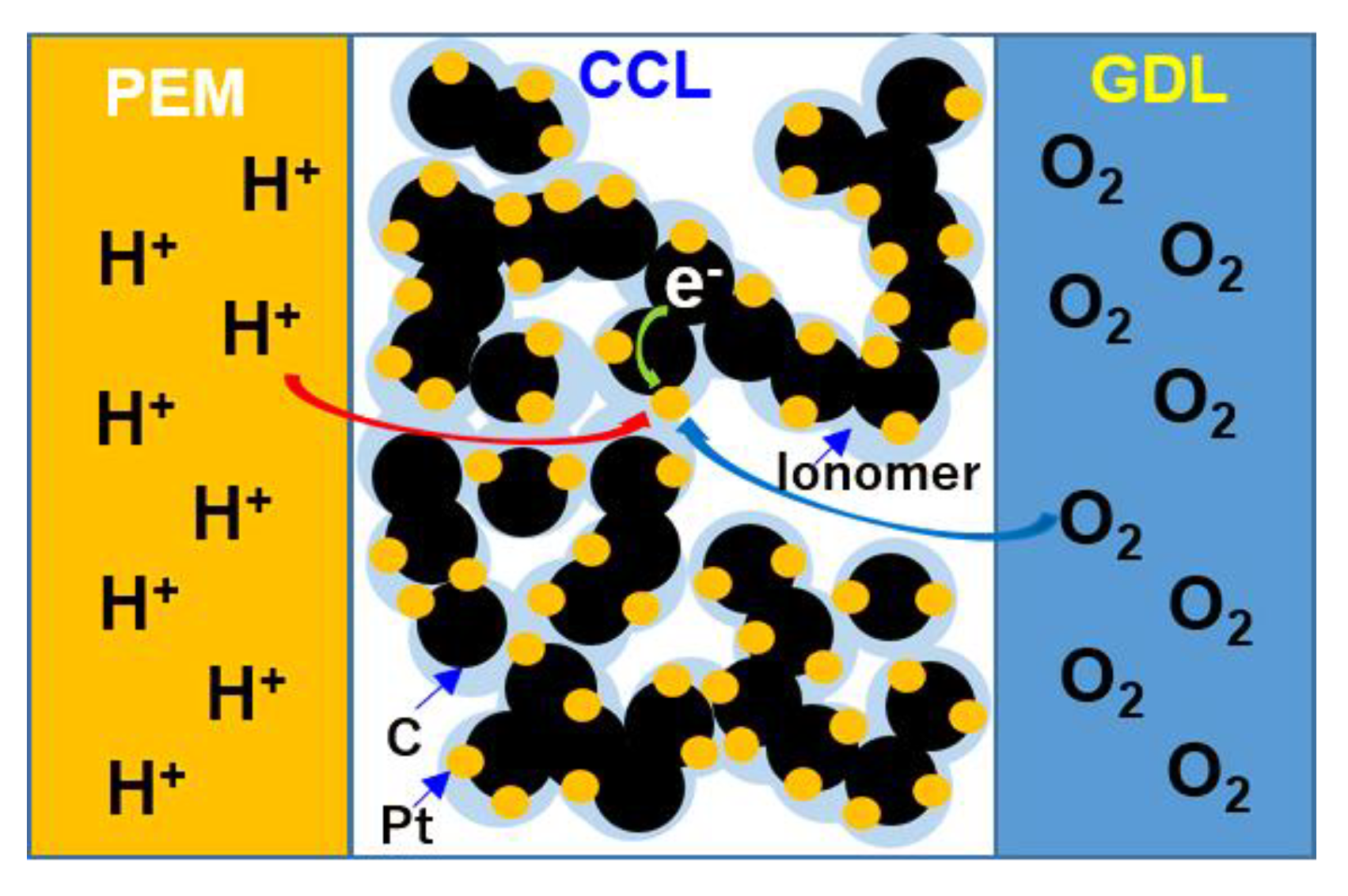
:1. Introduction
| Year | Material | Result | Ref |
|---|---|---|---|
| 2011–2015 | Ag/SPE/Pt atomic Catalyst ink MWCNT Lithium Nafion-Ultrathin film | Long cycle, stable, increased cell performance | [13,14,15,16,17,18] |
| 2016–2021 | Cellulose nano composite Catalyst ink Yttria-stabilized- zirconiaSolvent | Low cost, increased cell performance | [19,20,21,22,23] |
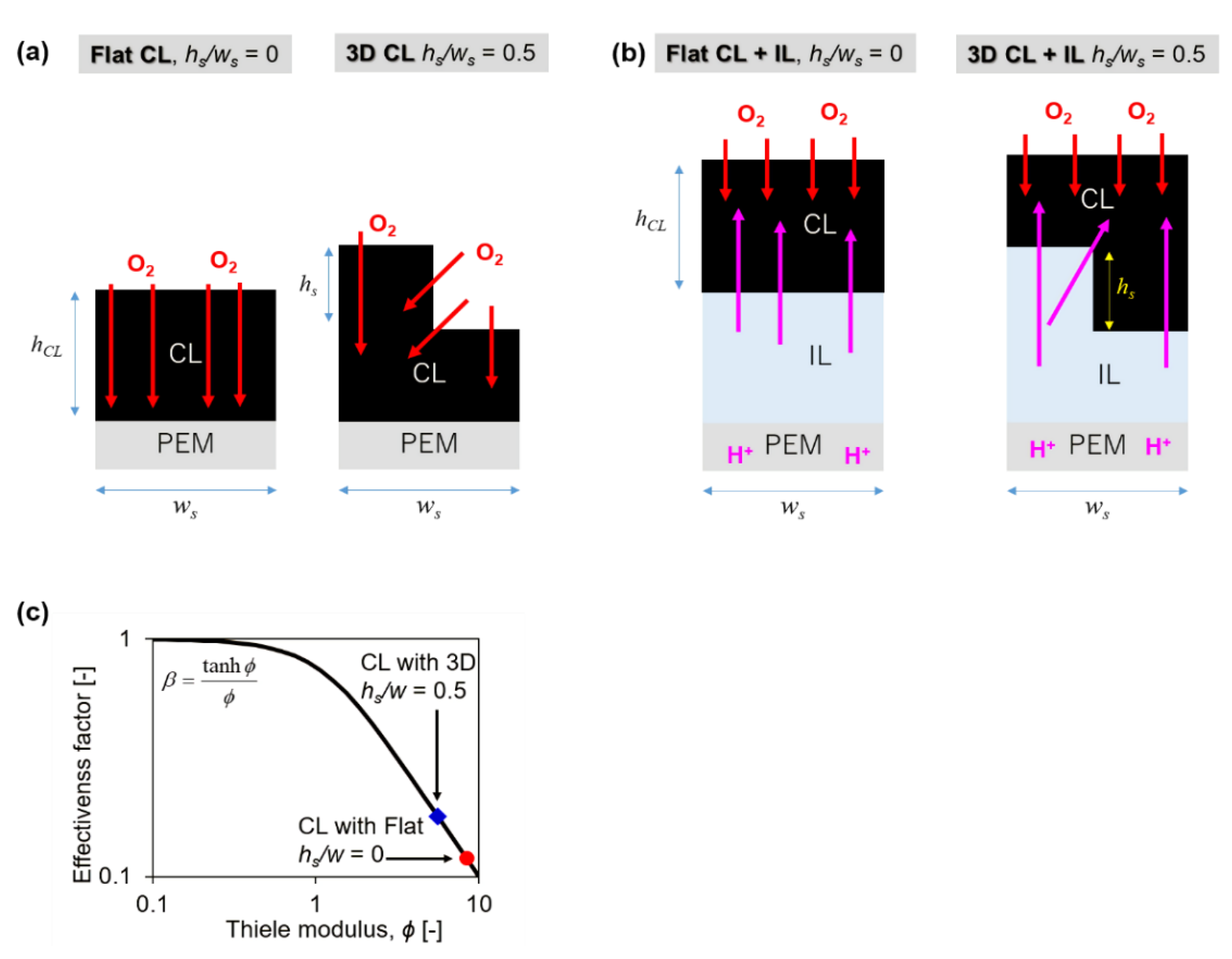
2. Theoretical Estimation
3. Experimental Section
3.1. Preparation of MEA
3.2. Characterization and Electrochemical Measurements of CCLs
4. Results and Discussion
4.1. Structural Characterization
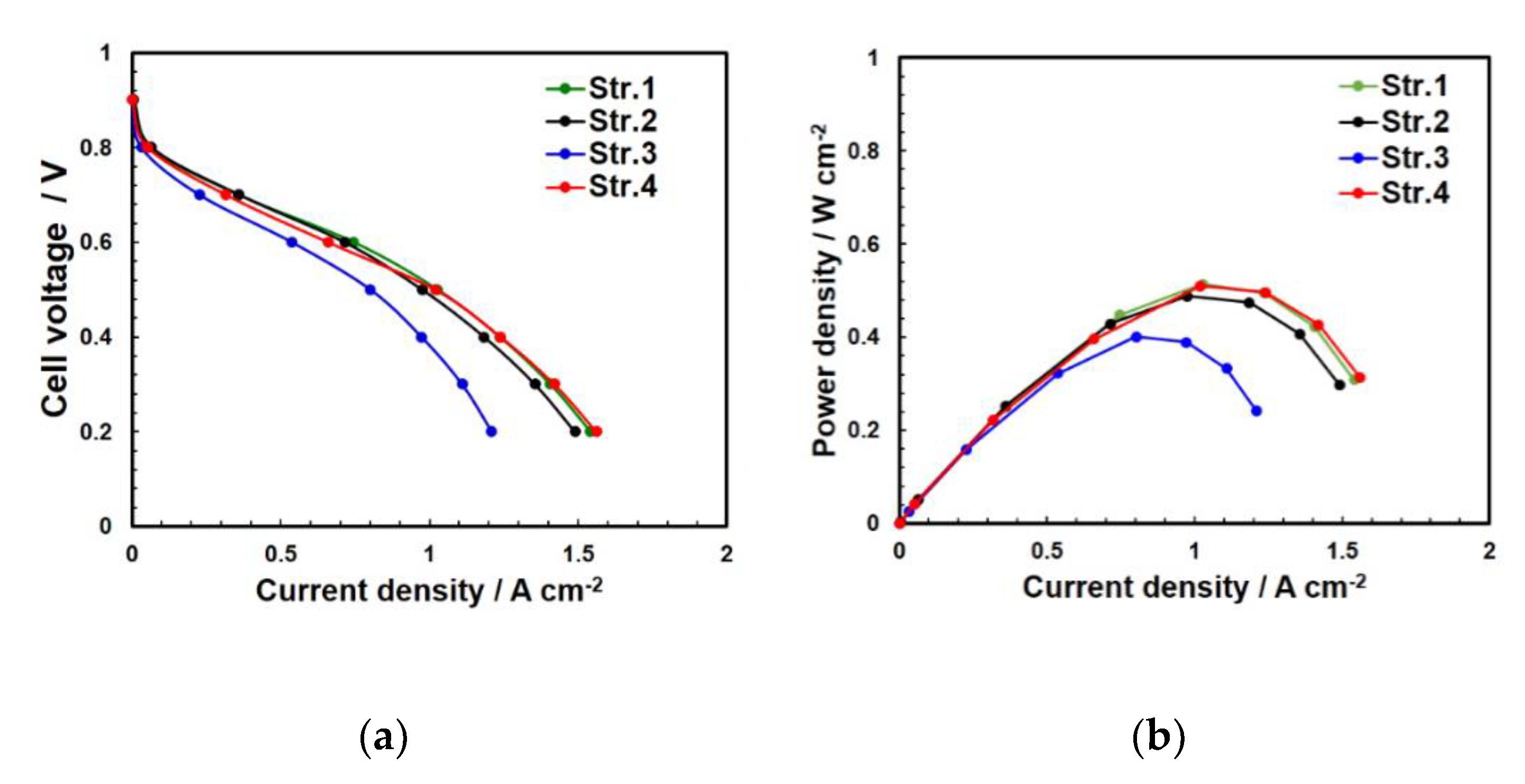
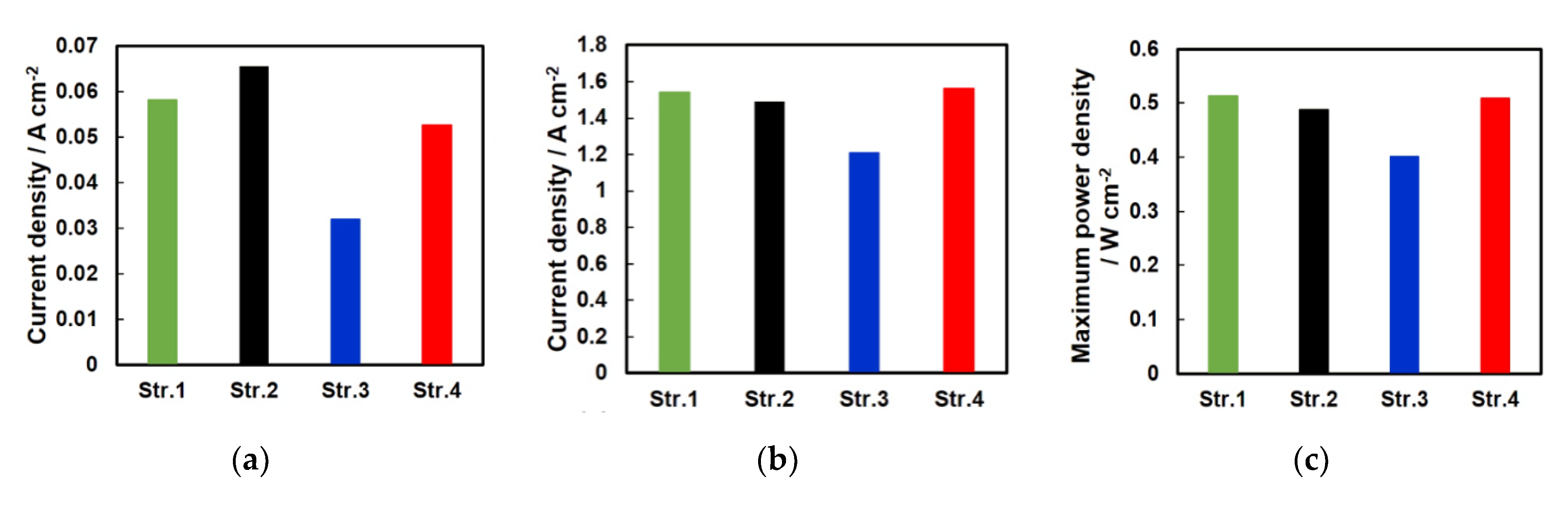
4.2. Electrochemical Analysis of the Two Structures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández, R.; Ferreira-Aparicio, P.; Daza, L. PEMFC Electrode Preparation: Influence of the Solvent Composition and Evaporation Rate on the CatalytILayer Micropattern. J. Power Source 2005, 151, 18–24. [Google Scholar] [CrossRef]
- Ngo, T.T.; Yu, T.L.; Lin, H.L. Influence of the Composition of Isopropyl Alcohol/Water Mixture Solvents in Catalyst Ink Solutions on Proton Exchange Membrane Fuel Cell Performance. J. Power Source 2013, 225, 293–303. [Google Scholar] [CrossRef]
- So, M.; Ohnishi, T.; Park, K.; Ono, M.; Tsuge, Y.; Inoue, G. The Effect of Solvent and Ionomer on Agglomeration in Fuel Cell Catalyst Inks: Simulation by the Discrete Element Method. Int. J. Hydrogen Energy 2019, 44, 28984–28995. [Google Scholar] [CrossRef]
- Suzuki, T.; Tsushima, S.; Hirai, S. Fabrication and Performance Evaluation of Structurally-Controlled PEMFC Catalyst Layers by Blending Platinum-Supported and Stand-Alone Carbon Black. J. Power Source 2013, 233, 269–276. [Google Scholar] [CrossRef]
- Mao, Q.; Sun, G.; Wang, S.; Sun, H.; Wang, G.; Gao, Y.; Ye, A.; Tian, Y.; Xin, Q. Comparative Studies of Configurations and Preparation Methods for Direct Methanol Fuel Cell Electrodes. Electrochim. Acta 2007, 52, 6763–6770. [Google Scholar] [CrossRef]
- Kim, K.-H.; Lee, K.-Y.; Kim, H.-J.; Cho, E.; Lee, S.-Y.; Lim, T.-H.; Yoon, S.P.; Hwang, I.C.; Jang, J.H. The Effects of Nafion® Ionomer Content in PEMFC MEAs Prepared by a Catalyst-Coated Membrane (CCM) Spraying Method. Int. J. Hydrogen Energy 2010, 35, 2119–2126. [Google Scholar] [CrossRef]
- Chaparro, A.M.; Gallardo, B.; Folgado, M.A.; Martín, A.J.; Daza, L. PEMFC Electrode Preparation by Electrospray: Optimization of Catalyst Load and Ionomer Content. Catal. Today 2009, 143, 237–241. [Google Scholar] [CrossRef]
- Wang, Z.; Nagao, Y. Effects of Nafion Impregnation Using Inkjet Printing for Membrane Electrode Assemblies in Polymer Electrolyte Membrane Fuel Cells. Electrochim. Acta 2014, 129, 343–347. [Google Scholar] [CrossRef]
- Tai, X.Y.; Zhakeyev, A.; Wang, H.; Jiao, K.; Zhang, H.; Xuan, J. Accelerating Fuel Cell Development with Additive Manufacturing Technologies: State of the Art, Opportunities and Challenges. Fuel Cells 2019, 19, 636–650. [Google Scholar] [CrossRef]
- Cannio, M.; Righi, S.; Santangelo, P.E.; Romagnoli, M.; Pedicini, R.; Carbone, A.; Gatto, I. Smart Catalyst Deposition by 3D printing for Polymer Electrolyte Membrane Fuel Cell manufacturing. Renew. Energy 2021, 163, 414–422. [Google Scholar] [CrossRef]
- Breitwieser, M.; Klose, C.; Klingele, M.; Hartmann, A.; Erben, J.; Cho, H.; Kerres, J.; Zengerle, R.; Thiele, S. Simple Fabrication of 12 μm Thin Nanocomposite Fuel Cell Membranes by Direct Electrospinning and Printing. J. Power Source 2017, 337, 137–144. [Google Scholar] [CrossRef]
- Towne, S.; Viswanathan, V.; Holbery, J.; Rieke, P. Fabrication of Polymer Electrolyte Membrane Fuel Cell MEAs Utilizing Inkjet Print Technology. J. Power Source 2007, 171, 575–584. [Google Scholar] [CrossRef]
- Wong, K.V.; Hernandez, A. A Review of Additive Manufacturing. ISRN Mech. Eng. 2012, 208760. [Google Scholar] [CrossRef] [Green Version]
- Yazdanpour, M.; Esmaeilifar, A.; Rowshanzamir, S. Effects of hot pressing conditions on the performance of Nafion membranes coated by ink-jet printing of Pt/MWCNTs electrocatalyst for PEMFCs. Int. J. Hydrogen Energy 2012, 37, 11290–11298. [Google Scholar] [CrossRef]
- Mohapatra, S.R.; Tsuruoka, T.; Hasegawa, T.; Terabe, K.; Aono, M. Flexible resistive switching memory using inkjet printing of a solid polymer electrolyte. AIP Adv. 2012, 2, 022144. [Google Scholar] [CrossRef]
- Guo, Y.; Ono, Y.; Nagao, Y. Modification for Uniform Surface of Nafion Ultrathin Film Deposited by Inkjet Printing. Langmuir 2015, 31, 10137–10144. [Google Scholar] [CrossRef]
- Ferrari, S.; Loveridge, M.; Beattie, S.D.; Jahn, M.; Dashwood, R.J.; Bhagat, R. Latest advances in the manufacturing of 3D rechargeable lithium microbatteries. J. Power Source 2015, 286, 25–46. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.; Domican, K.; Karan, K.; Bhattacharjee, S.; Secanell, M. Analysis of low platinum loading thin polymer electrolyte fuel cell electrodes prepared by inkjet printing. Electrochim. Acta 2014, 156, 289–300. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Singh, M.; Haring, A.P.; Tong, Y.; Cesewski, E.; Ball, E.; Jasper, R.; Davis, E.M.; Johnson, B.N. Additive Manufacturing of Mechanically Isotropic Thin Films and Membranes via Microextrusion 3D Printing of Polymer Solutions. ACS Appl. Mater. Interfaces 2019, 11, 6652–6661. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Yu, F.; Zhang, W.; Meng, X.; Yang, N.; Liu, S. A novel fabrication of yttria-stabilized-zirconia dense electrolyte for solid oxide fuel cells by 3D printing technique. Int. J. Hydrogen Energy 2019, 44, 6182–6191. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.; McMurtrey, M.D.; Jerred, N.D.; Liou, F.; Li, J. Additive manufacturing for energy: A review. Appl. Energy 2021, 282, 116041. [Google Scholar] [CrossRef]
- Blyweert, P.; Nicolas, V.; Fierro, V.; Celzard, A. 3D printing of carbon-based materials: A review. Carbon N. Y. 2021, 183, 449–485. [Google Scholar] [CrossRef]
- Calvert, P. Inkjet Printing for Materials and Devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Farandos, N.M.; Kleiminger, L.; Li, T.; Hankin, A.; Kelsall, G.H. Three-dimensional Inkjet Printed Solid Oxide Electrochemical Reactors. I. Yttria-stabilized Zirconia Electrolyte. Electrochim. Acta 2016, 213, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Wei, Y.; So, M.; Noh, T.H.; Kimura, N.; Tsuge, Y.; Inoue, G. Influence of Surface Structure on Performance of Inkjet Printed Cathode Catalyst Layers for Polymer Electrolyte Fuel Cells. J. Electrochem. Energy Convers. Storage 2022, 19, 010910. [Google Scholar] [CrossRef]
- He, G.; Li, Z.; Li, Y.; Li, Z.; Wu, H.; Yang, X.; Jiang, Z. Zwitterionic Microcapsules as Water Reservoirs and Proton Carriers within a Nafion Membrane to Confer High Proton Conductivity under Low Humidity. ACS Appl. Mater. Interfaces 2014, 6, 5362–5366. [Google Scholar] [CrossRef]
- Li, Z.; He, G.; Zhang, B.; Cao, Y.; Wu, H.; Jiang, Z.; Tiantian, Z. Enhanced Proton Conductivity of Nafion Hybrid Membrane under Different Humidities by Incorporating Metal–Organic Frameworks with High Phytic Acid Loading. ACS Appl. Mater. Interfaces 2014, 6, 9799–9807. [Google Scholar] [CrossRef]
- Faulkner, L.R.; Bard, A.J. Electrochemical Methods: Fundamentals and Applications; John Wiley and Sons: New York, NY, USA, 2002. [Google Scholar]
- Inoue, G.; Yokoyama, K.; Ooyama, J.; Terao, T.; Tokunaga, T.; Kubo, N.; Kawase, M. Theoretical Examination of Effective Oxygen Diffusion Coefficient and Electrical Conductivity of Polymer Electrolyte Fuel Cell Porous Components. J. Power Sources 2016, 327, 610–621. [Google Scholar] [CrossRef]
- O’Neil, K.; Meyers, J.P.; Darling, R.M.; Perry, M.L. Oxygen Gain Analysis for Proton Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2012, 37, 373–382. [Google Scholar] [CrossRef]
- Inoue, G.; Ohnishi, T.; So, M.; Park, K.; Ono, M.; Tsuge, Y. Simulation of carbon black aggregate and evaluation of ionomer structure on carbon in catalyst layer of polymer electrolyte fuel cell. J. Power Source 2019, 439, 227060. [Google Scholar] [CrossRef]
- Choi, S.; Stassi, S.; Pisano, A.P.; Zohdi, T.I. Coffee-Ring Effect-Based Three Dimensional Patterning of Micro/Nanoparticle Assembly with a Single Droplet. Langmuir 2010, 26, 11690–11698. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, H.; Tseng, C.J.; Huang, T.W.; Chen, S.Y. Pulsed Laser Deposition of Platinum Nanoparticles as a Catalyst for High-Performance PEM Fuel Cells. Catalysts 2016, 6, 180. [Google Scholar] [CrossRef] [Green Version]
- Sabarirajan, D.C.; Liu, J.; Qi, Y.; Perego, A.; Haug, A.T.; Zenyuk, I.V. Determining Proton Transport in Pseudo Catalyst Layers Using Hydrogen Pump DC and AC Techniques. J. Electrochem. Soc. 2020, 167, 084521. [Google Scholar] [CrossRef]



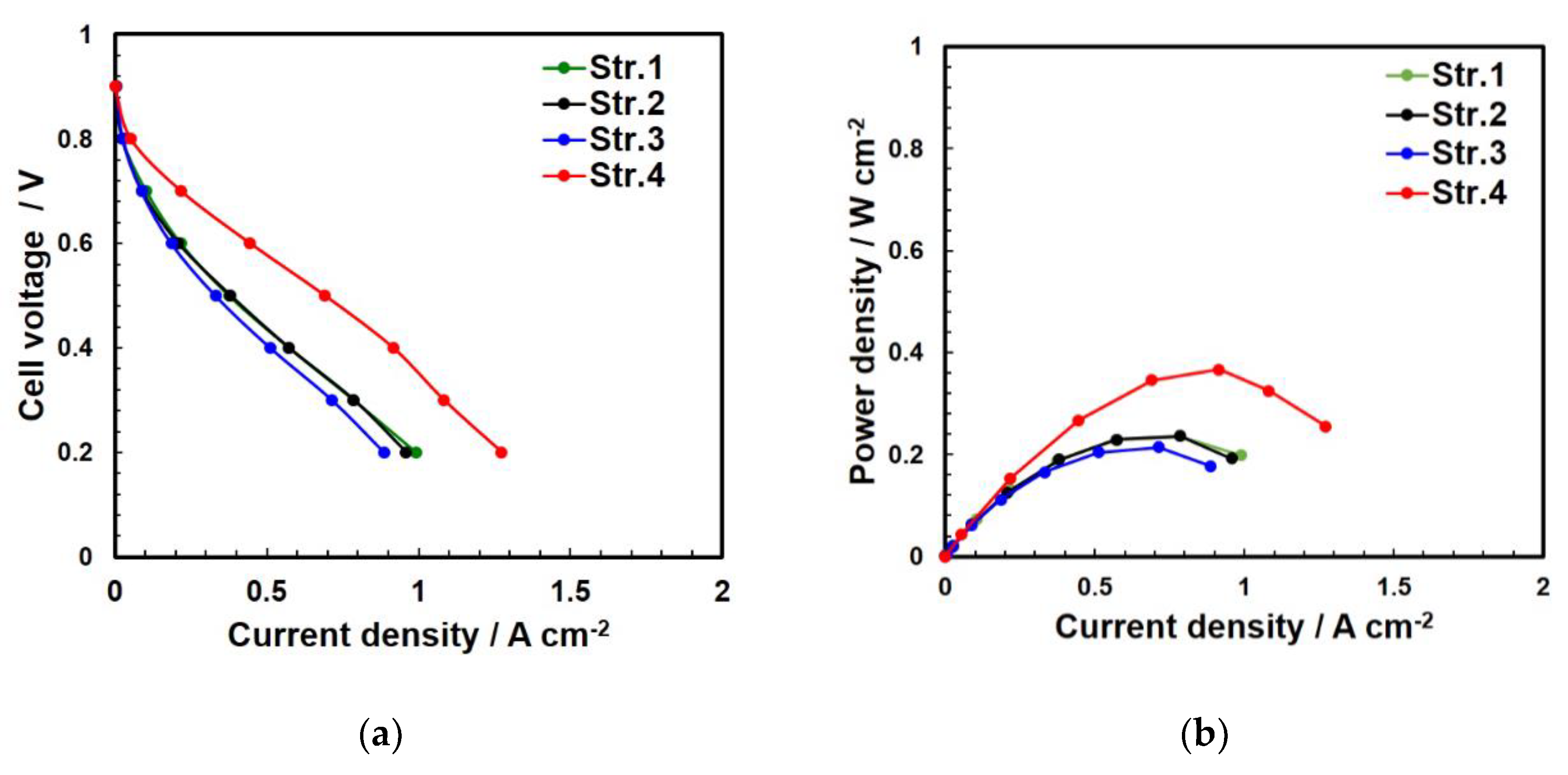
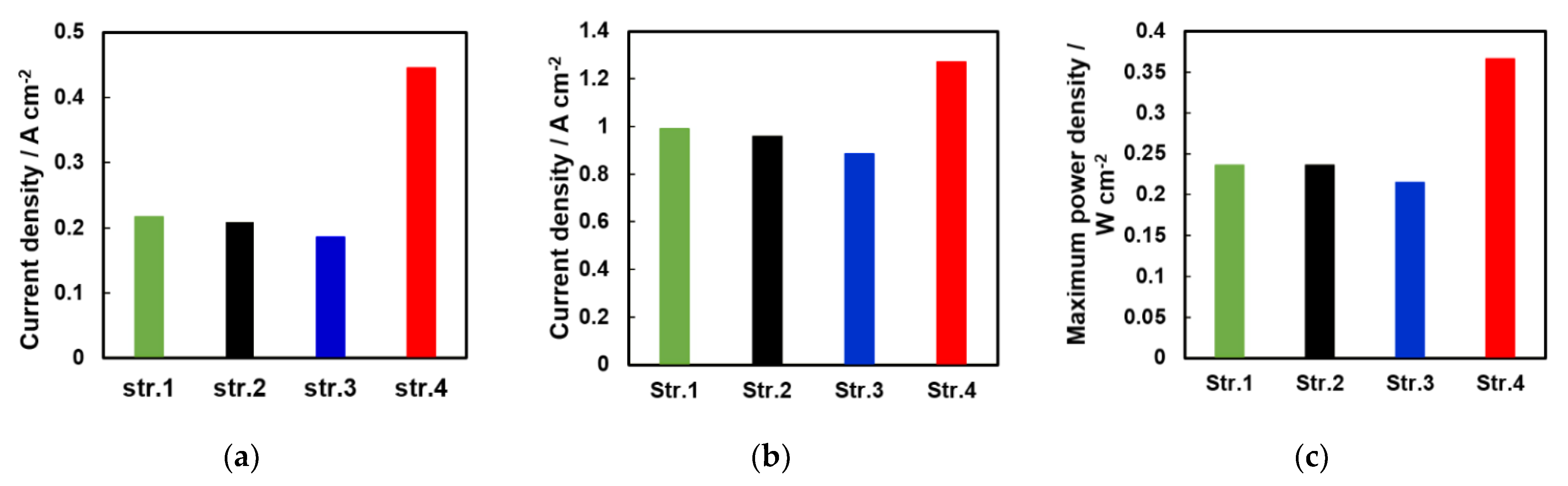


| Pattern | IL | CL |
|---|---|---|
| Str.1 (IL W/O + CL 3D) | - | 3D |
| Str.2 (IL Flat + CL Flat) | Flat | Flat |
| Str.3 (IL 3D + CL Flat) | 3D | Flat |
| Str.4 (IL 3D + CL 3D) | 3D | 3D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, T.; Park, K.; Gao, R.; Kimura, N.; Inoue, G.; Tsuge, Y. Effect of Double-Sided 3D Patterned Cathode Catalyst Layers on Polymer Electrolyte Fuel Cell Performance. Energies 2022, 15, 1179. https://doi.org/10.3390/en15031179
Noh T, Park K, Gao R, Kimura N, Inoue G, Tsuge Y. Effect of Double-Sided 3D Patterned Cathode Catalyst Layers on Polymer Electrolyte Fuel Cell Performance. Energies. 2022; 15(3):1179. https://doi.org/10.3390/en15031179
Chicago/Turabian StyleNoh, Taehyoung, Kayoung Park, Ruijing Gao, Naoki Kimura, Gen Inoue, and Yoshifumi Tsuge. 2022. "Effect of Double-Sided 3D Patterned Cathode Catalyst Layers on Polymer Electrolyte Fuel Cell Performance" Energies 15, no. 3: 1179. https://doi.org/10.3390/en15031179
APA StyleNoh, T., Park, K., Gao, R., Kimura, N., Inoue, G., & Tsuge, Y. (2022). Effect of Double-Sided 3D Patterned Cathode Catalyst Layers on Polymer Electrolyte Fuel Cell Performance. Energies, 15(3), 1179. https://doi.org/10.3390/en15031179







