Evaluation of the Melting Gasification Process for Recovery of Energy and Resources from Automobile Shredder Residues
Abstract
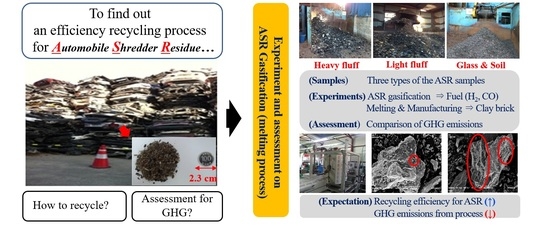
1. Introduction
2. Materials and Methods
2.1. Thermochemical Analysis of the ASR
2.2. ASR Gasification in a Fixed-Bed Reactor
2.3. Manufacturing Process of Clay Brick Using the Melting Slag
2.4. Estimation of GHG Emissions in the ASR Melting Gasification Process
3. Results and Discussion
3.1. Thermochemical Characteristics of the ASR
3.2. Results for ASR Gasification in a Fixed-Bed Reactor
3.3. Quality Assessment of Clay Brick Manufactured from Melting Slag
3.4. GHG Emissions from the ASR Melting Gasification Process
4. Conclusions
- 1.
- The elemental analysis revealed that the ASR was composed of 52.75 wt.% carbon, 7.02 wt.% hydrogen, 15.81 wt.% nitrogen and 1.78 wt.% oxygen, while the proximate analysis indicated that the ASR contained 1.17 wt.% moisture, 63.90 wt.% volatile compounds, 16.13 wt.% ash, and 18.80 wt.% fixed carbon. Moreover, the ASR is more efficient as an alternative fuel compared to other combustible wastes, since it has higher levels of combustible compounds and a lower moisture content.
- 2.
- In the ASR gasification process, the tar and ash contents decreased by approximately 10 wt.% with increasing temperature and ER. Additionally, the cold-gas efficiency levels and HHV also decreased with an increase in the ER. In particular, the HHV of the gas produced at a lower ER and temperature was higher than that of the gas produced at a higher ER and temperature because there was less oxidation and thermal cracking at a lower ER and temperature. However, the dry gas yield increased from 0.64 Nm3/kg to 1.20 Nm3/kg when the temperature increased from 800 °C to 1200 °C and the ER increased from 0.1 to 0.5, with the carbon conversion exhibiting the same trend. Consequently, considering economy and efficiency, the optimum conditions for the ASR gasification were determined to be a temperature of 1200 °C and an ER of 0.5.
- 3.
- From the results of melting process, the highest compressive strength was shown to be 153.35 N/mm2 when the melting slag content is 10 wt.% at a melting temperature of 1300 °C. According to the results, it was concluded that the optimum melting slag content is 10 wt.% and the optimal melting temperature is 1300 °C. However, the leaching test has to be performed before fixing the melting slag content. In some application cases, the amount of heavy metals could be measured since the manufactured clay brick exhibited foaming, causing a reduction in the compressive strength.
- 4.
- The ASR-gasification process has been proven to be a low GHG emission technology with high energy efficiency. Additionally, even though the ASR residues were melted and fired for clay-brick manufacturing, the GHG emissions remained approximately ten times higher than those produced in the ASR incineration process. It reveals that the purity of carbon dioxide in the flue-gas from the gasification plant was higher than that of the incineration plant. In terms of the operation cost of the carbon capture process for GHG reduction, the gasification plant would be more efficient than an incineration plant. In addition, to identify this process as an eco-friendly technology, a further analysis of the emission levels of dioxins and leaching tests for chlorine and heavy metals are required.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korea Energy Management Corporation. New & Renewable Energy RD&D Strategy 2030—Waste Part, 1–14. 2007. Available online: https://www.energy.or.kr/web/kem_home_new/info/data/open/kem_view.asp?q=13726 (accessed on 7 January 2008).
- Geyer, R.; Jampeck, J.; Law, K. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Euromap. Plastic Resin Production and Consumption in 63 Countries Worldwide. 2016. Available online: https://www.pagder.org/images/files/euromappreview.pdf (accessed on 8 December 2016).
- Lee, K.B. Studies on Solidification and Melting Characteristics for Developing Energy Recovery System of Automobile Shredder Residue. Master’s Thesis, Yonsei University, Seoul, Korea, 2012. [Google Scholar]
- Cossu, R.; Lai, T. Automotive shredder residue (ASR) management: An overview. Waste Manag. 2015, 45, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, I.; Van Caneghem, J.; Block, C.; Baeyens, J.; Vandecasteele, C. Automotive shredder residue (ASR): Reviewing its production from end-of-life vehicles (ELVs) and its recycling, energy or chemical’s valorisation. J. Hazard. Mater. 2011, 190, 8–27. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council. Directive 2000/53/EC of the European Parliament and of the Council of 18 September 2000 on End-of Life Vehicles. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:02fa83cf-bf28-4afc-8f9f-eb201bd61813.0005.02/DOC_1&format=PDF (accessed on 29 May 2020).
- Cullis, C.F.; Hirschler, M.M. The Combustion of Organic Polymers, 5; Oxford University Press: Jericho, NY, USA, 1981. [Google Scholar]
- Mirabile, D.; Pistelli, M.I.; Marchesini, M.; Falciani, R.; Chiappelli, L. Thermal valorization of automotive shredder residue: Injection in blast furnace. Waste Manag. 2002, 22, 841–851. [Google Scholar] [CrossRef]
- Day, M.; Shen, Z.; Cooney, J.D. Pyrolysis of automotive shredder residue: An analysis of the products of a commercial screw kiln process. J. Anal. Appl. Pyrol. 1996, 37, 49–67. [Google Scholar] [CrossRef][Green Version]
- Day, M.; Shen, Z.; Cooney, J.D. Pyrolysis of auto shredder residue: Experiments with a laboratory screw kiln reactor. J. Anal. Appl. Pyrol. 1999, 51, 181–200. [Google Scholar] [CrossRef]
- Donaj, P.; Kaminsky, W. Recycling of polyolefins by pyrolysis in a fluidized bed reactor. In Proceedings of the 17th European Biomass Conference, Hamburg, Germany, 29 June–3 July 2009. paper No.OB4.4. [Google Scholar]
- Forsgren, C. Microwave pyrolysis a new recycling tool. In Proceedings of the 26th Annual International Conference on Incineration and Thermal Treatment Technologies, Phoenix, AZ, USA, 14–18 May 2007. [Google Scholar]
- Galvagno, S.; Fortuna, F.; Cornacchia, G.; Casu, S.; Coppola, T.; Sharma, V.K. Pyrolysis process for treatment of automotive shredder residue: Preliminary experimental results. Energy Convers. Manag. 2001, 42, 573–586. [Google Scholar] [CrossRef]
- Harder, M.K.; Forton, O.T. A critical review of developments in the pyrolysis of automotive shredder residue. J. Anal. Appl. Pyrol. 2007, 79, 387–394. [Google Scholar] [CrossRef]
- Joung, H.T.; Kim, K.H.; Seo, Y.C. Effects of oxygen, catalyst and PVC on the formation of PCDDs, PCDFs and dioxin-like PCbs in pyrolysis products of automotive residues. Chemosphere 2006, 65, 1481–1489. [Google Scholar] [CrossRef]
- Kondoh, M.; Hamai, M.; Yamaguchi, M.; Mori, S. Study of gasification characteristics of automobile shredder residue. JSAE Rev. 2001, 2, 234–236. [Google Scholar] [CrossRef]
- Kurose, K.; Okuda, T.; Nishijima, W.; Okada, M. Heavy metals removal from automotive shredder residues (ASR). J. Hazard. Mater. 2006, 137, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Marco, I.; Caballero, B.M.; Cabrero, M.A.; Laresgoiti, M.F.; Torres, A.; Chomon, M.J. Recycling of automotive shredder residues by means of pyrolysis. J. Anal. Appl. Pyrol. 2007, 79, 403–408. [Google Scholar] [CrossRef]
- Srogi, K. An overview of current processes for the thermochemical treatment of automobile shredder residue. Clean Technol. Environ. Policy 2008, 10, 235–244. [Google Scholar] [CrossRef]
- Selvarajoo, A. Slow pyrolysis of Durio zibethinus rind and the influence of carbonization temperature on biochar properties. Mater. Sci. Eng. 2021, 1092, 012042. [Google Scholar] [CrossRef]
- Donaj, P.; Kubik, K.; Swiderski, A.; Yang, W.; Blasiak, W.; Forsgren, C. Assessment of ASR treatment using pyrolysis and reforming of its residences for small scale electricity generation systems. In Proceedings of the 27th Annual International Conference on Thermal Treatment Technologies, Montreal, QC, Canada, 12–16 May 2008. [Google Scholar]
- Kantarelis, E.; Donaj, P.; Yang, W.; Zabaniotou, A. Sustainable valorization of plastic wastes for energy with environmental safety via high-temperature pyrolysis (HTP) and high-temperature steam gasification (HTSG). J. Hazard. Mater. 2009, 167, 675–684. [Google Scholar] [CrossRef]
- Kubik, K. Reforming of Car Residues Pyrolysis Products into High-Purity Synthetic Gas for Small-Scale Electricity Generation. Master’s Thesis, Royal Institute of Technology, Stockholm, Sweden, 2008. [Google Scholar]
- Malkow, T. Novel and innovative pyrolysis and gasification technologies for energy efficient and environmentally sound MSW disposal. Waste Manag. 2004, 24, 53–79. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Hwang, H. Gasification of Wood Pellet Using Multi-Stage Reactor System. Master’s Dissertation, Seoul National University of Science and Technology, Seoul, Korea, 2011; pp. 36–44. [Google Scholar]
- Jung, H.T. Study on the Pyrolysis Characteristics of Automobile Shredder Residue. Master’s Thesis, Yonsei University, Seoul, Korea, 2003. [Google Scholar]
- Donaj, P.; Yang, W.; Błasiak, W.; Forsgren, C. Recycling of automobile shredder residue with a microwave pyrolysis combined with high temperature steam gasification. J. Hazard. Mater. 2010, 182, 80–89. [Google Scholar] [CrossRef]
- Kim, K.H.; Joung, H.T.; Nam, H.; Seo, Y.C.; Hong, J.H.; Yoo, T.W.; Lim, B.S.; Park, J.H. Management status of end-of-life vehicles and characteristics of automobile shredder residues on Korea. Waste Manag. 2004, 24, 533–540. [Google Scholar] [CrossRef]
- Endoh, S.; Takahashi, K.; Lee, J.R.; Ohya, H. Mechanical treatment of automobile shredder residue for its application as a fuel. J. Mater. Cycles Waste Manag. 2006, 8, 88–94. [Google Scholar] [CrossRef]
- Yang, W.S.; Cho, S.J.; Lee, K.B.; Seo, Y.C.; Kim, W.H. Studies on physicochemical and melting characteristics of automobile shredder residue for enhancing end of life vehicles (ELVs) recycling rate. In Proceedings of the Annual Conference of Japan Society of Material Cycles and Waste Management, Sendai, Japan, 22–24 October 2012. Japan Society of Material Cycles and Waste Management. [Google Scholar]
- Korean Standards Association. Concrete Interlocking Block for Side Walk and Road. KS F, 4419. 2009. Available online: https://e-ks.kr/streamdocs/view/sd;streamdocsId=72059225013305044 (accessed on 13 November 2017).
- Korean Standards Association. Clay Brick. KS L, 4201. 2012. Available online: https://e-ks.kr/streamdocs/view/sd;streamdocsId=72059203168086519 (accessed on 7 July 2015).
- Korean Standards Association. Concrete Curbs. KS F, 4006. 2013. Available online: https://e-ks.kr/streamdocs/view/sd;streamdocsId=72059207383841907 (accessed on 7 July 2015).
- Korean Standards Association. Preast Concrete Paving Flags. KS F, 4001. 2015. Available online: https://e-ks.kr/streamdocs/view/sd;streamdocsId=72059225017138675 (accessed on 7 July 2015).
- Ministry of the Environment. The Manual for Estimating a GHG Emission Amount -Waste Part, 70–301. 2012. Available online: https://www.keco.or.kr/kr/business/climate/communityid/187/view.do?p=1&idx=328&f=1&q= (accessed on 4 December 2012).
- Kim, S.W.; Koo, B.S.; Ryu, J.W.; Lee, J.S.; Kim, C.J.; Lee, D.H.; Kim, G.R.; Choi, S. Bio-oil from the pyrolysis of palm and Jatropha wastes in a fluidized bed. Fuel Processing Technol. 2013, 108, 118–124. [Google Scholar] [CrossRef]
- Sulaiman, F.; Abdullah, N. Optimum conditions for maximizing pyrolysis liquids of oil palm empty fruit bunches. Energy 2011, 36, 2352–2359. [Google Scholar] [CrossRef]
- Cho, S.J. Studies on Gasification and Melting Characteristics of Waste and Biomass. Ph.D. Dissertation, Yonsei University, Seoul, Korea, 2012. [Google Scholar]
- Yoo, H.M.; Chung, T.H.; Lee, S.Y.; Park, S.W.; Seo, Y.C. A study on applicability for air pollutant materials sources: Characteristics comparison of carbon dioxide isotope with conbustion engines. J. Korea Soc. Waste Manag. 2019, 36, 644–651. [Google Scholar] [CrossRef]
- Cuiping, L.; Chuangzhi, W.; Yanyongjie, H.H.; Haitao, H. Chemical elemental characteristics of biomass fuels in China. Biomass Bioenergy 2004, 27, 119–130. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, Y.K.; Kang, B.S.; Park, H.J.; Lee, K.H.; Kim, E.Y.; Kim, S.D.; Song, D.K.; Kim, Y.C. Production of Clean Bio-Fuel from Rice Straw by Flash Pyrolysis and Catalytic Upgrading; The University of Seoul: Seoul, Korea, 2005. [Google Scholar]
- Lee, J.G.; Kim, J.H.; Lee, S.H.; Choi, Y.C.; Kim, Y.G.; Yoo, K.S.; Lee, S.H. Development of fluidized bed reactor for the pyrolysis and gasification of agricultural and forestry wastes. Korea Inst. Energy Res. 2005, 19–73. [Google Scholar]
- Iliuta, I.; Leclerc, A.; Larachi, F. Allothermal steam gasification of biomass in cyclic multi-compartment bubbling fluidized-bed gasifier/combustor–New reactor concept. Bioresour. Technol. 2010, 101, 3194–3208. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Park, J.K.; Seo, Y.C.; Lee, J.S.; Yoo, H.M.; Yang, W.S.; Park, S.W.; Han, B.H.; Choi, H.S.; Cho, S.J.; Lee, K.B.; et al. Studies on physicochemical characteristics and optimal melting condition of automobile shredder residue in a melting furnace. J. Korea Soc.Waste Manag. 2013, 30, 189–198. [Google Scholar] [CrossRef]
- Shen, L.; Gao, Y.; Xiao, J. Simulation of hydrogen production from biomass gasification in interconnected fluidized beds. Biomass Bioenergy 2008, 32, 120–127. [Google Scholar] [CrossRef]
- Kim, M.J.; Ryu, H.L.; Lee, W.K. Coal gasification in a fluidized bed at atmospheric pressure. J. Korean Inst. Chem. Eng. 1983, 21, 20–27. [Google Scholar]
- Kaewluan, S.; Pipatmanomai, S. Gasification of high moisture rubber woodchip with rubber waste in a bubbling fluidized bed. Fuel Process. Technol. 2011, 92, 671–677. [Google Scholar] [CrossRef]
- Lee, H.Y. Characteristics and heavy metal leaching of ash generated from incineration of automobile shredder residue. J. Hazard. Mater. 2007, 147, 570–575. [Google Scholar] [CrossRef] [PubMed]








| Materials | Heavy Fluff (wt. %) | Light Fluff (wt. %) | Glass & Soil (wt. %) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| This Study | Kuen-Song Lin et al., 2010 | K.-H. Kim et al., 2004 | I. de Marco et al., 2007 | This Study | Kuen-Song Lin et al., 2010 | K.-H. Kim et al., 2004 | I. de Marco et al., 2007 | This Study | |
| Metals | 0.80 | - | 0.20 | - | 1.50 | - | 1.00 | - | 0.20 |
| Rubbers | 22.10 | 32.47 | 22.20 | 35.10 | 3.80 | 3.52 | 3.80 | 4.10 | 5.80 |
| Resins | 16.60 | 0.28 | 1.50 | 10.50 | 37.50 | 1.12 | 20.60 | 8.00 | 6.40 |
| Wires | 14.00 | 0.65 | 20.10 | 0.70 | 2.90 | 0.33 | 2.90 | 0.40 | 11.50 |
| Thermosetting plastics | 7.20 | 2.96 | 33.80 | 1.40 | 21.30 | 1.65 | 24.10 | 1.20 | 0.30 |
| Thermo plastics | 33.80 | 29.41 | 27.60 | 24.10 | 8.20 | 7.50 | 27.30 | ||
| Woods | 0.10 | 4.74 | 0.020 | 5.60 | 0.20 | 0.57 | 0.030 | - | 0.10 |
| Papers | 2.10 | - | 2.00 | - | 1.00 | - | 1.00 | - | 0.00 |
| Soils | 1.80 | 7.84 | - | 6.10 | 6.90 | 70.45 | - | 75.00 | 17.20 |
| Glass | 1.50 | - | 0.80 | - | 31.20 | ||||
| others | - | 21.65 | 20.18 | 13.00 | 14.16 | 46.57 | 3.80 | - | |
| Sum | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Material | Analysis | Instrument | Method |
|---|---|---|---|
 | Elemental Analysis | - EA 1112, Thermo Fischer Scientific - EA1110, CE Instrument | ASTM D 5373 |
| Proximate Analysis | TGA-701, LECO | ASTM D 3172 | |
| Thermo-gravity Analysis | TGA-701, LECO | ASTM E 1131 | |
| Heating Value | AC-600, LECO | ASTM D 4809 |
| Parameter | Unit | Value |
|---|---|---|
| Capacity | kg/h | 1 |
| Feeding Rate | g/min | 10 |
| Setting Temperature Range | °C | 800/1000/1200 |
| ER * (Equivalence Ratio) | - | 0.1~0.5 |
| Oxygen (Flow rate) | L/min | 1.440~7.201 |
| Conditions | Column | |
|---|---|---|
| Molsieve 5A PLOT | PLOT Q | |
| Gas Inlet Temperature (°C) | 45 | 95 |
| Injector Temperature (°C) | 55 | 95 |
| Column Temperature (°C) | 60 | 100 |
| Column Pressure (kPa [psi]) | 138 (20) | 207 (30) |
| Post Run Pressure (kPa [psi]) | 138 (20) | 276 (40) |
| Carrier gas | Argon | Helium |
| Sampling Time (s) | 10 | 10 |
| Inject time (ms) | 30 | 30 |
| Run Time (s) | 240 | 180 |
| Peak | H2, O2, N2, CH4, CO | N2, CH4, CO2, C2H6, C3H8 |
| Classification | Kaolin | Feldspar | Clay | Melting Slag (°C) | ||
|---|---|---|---|---|---|---|
| 1300 | 1350 | 1400 | ||||
| Standard | 70 | 10 | 20 | 0 | ||
| A | 69 | 10 | 20 | 1 | ||
| B | 67 | 10 | 20 | 3 | ||
| C | 65 | 10 | 20 | 5 | ||
| D | 63 | 10 | 20 | 7 | ||
| E | 60 | 10 | 20 | 10 | ||
| Analysis Items | KS Standard | Specifications | Value |
|---|---|---|---|
| Absorption | KS F 4201, KS F 4001, KS F 4006, KS F 4419 | - | - |
| Compressive Strength | Max. Capacity | 1 mN (100 tf) | |
| Min. Gradation | 100 N (10 kgf) | ||
| Test Space | 160~310 mm | ||
| Ram Stroke | 25 mm | ||
| Dimension | 1160 × 560 × 1230 (H)mm | ||
| Hydraulic Pump | Rotary Plunger Pump |
| Elemental Analysis [wt.%] | HHV [kJ/kg] | ||||||
|---|---|---|---|---|---|---|---|
| C | H | O | N | S | Cl | ||
| ASR | 52.75 | 7.02 | 1.78 | 15.81 | 0.71 | 1.37 | 21,680 |
| Sawdust | 45.93 | 6.65 | 45.96 | 0.68 | 0.16 | 0.14 | 17,623 |
| Plastic | 80.16 | 12.34 | 0.16 | 0.73 | 0.00 | 2.76 | 34,973 |
| SRF | 50.57 | 6.15 | 37.67 | 0.41 | N.D. | 0.20 | 18,887 |
| Proximate Analysis [wt.%] | ||||
|---|---|---|---|---|
| Moisture | Volatile | Fixed-Carbon | Ash | |
| ASR | 1.17 | 63.90 | 18.80 | 16.13 |
| Sawdust | 6.27 | 78.11 | 15.04 | 0.58 |
| Plastic | 0.05 | 86.52 | 6.66 | 6.77 |
| SRF | 18.67 | 70.88 | 2.94 | 7.50 |
| Carbon Conversion (%) | |
| Cold-gas Efficiency (%) | HHV ** of produced Gas (kJ/kg) HHV of feedstock (kJ/kg) × 100 |
| Process | Factor | CO2 | CH4 | N2O | GHG |
|---|---|---|---|---|---|
| ASR incineration | IEF * | 0.033323 | 0.000378 | 0.000290 | 0.03657239 |
| Reference value | 0.002574 | 2.52 × 10−9 | 7.40 × 10−6 | ||
| ASR gasification (melting & firing) | Gasifier IEF * | 0.033323 | 0.000378 | 0.000290 | 0.304309 |
| Experimental result | 0.019545 | 0.000707 | - | ||
| Furnaces IEF * | 0.245152 | 0.002777 | 0.002136 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.-M.; Lee, S.-Y.; Cho, S.-J.; Seo, Y.-C.; Jang, H.-N. Evaluation of the Melting Gasification Process for Recovery of Energy and Resources from Automobile Shredder Residues. Energies 2022, 15, 1248. https://doi.org/10.3390/en15031248
Yoo H-M, Lee S-Y, Cho S-J, Seo Y-C, Jang H-N. Evaluation of the Melting Gasification Process for Recovery of Energy and Resources from Automobile Shredder Residues. Energies. 2022; 15(3):1248. https://doi.org/10.3390/en15031248
Chicago/Turabian StyleYoo, Heung-Min, Sang-Yeop Lee, Sung-Jin Cho, Yong-Chil Seo, and Ha-Na Jang. 2022. "Evaluation of the Melting Gasification Process for Recovery of Energy and Resources from Automobile Shredder Residues" Energies 15, no. 3: 1248. https://doi.org/10.3390/en15031248
APA StyleYoo, H.-M., Lee, S.-Y., Cho, S.-J., Seo, Y.-C., & Jang, H.-N. (2022). Evaluation of the Melting Gasification Process for Recovery of Energy and Resources from Automobile Shredder Residues. Energies, 15(3), 1248. https://doi.org/10.3390/en15031248