A New Graphitic Nitride and Reduced Graphene Oxide-Based Sulfur Cathode for High-Capacity Lithium-Sulfur Cells
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of RGO/GN Composite
2.3. Preparation of S@RGO/GN Cathode
2.4. Materials Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2011, 11, 19–29. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable lithium-sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in lithium-sulfur batteries based on multifunctional cathodes and elec-trolytes. Nat. Energy 2016, 1, 16132. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Xu, J.; Guo, Z. Advances in Polar Materials for Lithium–Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1707520. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Dudney, N.J.; Howe, J.Y. Hierarchically Structured Sulfur/Carbon Nanocomposite Material for High-Energy Lithium Battery. Chem. Mater. 2009, 21, 4724–4730. [Google Scholar] [CrossRef]
- Schuster, J.; He, G.; Mandlmeier, B.; Yim, T.; Lee, K.T.; Bein, T.; Nazar, L.F. Spherical Ordered Mesoporous Carbon Nanoparticles with High Porosity for Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2012, 51, 3591–3595. [Google Scholar] [CrossRef]
- Chen, S.; Sun, B.; Xie, X.; Mondal, A.K.; Huang, X.; Wang, G. Multi-chambered micro/mesoporous carbon nanocubes as new polysulfides reservoirs for lithium-sulfur batteries with long cycle life. Nano Energy 2015, 16, 268–280. [Google Scholar] [CrossRef]
- Li, G.; Sun, J.; Hou, W.; Jiang, S.; Huang, Y.; Geng, J. Three-dimensional porous carbon composites containing high sulfur na-noparticle content for high-performance lithium-sulfur batteries. Nat. Commun. 2016, 7, 10601. [Google Scholar] [CrossRef]
- Zheng, Z.; Guo, H.; Pei, F.; Zhang, X.; Chen, X.; Fang, X.; Wang, T.; Zheng, N. High Sulfur Loading in Hierarchical Porous Carbon Rods Constructed by Vertically Oriented Porous Graphene-Like Nanosheets for Li-S Batteries. Adv. Funct. Mater. 2016, 26, 8952–8959. [Google Scholar] [CrossRef]
- Wu, R.; Chen, S.; Deng, J.; Huang, X.; Song, Y.; Gan, R.; Wan, X.; Wei, Z. Hierarchically porous nitrogen-doped carbon as cathode for lithium–sulfur batteries. J. Energy Chem. 2018, 27, 1661–1667. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Yuan, H.; Qiu, X.; Chen, L.; Zhu, W. Improvement of cycle property of sulfur-coated multi-walled carbon nanotubes composite cathode for lithium/sulfur batteries. J. Power Sources 2009, 189, 1141–1146. [Google Scholar] [CrossRef]
- Dörfler, S.; Hagen, M.; Althues, H.; Tübke, J.; Kaskel, S.; Hoffmann, M.J. High capacity vertical aligned carbon nanotube/sulfur composite cathodes for lithium–sulfur batteries. Chem. Commun. 2012, 48, 4097–4099. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Wang, D.; Luo, Y.; Wang, K.; Kong, W.; Wu, Y.; Zhang, L.; Jiang, K.; Li, Q.; Zhang, Y.; et al. Sulfur Embedded in a Mesoporous Carbon Nanotube Network as a Binder-Free Electrode for High-Performance Lithium–Sulfur Batteries. ACS Nano 2015, 10, 1300–1308. [Google Scholar] [CrossRef]
- Yuan, Z.; Peng, H.-J.; Huang, J.-Q.; Liu, X.-Y.; Wang, D.-W.; Cheng, X.-B.; Zhang, Q. Electrodes: Hierarchical free-standing car-bon-nanotube paper electrodes with ultrahigh sulfur-loading for lithium-sulfur batteries. Adv. Funct. Mater. 2014, 24, 6244. [Google Scholar] [CrossRef]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene Oxide as a Sulfur Immobilizer in High Performance Lithium/Sulfur Cells. J. Am. Chem. Soc. 2011, 133, 18522–18525. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Z.; Lu, L.; Choucair, M.; Stride, J.A.; Xu, X.; Liu, H.-K. Sulfur-graphene composite for rechargeable lithium batteries. J. Power Sources 2011, 196, 7030–7034. [Google Scholar] [CrossRef]
- Yuan, S.; Guo, Z.; Wang, L.; Hu, S.; Wang, Y.; Xia, Y. Leaf-Like Graphene-Oxide-Wrapped Sulfur for High-Performance Lithium-Sulfur Battery. Adv. Sci. 2015, 2, 1500071. [Google Scholar] [CrossRef]
- Hu, G.; Xu, C.; Sun, Z.; Wang, S.; Cheng, H.-M.; Li, F.; Ren, W. 3D graphene foam–reduced-graphene-oxide hybrid nested hier-archical networks for high-performance Li–S batteries. Adv. Mater. 2016, 28, 1603–1609. [Google Scholar] [CrossRef]
- Chen, L.; Rago, N.L.D.; Bloom, I.D.; Shaw, L.L. New insights into the electrode mechanism of lithium sulfur batteries via air-free post-test analysis. Chem. Commun. 2016, 52, 9913–9916. [Google Scholar] [CrossRef]
- Chabu, J.M.; Zeng, K.; Jin, G.; Zhang, M.; Li, Y.; Liu, Y.-N. Simple approach for the preparation of nitrogen and sulfur codoped carbon dots/reduced graphene oxide as host for high-rate lithium sulfur batteries. Mater. Chem. Phys. 2019, 229, 226–231. [Google Scholar] [CrossRef]
- Shi, J.-L.; Tang, C.; Huang, J.-Q.; Zhu, W.; Zhang, Q. Effective exposure of nitrogen heteroatoms in 3D porous graphene framework for oxygen reduction reaction and lithium–sulfur batteries. J. Energy Chem. 2018, 27, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Peng, H.-J.; Li, B.-Q.; Xie, J.; Kong, L.; Zhao, M.; Chen, X.; Huang, J.-Q.; Zhang, Q. Conductive and Catalytic Triple-Phase Interfaces Enabling Uniform Nucleation in High-Rate Lithium-Sulfur Batteries. Adv. Energy Mater. 2018, 9, 1802768. [Google Scholar] [CrossRef]
- Li, Z.; Wu, H.B.; Lou, X.W. Rational designs and engineering of hollow micro-/nanostructures as sulfur hosts for advanced lithium-sulfur batteries. Energy Environ. Sci. 2016, 9, 3061–3070. [Google Scholar] [CrossRef] [Green Version]
- Hart, C.J.; Cuisinier, M.; Liang, X.; Kundu, D.; Garsuch, A.; Nazar, L.F. Rational design of sulphur host materials for Li–S batteries: Correlating lithium polysulphide adsorptivity and self-discharge capacity loss. Chem. Commun. 2014, 51, 2308–2311. [Google Scholar] [CrossRef]
- Pang, Q.; Tang, J.; Huang, H.; Liang, X.; Hart, C.; Tam, K.C.; Nazar, L.F. A Nitrogen and Sulfur Dual-Doped Carbon Derived from Polyrhodanine@Cellulose for Advanced Lithium-Sulfur Batteries. Adv. Mater. 2015, 27, 6021–6028. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, S.; Huang, C.; Wang, X.; Cai, S.; Xiang, K.; Zhang, Y.; Xue, J. Honeycomb-like Nitrogen and Sulfur Dual-Doped Hierarchical Porous Biomass-Derived Carbon for Lithium-Sulfur Batteries. ChemSusChem 2017, 10, 1803–1812. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Ashuri, M.; Liu, C.; Shaw, L.L. Li2S encapsulated by nitrogen-doped carbon for lithium sulfur batteries. J. Mater. Chem. A 2014, 2, 18026–18032. [Google Scholar] [CrossRef]
- Zheng, J.; Tian, J.; Wu, D.; Gu, M.; Xu, W.; Wang, C.; Gao, F.; Engelhard, M.H.; Zhang, J.-G.; Liu, J.; et al. Lewis acid-base inter-actions between polysulfides and metal-organic framework in lithium-sulfur batteries. Nano Lett. 2014, 14, 2345–2352. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, W.; Chen, Y.; Fan, H.; Su, D.; Wang, G. MOF-derived porous N–Co3O4@N–C nanododecahedra wrapped with reduced graphene oxide as a high capacity cathode for lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 2797–2807. [Google Scholar] [CrossRef]
- Seh, Z.W.; Li, W.; Cha, J.J.; Zheng, G.; Yang, Y.; McDowell, M.T.; Hsu, P.-C.; Cui, Y. Sulphur–TiO2 yolk-shell nanoarchitecture with internal void space for long-cycle lithium–sulphur batteries. Nat. Commun. 2013, 4, 1331. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Guan, B.Y.; Zhang, J.; Lou, X.W.D. A Compact Nanoconfined Sulfur Cathode for High-Performance Lithium-Sulfur Batteries. Joule 2017, 1, 576–587. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, J.; Guan, B.; Wang, D.; Liu, L.-M.; Lou, X.W. A sulfur host based on titanium monoxide@carbon hollow spheres for advanced lithium–sulfur batteries. Nat. Commun. 2016, 7, 13065. [Google Scholar] [CrossRef] [Green Version]
- Yao, W.; Chu, C.; Zheng, W.; Zhan, L.; Wang, Y. “Pea-pod-like” nitrogen-doped hollow porous carbon cathode hosts decorated with polar titanium dioxide nanocrystals as efficient polysulfide reservoirs for advanced lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 18191–18205. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Kim, H.M.; Lee, S.-K.; Lee, J.-H.; Abouimrane, A.; Khaleel, M.A.; Belharouak, I.; Manthiram, A.; Sun, Y.-K. High-energy, high-rate, lithium–sulfur batteries: Synergetic effect of hollow TiO2-webbed carbon nanotubes and a dual functional car-bon-paper interlayer. Adv. Energy Mater. 2016, 6, 1501480. [Google Scholar] [CrossRef]
- Pang, Q.; Nazar, L.F. Long-Life and high-areal-capacity Li–S batteries enabled by a light-weight polar host with intrinsic pol-ysulfide adsorption. ACS Nano 2016, 10, 4111–4118. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Duan, L.; Li, X.; Ji, L.; Geng, Z.; Huang, K.; Lu, L.; Zhou, L.; Liu, Z.; et al. A Graphene-like Oxygenated Carbon Nitride Material for Improved Cycle-Life Lithium/Sulfur Batteries. Nano Lett. 2015, 15, 5137–5142. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Kulisch, J.; Nazar, L.F. A Comprehensive Approach toward Stable Lithium–Sulfur Batteries with High Volumetric Energy Density. Adv. Energy Mater. 2016, 7, 1601630. [Google Scholar] [CrossRef]
- Liang, X.; Hart, C.J.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L.F. A highly efficient polysulfide mediator for lithium–sulfur batteries. Nat. Commun. 2015, 6, 5682. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Nazar, L.F. In situ reactive assembly of scalable core-shell sulfur–MnO2 composite cathodes. ACS Nano 2016, 10, 4192–4198. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Zhao, G.; Yang, G.; Niu, G.; Chen, M.; Diao, G. Dual-core–shell-structured S@C@MnO2 nanocomposite for highly stable lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 34793–34803. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Konstantinov, K.; Zhao, L.; Ng, S.; Wang, G.; Guo, Z.; Liu, H.K. Sulphur-polypyrrole composite positive electrode materials for rechargeable lithium batteries. Electrochim. Acta 2006, 51, 4634–4638. [Google Scholar] [CrossRef]
- Li, X.; Yuan, L.; Liu, D.; Li, Z.; Chen, J.; Yuan, K.; Xiang, J.; Huang, Y. High sulfur-containing organosulfur polymer composite cathode embedded by monoclinic S for lithium sulfur bat-teries. Energy Storage Mater. 2020, 26, 570–576. [Google Scholar] [CrossRef]
- Wang, C.; Yi, Y.; Li, H.; Wu, P.; Li, M.; Jiang, W.; Chen, Z.; Li, H.; Zhu, W.; Dai, S. Rapid gas-assisted exfoliation promises V2O5 nanosheets for high performance lithium-sulfur batteries. Nano Energy 2019, 67, 104253. [Google Scholar] [CrossRef]
- Sun, Z.; Xiao, M.; Wang, S.; Han, D.; Song, S.; Chen, G.; Meng, Y. Sulfur-rich polymeric materials with semi-interpenetrating network structure as a novel lithium–sulfur cathode. J. Mater. Chem. A 2014, 2, 9280–9286. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Ahn, H.; Kim, O.; Park, M.J. Synthesis of three-dimensionally interconnected sulfur-rich polymers for cathode materials of high-rate lithium–sulfur batteries. Nat. Commun. 2015, 6, 7278. [Google Scholar] [CrossRef] [Green Version]
- Je, S.H.; Hwang, T.H.; Talapaneni, S.N.; Buyukcakir, O.; Kim, H.J.; Yu, J.-S.; Woo, S.-G.; Jang, M.C.; Son, B.K.; Coskun, A.; et al. Rational Sulfur Cathode Design for Lithium–Sulfur Batteries: Sulfur-Embedded Benzoxazine Polymers. ACS Energy Lett. 2016, 1, 566–572. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Hwang, T.H.; Je, S.H.; Buyukcakir, O.; Choi, J.W.; Coskun, A. Elemental-Sulfur-Mediated Facile Synthesis of a Covalent Triazine Framework for High-Performance Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2016, 55, 3106–3111. [Google Scholar] [CrossRef]
- Je, S.H.; Kim, H.J.; Kim, J.; Choi, J.W.; Coskun, A. Perfluoroaryl-Elemental Sulfur SNAr Chemistry in Covalent Triazine Frameworks with High Sulfur Contents for Lithium–Sulfur Batteries. Adv. Funct. Mater. 2017, 27, 1703947. [Google Scholar] [CrossRef]
- Seh, Z.W.; Zhang, Q.; Li, W.; Zheng, G.; Yao, H.; Cui, Y. Stable cycling of lithium sulfide cathodes through strong affinity with a bifunctional binder. Chem. Sci. 2013, 4, 3673–3677. [Google Scholar] [CrossRef]
- Mohan, V.B.; Brown, R.; Jayaraman, K.; Bhattacharyya, D. Characterization of reduced graphene oxide: Effects of reduction variables on electrical conductivity. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2015, 193, 49–60. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Dong, W.; Lu, W.; Du, Z.; Chen, L. Monodispersed Sulfur Nanoparticles for Lithium–Sulfur Batteries with Theoretical Performance. Nano Lett. 2014, 15, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.-F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Dahl, J.A.; Maddux, B.; Hutchison, J.E. Toward Greener Nanosynthesis. Chem. Rev. 2007, 107, 2228–2269. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Xue, S.; Peng, S.; Jing, M.; Qian, X.; Shen, X.; Li, T.; Wang, Y. Synthesis of graphitic carbon nitride at different ther-mal-pyrolysis temperature of urea and its application in lithium-sulfur batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 17921–17930. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, H.; Yu, Y.; Chen, Y.; Yan, J.; Li, X.; Zhang, H. Trithiocyanuric acid-derived g–C3N4 for anchoring the polysulfide in Li–S batteries application. J. Energy Chem. 2020, 43, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Liang, Q.; Han, J.; Tao, Y.; Liu, D.; Zhang, C.; Lv, W.; Yang, Q.-H. Catalyzing polysulfide conversion by g-C3N4 in a graphene network for long-life lithium-sulfur batteries. Nano Res. 2018, 11, 3480–3489. [Google Scholar] [CrossRef]
- Li, C.; Gao, K.; Zhang, Z. Graphitic carbon nitride as polysulfide anchor and barrier for improved lithium–sulfur batteries. Nanotechnology 2018, 29, 465401. [Google Scholar] [CrossRef]
- Toby, B.; von Dreele, R. GSAS-II: The genesis of a modern open-source all-purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Wang, Z.; Dawson, G.; Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wu, G.; Chen, W. Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production. Nanoscale 2012, 4, 5300–5303. [Google Scholar] [CrossRef] [PubMed]
- Shcherban, N.D.; Mäki-Arvela, P.; Aho, A.; Sergiienko, S.A.; Yaremov, P.S.; Eränen, K.; Murzin, D.Y. Melamine-derived graphitic carbon nitride as a new effective metal-free catalyst for Knoevenagel condensation of benzaldehyde with ethylcyanoacetate. Catal. Sci. Technol. 2018, 8, 2928–2937. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Shen, L.; Ma, Y.; Lei, W.; Cui, Q.; Zou, G. Preparation and characterization of graphitic carbon nitride through pyrolysis of melamine. Appl. Phys. A 2008, 94, 387–392. [Google Scholar] [CrossRef]
- Dunya, H.; Yue, Z.; Ashuri, M.; Mei, X.; Lin, Y.; Kucuk, K.; Aryal, S.; Segre, C.U.; Mandal, B.K. A new graphitic carbon nitride-coated dual Core–Shell sulfur cathode for highly stable lithium–sulfur cells. Mater. Chem. Phys. 2020, 246, 122842. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Azizi, O.; El-Boher, A.; Gahl, J.; Bok, S. Facile Synthesis and Characterization of Reduced Graphene Oxide Produced with Green and Conventional Reductants. ECS J. Solid State Sci. Technol. 2018, 7, M173–M179. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 73. [Google Scholar] [CrossRef] [Green Version]
- Shruthi, G.; Baishali, G.; Radhakrishna, V.; Verma, P. Reducing graphene oxide using hydroiodic acid fumes and low temperature annealing for enhanced electrical conductivity. Graphene Technol. 2020, 5, 19–25. [Google Scholar] [CrossRef]
- Yin, F.; Ren, J.; Zhang, Y.; Tan, T.; Chen, Z. A PPy/ZnO functional interlayer to enhance electrochemical performance of lithium/sulfur batteries. Nanoscale Res. Lett. 2018, 13, 307. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, G.; Li, J.; Zhang, Y.; Wook, A.; Yu, A.; Chen, Z. Structural and chemical synergistic encapsulation of polysulfides enables ultralong-life lithium–sulfur batteries. Energy Environ. Sci. 2016, 9, 2533–2538. [Google Scholar] [CrossRef]
- Medenbach, L.; Escher, I.; Köwitsch, N.; Armbrüster, M.; Zedler, L.; Dietzek, B.; Adelhelm, P. Sulfur Spillover on Carbon Materials and Possible Impacts on Metal-Sulfur Batteries. Angew. Chem. Int. Ed. 2018, 57, 13666–13670. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, J.; Bai, A.; Li, P.; Li, J.; Zhang, X.; Yu, M.; Wang, J.; Sun, H. Preparation of a Nitrogen-Doped Reduced Graphene Oxide-Modified Graphite Felt Electrode for VO2+/VO2+ Re-action by Freeze-Drying and Pyrolysis Method. J. Chem. 2019, 2019, 8958946. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, Y.; Zhang, F.; Liu, C.; Shaw, L.L. PVP-assisted synthesis of uniform carbon-coated Li2S/C.B. for high-performance lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2015, 7, 25748–25756. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Z.; Zhang, H.; Tan, H.; Yu, J.; Wu, J. Mesoporous β-MnO2/sulfur composite as cathode material for Li–S batteries. Electrochim. Acta 2013, 106, 307–311. [Google Scholar] [CrossRef]


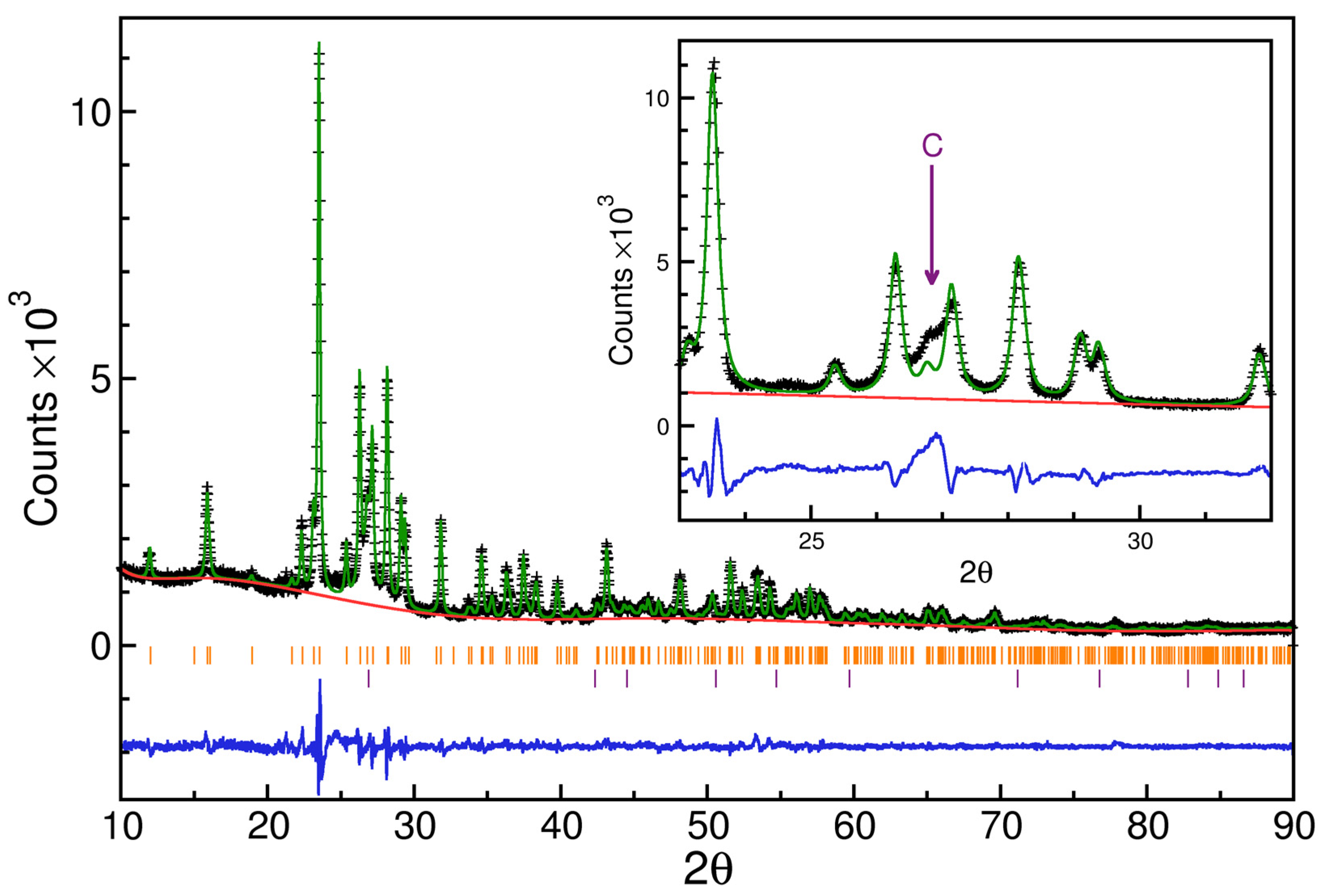
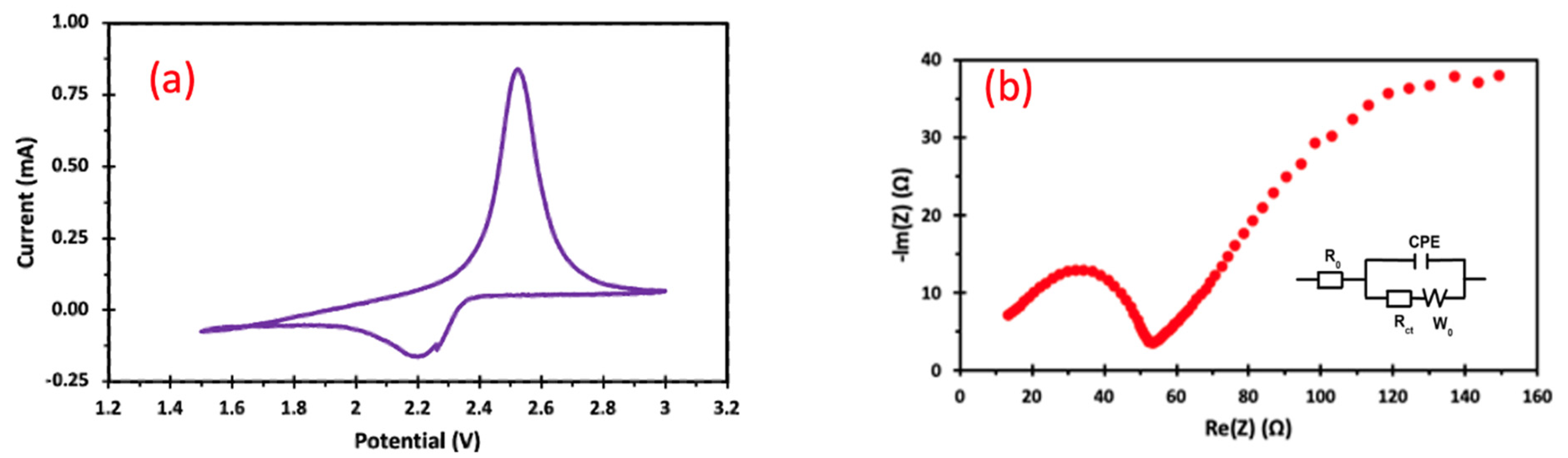
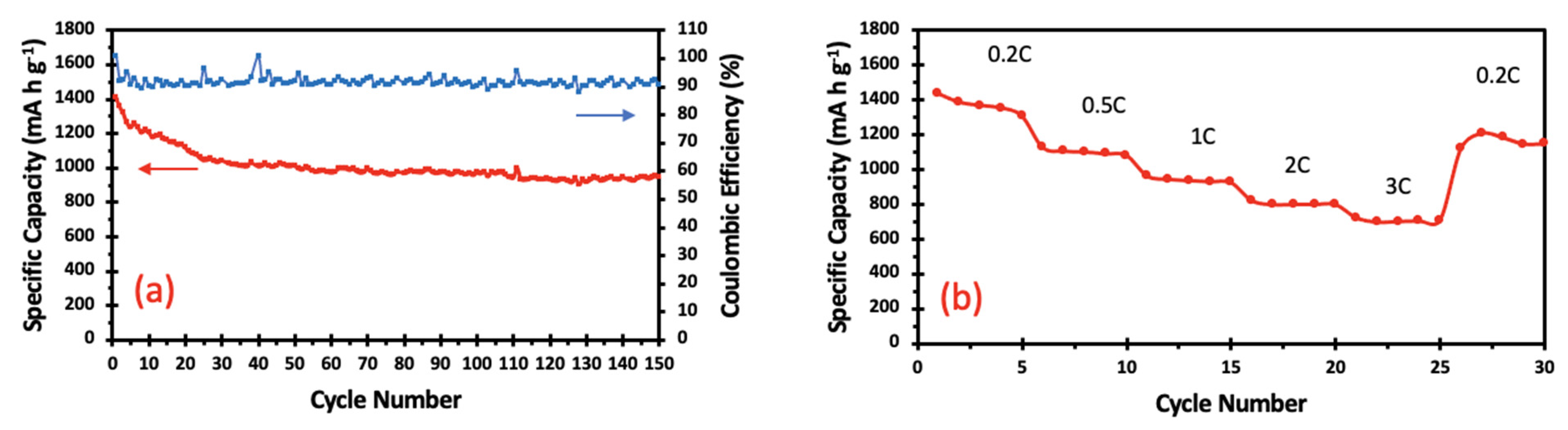
| Sample | Atomic Number | Element | Atomic Concentration +/− 1% | Weight Concentration +/− 1% |
|---|---|---|---|---|
| Void@RGO/GN | 6 | C | 57 | 52 |
| 7 | N | 25 | 26 | |
| 8 | O | 18 | 22 | |
| S@RGO/GN | 16 | S | 46 | 65 |
| 8 | O | 28 | 20 | |
| 6 | C | 22 | 12 | |
| 7 | N | 4 | 2 |
| Atom | x | y | z | B |
|---|---|---|---|---|
| S1 | 0.8572(5) | 0.9536(3) | −0.0499(2) | 0.06 |
| S2 | 0.7852(5) | 0.0318(4) | 0.0751(2) | 0.06 |
| S3 | 0.7090(6) | 0.9821(3) | 0.0050(2) | 0.06 |
| S4 | 0.7848(4) | 0.9072(4) | 0.1298(2) | 0.06 |
| Structure | Space group | Weight fraction % | R-factor % | |
| Alpha-sulfur (8) | Fddd | 71 | 3.6 | |
| Graphite | P63/mm | 29 | 2.4 | |
| Refinement weighted R-factor | 6.3 | |||
| Cathode | Rate | Initial Capacity (mA h g−1) | Reversible Capacity (mA h g−1) | Cycle Number | Reference |
|---|---|---|---|---|---|
| Sulfur/CN-550 | 0.39 mA cm−2 | 1262 | 605 | 500 | [56] |
| S/g-C3N4 | 0.2 C | 1200 | 806 | 100 | [57] |
| 0.5 C | 1092 | 601 | 500 | ||
| 2 C | 875 | 580 | 300 | ||
| S/CNG-10 | 100 mA g−1 | 1075 | 505 | 600 | [58] |
| hrGO/S-GCM | 0.1 C | 1158 | 575 | 100 | [59] |
| S@HCS@g-C3N4 | 0.2 C | 1420 | 885 | 100 | [65] |
| 1 C | 953 | 719 | 500 | ||
| S@RGO/GN | 0.2 C | 1415 | 951 | 150 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzanowicz, A.M.; Lee, Y.; Lin, H.; Marques, O.J.J.; Segre, C.U.; Mandal, B.K. A New Graphitic Nitride and Reduced Graphene Oxide-Based Sulfur Cathode for High-Capacity Lithium-Sulfur Cells. Energies 2022, 15, 702. https://doi.org/10.3390/en15030702
Suzanowicz AM, Lee Y, Lin H, Marques OJJ, Segre CU, Mandal BK. A New Graphitic Nitride and Reduced Graphene Oxide-Based Sulfur Cathode for High-Capacity Lithium-Sulfur Cells. Energies. 2022; 15(3):702. https://doi.org/10.3390/en15030702
Chicago/Turabian StyleSuzanowicz, Artur M., Youngjin Lee, Hao Lin, Otavio J. J. Marques, Carlo U. Segre, and Braja K. Mandal. 2022. "A New Graphitic Nitride and Reduced Graphene Oxide-Based Sulfur Cathode for High-Capacity Lithium-Sulfur Cells" Energies 15, no. 3: 702. https://doi.org/10.3390/en15030702







