Oxidized Nickel to Prepare an Inorganic Hole Transport Layer for High-Efficiency and Stability of CH3NH3PbI3 Perovskite Solar Cells
Abstract
:1. Introduction
2. Experimental and Methods
2.1. Device Structure and Fabrication
2.2. Equipment Specification
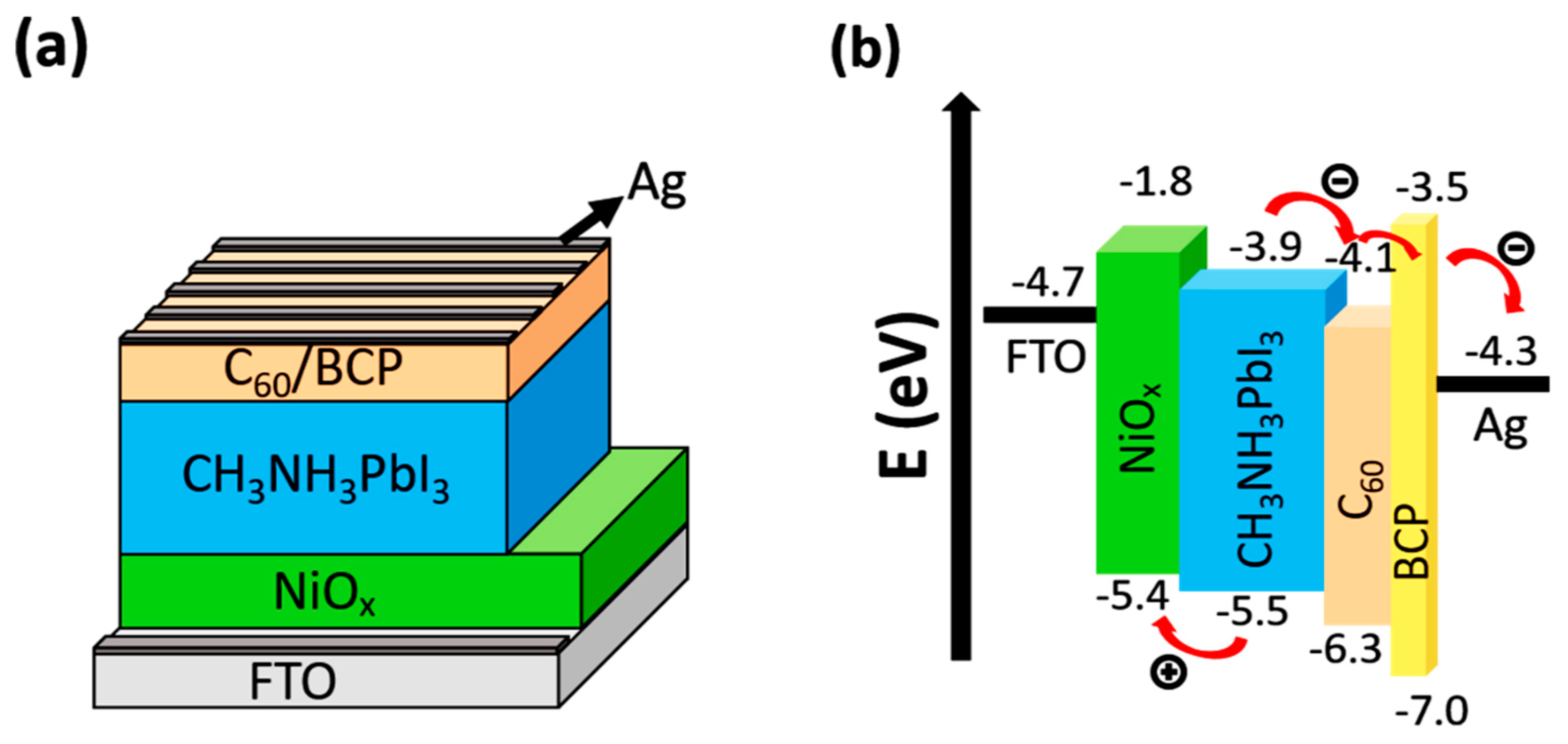
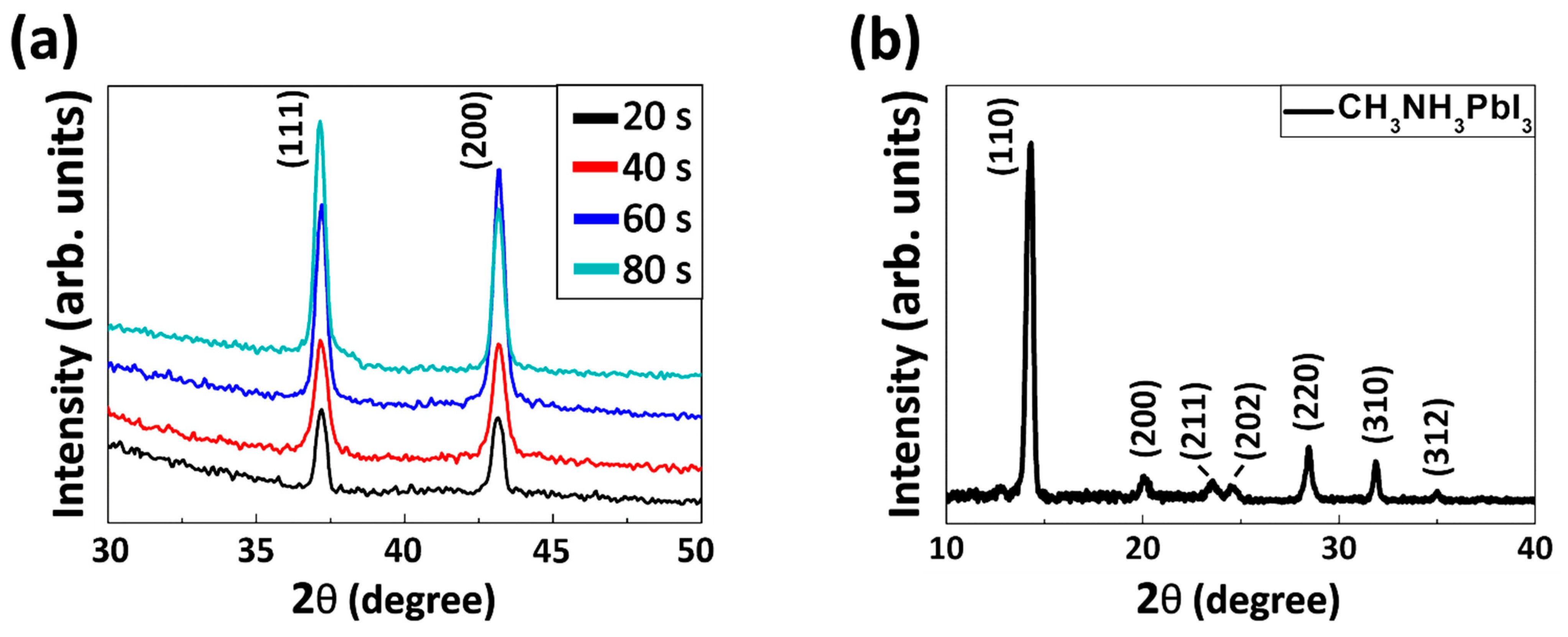
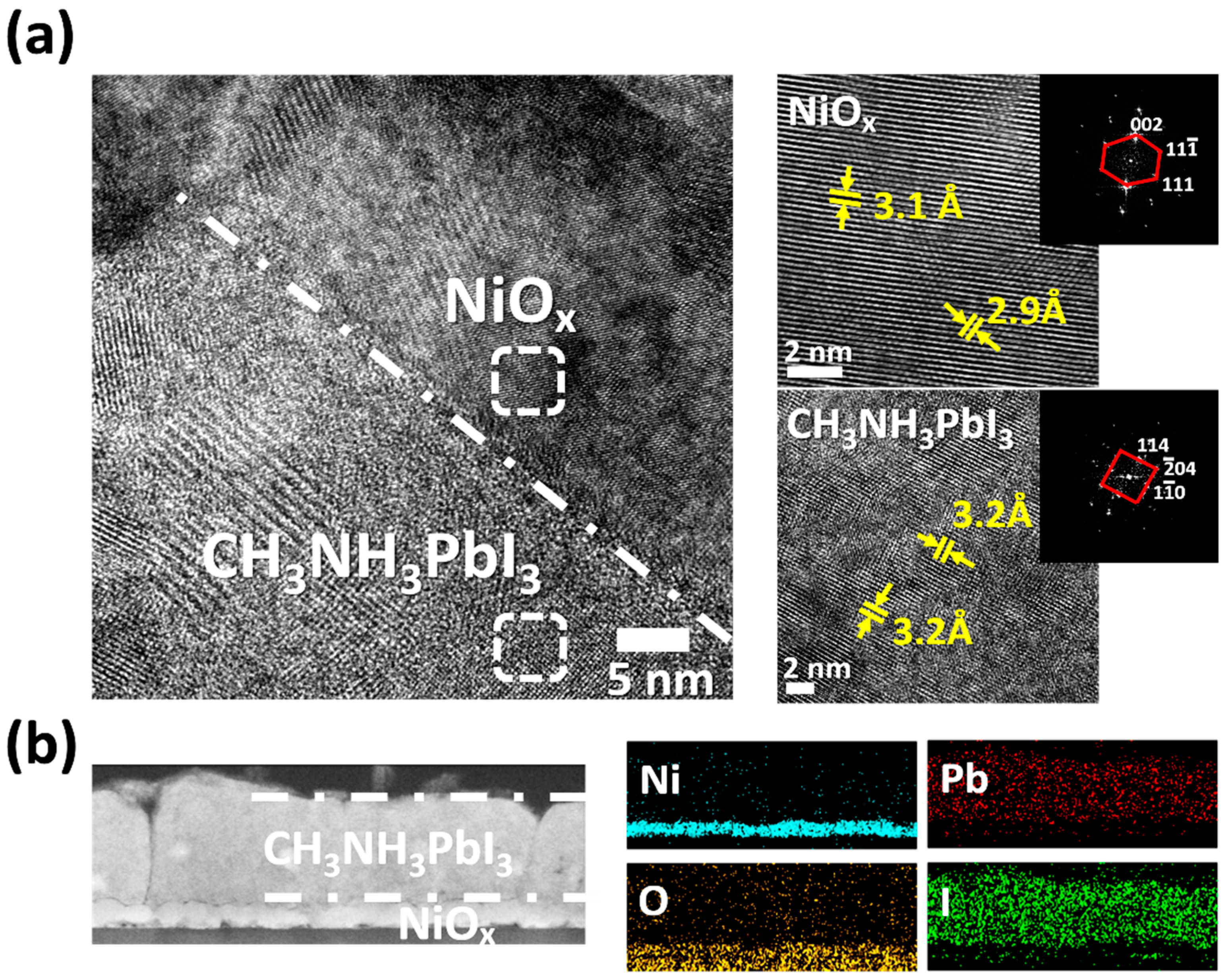
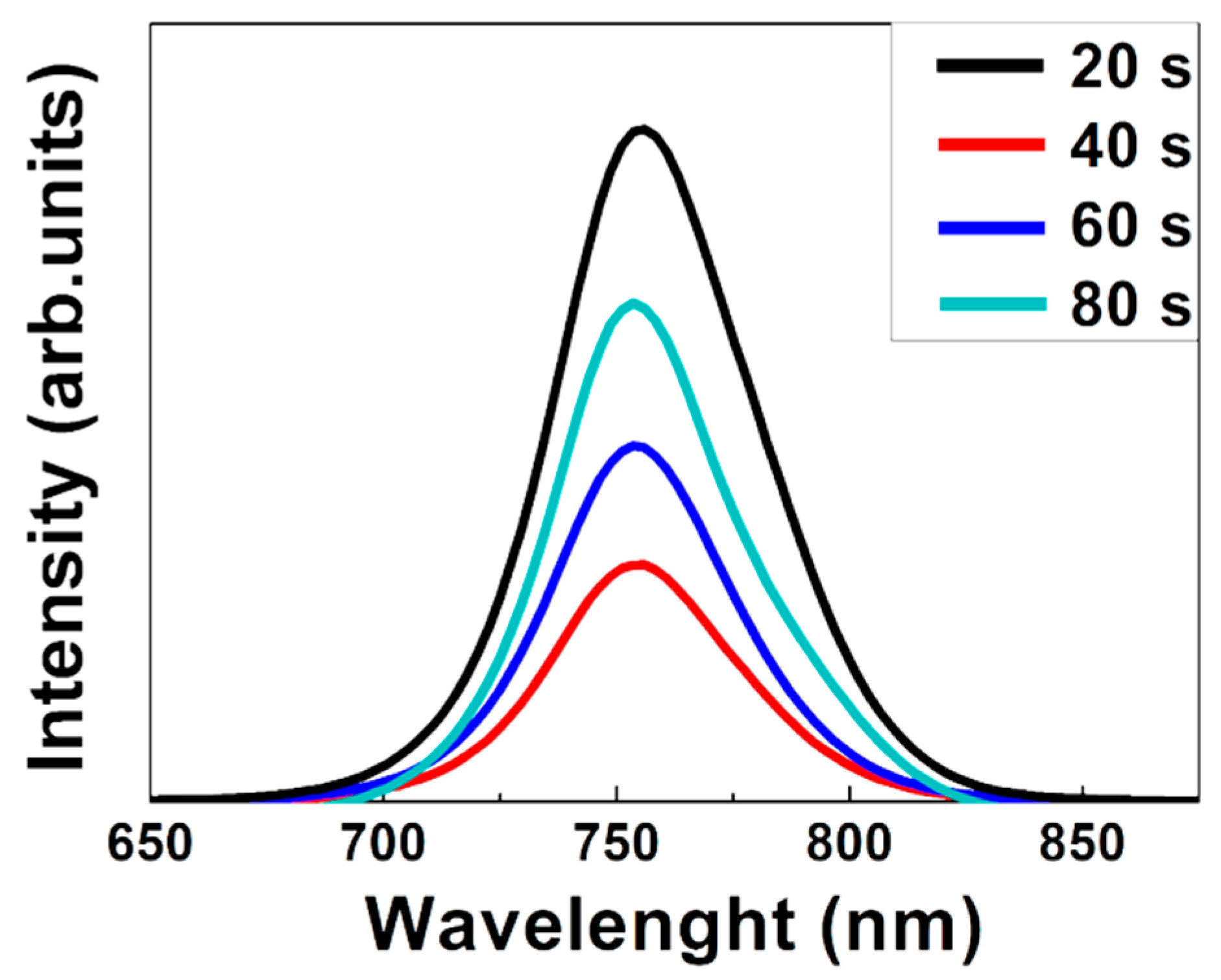
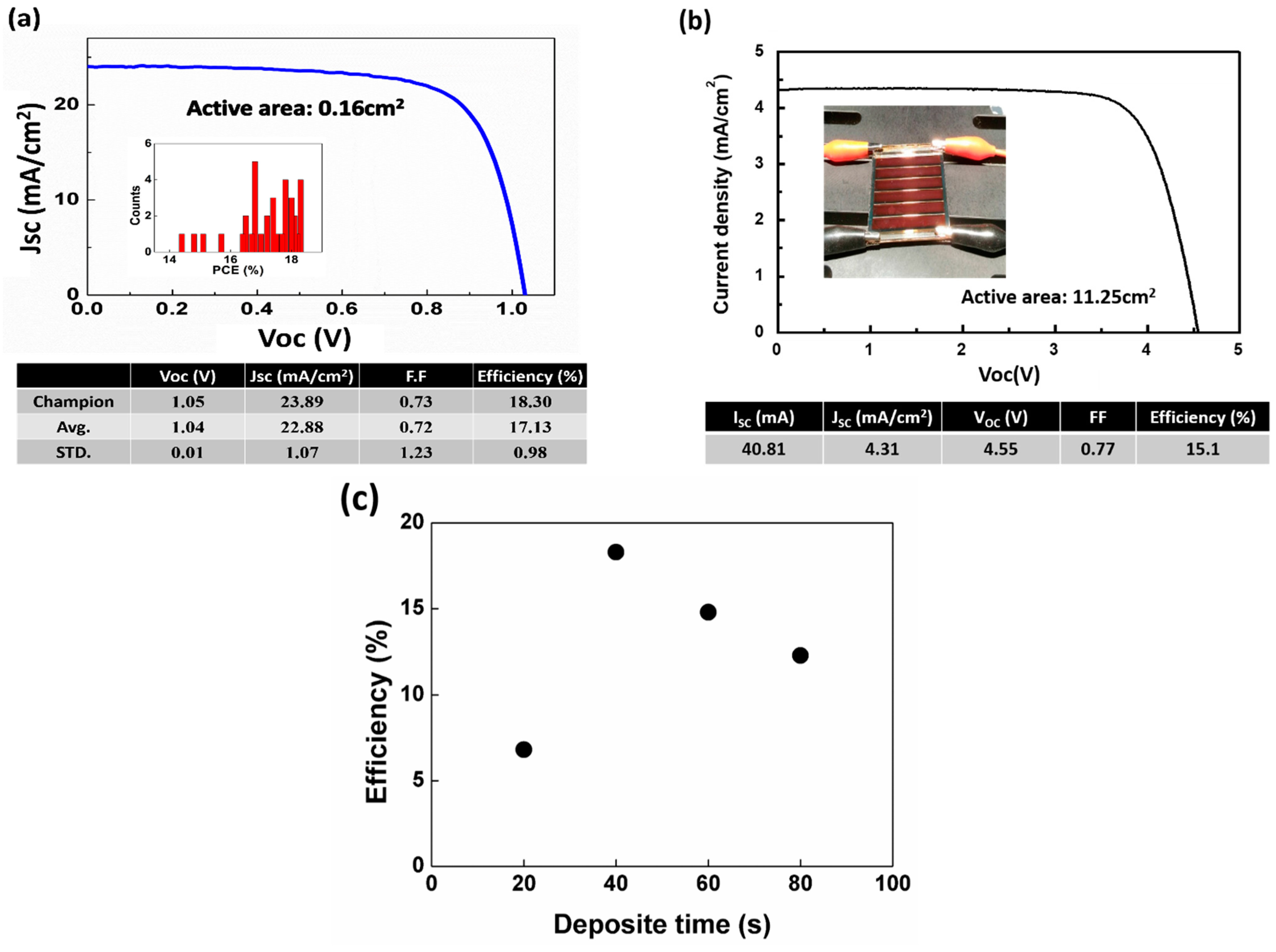
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Sadegh, F.; Akin, S.; Moghadam, M.; Keshavarzi, R.; Mirkhani, V.; Ruiz-Preciado, M.A.; Akman, E.; Zhang, H.; Amini, M.; Tangestaninejad, S.; et al. Copolymer-Templated Nickel Oxide for High-Efficiency Mesoscopic Perovskite Solar Cells in Inverted Architecture. Adv. Funct. Mater. 2021, 31, 2102237. [Google Scholar] [CrossRef]
- Akman, E.; Akin, S. Poly(N,N′-bis-4-butylphenyl-N,N′-bisphenyl)benzidine-Based Interfacial Passivation Strategy Promoting Efficiency and Operational Stability of Perovskite Solar Cells in Regular Architecture. Adv. Mater. 2021, 33, 2006087. [Google Scholar] [CrossRef] [PubMed]
- Akman, E.; Shalan, A.E.; Sadegh, F.; Akin, S. Moisture-Resistant FAPbI3 Perovskite Solar Cell with 22.25 % Power Conversion Efficiency through Pentafluorobenzyl Phosphonic Acid Passivation. ChemSusChem 2021, 14, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, T.; Akman, E.; Shalan, A.E.; Akin, S. Composition engineering of operationally stable CsPbI2Br perovskite solar cells with a record efficiency over 17%. Nano Energy 2021, 87, 106157. [Google Scholar] [CrossRef]
- Shalan, A.E.; Akman, E.; Sadegh, F.; Akin, S. Efficient and Stable Perovskite Solar Cells Enabled by Dicarboxylic Acid-Supported Perovskite Crystallization. J. Phys. Chem. Lett. 2021, 12, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Park, N.G. Perovskite solar cells: From materials to devices. Small 2015, 11, 10–25. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Sci. Adv. 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, H.; Zhu, Z.; Jiang, K.; Zhang, T.; Zhao, N.; Yang, S.; Yan, H. High performance inverted structure perovskite solar cells based on a PCBM:polystyrene blend electron transport layer. J. Mater. Chem. A 2015, 3, 9098–9102. [Google Scholar] [CrossRef]
- Liang, P.W.; Chueh, C.C.; Williams, S.T.; Jen, A.Y. Roles of Fullerene-Based Interlayers in Enhancing the Performance of Organometal Perovskite Thin-Film Solar Cells. Adv. Energy Mater. 2015, 5, 1402321. [Google Scholar] [CrossRef]
- Yin, X.; Yao, X.; Luo, Q.; Dai, X.; Zhou, Y.; Zheng, Y.; Zhou, Y.; Luo, S.; Li, J.; Wang, N.; et al. High Efficiency Inverted Planar Perovskite Solar Cells with Solution-Processed NiOx Hole Contact. ACS Appl. Mater. Interfaces 2017, 9, 2439–2448. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, K.F.; Chiu, K.Y.; Tsai, C.L.; Cheng, H.M.; Yeh, S.C.; Wu, W.T.; Chen, W.N.; Chen, C.T.; Chen, S.H.; et al. Improving the efficiency of CH3NH3PbI3 based photovoltaics by tuning the work function of the PEDOT:PSS hole transport layer. Sol. Energy 2015, 122, 892–899. [Google Scholar] [CrossRef]
- Kim, S.K.; Seok, H.J.; Choi, D.H.; Nam, S.J.; Kim, S.C.; Kim, H.K. Comparison of NiOx thin film deposited by spin-coating or thermal ecaporation for application as a hole transport layer of perovskite solar cells. RSC Adv. 2020, 10, 43847–43852. [Google Scholar] [CrossRef]
- D’Amario, L.; Fohlinger, J.; Boschloo, G.; Hammarstrom, L. Unveiling hole trapping and surface dynamics of NiO nanoparticles. Chem. Sci. 2018, 9, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.I.; Shahiduzzaman, M.; Ahmed, S.; Huqe, M.R.; Qarony, W.; Saleque, A.M.; Akhtaruzzaman, M.; Knipp, D.; Tsang, T.H.; Taima, T.; et al. Near field control for enhanced photovoltaic performance and photostability in perovskite solar cells. Nano Energy 2021, 89, 106388. [Google Scholar] [CrossRef]
- Hossain, M.I.; Shahiduzzaman, M.; Saleque, A.M.; Huqe, M.R.; Qarony, W.; Ahmed, S.; Akhtaruzzaman, M.; Knipp, D.; Tsang, Y.H.; Taima, T.; et al. Improved Nanophotonic Front Contact Design for High Performance Perovskite Single-Junction and Perovskite/Perovskite Tandem Solar Cells. Sol. RRL 2021, 5, 2100509. [Google Scholar] [CrossRef]
- Hossain, M.I.; Saleque, A.M.; Ahmed, S.; Saidjafarzoda, I.; Shahiduzzaman, M.; Qarony, W.; Knipp, D.; Biyikli, N.; Tsang, Y.H. Perovskite/perovskite planer tandem solar cells: A comprehensive guideline for reaching energy conversion efficiency beyond 30%. Nano Energy 2021, 79, 105400. [Google Scholar] [CrossRef]
- Muslih, W.Y.; Shahiduzzaman, M.; Akhtaruzzaman, M.; Hossain, M.I.; Wang, L.; Alkhammash, H.I.; Alharthi, S.S.; Nakano, M.; Karakawa, M.; Aminuzzaman, M. Reproducible perovskite solar cells using a simple solvent mediated sol−gel synthesized NiOx hole transport layer. Appl. Phys. 2022, 15, 015504. Appl. Phys. 2022, 15, 015504. [Google Scholar] [CrossRef]
- Hossain, M.I.; Hasan, A.K.M.; Qarony, W.; Shahiduzzaman, M.; Islam, M.A.; Ishikawa, Y.; Uraoka, Y.; Amin, N.; Knipp, D.; Akhtaruzzaman, A.; et al. Electrical and Optical Properties of Nickel- Oxide Films for Efficient Perovskite Solar Cells. Small Methods 2020, 4, 2000454. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.Z.; Feng, X.Y.; Djurišic´, A.; Chan, W.K.; He, Z.B. Cesium Doped NiOx as an Efficient Hole Extraction Layer for Inverted Planar Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1700722. [Google Scholar] [CrossRef]
- Yan, W.; Ye, S.; Li, Y.; Sun, W.; Rao, H.; Liu, Z.; Bian, Z.; Huang, C. Hole-Transporting Materials in Inverted Planar Perovskite Solar Cells. Adv. Energy Mater. 2016, 6, 1600474. [Google Scholar] [CrossRef]
- Li, M.H.; Shen, P.S.; Wang, K.C.; Guo, T.F.; Chen, P. Inorganic p-type contact materials for perovskite-based solar cells. J. Mater. Chem. A 2015, 3, 9011–9019. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Fan, J.; Djurišic´, A.; Liu, F.; Tam, H.W.; Ng, A.; Surya, C.; Chan, W.K.; Wang, D.; et al. Understanding the Doping Effect on NiO: Toward High-Performance Inverted Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1703519. [Google Scholar] [CrossRef]
- Jeng, J.Y.; Chen, K.C.; Chiang, T.Y.; Lin, P.Y.; Tsai, T.D.; Chang, Y.C.; Guo, T.F.; Chen, P.; Wen, T.C.; Hsu, Y.J. Nickel Oxide Electrode Interlayer in CH3NH3PbI3 Perovskite/PCBM Planar-Heterojunction Hybrid Solar Cells. Adv. Mater. 2014, 26, 4107–4113. [Google Scholar] [CrossRef] [PubMed]
- Corani, A.; Li, M.H.; Shen, P.S.; Chen, P.; Guo, T.F.; Nahhas, A.E.; Zheng, K.; Yartsev, A.; Sundström, V.; Ponseca, C.S., Jr. Ultrafast Dynamics of Hole Injection and Recombination in Organometal Halide Perovskite Using Nickel Oxide as p-Type Contact Electrode. J. Phys. Chem. Lett. 2016, 7, 1096–1101. [Google Scholar] [CrossRef]
- Zhang, B.; Su, J.; Guo, X.; Zhou, L.; Lin, Z.; Feng, L.; Zhang, J.; Chang, J.; Hao, Y. NiO/Perovskite Heterojunction Contact Engineering for Highly Efficient and Stable Perovskite Solar Cells. Adv. Sci. 2020, 7, 1903044. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Meng, L.; Song, T.B.; Guo, T.F.; Yang, Y.; Chang, W.H.; Hong, Z.; Chen, H.; Zhou, H.; Chen, Q.; et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotechnol. 2016, 11, 75–81. [Google Scholar] [CrossRef]
- Yin, X.; Han, J.; Zhou, Y.; Gu, Y.; Tai, M.; Nan, H.; Zhou, Y.; Li, J.; Lin, H. Critical roles of potassium in charge-carrier balance and diffusion induced defect passivation for efficient inverted perovskite solar cells. J. Mater. Chem. A 2019, 7, 5666–5676. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Ok, S.; Choe, Y. Effects of pentacene-doped PEDOT: PSS as a hole-conducting layer on the performance characteristics of polymer photovoltaic cells. Nanoscale Res. Lett. 2012, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Ukoba, K.O.; Eloka-Eboka, A.E.; Inambao, F.L. Review of nanostructured NiO thin film deposition using the spray pyrolysis technique. Renew. Sustain. Energy Rev. 2018, 82, 2900–2915. [Google Scholar] [CrossRef]
- Qiu, Z.; Gong, H.; Zheng, G.; Yuan, S.; Zhang, H.; Zhu, X.; Zhou, H.; Cao, B. Enhanced physical properties of pulsed laser deposited NiO films via annealing and lithium doping for improving perovskite solar cell efficiency. J. Mater. Chem. C 2017, 5, 7084–7094. [Google Scholar] [CrossRef]
- Liu, Z.; Chang, J.; Lin, Z.; Zhou, L.; Yang, Z.; Chen, D.; Zhang, C.; Liu, S.; Hao, Y. High-Performance Planar Perovskite Solar Cells Using Low Temperature, Solution–Combustion-Based Nickel Oxide Hole Transporting Layer with Efficiency Exceeding 20%. Adv. Energy Mater. 2018, 8, 1703432. [Google Scholar] [CrossRef]
- Zhong, Y.; Suzuki, K.; Inoue, D.; Hashizume, D.; Izawa, S.; Hashimoto, K.; Koganezawa, T.; Tajima, K. Interface-induced crystallization and nanostructure formation of [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) in polymer blend films and its application in photovoltaics. J. Mater. Chem. A 2016, 4, 3335–3341. [Google Scholar] [CrossRef]
- Hsu, C.C.; Yu, S.M.; Lee, K.M.; Lin, C.J.; Cheng, H.C.; Chen, F.R. Solid-state reaction process for high-quality organometallic halide perovskite thin film. Sol. Energy Mater Sol. Cells 2021, 227, 111014. [Google Scholar] [CrossRef]
- Qarony, W.; Hossain, M.I.; Jovanov, V.; Salleo, A.; Knipp, D. Influence of Perovskite Interface Morphology on the Photon Management in Perovskite/Silicon Tandem Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 15080–15086. [Google Scholar] [CrossRef]
- Hossain, M.I.; Hongsingthong, A.; Qarony, W.; Sichanugrist, P.; Konagai, M.; Salleo, A.; Knipp, D.; Tsang, Y.H. Optics of Perovskite Solar Cell Front Contacts. ACS Appl. Mater. Interfaces 2019, 11, 14693–14701. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.I.; Khan, H.A.; Kozawa, M.; Qarony, W.; Salleo, A.; Hardeberg, J.Y.; Fujiwara, H.; Tsang, Y.H.; Knipp, D. Perovskite Color Detectors: Approaching the Efficiency Limit. ACS Appl. Mater. Interfaces 2020, 12, 47831–47839. [Google Scholar] [CrossRef]
- Wheeler, S.; Deledalle, F.; Tokmoldin, N.; Kirchartz, T.; Nelson, J.; Durrant, J.R. Influence of Surface Recombination on Charge-Carrier Kinetics in Organic Bulk Heterojunction Solar Cells with Nickel Oxide Interlayers. Phys. Rev. Appl. 2015, 4, 024020. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Guo, Y.; Yuan, S.; Zhao, J.S.; Liang, X.M.; Qin, Y.; Zhang, J.P.; Ai, X.C. Modification of NiOx hole transport layer for acceleration of charge extraction in inverted perovskite solar cells. RSC Adv. 2020, 10, 12289–12296. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-C.; Yu, S.-M.; Lee, K.-M.; Lin, C.-J.; Liou, B.-Y.; Chen, F.-R. Oxidized Nickel to Prepare an Inorganic Hole Transport Layer for High-Efficiency and Stability of CH3NH3PbI3 Perovskite Solar Cells. Energies 2022, 15, 919. https://doi.org/10.3390/en15030919
Hsu C-C, Yu S-M, Lee K-M, Lin C-J, Liou B-Y, Chen F-R. Oxidized Nickel to Prepare an Inorganic Hole Transport Layer for High-Efficiency and Stability of CH3NH3PbI3 Perovskite Solar Cells. Energies. 2022; 15(3):919. https://doi.org/10.3390/en15030919
Chicago/Turabian StyleHsu, Chien-Chung, Sheng-Min Yu, Kun-Mu Lee, Chuan-Jung Lin, Bo-Yi Liou, and Fu-Rong Chen. 2022. "Oxidized Nickel to Prepare an Inorganic Hole Transport Layer for High-Efficiency and Stability of CH3NH3PbI3 Perovskite Solar Cells" Energies 15, no. 3: 919. https://doi.org/10.3390/en15030919
APA StyleHsu, C.-C., Yu, S.-M., Lee, K.-M., Lin, C.-J., Liou, B.-Y., & Chen, F.-R. (2022). Oxidized Nickel to Prepare an Inorganic Hole Transport Layer for High-Efficiency and Stability of CH3NH3PbI3 Perovskite Solar Cells. Energies, 15(3), 919. https://doi.org/10.3390/en15030919




