Effect of MXene Loaded on g-C3N4 Photocatalyst for the Photocatalytic Degradation of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
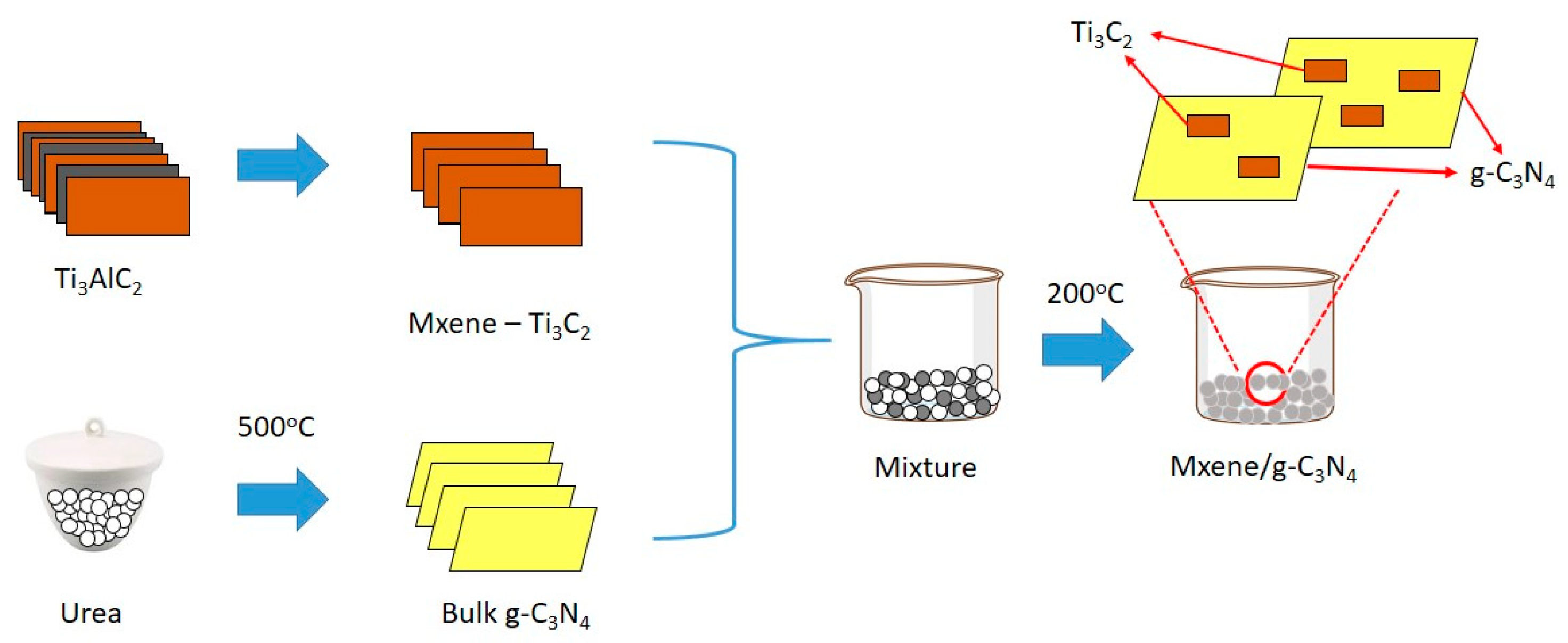
2.2. Photocatalyst Preparation
2.3. Physicochemical Characterization
2.4. Photocatalytic Degradation Testing
3. Results
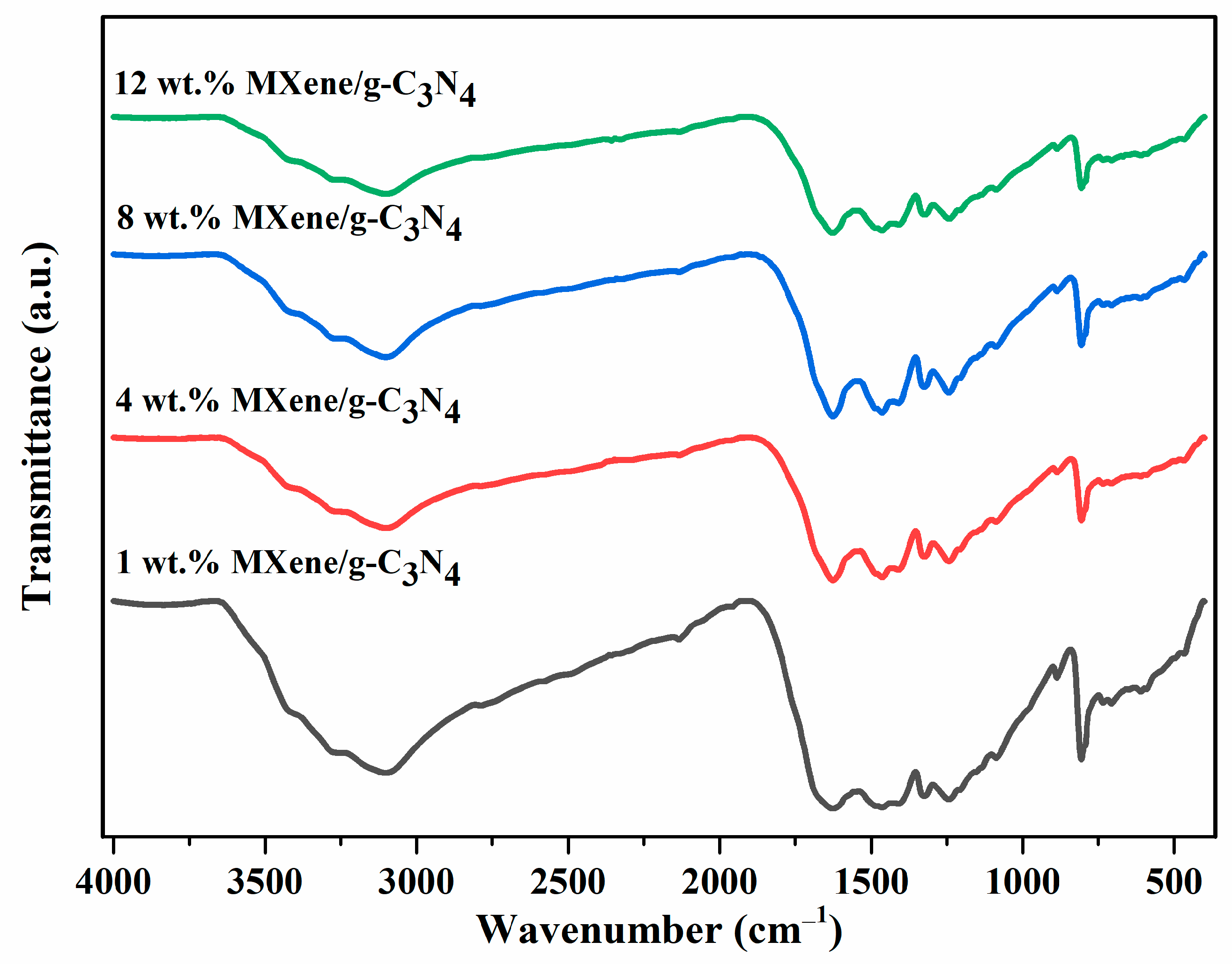
3.1. FTIR Analysis
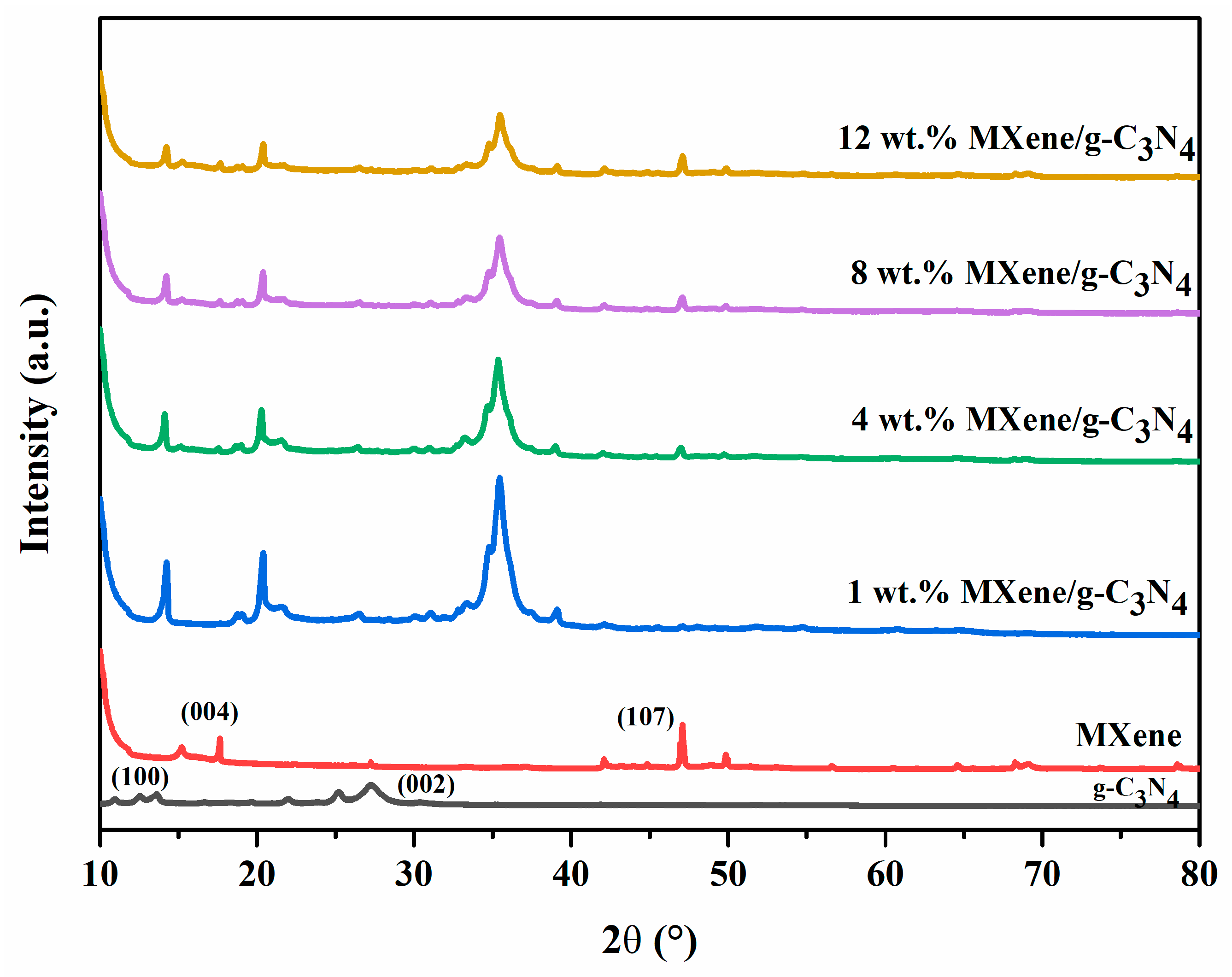
3.2. XRD Analysis
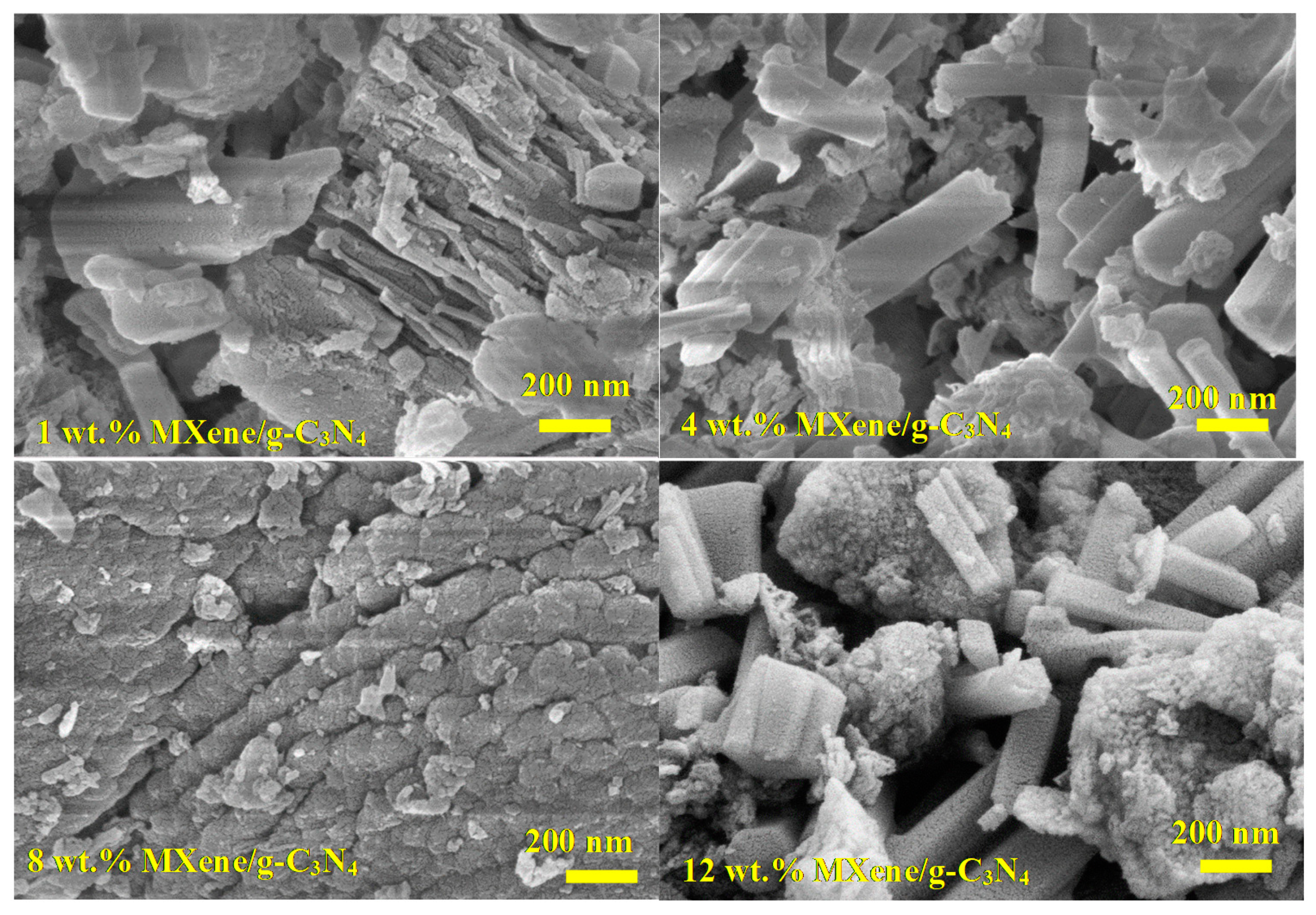
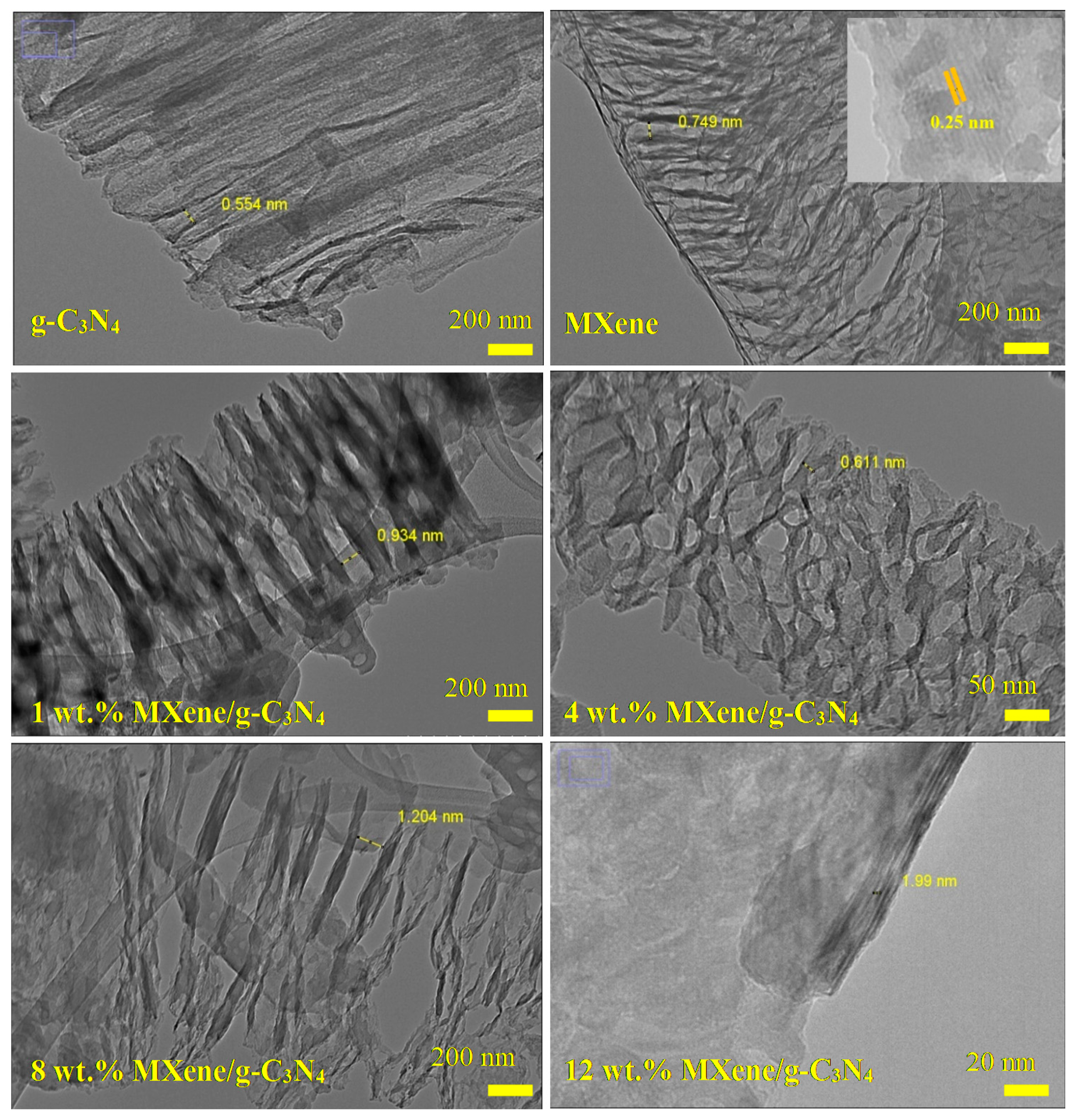
3.3. Morphological Analysis
3.4. BET Surface Area and Porosity Analysis
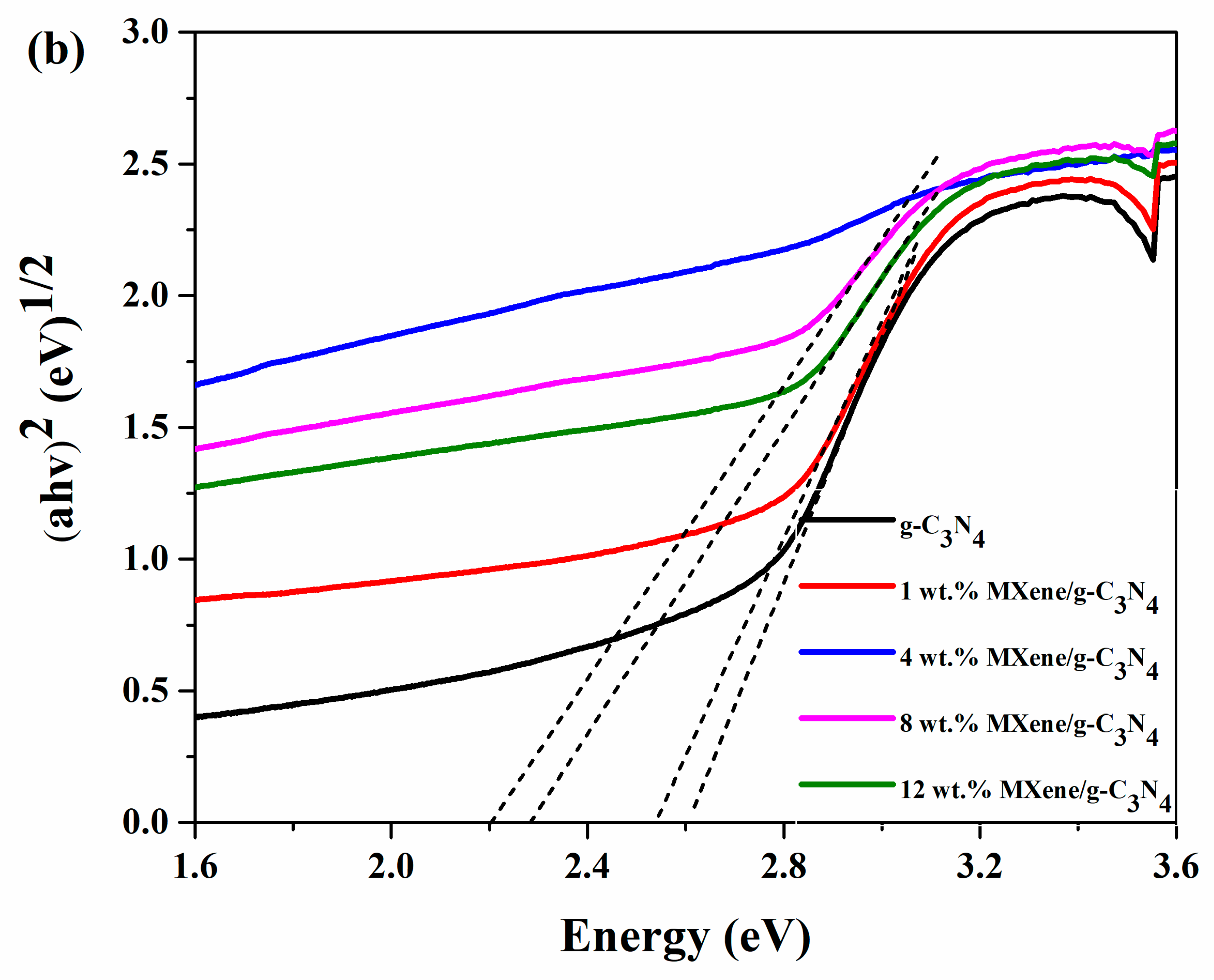
3.5. Optical Properties
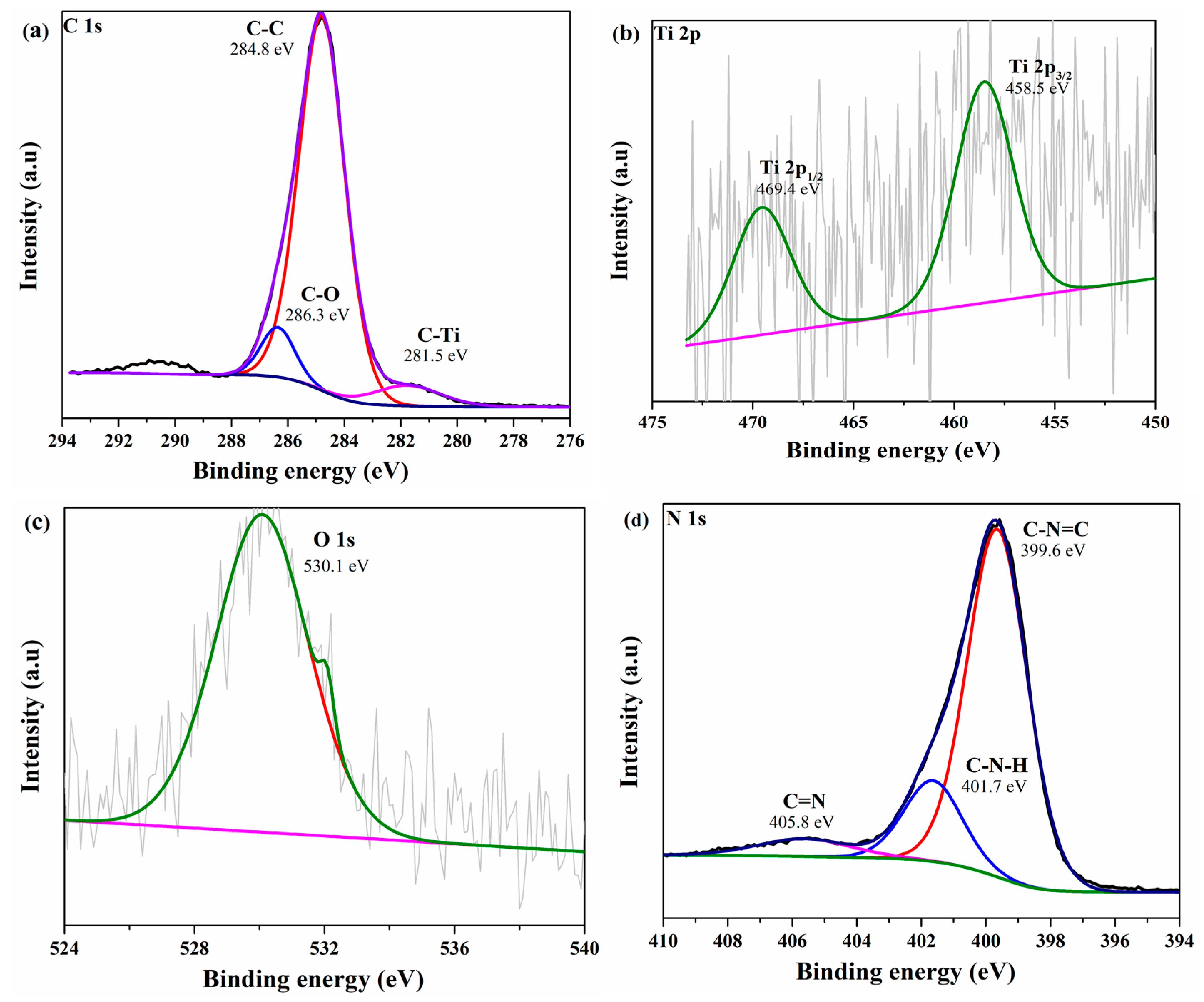
3.6. XPS Analysis
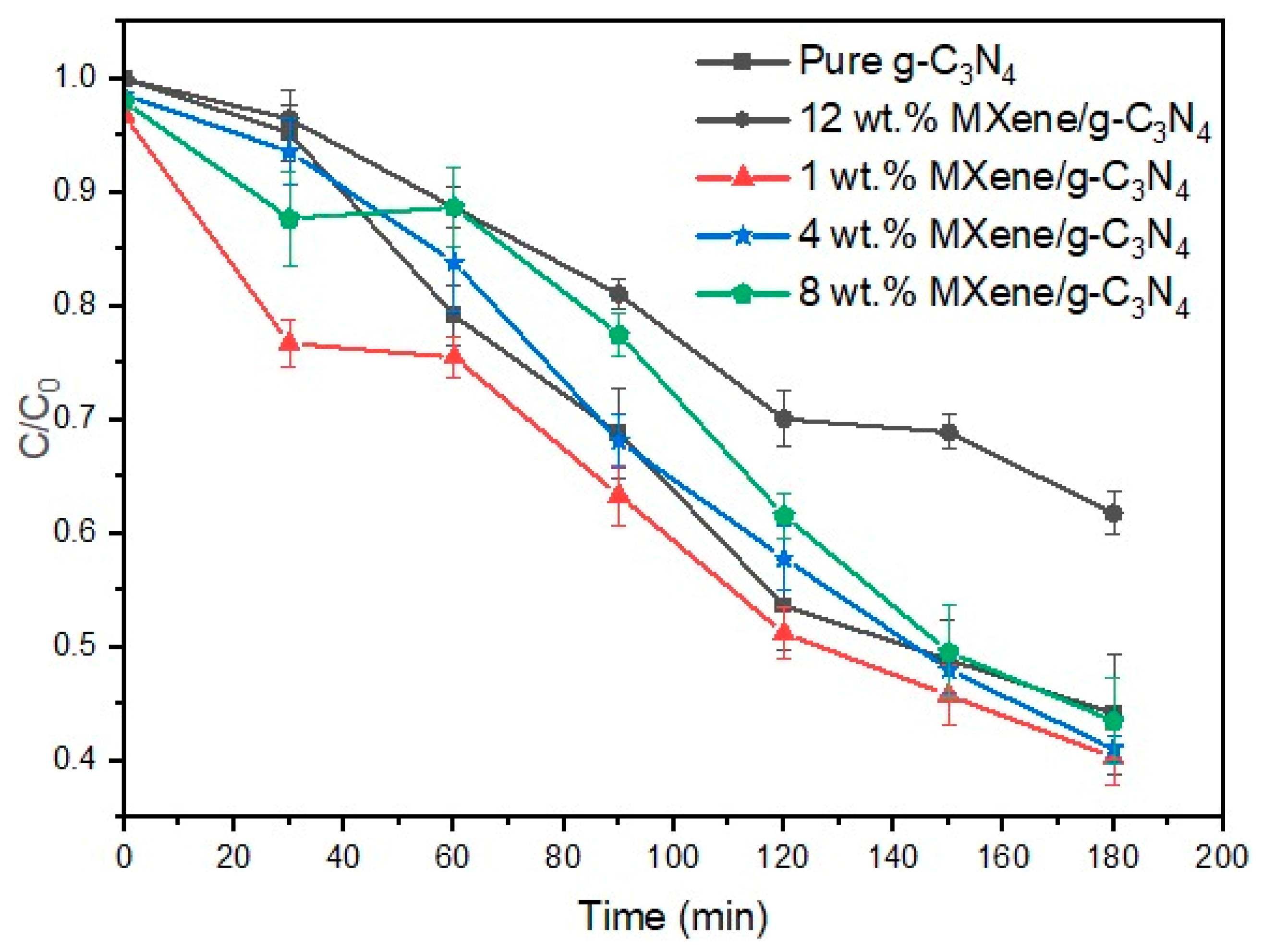
3.7. Photocatalytic Degradation Performance Testing
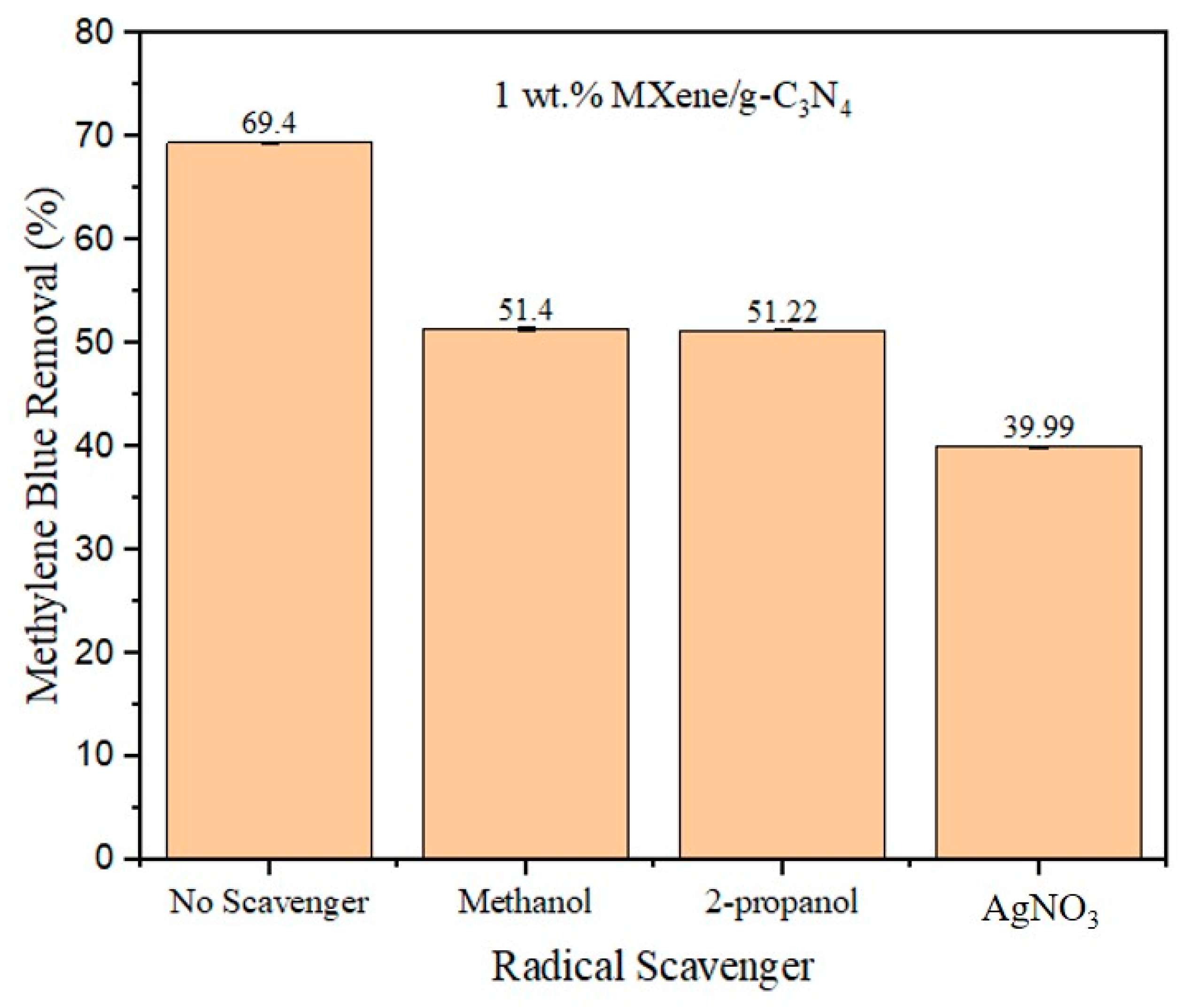
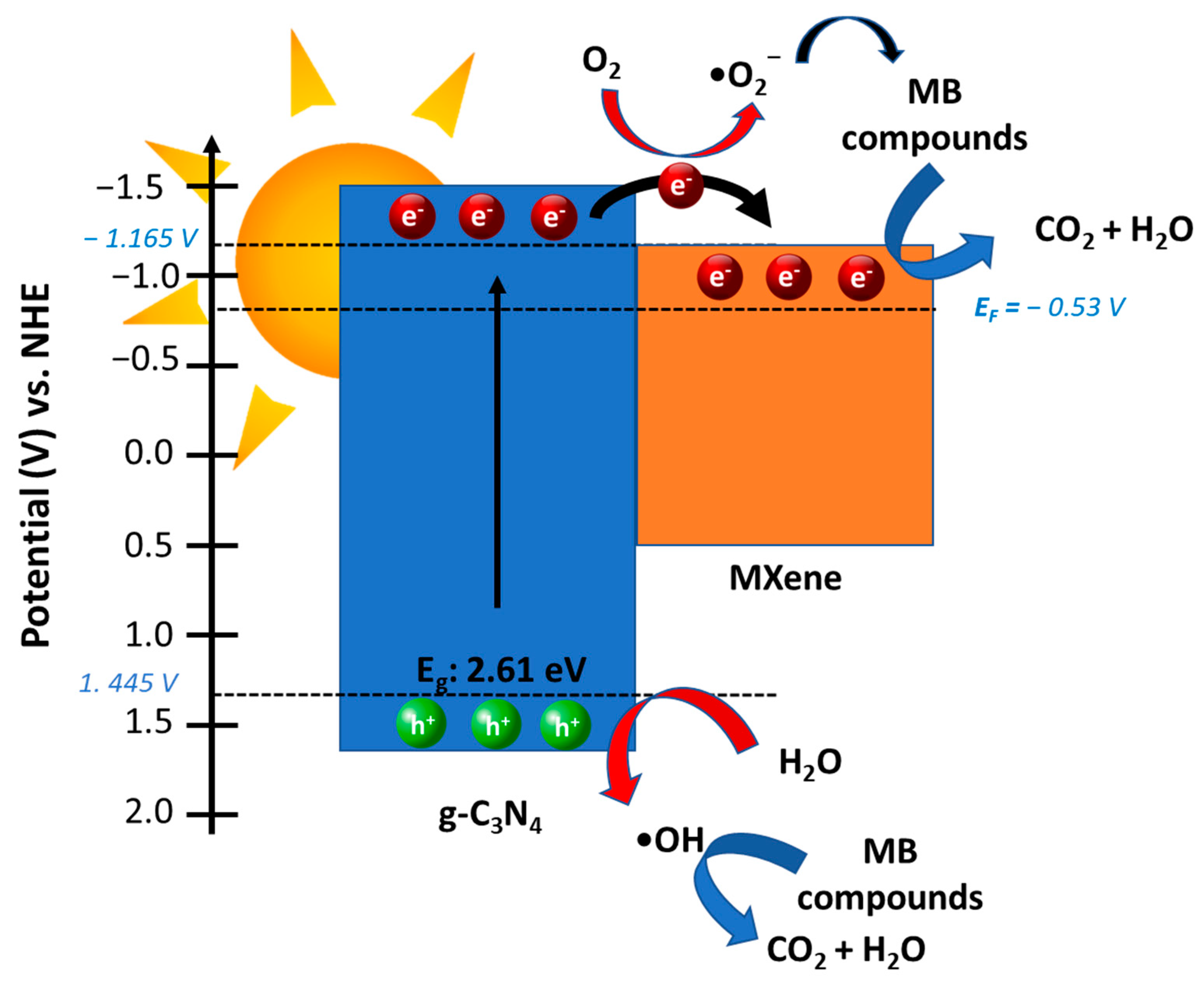
3.8. Possible Route for Photodegradation of Methylene Blue over MXene/g-C3N4 Heterostructure Photocatalyst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desore, A.; Narula, S.A. An overview on corporate response towards sustainability issues in textile industry. Environ. Dev. Sustain. 2018, 20, 1439–1459. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Mengting, Z.; Fu, D.; Yeap, S.K.; Othman, M.H.D.; Avtar, R.; Ouyang, T. Functionalizing TiO2 with graphene oxide for enhancing photocatalytic degradation of methylene blue (MB) in contaminated wastewater. J. Environ. Manag. 2020, 270, 110871. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Wanag, A.; Kapica-Kozar, J.; Kowalczyk, Ł.; Zgrzebnicki, M.; Tryba, B.; Przepiórski, J.; Morawski, A.W. Methylene blue decomposition on TiO2/reduced graphene oxide hybrid photocatalysts obtained by a two-step hydrothermal and calcination synthesis. Catal. Today 2020, 357, 630–637. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Frebillot, C.; Kaddoury, Y.; Sufian, S.; Ong, W.J. Bifunctional Z-Scheme Ag/AgVO3/g-C3N4 photocatalysts for expired ciprofloxacin degradation and hydrogen production from natural rainwater without using scavengers. J. Environ. Manag. 2020, 270, 110803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Xiong, W.; Wang, L. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere 2019, 214, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Matafonova, G.; Batoev, V. Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: A review. Water Res. 2018, 132, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Raizada, P.; Sudhaik, A.; Singh, P. Photocatalytic water decontamination using graphene and ZnO coupled photocatalysts: A review. Mater. Sci. Energy Technol. 2019, 2, 509–525. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Bacho, N.; Sufian, S.; Ng, Y.H. Photocatalytic degradation of phenol wastewater over Z-scheme g-C3N4/CNT/BiVO4 heterostructure photocatalyst under solar light irradiation. J. Mol. Liq. 2018, 277, 977–988. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K. State-of-the-art review of morphological advancements in graphitic carbon nitride (g-CN) for sustainable hydrogen production. Renew. Sustain. Energy Rev. 2021, 135, 110235. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, R.; Zhang, W.; Shi, S.; Zhu, W.; Liu, M.; Yang, C.; Xie, X.; Wang, J. Graphitic carbon nitride (g-C3N4)-based nanostructured materials for photodynamic inactivation: Synthesis, efficacy and mechanism. Chem. Eng. J. 2021, 404, 126528. [Google Scholar] [CrossRef]
- Huang, D.; Yan, X.; Yan, M.; Zeng, G.; Zhou, C.; Wan, J.; Cheng, M.; Xue, W. Graphitic Carbon Nitride-Based Heterojunction Photoactive Nanocomposites: Applications and Mechanism Insight. ACS Appl. Mater. Interfaces 2018, 10, 21035–21055. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, S.; Li, Y.; Fan, J.; Lv, K. MXenes as noble-metal-alternative co-catalysts in photocatalysis. Chin. J. Catal. 2020, 42, 3–14. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, R.; Huang, J.; Jiang, Y.; Zhao, C.; Yang, X. In situ construction of protonated g-C3N4/Ti3C2 MXene Schottky heterojunctions for efficient photocatalytic hydrogen production. Chin. J. Catal. 2021, 42, 107–114. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Ullah, H.; Tahir, A.A.; Li, X.; Ng, Y.H.; Sufian, S. Superior photoelectrocatalytic performance of ternary structural BiVO4/GQD/g-C3N4 heterojunction. J. Colloid Interface Sci. 2021, 586, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, D.; Sun, Y.; Meng, X.; Gao, Y.; Dall’Agnese, Y.; Chen, G.; Wang, X.F. G-C3N4/Ti3C2TX (MXenes) composite with oxidized surface groups for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 9124–9131. [Google Scholar] [CrossRef]
- Li, H.; Tian, H.; Wang, X.; Pi, M.; Wei, S.; Zhu, H.; Zhang, D.; Chen, S. Self-Coupled g-C3N4 van der Waals Heterojunctions for Enhanced Photocatalytic Hydrogen Production. ACS Appl. Energy Mater. 2019, 2, 4692–4699. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, X.; Li, Y.; Jin, Z. Performance of WO3/g-C3N4 heterojunction composite boosting with NiS for photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 499, 143862. [Google Scholar] [CrossRef]
- Jing, H.; Ou, R.; Yu, H.; Zhao, Y.; Lu, Y.; Huo, M.; Huo, H.; Wang, X. Engineering of g-C3N4 nanoparticles/WO3 hollow microspheres photocatalyst with Z-scheme heterostructure for boosting tetracycline hydrochloride degradation. Sep. Purif. Technol. 2021, 255, 117646. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Tran, N.M.; Tri, N.N.; Sreedhar, A.; Noh, J.S. Highly surface-active Si-doped TiO2/Ti3C2Tx heterostructure for gas sensing and photodegradation of toxic matters. Chem. Eng. J. 2021, 425, 131437. [Google Scholar] [CrossRef]
- Huang, K.; Li, C.; Wang, L.; Wang, W.; Meng, X. Layered Ti3C2 MXene and silver co-modified g-C3N4 with enhanced visible light-driven photocatalytic activity. Chem. Eng. J. 2021, 425, 131493. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Safaei, J.; Ismail, A.F.; Khalid, M.N.; Mohd Jailani, M.F.A.; Noh, M.F.M.; Arzaee, N.A.; Zhou, D.; Sagu, J.S.; Teridi, M.A.M. Boosting photocatalytic activities of BiVO4 by creation of g-C3N4/ZnO@BiVO4 Heterojunction. Mater. Res. Bull. 2020, 125, 110779. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Zhao, X.; Zhang, J.; Ao, Z.; Liu, W.; Wu, H.; Shi, L.; Yin, Y.; Xu, X.; et al. Efficient photocatalytic overall water splitting on metal-free 1D SWCNT/2D ultrathin C3N4 heterojunctions via novel non-resonant plasmonic effect. Appl. Catal. B Environ. 2020, 278, 119312. [Google Scholar] [CrossRef]
- Han, X.; An, L.; Hu, Y.; Li, Y.; Hou, C.; Wang, H.; Zhang, Q. Ti3C2 MXene-derived carbon-doped TiO2 coupled with g-C3N4 as the visible-light photocatalysts for photocatalytic H2 generation. Appl. Catal. B Environ. 2020, 265, 118539. [Google Scholar] [CrossRef]
- Cao, B.; Wan, S.; Wang, Y.; Guo, H.; Ou, M.; Zhong, Q. Highly-efficient visible-light-driven photocatalytic H2 evolution integrated with microplastic degradation over MXene/ZnxCd1-xS photocatalyst. J. Colloid Interface Sci. 2022, 605, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, L.; Ni, C.; He, E.; Yu, L.; Li, X. 3D/2D MOF-derived CoCeOx/g-C3N4 Z-scheme heterojunction for visible light photocatalysis: Hydrogen production and degradation of carbamazepine. J. Alloys Compd. 2022, 890, 161786. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Harish, S.; Archana, J.; Navaneethan, M. Fabrication of novel hybrid Z-Scheme WO3@g-C3N4@MWCNT nanostructure for photocatalytic degradation of tetracycline and the evaluation of antimicrobial activity. Chemosphere 2022, 287, 132050. [Google Scholar] [CrossRef] [PubMed]
- Thirumal, V.; Yuvakkumar, R.; Kumar, P.S.; Ravi, G.; Keerthana, S.P.; Velauthapillai, D. Facile single-step synthesis of MXene@CNTs hybrid nanocomposite by CVD method to remove hazardous pollutants. Chemosphere 2022, 286, 131733. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, D.; Chen, W.; Tang, Y.; Wang, X.; Li, L.; Wang, J. Oxygen-vacancy-embedded 2D/2D NiFe-LDH/MXene Schottky heterojunction for boosted photodegradation of norfloxacin. Appl. Surf. Sci. 2021, 572, 151432. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Ullah, H.; Bashiri, R.; Mohamed, N.M.; Sufian, S.; Ng, Y.H. Experimental and DFT Insights on Microflower g-C3N4/BiVO4 Photocatalyst for Enhanced Photoelectrochemical Hydrogen Generation from Lake water. ACS Sustain. Chem. Eng. 2020, 8, 9393–9403. [Google Scholar] [CrossRef]
- Tan, H.L.; Du, A.; Amal, R.; Ng, Y.H. Decorating platinum on nitrogen-doped graphene sheets: Control of the platinum particle size distribution for improved photocatalytic H2 generation. Chem. Eng. Sci. 2018, 194, 85–93. [Google Scholar] [CrossRef]
- Liu, W.; Sun, M.; Ding, Z.; Gao, B.; Ding, W. Ti3C2 MXene embellished g-C3N4 nanosheets for improving photocatalytic redox capacity. J. Alloys Compd. 2021, 877, 160223. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Ge, J.; Zhao, C.; Zhao, Q.; Zhang, F.; Ni, T.; Wu, W. 3D interconnected g-C3N4 hybridized with 2D Ti3C2 MXene nanosheets for enhancing visible light photocatalytic hydrogen evolution and dye contaminant elimination. Appl. Surf. Sci. 2021, 579, 152180. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, M.; Zhan, R.; Wang, X.; Peng, G.; Niu, J. Ti3C2 MXene-induced interface electron separation in g-C3N4/Ti3C2 MXene/MoSe2 Z-scheme heterojunction for enhancing visible light-irradiated enoxacin degradation. Sep. Purif. Technol. 2021, 275, 119194. [Google Scholar] [CrossRef]
- Liu, N.; Lu, N.; Su, Y.; Wang, P.; Quan, X. Fabrication of g-C3N4/Ti3C2 composite and its visible-light photocatalytic capability for ciprofloxacin degradation. Sep. Purif. Technol. 2019, 211, 782–789. [Google Scholar] [CrossRef]
- Chang, F.; Xie, Y.; Li, C.; Chen, J.; Luo, J.; Hu, X.; Shen, J. A facile modification of g-C3N4 with enhanced photocatalytic activity for degradation of methylene blue. Appl. Surf. Sci. 2013, 280, 967–974. [Google Scholar] [CrossRef]
- Regmi, C.; Kshetri, Y.K.; Ray, S.K.; Pandey, R.P.; Lee, S.W. Utilization of visible to NIR light energy by Yb+3, Er+3 and Tm+3 doped BiVO4 for the photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2017, 392, 61–70. [Google Scholar] [CrossRef]
- Łęcki, T.; Zarębska, K.; Sobczak, K.; Skompska, M. Photocatalytic degradation of 4-chlorophenol with the use of FTO/TiO2/SrTiO3 composite prepared by microwave-assisted hydrothermal method. Appl. Surf. Sci. 2019, 470, 991–1002. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Sufian, S.; Bashiri, R.; Mohamed, N.M.; Ramli, R.M. Synergistic effects of pH and calcination temperature on enhancing photodegradation performance of m-BiVO4. J. Taiwan Inst. Chem. Eng. 2017, 81, 305–315. [Google Scholar] [CrossRef]
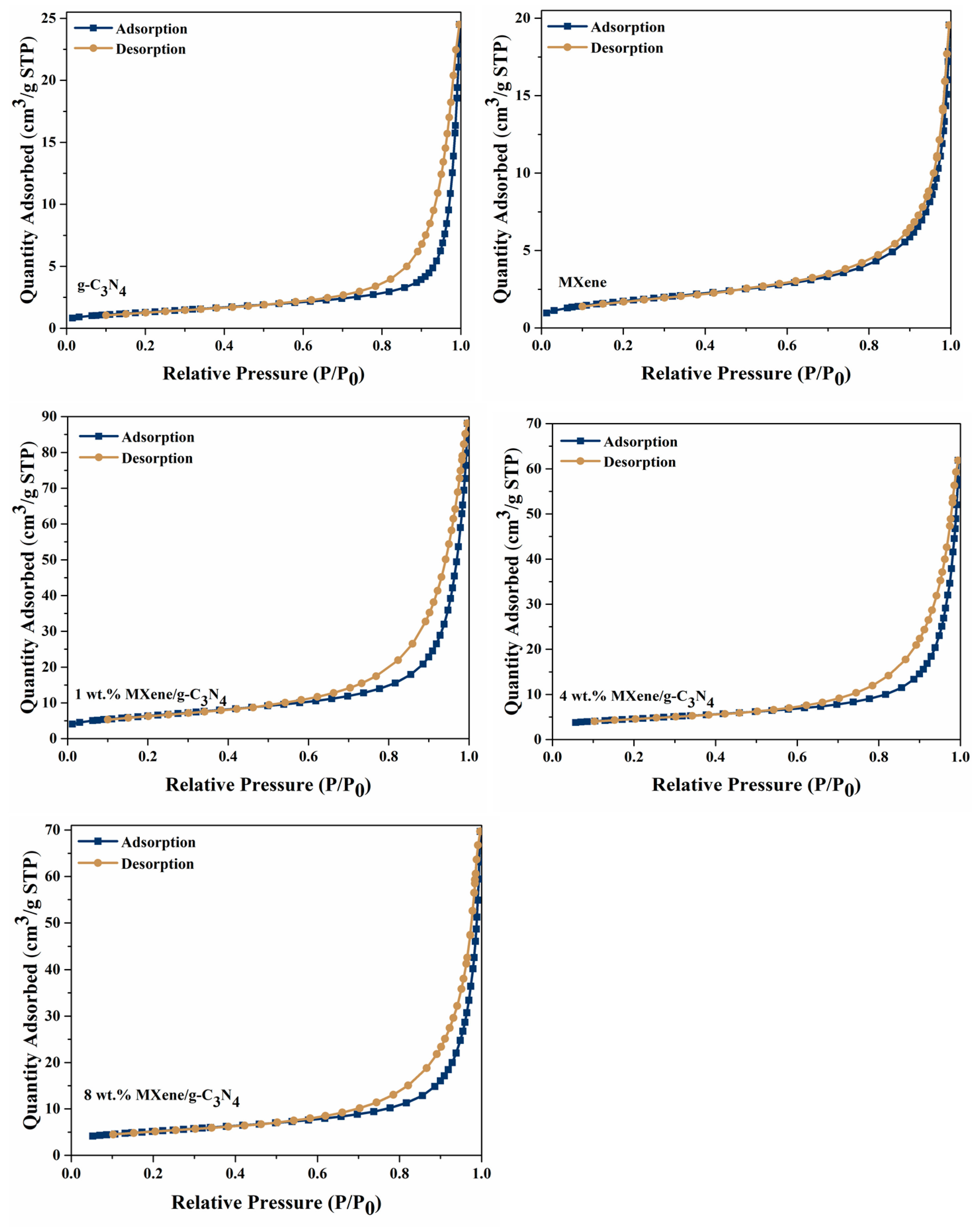

| Photocatalyst | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| g-C3N4 | 4.64 | 0.033 | 28.09 |
| MXene | 6.26 | 0.027 | 16.96 |
| 1 wt.% MXene/g-C3N4 | 22.58 | 0.123 | 21.88 |
| 4 wt.% MXene/g-C3N4 | 15.66 | 0.036 | 9.377 |
| 8 wt.% MXene/g-C3N4 | 17.73 | 0.039 | 8.826 |
| 12 wt.% MXene/g-C3N4 | 13.70 | 0.033 | 9.535 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasri, M.S.I.; Samsudin, M.F.R.; Tahir, A.A.; Sufian, S. Effect of MXene Loaded on g-C3N4 Photocatalyst for the Photocatalytic Degradation of Methylene Blue. Energies 2022, 15, 955. https://doi.org/10.3390/en15030955
Nasri MSI, Samsudin MFR, Tahir AA, Sufian S. Effect of MXene Loaded on g-C3N4 Photocatalyst for the Photocatalytic Degradation of Methylene Blue. Energies. 2022; 15(3):955. https://doi.org/10.3390/en15030955
Chicago/Turabian StyleNasri, Muhammad Syahmi Irfan, Mohamad Fakhrul Ridhwan Samsudin, Asif Ali Tahir, and Suriati Sufian. 2022. "Effect of MXene Loaded on g-C3N4 Photocatalyst for the Photocatalytic Degradation of Methylene Blue" Energies 15, no. 3: 955. https://doi.org/10.3390/en15030955






