Methanol Production in the Brayton Cycle
Abstract
:1. Introduction
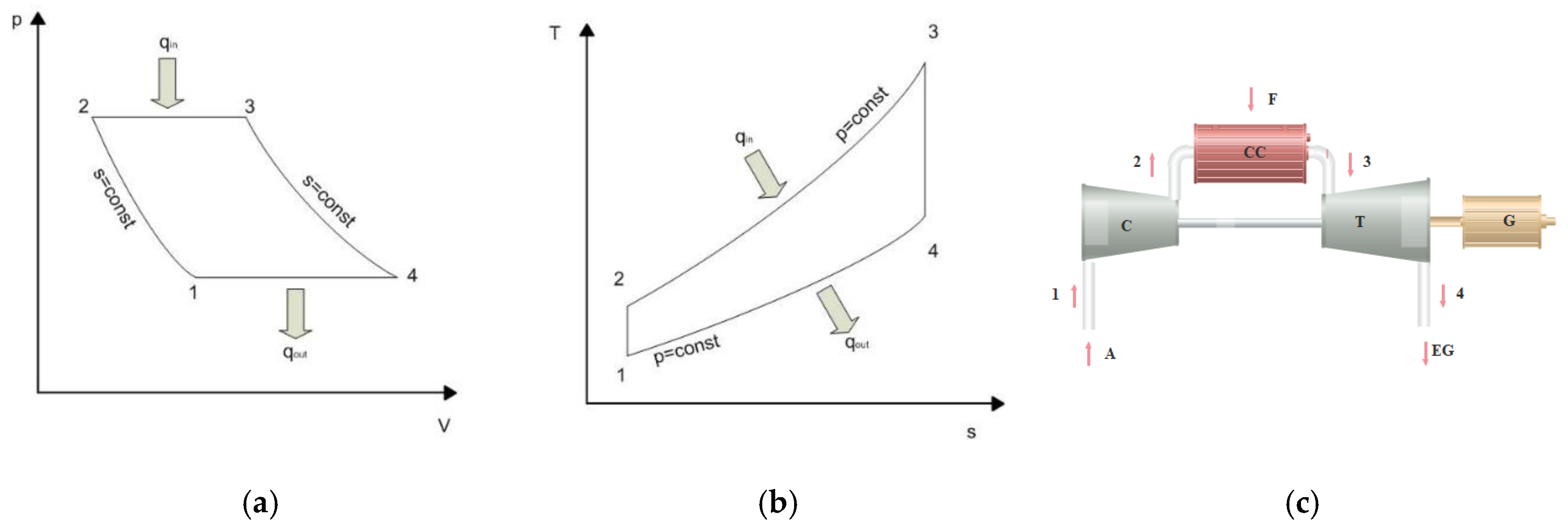
2. A System for the Methanol Production in the Gas Turbine Cycle
3. Methodology of Calculations
4. The Results of the Analyses
- total amount of heat from heat exchangers HX1, HX2, HX3 ( );
- gas turbine power (NT);
- total power of compressors C (ΣNc);
- yield (;
- efficiency of the chemical conversion ();
- reactor efficiency ().
5. Summary
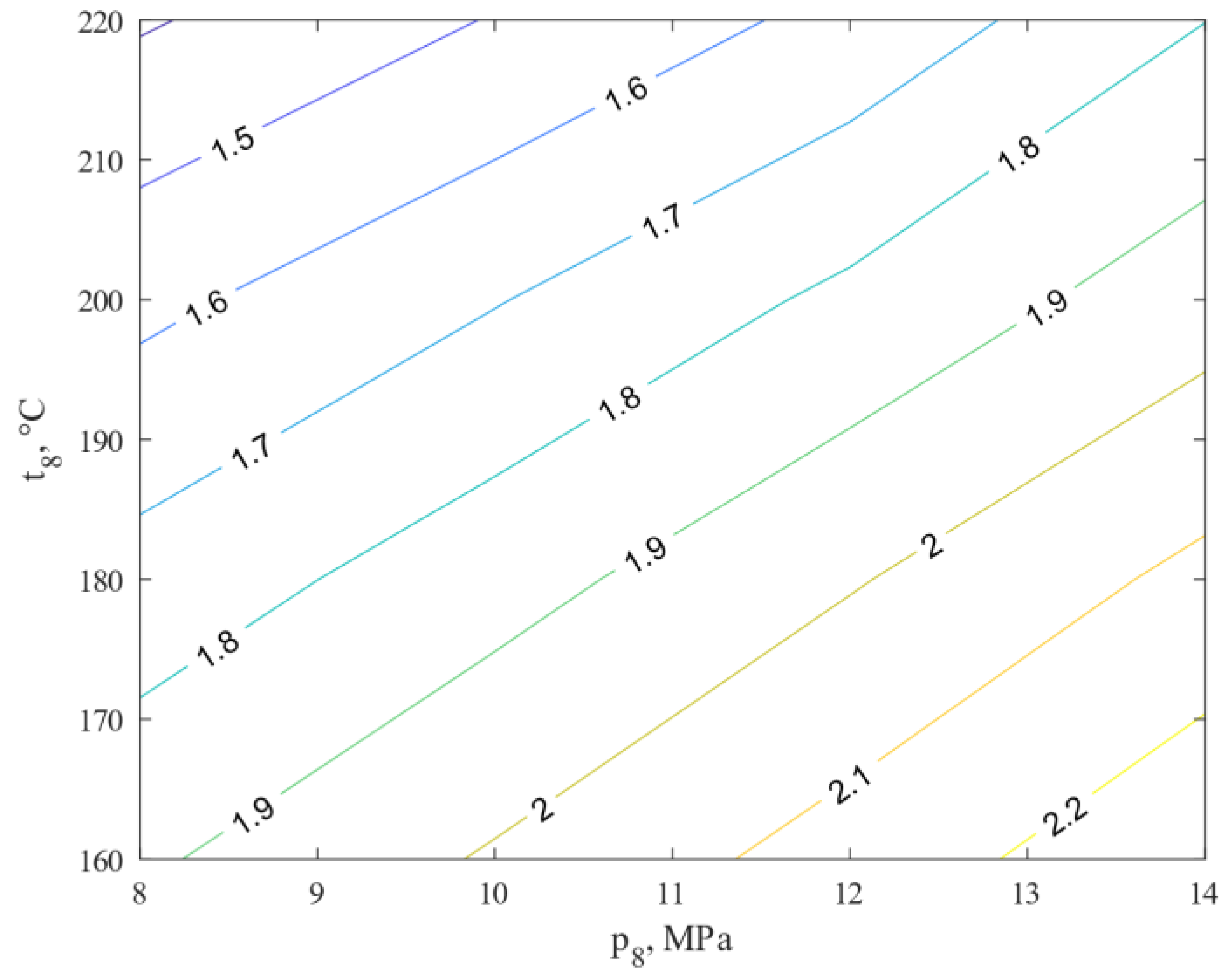
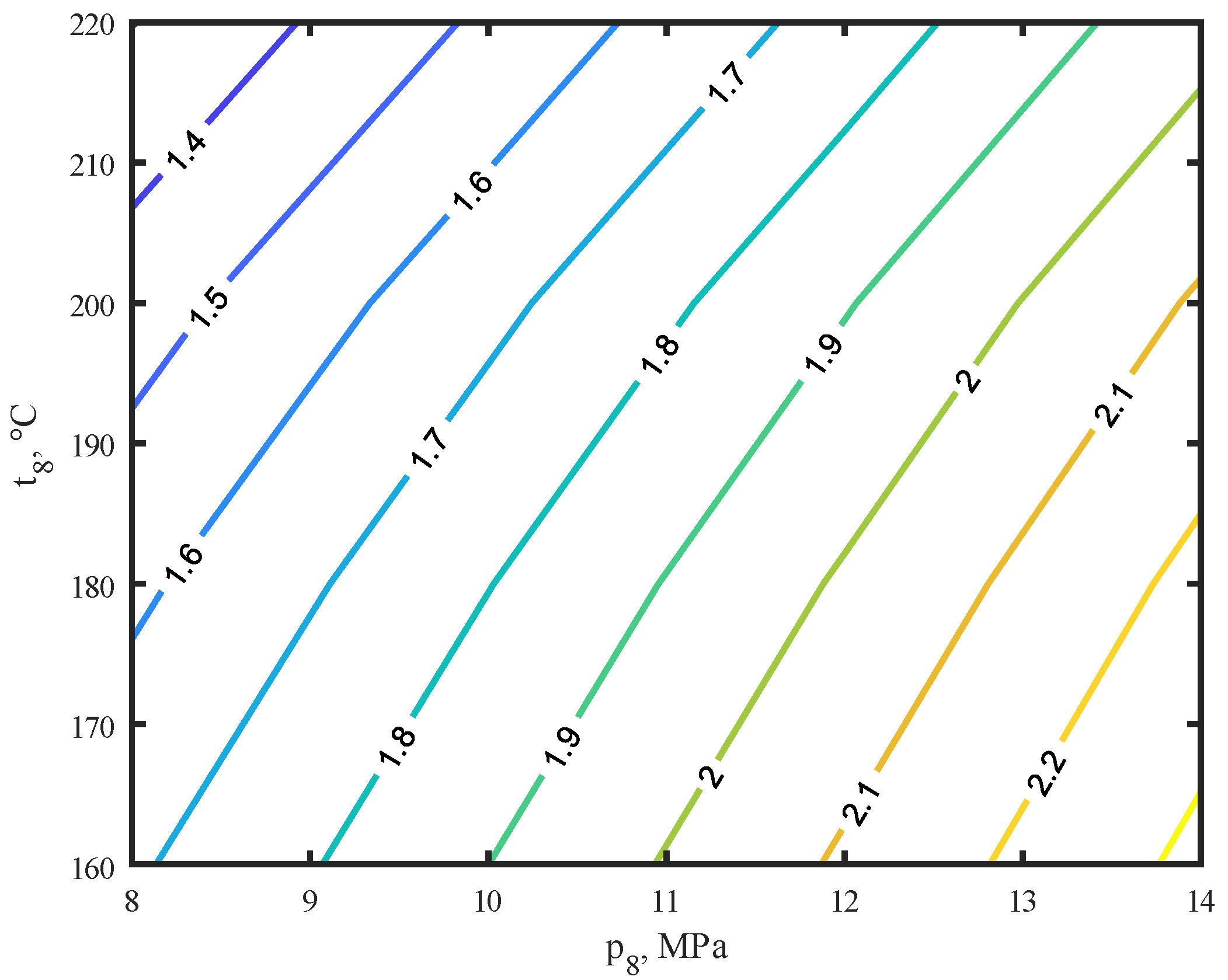
- The power values of the compressor, ΣNc, and the NT turbine increase with the increase of temperature, t8, and pressure, p8, prevailing in the reactor, reaching the highest value of 14 MPa and 220 °C and are, respectively, ΣNc = 2.97 kW and NT = 1.96 kW.
- The amount of heat exchanged in all exchangers reached the highest value for 14 MPa and 160 °C, and equal 2.28 kW.
- The efficiency of the methanol production process, η, and the efficiency of the methanol reactor increase with increasing pressure, p8, and decreasing temperature, t8. This represents the expected development towards higher reactor pressures.
- Inserting an additional heat exchanger preceding the expander, which heats the medium to 560 °C, will increase the expander’s power so much that it will cover the compressor’s electricity demand.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Nomenclature
| amount of heat, kW; | |
| reactor efficiency, -; | |
| HHVCH3OH | 22,341.21 kJ/kg; |
| HHVH2 | 142,327 kJ/kg; |
| NT | gas turbine power, kW; |
| p | pressure, MPa; |
| t | temperature, °C; |
| uCH3OH | pure methanol yield, kgCH3OH/kgH2; |
| uCH3OH,stec | pure methanol yield kgCH3OH/kgH2; |
| ΣNc | total power of compressors, kW; |
| enthalpy of the medium, kJ/kg; | |
| efficiency of the chemical conversion, -; | |
| the flow of the medium, kg/s. |
Subscripts
| . | separator of subscripts; |
| 1 ÷ 17 | numbers in characteristic points of installations; |
| CH3OH | methanol; |
| H2 | hydrogen; |
| CO2 | carbon dioxide; |
| CO | carbon monoxide; |
| H2O | water. |
Abbreviations
| A | air; |
| C | compressor; |
| CC | combustion chamber; |
| F | fuel; |
| T | gas turbine; |
| G | generator; |
| EG | exhaust gas; |
| HX | heat exchanger; |
| R | methanol synthesis reactor; |
| S | separator; |
| MR | separation membrane; |
| G | generator; |
| M | motor; |
| CO2 | carbon dioxide; |
| H2 | hydrogen; |
| H2O | water; |
| CH3OH | methanol. |
References
- Vazquez, D.; Guillén-Gosálbez, G. Process design within planetary boundaries: Application to CO2 based methanol production. Chem. Eng. Sci. 2021, 246, 116891. [Google Scholar] [CrossRef]
- Eghbali, P.; Nişancı, B.; Metin, Ö. Graphene hydrogel supported palladium nanoparticles as an efficient and reusable heterogeneous catalysts in the transfer hydrogenation of nitroarenes using ammonia borane as a hydrogen source. Pure Appl. Chem. 2018, 90, 327–335. [Google Scholar] [CrossRef]
- Eghbali, P.; Gurbuzm, M.; Erturk, A.; Metin, Ö. In situ synthesis of dendrimer-encapsulated palladium(0) nanoparticles as catalysts for hydrogen production from the methanolysis of ammonia borane. Int. J. Hydrogen Energy 2020, 45, 26274–26285. [Google Scholar] [CrossRef]
- Coskun Avci, A.; Toklu, E. A new analysis of two phase flow on hydrogen production from water electrolysis. Int. J. Hydrogen Energy 2022, 47, 6986–6995. [Google Scholar] [CrossRef]
- Ravikumar, D.; Keoleian, G.; Miller, S. The environmental opportunity cost of using renewable energy for carbon capture and utilization for methanol production. Appl. Energy 2020, 279, 115770. [Google Scholar] [CrossRef]
- Berggren, M. Global methanol—State of the industry. In Proceedings of the 22nd IMPCA Asian Methanol Conference, Singapore, 5–7 November 2019. [Google Scholar]
- Osman, M.; Zaabout, A.; Cloete, S.; Amini, S. Pressurized chemical looping methane reforming to syngas for efficient methanol production: Experimental and process simulation study. Adv. Appl. Energy 2021, 4, 100069. [Google Scholar] [CrossRef]
- Schorn, F.; Breuer, J.; Samsun, R.; Schnorbus, T.; Heuser, B.; Peters, R.; Stolten, D. Methanol as a renewable energy carrier: An assessment of production and transportation costs for selected global locations. Adv. Appl. Energy 2021, 3, 100050. [Google Scholar] [CrossRef]
- Kotowicz, J.; Węcel, D.; Brzęczek, M. Analysis of the work of a “renewable” methanol production installation based ON H2 from electrolysis and CO2 from power plants. Energy 2021, 221, 119538. [Google Scholar] [CrossRef]
- Chen, C.; Yang, A. Power-to-methanol: The role of process flexibility in the integration of variable renewable energy into chemical production. Energy Convers. Manag. 2021, 228, 113673. [Google Scholar] [CrossRef]
- Bos, M.; Kersten, S.; Brilman, D. Wind power to methanol: Renewable methanol production using electricity, electrolysis of water and CO2 air capture. Appl. Energy 2020, 264, 114672. [Google Scholar] [CrossRef]
- Cui, X.; Kær, S.; Nielsen, M. Energy analysis and surrogate modeling for the green methanol production under dynamic operating conditions. Fuel 2022, 307, 121924. [Google Scholar] [CrossRef]
- Iwakiri, I.; Miguel, C.; Madeira, L. Modeling and simulation of a steam-selective membrane reactor for power-to-methanol. Comput. Chem. Eng. 2022, 156, 107555. [Google Scholar] [CrossRef]
- Kiss, A.; Pragt, J.; Vos, H.; Bargeman, G.; de Groot, M. Novel efficient process for methanol synthesis by CO2 hydrogenation. Chem. Eng. J. 2016, 284, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Lee, H.; Lim, D.; Brigljević, B.; Cho, W.; Cho, H.-S.; Kim, C.-H.; Lim, H. Renewable methanol synthesis from renewable H2 and captured CO2: How can power-to-liquid technology be economically feasible? Appl. Energy 2020, 279, 115827. [Google Scholar] [CrossRef]
- Gou, X.; Zhang, H.; Li, G.; Cao, Y.; Zhang, Q. Dynamic simulation of a gas turbine for heat recovery at varying load and environment conditions. Appl. Therm. Eng. 2021, 195, 117014. [Google Scholar] [CrossRef]
- Kralj, A.; Glavic, P. Optimization of a gas turbine in the methanol process, using the NLP model. Appl. Therm. Eng. 2007, 27, 1799–1805. [Google Scholar] [CrossRef]
- Carapellucci, R.; Milazzo, A. Repowering combined cycle power plants by a modified STIG configuration. Energy Convers. Manag. 2007, 48, 1590–1600. [Google Scholar] [CrossRef]
- Vadlamudi, T.; Kommineni, R.; Katuru, B. Exploration of turbine blade cooling strategies for performance boosting and CO2 emissions reduction of combined cycle with steam injection based gas turbine. Int. J. Ambient Energy 2020. [Google Scholar] [CrossRef]
- Rad, E.; Kazemiani-Najafabadi, P. Thermo-environmental and economic analyses of an integrated heat recovery steam-injected gas turbine. Energy 2017, 141, 1940–1954. [Google Scholar] [CrossRef]
- Kotowicz, J.; Brzęczek, M. Analysis of increasing efficiency of modern combined cycle power plant: A case study. Energy 2018, 153, 90–99. [Google Scholar] [CrossRef]
- Kotowicz, J.; Brzęczek, M. Comprehensive multivariable analysis of the possibility of an increase in the electrical efficiency of a modern combined cycle power plant with and without a CO2 capture and compression installations study. Energy 2019, 175, 1100–1120. [Google Scholar] [CrossRef]
- Kotowicz, J.; Brzęczek, M.; Job, M. The thermodynamic and economic characteristics of the modern combined cycle power plant with gas turbine steam cooling. Energy 2018, 164, 359–376. [Google Scholar] [CrossRef]
- Salilew, W.; Karim, Z.; Baheta, A. Review on gas turbine condition based diagnosis method. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Tahan, M.; Muhammad, M.; Karim, Z. A multi-nets ANN model for real-time performance-based automatic fault diagnosis of industrial gas turbine engines. J. Braz. Soc. Mech. Sci. Eng. 2017, 39, 2865–2876. [Google Scholar] [CrossRef]
- Greeff, I.; Visser, J.A.; Ptasiński, K.; Janssen, F. Utilisation of reactor heat in methanol synthesis to reduce compressor duty—Application of power cycle principles and simulation tools. Appl. Therm. Eng. 2002, 22, 1549–1558. [Google Scholar] [CrossRef]
- Tola, V.; Lonis, F. Low CO2 emissions chemically recuperated gas turbines fed by renewable methanol. Appl. Energy 2021, 298, 117146. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, X.; Hong, H.; Han, W. An innovative gas turbine cycle with methanol-fueled chemical-looping combustion. J. Eng. Gas Turbine Power 2009, 131, 061701. [Google Scholar] [CrossRef]
- Eisavi, B.; Ranjbar, F.; Nami, H.; Chitsaz, A. Low-carbon biomass-fueled integrated system for power, methane and methanol production. Energy Convers. Manag. 2022, 253, 115163. [Google Scholar] [CrossRef]
- Kotowicz, J.; Brzęczek, M. Methods to increase the efficiency of production and purification installations of renewable methanol. Renew. Energy 2021, 177, 568–583. [Google Scholar] [CrossRef]
- Aydin, M.; Dincer, I. An assessment study on various clean hydrogen production methods. Energy 2022, 245, 123090. [Google Scholar] [CrossRef]
- Dar, A.; Hameed, J.; Huo, C.; Sarfraz, M.; Albasher, G.; Wang, C.; Nawaz, A. Recent optimization and panelizing measures for green energy projects; insights into CO2 emission influencing to circular economy. Fuel 2022, 314, 12094. [Google Scholar] [CrossRef]
- Altayib, K.; Dincer, I. Development of an integrated hydropower system with hydrogen and methanol production. Energy 2022, 240, 122780. [Google Scholar] [CrossRef]






| Parameter, Unit | Symbol | Value |
|---|---|---|
| Hydrogen pressure at the inlet system inlet, MPa | p1 | 2.5 |
| Carbon dioxide pressure at the system inlet, MPa | p2 | 0.15 |
| Hydrogen temperature at the inlet to the installation, °C | t1 | 25 |
| Temperature of carbon dioxide at the inlet to the installation, °C | t2 | 25 |
| Pressure at the outlet of the gas turbine, - | p10 | 150 |
| Isentropic efficiency of a gas turbine, - | ηiT | 0.9 |
| Mechanical efficiency of a gas turbine, - | ηmT | 0.99 |
| Isentropic efficiency of compressors, - | ηiC | 0.88 |
| Mechanical efficiency of compressors, - | ηmC | 0.995 |
| Electric efficiency of the engine, - | ηmel | 0.95 |
| Mechanical efficiency of the engine, - | ηmm | 0.998 |
| Generator nominal efficiency, - | ηng | 0.9856 |
| Lp | m [kg/h] | p [MPa] | t [°C] | i [kJ/kg] | X [-] | CH3OH | H2 | CO2 | CO | H2O |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.45 | 2.5 | 25 | 356.9 | 1 | 0 | 1 | 0 | 0 | 0 |
| 2 | 3.34 | 0.15 | 25 | 20.8 | 1 | 0 | 0 | 1 | 0 | 0 |
| 3 | 0.55 | 2.5 | 25 | 356.9 | 1 | 0 | 1 | 0 | 0 | 0 |
| 4 | 1.00 | 2.5 | 25 | 356.9 | 1 | 0 | 1 | 0 | 0 | 0 |
| 5 | 7.28 | 2.5 | 272.9 | 257.0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 6 | 8.28 | 2.5 | 107.5 | 269.0 | 1 | 0 | 0.12 | 0.88 | 0 | 0 |
| 7 | 8.28 | 14 | 342.6 | 890.5 | 1 | 0 | 0.12 | 0.88 | 0 | 0 |
| 8 | 8.28 | 14 | 160 | 404.6 | 1 | 0 | 0.12 | 0.88 | 0 | 0 |
| 9 | 8.28 | 14 | 290.6 | 649.4 | 1 | 0.28 | 0.07 | 0.48 | 0 | 0.01 |
| 10 | 8.28 | 0.15 | 43.7 | −158.5 | 0.9 | 0.28 | 0.07 | 0.48 | 0 | 0.01 |
| 11 | 8.28 | 0.15 | 15 | −349.0 | 0.8 | 0.28 | 0.07 | 0.48 | 0 | 0.01 |
| 12 | 1.28 | 0.15 | 15.0 | 63.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13 | 7.00 | 0.15 | 15 | 31.9 | 1 | 0.34 | 0.08 | 0.56 | 0 | 0.01 |
| 14 | 7.00 | 0.15 | 15 | 12.4 | 1 | 0 | 0 | 1 | 0 | 0 |
| 15 | 0.55 | 0.15 | 15 | 213.9 | 1 | 0 | 1 | 0 | 0 | 0 |
| 16 | 0.55 | 2.5 | 126.6 | 1821.4 | 1 | 0 | 1 | 0 | 0 | 0 |
| 17 | 2.32 | 0.15 | 15 | 22.6 | 1 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotowicz, J.; Brzęczek, M.; Walewska, A.; Szykowska, K. Methanol Production in the Brayton Cycle. Energies 2022, 15, 1480. https://doi.org/10.3390/en15041480
Kotowicz J, Brzęczek M, Walewska A, Szykowska K. Methanol Production in the Brayton Cycle. Energies. 2022; 15(4):1480. https://doi.org/10.3390/en15041480
Chicago/Turabian StyleKotowicz, Janusz, Mateusz Brzęczek, Aleksandra Walewska, and Kamila Szykowska. 2022. "Methanol Production in the Brayton Cycle" Energies 15, no. 4: 1480. https://doi.org/10.3390/en15041480
APA StyleKotowicz, J., Brzęczek, M., Walewska, A., & Szykowska, K. (2022). Methanol Production in the Brayton Cycle. Energies, 15(4), 1480. https://doi.org/10.3390/en15041480







