Performance Degradation of a Shell-and-Tube Heat Exchanger Due to Tar Deposition
Abstract
:1. Introduction
2. Conjugate Heat Transfer Methodology
Operating Conditions and Model Setup
3. Clean Heat Exchanger Performance
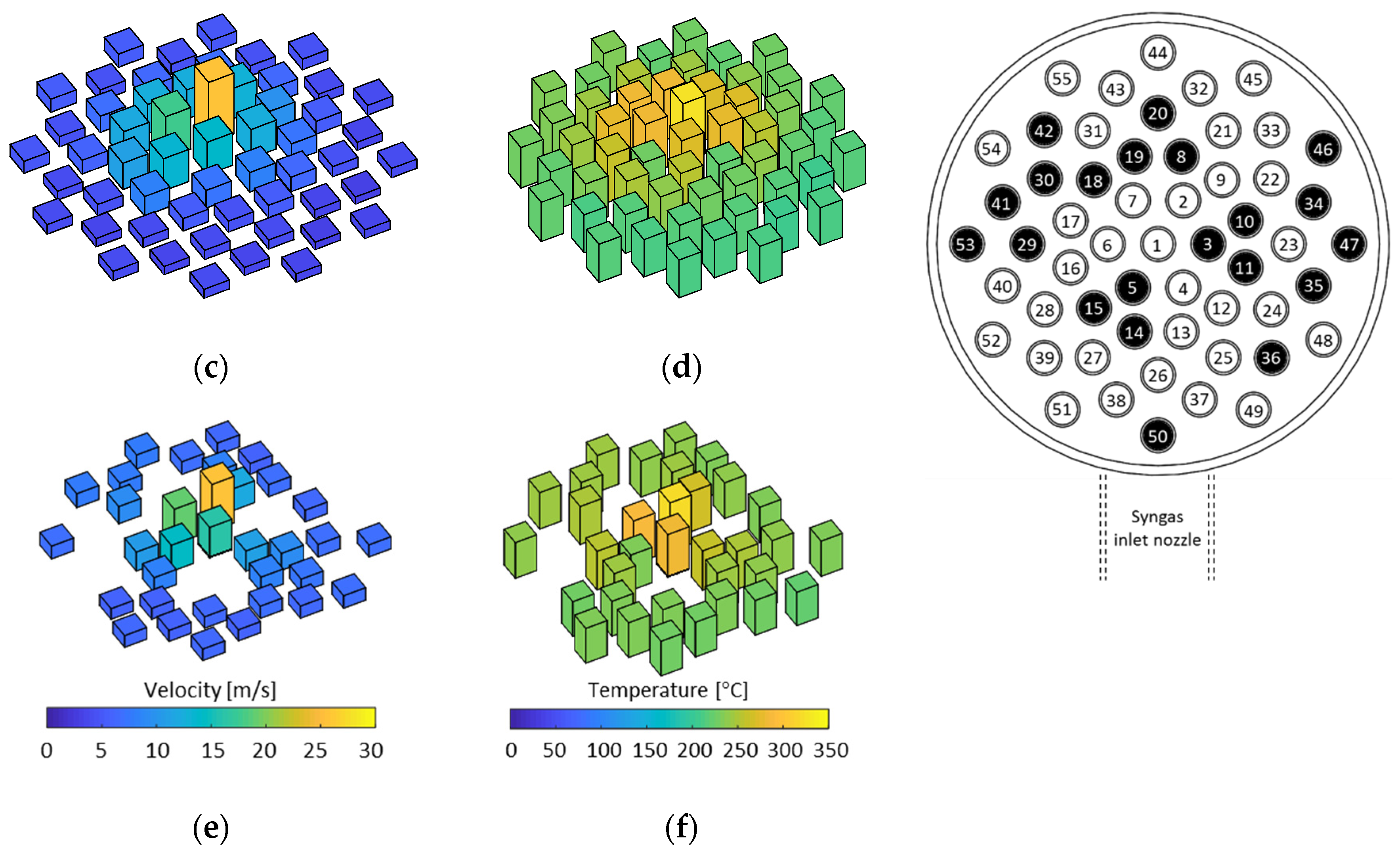
3.1. Flow Distribution Analysis
3.2. Temperature Distribution Analysis
4. Tar Condensation Assessment
4.1. Simulation Strategy
4.2. Potential Tar Deposition Zones
5. Fouled Heat Exchanger Analysis
5.1. On-Field Fouling Observations
5.2. Performance Deterioration Assessment
5.3. Flow Distribution Modification
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Srirangan, K.; Akawi, L.; Moo-Young, M.; Chou, C.P. Towards sustainable production of clean energy carriers from biomass resources. Appl. Energy 2012, 100, 172–186. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Heidenreich, S.; Foscolo, P.U. New concepts in biomass gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95. [Google Scholar] [CrossRef]
- Ahrenfeldt, J.; Thomsen, T.P.; Henriksen, U.; Clausen, L.R. Biomass gasification cogeneration—A review of state of the art technology and near future perspectives. Appl. Therm. Eng. 2013, 50, 1407–1417. [Google Scholar] [CrossRef] [Green Version]
- Belgiorno, V.; De Feo, G.; Della Rocca, C.; Napoli, R.M.A. Energy from gasification of solid wastes. Waste Manag. 2003, 23, 1–15. [Google Scholar] [CrossRef]
- Asadullah, M. Biomass gasification gas cleaning for downstream applications: A comparative critical review. Renew. Sustain. Energy Rev. 2014, 40, 118–132. [Google Scholar] [CrossRef]
- Han, J.; Kim, H. The reduction and control technology of tar during biomass gasification/pyrolysis: An overview. Renew. Sustain. Energy Rev. 2008, 12, 397–416. [Google Scholar] [CrossRef]
- Li, C.; Suzuki, K. Tar property, analysis, reforming mechanism and model for biomass gasification—An overview. Renew. Sustain. Energy Rev. 2009, 13, 594–604. [Google Scholar] [CrossRef]
- Morf, P.O. Secondary Reactions of Tar during Thermochemical Biomass Conversion. Ph.D. Thesis, Swiss Federal Institute of Technology, Zurich, Switzerland, 2001. [Google Scholar]
- Milne, T.A.; Evans, R.J.; Abatzoglou, N. Biomass Gasifier “Tars”: Their Nature, Formation and Conversion; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 1998. [Google Scholar]
- Evans, R.J.; Milne, T.A. Molecular Characterization of the Pyrolysis of Biomass. 1. Fundamentals. Energy Fuels 1987, 1, 123–137. [Google Scholar] [CrossRef]
- Evans, R.J.; Milne, T.A. Molecular Characterization of the Pyrolysis of Biomass. 2. Applications. Energy Fuels 1987, 1, 311–319. [Google Scholar] [CrossRef]
- Evans, R.J.; Milne, T.A. Chemistry of Tar Formation and Maturation in the Thermochemical Conversion of Biomass. In Developments in the Thermochemical Biomass Conversion; Bridgwater, A.V., Boocock, D.G.B., Eds.; Springer: Dordrecht, The Netherlands, 1997; Volume 2. [Google Scholar]
- Kiel, J.H.A.; van Paasen, S.V.B.; Neeft, J.P.A.; Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G.; Meijer, R.; Berends, R.H.; Temmink, H.M.G.; Brem, G.; et al. Primary Measures to Reduce Tar Formation in Fluidised-Bed Biomass Gasifiers; Energy Research Centre of the Netherlands (ECN): Petten, The Netherlands, 2004. [Google Scholar]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Anis, S.; Zainal, Z.A. Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: A review. Renew. Sustain. Energy Rev. 2011, 15, 2355–2377. [Google Scholar] [CrossRef]
- Thapa, S.; Bhoi, P.R.; Kumar, A.; Huhnke, R.L. Effects of Syngas Cooling and Biomass Filter Medium on Tar Removal. Energies 2017, 10, 349. [Google Scholar] [CrossRef] [Green Version]
- Thapa, S.; Indrawan, N.; Bhoi, P.R.; Kumar, A.; Huhnke, R.L. Tar reduction in biomass syngas using heat exchanger and vegetable oil bubbler. Energy 2019, 175, 402–409. [Google Scholar] [CrossRef]
- Gambarotta, A.; Manganelli, M.; Morini, M. A model for filter diagnostics in a syngas-fed CHP plant. Energy Procedia 2018, 148, 400–407. [Google Scholar] [CrossRef]
- Casari, N.; Pinelli, M.; Suman, A.; Candido, A.; Morini, M. Deposition of syngas tar in fuel supplying duct of a biomass gasifier: A numerical study. Fuel 2020, 273, 117579. [Google Scholar] [CrossRef]
- Bergman, T.L.; Lavine, A.S.; Incropera, F.P.; DeWitt, D.P. Fundamentals of Heat and Mass Transfer, 8th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- ANSYS CFX User Manual—V 20.2.; ANSYS, Inc.: Canonsburg, PA, USA, 2020.
- Mohammadi, K.; Malayeri, M.R. Parametric study of gross flow maldistribution in a single-pass shell and tube heat exchanger in turbulent regime. Int. J. Heat Fluid Flow 2013, 44, 14–27. [Google Scholar] [CrossRef]
- Kim, M.I.; Lee, Y.; Kim, B.W.; Lee, D.H.; Song, W.S. CFD modeling of shell-and-tube heat exchanger header for uniform distribution among tubes. Korean J. Chem. Eng. 2009, 26, 359–363. [Google Scholar] [CrossRef]
- Dufour, A.; Masson, E.; Girods, P.; Rogaume, Y.; Zoulalian, A. Evolution of Aromatic Tar Composition in Relation to Methane and Ethylene from Biomass Pyrolysis-Gasification. Energy Fuels 2011, 25, 4182–4189. [Google Scholar] [CrossRef]
- Vasudevan, T.V.; Gokhale, A.J.; Mahalingam, R. Phoretic Phenomena in Tar Vapor-Particulate Mixture Separation from Fuel Gas Streams. Can. J. Chem. Eng. 1985, 63, 903–910. [Google Scholar] [CrossRef]
- Schiller, L.; Naumann, A. A drag coefficient correlation. Z. VDI 1933, 77, 318–320. [Google Scholar]
- Gosman, A.D.; Ioannides, E. Aspects of computer simulation of liquid-fueled combustors. J. Energy 1983, 7, 482–490. [Google Scholar] [CrossRef]
- Ranz, W.E.; Marshall, W.R. Evaporation from drops: Parts I. Chem. Eng. Prog. 1952, 48, 141–146. [Google Scholar]
- Ranz, W.E.; Marshall, W.R. Evaporation from drops: Parts II. Chem. Eng. Prog. 1952, 48, 173–180. [Google Scholar]
- Euh, S.H.; Kafle, S.; Lee, S.Y.; Lee, C.G.; Jo, L.; Choi, Y.S.; Oh, J.H.; Kim, D.H. Establishment and validation of tar fouling mechanism in wood pellet boiler using kinetic models. Appl. Therm. Eng. 2017, 127, 165–175. [Google Scholar] [CrossRef]

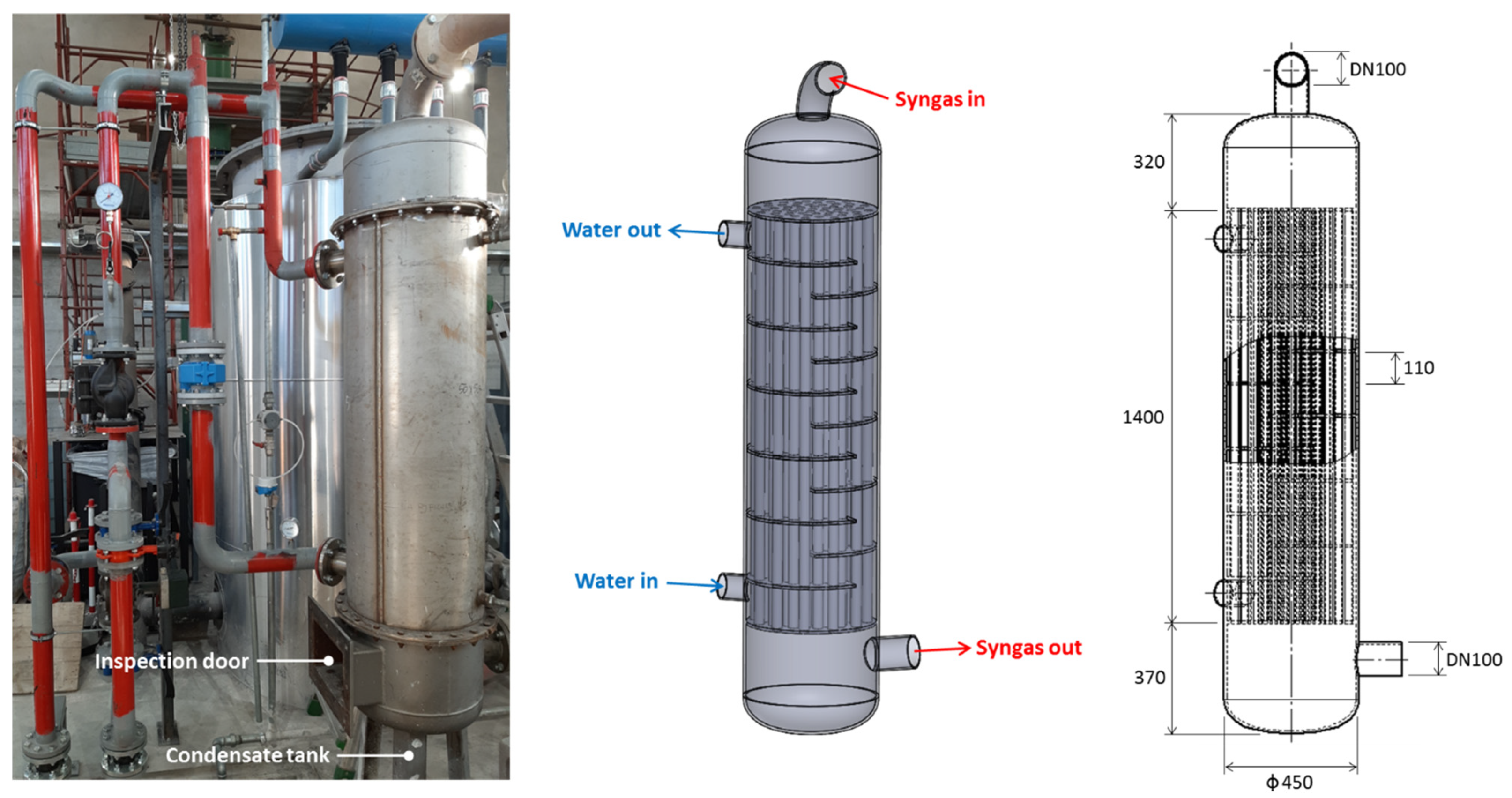
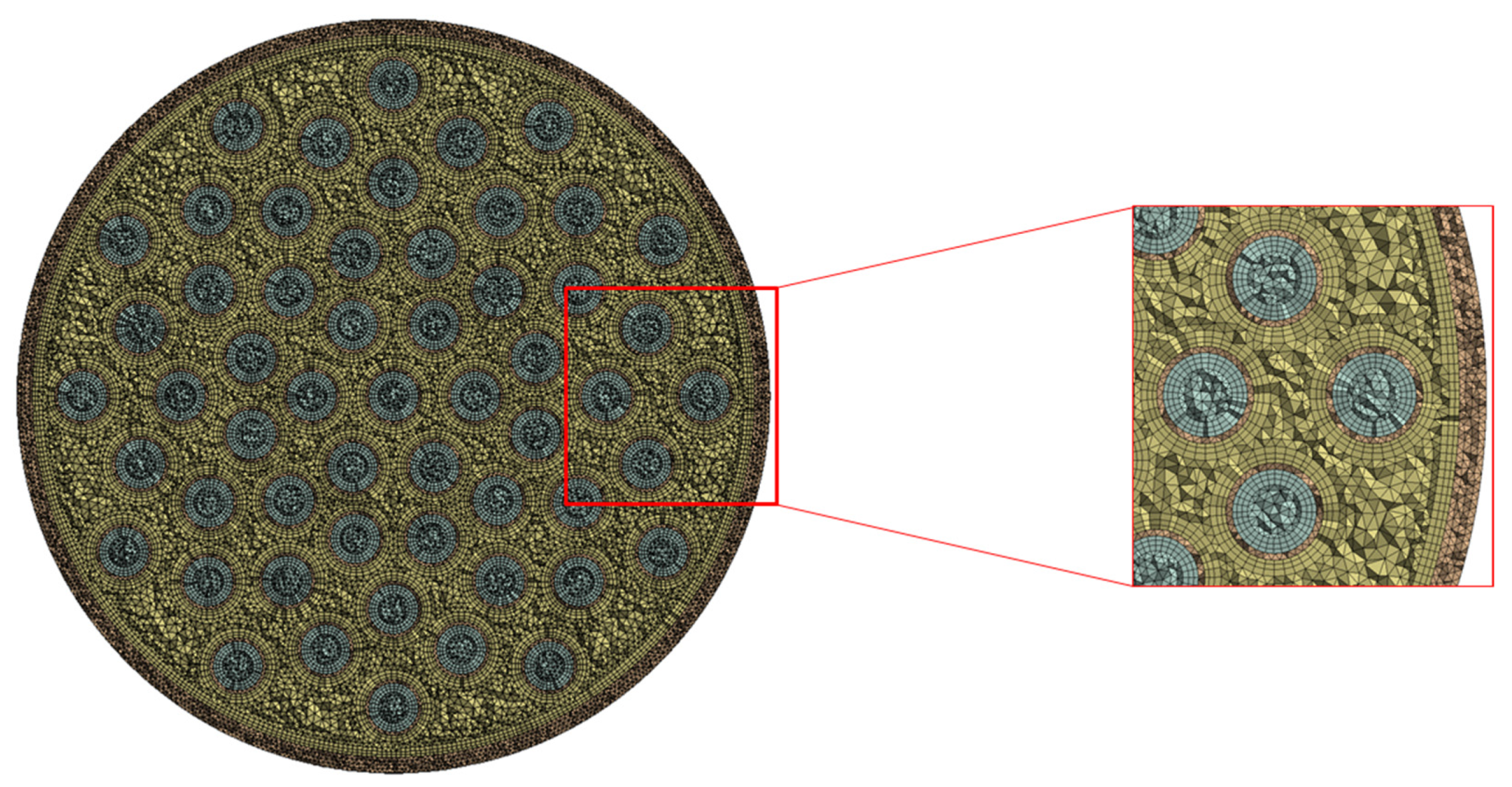









| Quantity | Value |
|---|---|
| Syngas mass flow rate [kg/s] | 0.10 |
| Syngas inlet relative pressure [Pa] | −15,000 |
| Syngas inlet temperature [°C] | 350 |
| Water volume flow rate [m3/h] | 11 |
| Water inlet temperature [°C] | 80 |
| Component | Molar Fraction |
|---|---|
| H2 | 0.150 |
| O2 | 0.020 |
| N2 | 0.420 |
| CH4 | 0.015 |
| CO | 0.230 |
| CO2 | 0.165 |
| Property | Syngas | Water |
|---|---|---|
| Density [kg/m3] | variable | 971.8 |
| Specific heat [J/(kg∙K)] | 1142 | 4197 |
| Dynamic viscosity [Pa∙s] | 1.655 × e−5 | 3.55 × e−4 |
| Thermal conductivity [W/(m∙K)] | 0.0244 | 0.670 |
| Tar Class | Major Compounds | Concentration [mg/m3] | Dew Point [°C] |
|---|---|---|---|
| Class 1 | Undetectable | 0 | - |
| Class 2 | Phenol, toluene | 1000 | 98.4 |
| Class 3 | m-Xylene | 1745 | −23.3 |
| Class 4 | Phenanthrene, naphthalene | 2250 | 108.4 |
| Class 5 | Pyrene | 5 | 162.6 |
| Configuration | Qsyngas [W] | Qwater [W] | Qair [W] | ε | ∆psyngas [Pa] |
|---|---|---|---|---|---|
| Clean | 21,404 | 18,193 (85.0%) | 3211 (15.0%) | 0.694 | 244 |
| Fouled | 21,053 | 17,806 (84.6%) | 3247 (15.4%) | 0.683 | 253 |
| Clogged | 20,796 | 17,526 (84.3%) | 3270 (15.7%) | 0.675 | 269 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldi, N.; Casari, N.; Pinelli, M.; Suman, A.; Vulpio, A. Performance Degradation of a Shell-and-Tube Heat Exchanger Due to Tar Deposition. Energies 2022, 15, 1490. https://doi.org/10.3390/en15041490
Aldi N, Casari N, Pinelli M, Suman A, Vulpio A. Performance Degradation of a Shell-and-Tube Heat Exchanger Due to Tar Deposition. Energies. 2022; 15(4):1490. https://doi.org/10.3390/en15041490
Chicago/Turabian StyleAldi, Nicola, Nicola Casari, Michele Pinelli, Alessio Suman, and Alessandro Vulpio. 2022. "Performance Degradation of a Shell-and-Tube Heat Exchanger Due to Tar Deposition" Energies 15, no. 4: 1490. https://doi.org/10.3390/en15041490
APA StyleAldi, N., Casari, N., Pinelli, M., Suman, A., & Vulpio, A. (2022). Performance Degradation of a Shell-and-Tube Heat Exchanger Due to Tar Deposition. Energies, 15(4), 1490. https://doi.org/10.3390/en15041490









