Comparison of Pyrolysis and Combustion Processes of Vinyl Floor Panels Using Thermogravimetric Analysis (TG-FTIR) in Terms of the Circular Economy
Abstract
:1. Introduction
Chemical Recycling
2. Material Characteristics and Methods
- Vinyl Floor Panel VP1
2.1. Physicochemical Analysis
2.2. TG-MS/FTIR Measurements
- Pyrolysis
- Combustion
3. Results and Discussion
3.1. Physicochemical Properties of the Sample
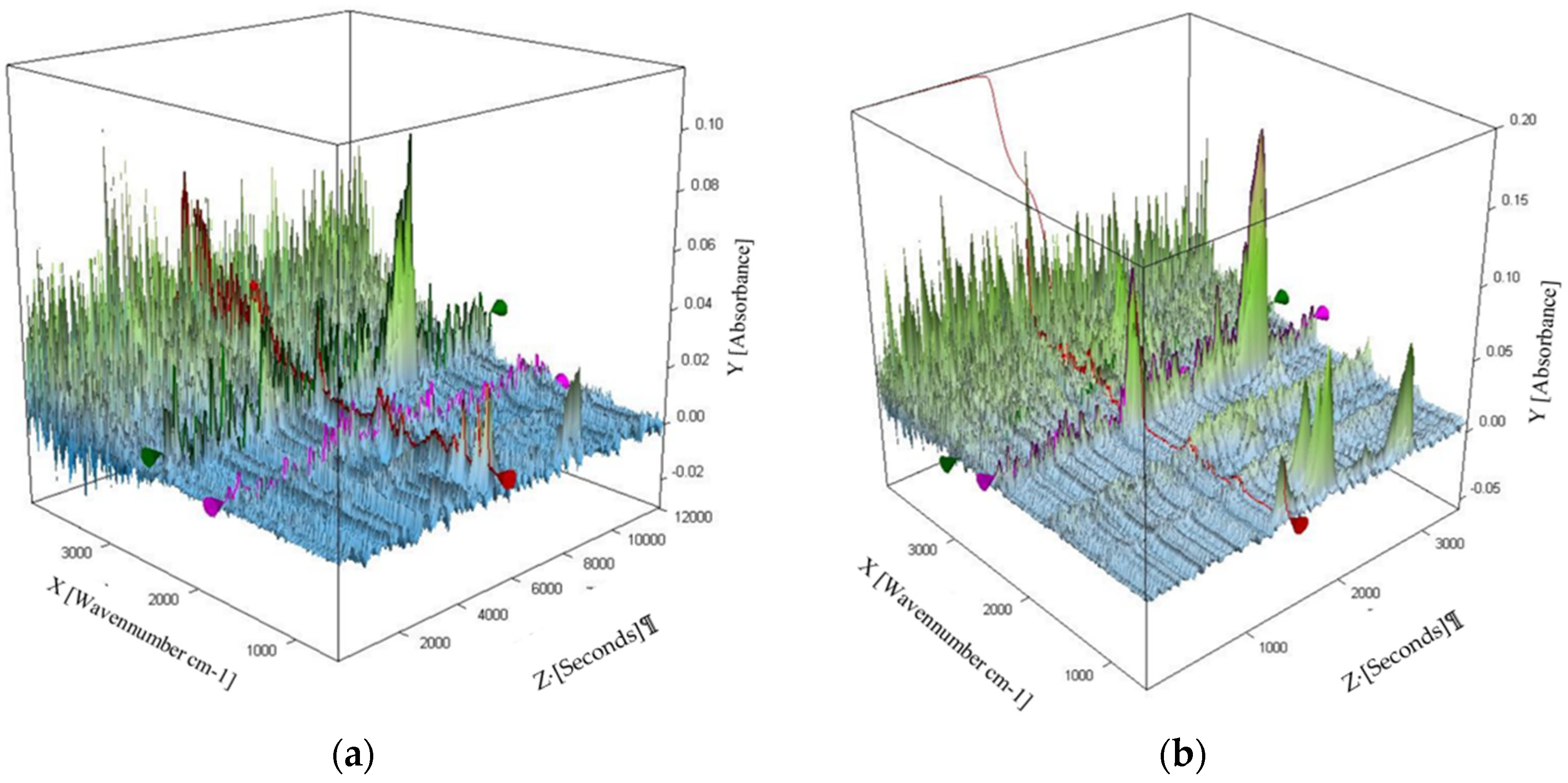
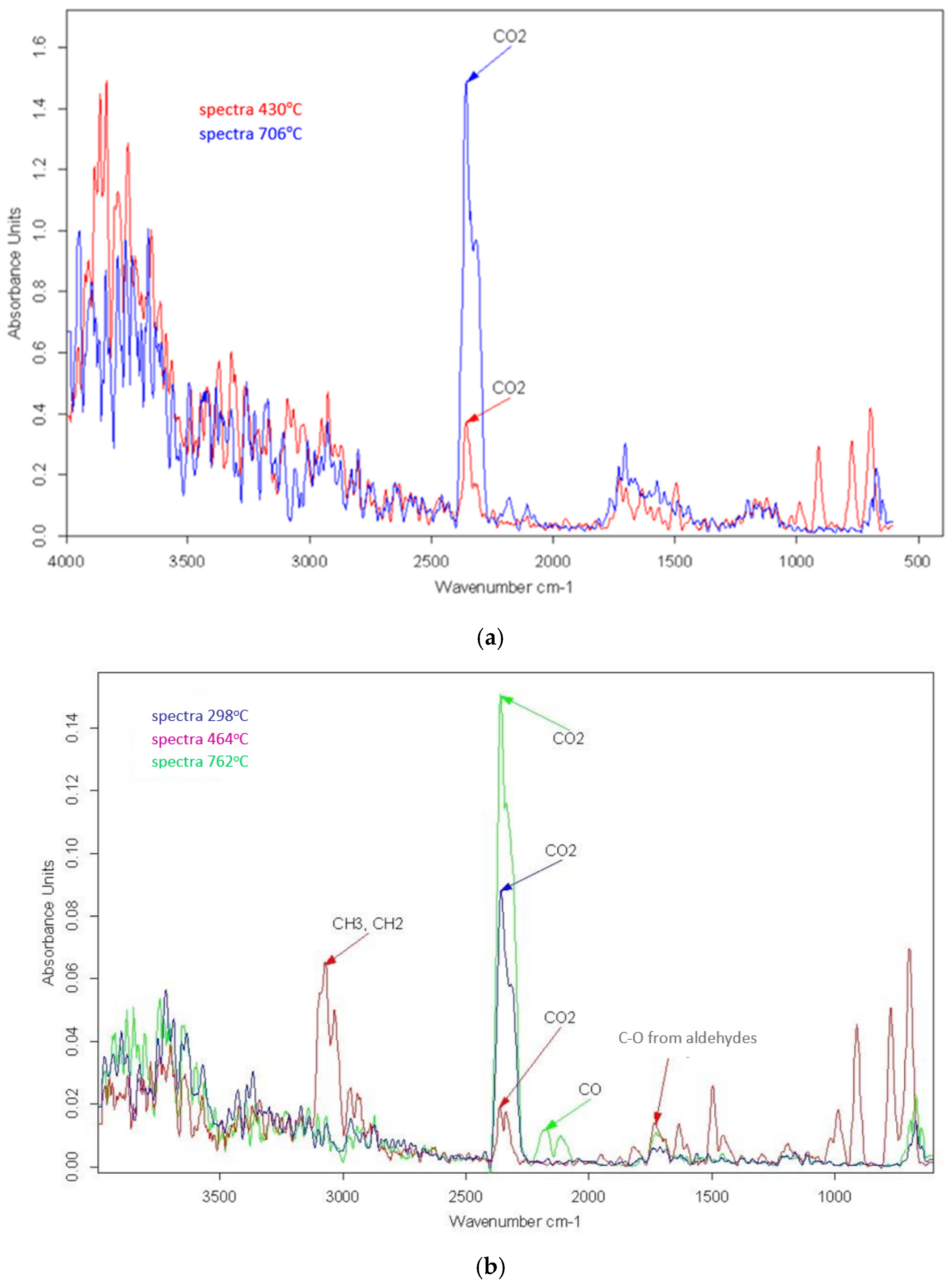
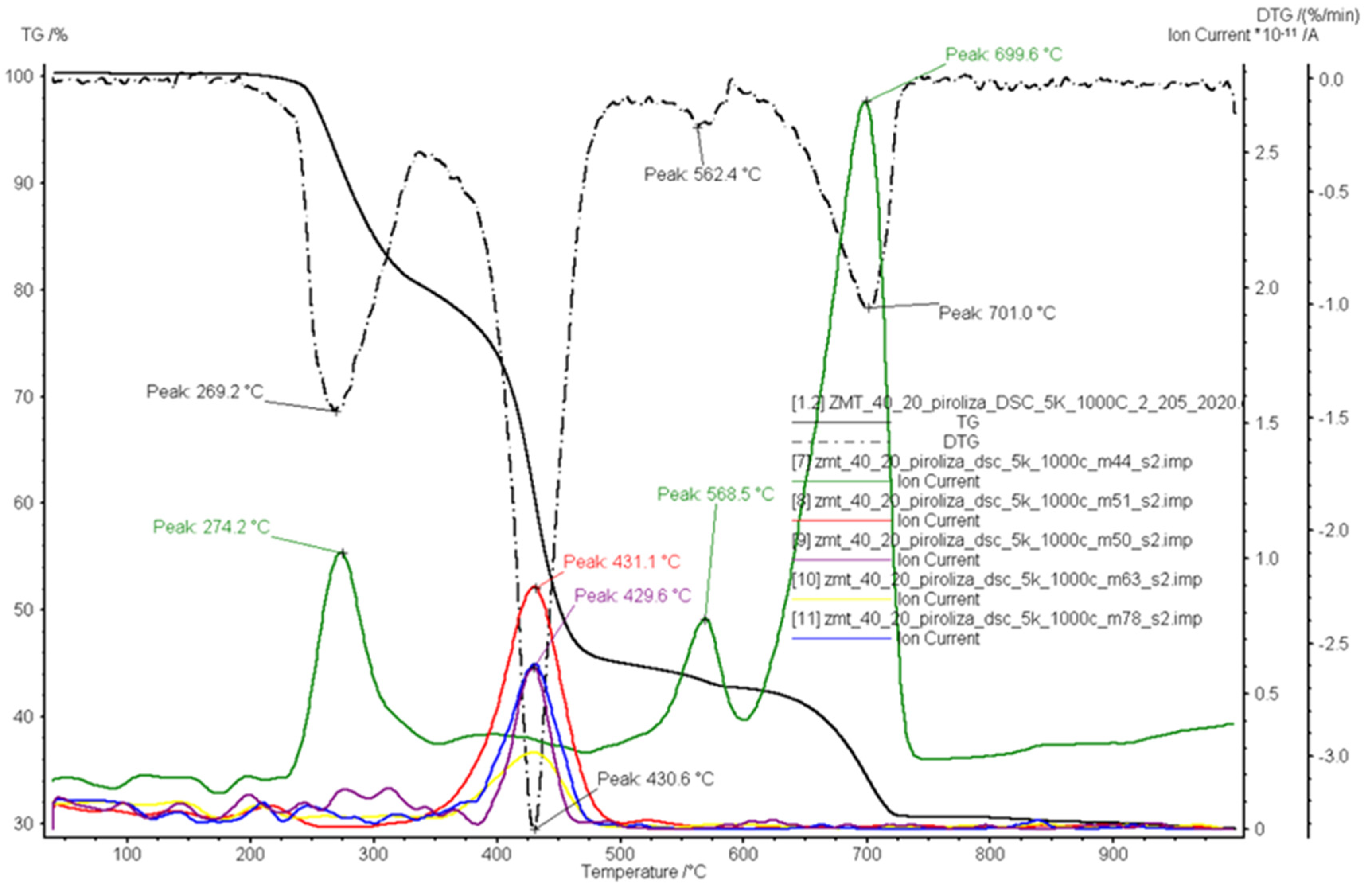
3.2. Pyrolysis
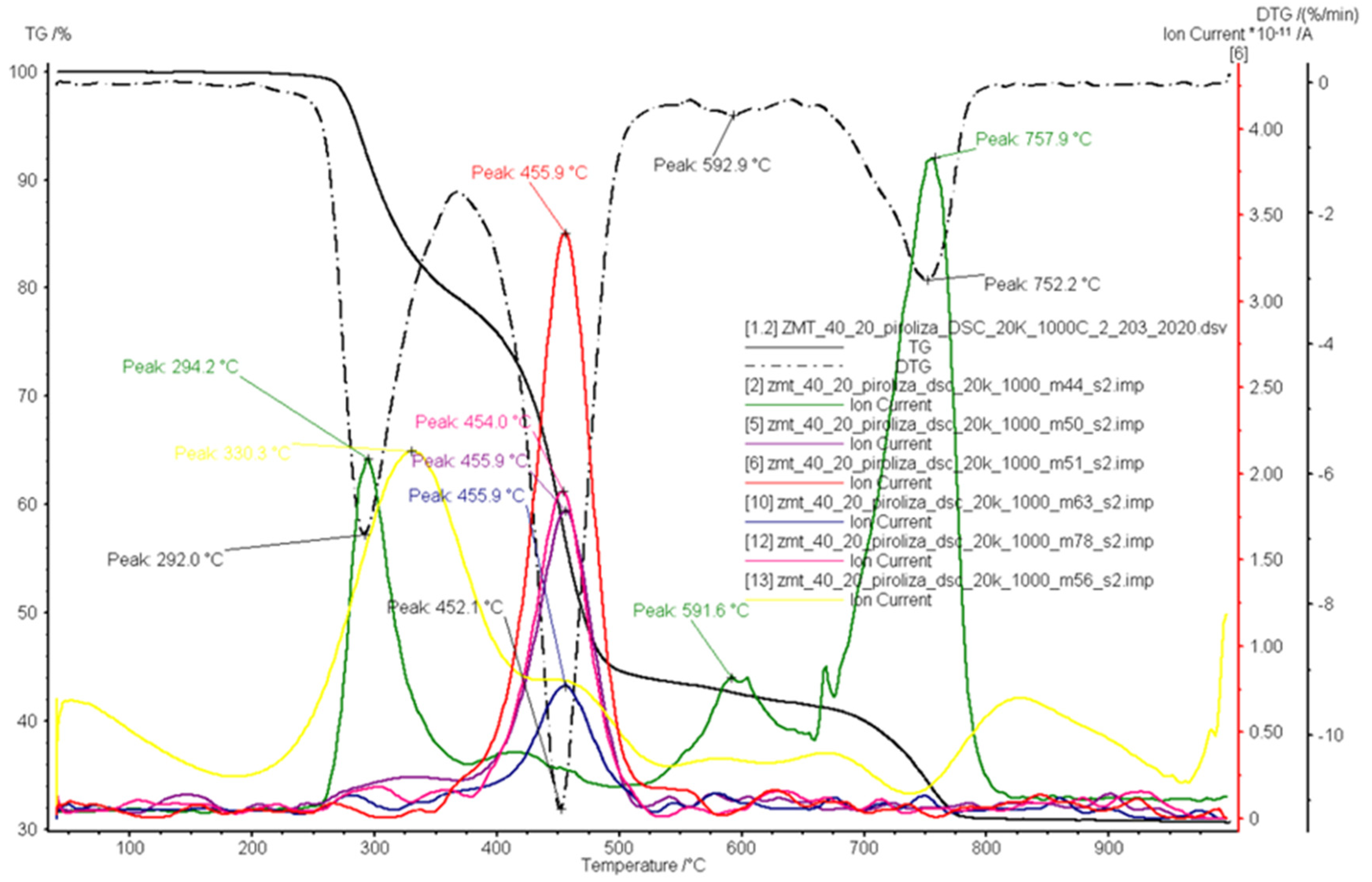
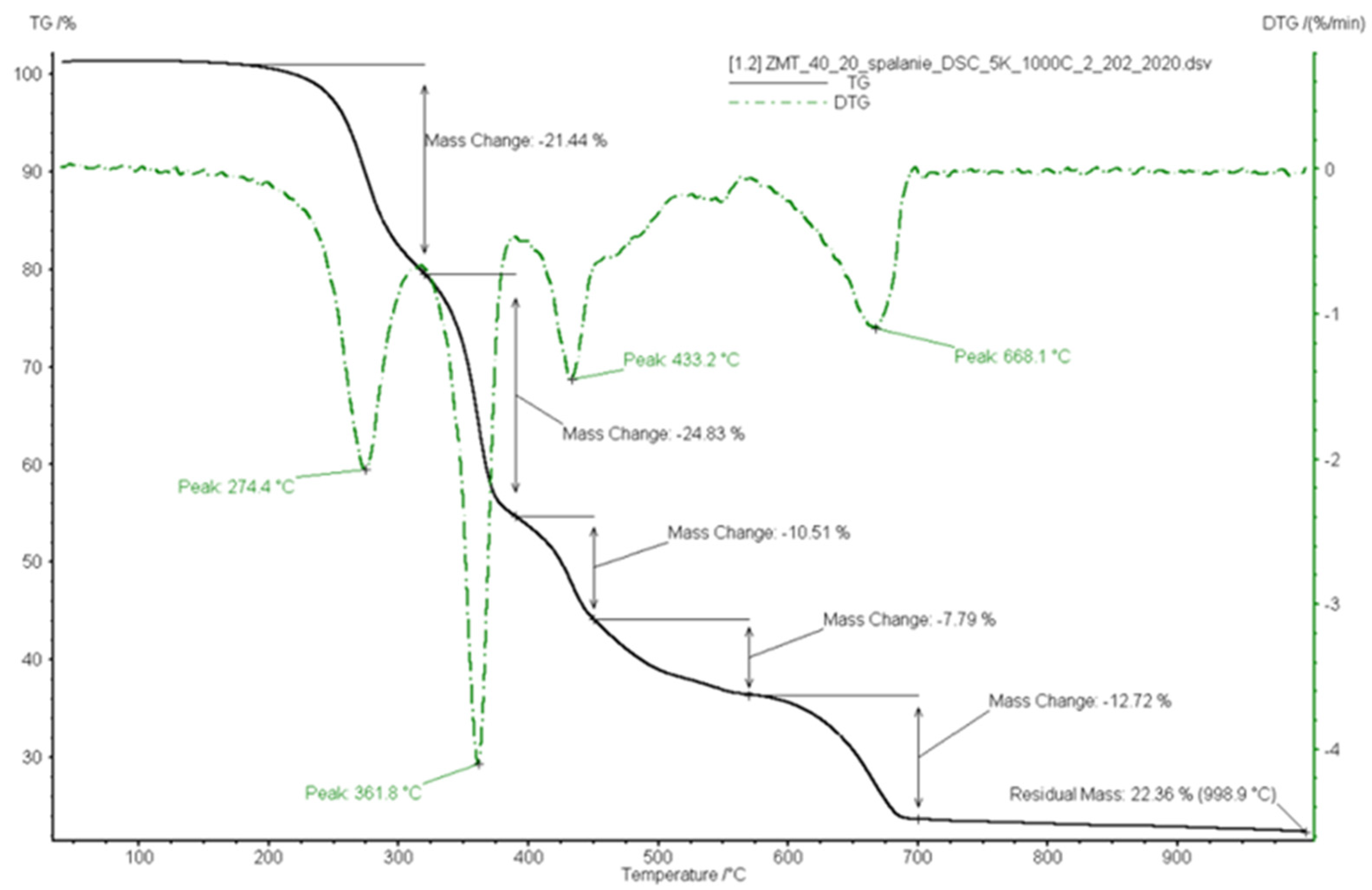
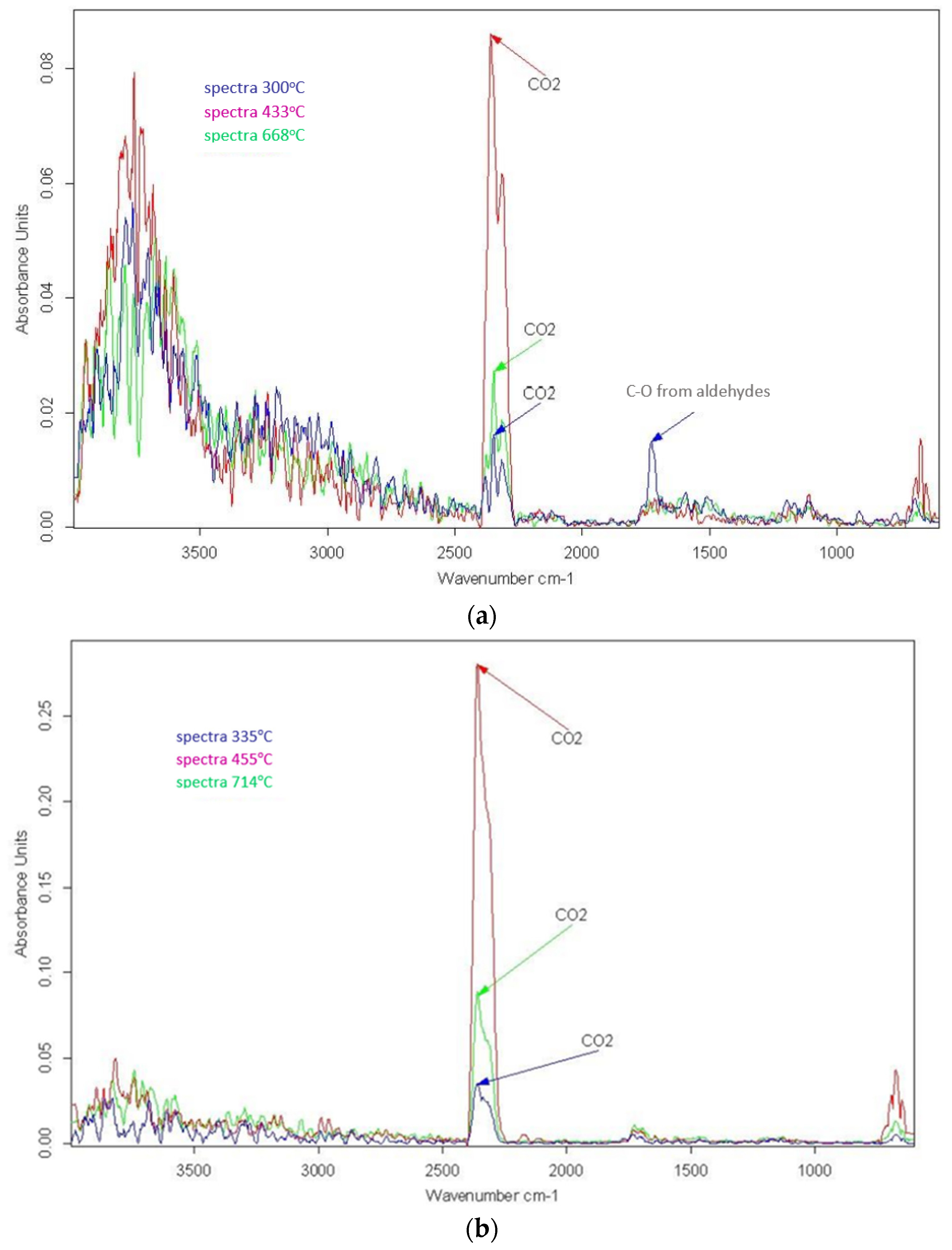
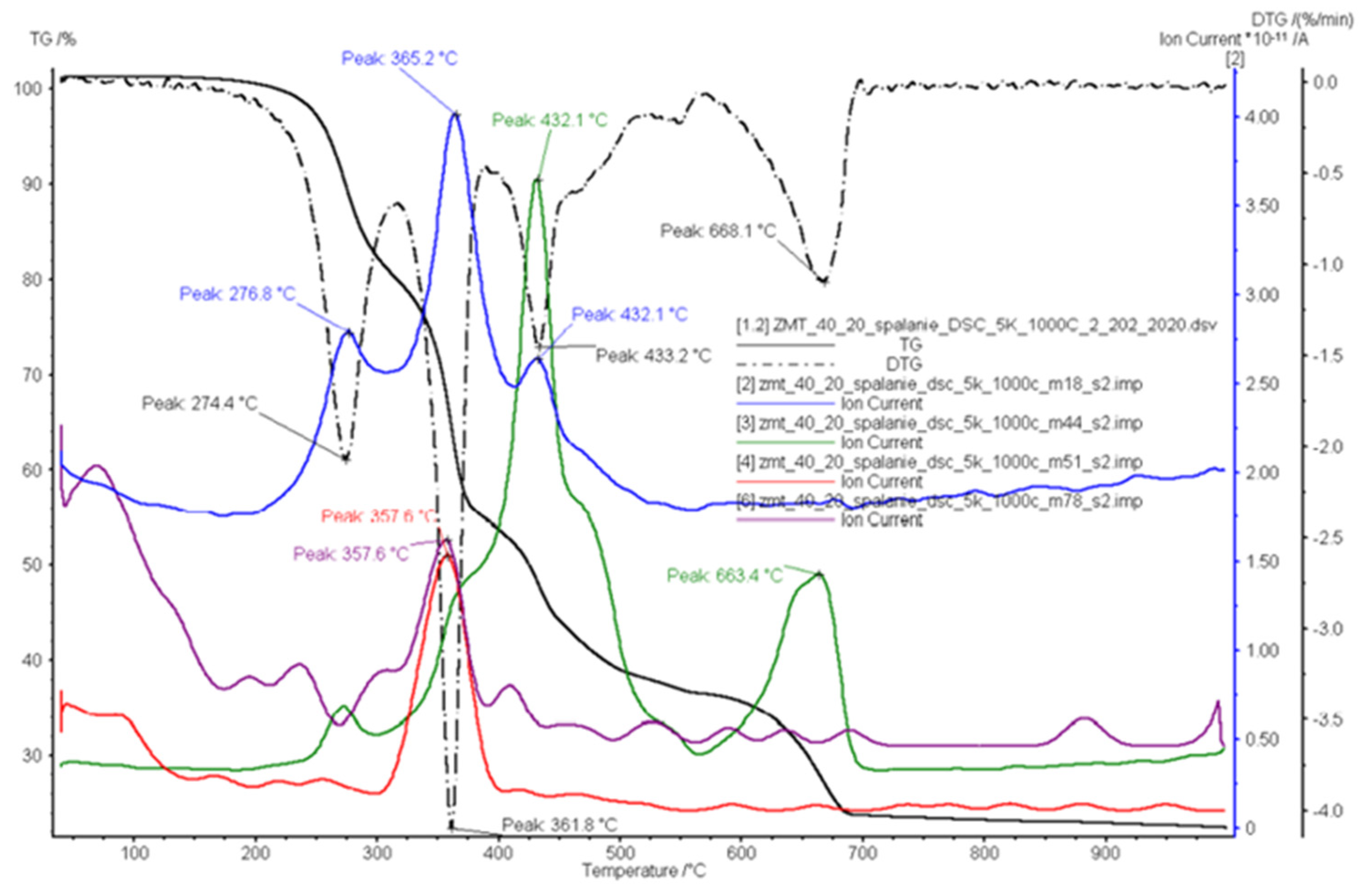
3.3. Combustion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| TG-MS | thermogravimetry-mass spectrometry |
| DTG | differential thermogravimetry |
| TG-FTIR | thermogravimetry-Fourier transform infrared spectroscopy |
| 3D-FTIR | three-dimensional thermogravimetric analysis-Fourier transform infrared |
| PVC | polyvinyl chloride |
References
- Plastics—The Facts 2019. An analysis of European Plastics Production, Demand and Waste Data. Available online: https://plasticseurope.org/wp-content/uploads/2021/10/2019-Plastics-the-facts.pdf (accessed on 28 July 2021).
- Zhu, H.; Jiang, X.; Yan, J.; Chi, Y.; Cen, K. TG-FTIR analysis of PVC thermal degradation and HCl removal. J. Anal. Appl. Pyrolysis 2008, 82, 1–9. [Google Scholar] [CrossRef]
- Huggett, C.; Levin, B.C. Toxicity of the Pyrolysis and Combustion Products of Poly (Vinyl Chlorides): A Literature Assess-ment. Fire Mater. 1987, 11, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Knümann, R.; Bockhorn, H. Investigation of the Kinetics of Pyrolysis of PVC by TG-MS-Analysis. Combust. Sci. Technol. 1994, 101, 285–299. [Google Scholar] [CrossRef]
- Lee, T.; Oh, J.-I.; Kim, T.; Tsang, D.C.; Kim, K.-H.; Lee, J.; Kwon, E.E. Controlling generation of benzenes and polycyclic aromatic hydrocarbons in thermolysis of polyvinyl chloride in CO2. Energy Convers. Manag. 2018, 164, 453–459. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, R.; Wang, X.; He, J.; Wang, J. Pyrolysis and Combustion of Polyvinyl Chloride (PVC) Sheath for New and Aged Cables via Thermogravimetric Analysis-Fourier Transform Infrared (TG-FTIR) and Calorimeter. Materials 2018, 11, 1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, L. Vinyl: The Plastic Found in (Almost) Everything. Available online: https://www.treehugger.com/vinyl-plastic-found-almost-everything-4847568 (accessed on 28 July 2021).
- Vinyl Flooring Market Size, Share & Trends Analysis Report By Product (Vinyl Sheets, Vinyl Tiles, Luxury Vinyl Tiles), By Application (Residential, Commercial), By Region, And Segment Forecasts, 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/vinyl-flooring-market (accessed on 28 July 2021).
- Czy Podłogi Winylowe Można Poddać Recyklingowi? 2021. Available online: Pl.EcoBuilderz.com (accessed on 4 November 2021).
- McKeen, L. Introduction to Plastics and Polymers. In The Effect of Sterilization Methods on Plastics and Elastomers, 4th ed.; William Andrew, an imprint of Elsevier: Oxford, UK, 2018; pp. 41–61. [Google Scholar]
- Gilbert, M.; Patrick, S. Poly(Vinyl Chloride). In Brydson’s Plastics Materials, 8th ed.; William Andrew, an imprint of Elsevier: Oxford, UK, 2017; pp. 329–388. [Google Scholar]
- Chaudhary, B.; Liotta, C.; Cogen, J.; Gilbert, M. Plasticized PVC. Ref. Modul. Mater. Sci. Mater. Eng. 2016. [Google Scholar] [CrossRef]
- Biron, M. Recycling Plastics: Advantages and Limitations of Use. In A Practical Guide to Plastics Sustainability; William Andrew, an imprint of Elsevier: Oxford, UK, 2020; pp. 411–467. [Google Scholar]
- Shrivastava, A. Additives for Plastics. In Introduction to Plastics Engineering; Elsevier: Oxford, UK, 2018; pp. 111–141. [Google Scholar] [CrossRef]
- BASF. We create chemistry. Available online: https://www.kongrespolskachemia.pl/files/84723514/basffactsheet-chemcyclingengot-2pl_1.pdf (accessed on 31 January 2022).
- Communication from the Commission to the European Parliament, the Council, the European Economic And Social Committee and the Committee of the Regions a European Strategy for Plastics in a Circular Economy COM/2018/028 final. Available online: https://op.europa.eu/en/publication-detail/-/publication/2df5d1d2-fac7-11e7-b8f5-01aa75ed71a1/language-en (accessed on 4 November 2021).
- Sobiecka, E. Thermal and physicochemical technologies used in hospital incineration fly ash utilization before landfill in Poland. J. Chem. Technol. Biotechnol. 2016, 91, 2457–2461. [Google Scholar] [CrossRef]
- Ciuła, J.; Kozik, V.; Generowicz, A.; Gaska, K.; Bak, A.; Paździor, M.; Barbusiński, K. Emission and Neutralization of Methane from a Municipal Landfill-Parametric Analysis. Energies 2020, 13, 6254. [Google Scholar] [CrossRef]
- Kicińska, A.; Caba, G. Leaching of Chlorides, Sulphates, and Phosphates from Ashes Formed as a Result of Burning Conventional Fuels, Alternative Fuels, and Municipal Waste in Household Furnaces. Energies 2021, 14, 3936. [Google Scholar] [CrossRef]
- Kosa, B.; Kicińska, A.; Filipowicz, M.; Dudek, M.; Olkuski, T.; Styszko, K. Coal from the waste disposal site of the Siersza mine (Trzebinia, Poland) and its properties as a possible alternative fuel. E3S Web Conf. 2016, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Ustawa o odpadach (Dz. U. 2013 poz. 23 z póz. zm). Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20130000021 (accessed on 26 July 2021).
- Zeller, M.; Netsch, N.; Richter, F.; Leibold, H.; Stapf, D. Chemical Recycling of Mixed Plastic Wastes by Pyrolysis—Pilot Scale Investigations. Chem. Ing. Tech. 2021, 93, 1763–1770. [Google Scholar] [CrossRef]
- Beneš, M.; Milanov, N.; Matuschek, G.; Kettrup, A.; Plaček, V.; Balek, V. Thermal degradation of PVC cable insulation studied by simultaneous TG-FTIR and TG-EGA methods. J. Therm. Anal. 2004, 78, 621–630. [Google Scholar] [CrossRef]
- Jaworski, T.; Kajda-Szcześniak, M. Research on the Kinetics of Pyrolysis of Wood-Based Panels in Terms of Waste Management. Energies 2019, 12, 3705. [Google Scholar] [CrossRef] [Green Version]
- Aboulkas, A.; El Harfi, K. Co-pyrolysis of olive residue with poly(vinyl chloride) using thermogravimetric analysis. J. Therm. Anal. Calorim. 2009, 95, 1007–1013. [Google Scholar] [CrossRef]
- Czégény, Z.; Jakab, E.; Bozi, J.; Blazsó, M. Pyrolysis of wood—PVC mixtures. Formation of chloromethane from lignocellulosic materials in the presence of PVC. J. Anal. Appl. Pyrol. 2015, 113, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Long, Y.; Meng, A.; Li, Q.; Zhang, Y. Interactions of three municipal solid waste components during co-pyrolysis. J. Anal. Appl. Pyrolysis 2015, 111, 265–271. [Google Scholar] [CrossRef]
- Bittencourt, P.R.S.; Scremin, F. Evolved Gas Analysis of PE:PVC Systems Thermodegradation Under Inert and Oxidizing Atmosphere. J. Polym. Environ. 2019, 27, 612–617. [Google Scholar] [CrossRef]
- Xiu, F.-R.; Lu, Y.; Qi, Y. DEHP degradation and dechlorination of polyvinyl chloride waste in subcritical water with alkali and ethanol: A comparative study. Chemosphere 2020, 249, 126138. [Google Scholar] [CrossRef]
- Nozue, K.; Tagaya, H. Chemical recycling of waste Poly Vinyl Chloride (PVC) by the liquid-phase treatment. J. Mater. Cycles Waste Manag. 2021, 23, 489–504. [Google Scholar] [CrossRef]
- Senhadji, Y.; Siad, H.; Escadeillas, G.; Benosman, A.S.; Chihaoui, R.; Mouli, M.; Lachemi, M. Physical, mechanical and thermal properties of lightweight composite mortars containing recycled polyvinyl chloride. Constr. Build. Mater. 2019, 195, 198–207. [Google Scholar] [CrossRef]
- Jaworski, T.; Kajda-Szcześniak, M. Study on the Similarity of the Parameters of Biomass and Solid Waste Fuel Combustion for the Needs of Thermal Power Engineering. Sustainability 2020, 12, 7894. [Google Scholar] [CrossRef]
- PN-EN 15934:2013-02; Determination of Moisture Content. Available online: https://sklep.pkn.pl/pn-en-15934-2013-02p.html (accessed on 1 July 2021).
- PN-EN ISO 21656:2021-08; Solid Secondary Fuels—Determination of Ash Content. Available online: https://sklep.pkn.pl/pn-en-iso-21656-2021-08e.html (accessed on 1 July 2021).
- PN-G-04516:1998; Solid Fuels—Determination of Volatile Matter Content. Available online: https://sklep.pkn.pl/pn-g-04516-1998p.html (accessed on 1 July 2021).
- PN-ISO 1928:2020-05; Solid Fuels—Determination of Combustion Heat in a Calorimetric Bomb and Calculation of Calorific Value. Available online: https://sklep.pkn.pl/pn-iso-1928-2020-05e.html (accessed on 1 July 2021).
- PN-G-04523:1992; Solid Fuels—Determination of Nitrogen Content by the Kjeldahl Method. Available online: https://sklep.pkn.pl/pn-g-04523-1992p.html (accessed on 1 July 2021).
- PN-EN 15407:2011; Methods for the Determination of Carbon (C), Hydrogen (H) and Nitrogen (N) Content. Available online: https://www.en-standard.eu/une-en-15407-2011-solid-recovered-fuels-methods-for-the-determination-of-carbon-chydrogen-h-and-nitrogen-n-content/ (accessed on 1 July 2021).
- PN-ISO 334:1997; Determination of Sulphur with the Eschki Method. Available online: https://sklep.pkn.pl/pn-iso-334-1997p.html (accessed on 1 July 2021).
- PN-ISO 587:2000; Determination of Chloride Using the Eschki Mixture. Available online: https://sklep.pkn.pl/pn-iso-587-2000p.html (accessed on 1 July 2021).
- Miranda, R.; Yang, J.; Roy, C.; Vasile, C. Vacuum pyrolysis of PVC I. Kinetic study. Polym. Degrad. Stab. 1999, 64, 127–144. [Google Scholar] [CrossRef]
- Miranda, R.; Yang, J.; Roy, C.; Vasile, C. Vacuum pyrolysis of commingled plastics containing PVC I. Kinetic study. Polym. Degrad. Stab. 2001, 72, 469–491. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Zhang, J.; Yang, S.; Zhang, Z.; Zhao, W. Thermal degradation of flame-retarded high-voltage cable sheath and insulation via TG-FTIR. J. Anal. Appl. Pyrolysis 2018, 134, 167–175. [Google Scholar] [CrossRef]
- Rajendran, S.; Uma, T. Conductivity studies on PVCrPMMA polymer blend electrolyte. Mater. Lett. 2000, 44, 242–247. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C.; Du, L.; Duan, Y. TG–FTIR and Py–GC/MS analysis on pyrolysis and combustion of pine sawdust. J. Anal. Appl. Pyrolysis 2013, 100, 26–32. [Google Scholar] [CrossRef]
- Singh, R.; Pant, D. Polyvinyl chloride degradation by hybrid (chemical and biological) modification. Polym. Degrad. Stab. 2016, 123, 80–87. [Google Scholar] [CrossRef]
- Feng, J.; Hao, J.; Du, J.; Yang, R. Using TGA/FTIR TGA/MS and cone calorimetry to understand thermal degradation and flame retardancy mechanism of polycarbonate filled with solid bisphenol A bis(diphenyl phosphate) and montmorillonite. Polym. Degrad. Stab. 2012, 97, 605–614. [Google Scholar] [CrossRef]
- Qin, L.; Han, J.; Zhao, B.; Wang, Y.; Chen, W.; Xing, F. Thermal degradation of medical plastic waste by in-situ FTIR, TG-MS and TG-GC/MS coupled analyses. J. Anal. Appl. Pyrolysis 2018, 136, 132–145. [Google Scholar] [CrossRef]
- Han, J.; Li, W.; Liu, D.; Qin, L.; Chen, W.; Xing, F. Pyrolysis characteristic and mechanism of waste tyre: A thermogravimetry-mass spectrometry analysis. J. Anal. Appl. Pyrolysis 2018, 129, 1–5. [Google Scholar] [CrossRef]
- Anthony, G.M. Kinetic and chemical studies of polymer cross-linking using thermal gravimetry and hyphenated methods. Degradation of polyvinylchloride. Polym. Degrad. Stab. 1999, 64, 353–357. [Google Scholar] [CrossRef]







| Parameter | VP1 |
|---|---|
| Total moisture, % mass | 0.49 |
| Combustible fraction, % dry mass | 62.67 |
| Ash, % dry mass | 37.33 |
| Volatile fraction, % dry mass | 42.49 |
| High Heating Value, MJ/kg dry mass | 10.41 |
| Low Heating Value, MJ/kg dry mass | 10.06 |
| Carbon, % dry mass | 32.39 |
| Hydrogen, % dry mass | 1.27 |
| Oxygen, % dry mass | 15.96 |
| Nitrogen, % dry mass | 0.15 |
| Sulphur, % dry mass | 0.29 |
| Chloride, % dry mass | 12.61 |
| Wavenumber, cm−1 | Assignment | Reference |
|---|---|---|
| 1800–1300; 4000–3500 | H2O | [7,44,45,46] |
| circa 2910–2930 | Alkanes C-H stretching | [44] |
| 2400–2260 | CO2 | [7,44] |
| 1458–1423 | C-H aliphatic bending bond | [44,47] |
| 1240 | C-H bending near Cl | [44,48] |
| 550–850 | C-Cl stretch | [7,44,45] |
| Ion Current Intensity m/z | Possible Products | Reference |
|---|---|---|
| 44 | carbon dioxide/propane(CO2/C3H8) | [49,50] |
| 50 | 1,3-butadiyne(C4H2) | [49,50] |
| 51 | vinylacetylene(C4H4); 1,3-butadiene(C4H6) | [49] |
| 56 | 1-butene/2-methylpropene(C4H8); butyl radical(C4H9) | [49,50] |
| 63 | 1,3-cyclopentadiene(C5H6) | [49] |
| 78 | benzene(C6H6) | [48,49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajda-Szcześniak, M.; Czop, M. Comparison of Pyrolysis and Combustion Processes of Vinyl Floor Panels Using Thermogravimetric Analysis (TG-FTIR) in Terms of the Circular Economy. Energies 2022, 15, 1516. https://doi.org/10.3390/en15041516
Kajda-Szcześniak M, Czop M. Comparison of Pyrolysis and Combustion Processes of Vinyl Floor Panels Using Thermogravimetric Analysis (TG-FTIR) in Terms of the Circular Economy. Energies. 2022; 15(4):1516. https://doi.org/10.3390/en15041516
Chicago/Turabian StyleKajda-Szcześniak, Małgorzata, and Monika Czop. 2022. "Comparison of Pyrolysis and Combustion Processes of Vinyl Floor Panels Using Thermogravimetric Analysis (TG-FTIR) in Terms of the Circular Economy" Energies 15, no. 4: 1516. https://doi.org/10.3390/en15041516







