An NMR-Based Method for Multiphase Methane Characterization in Coals
Abstract
:1. Introduction
2. Experimental Methods
2.1. Coal Sampling
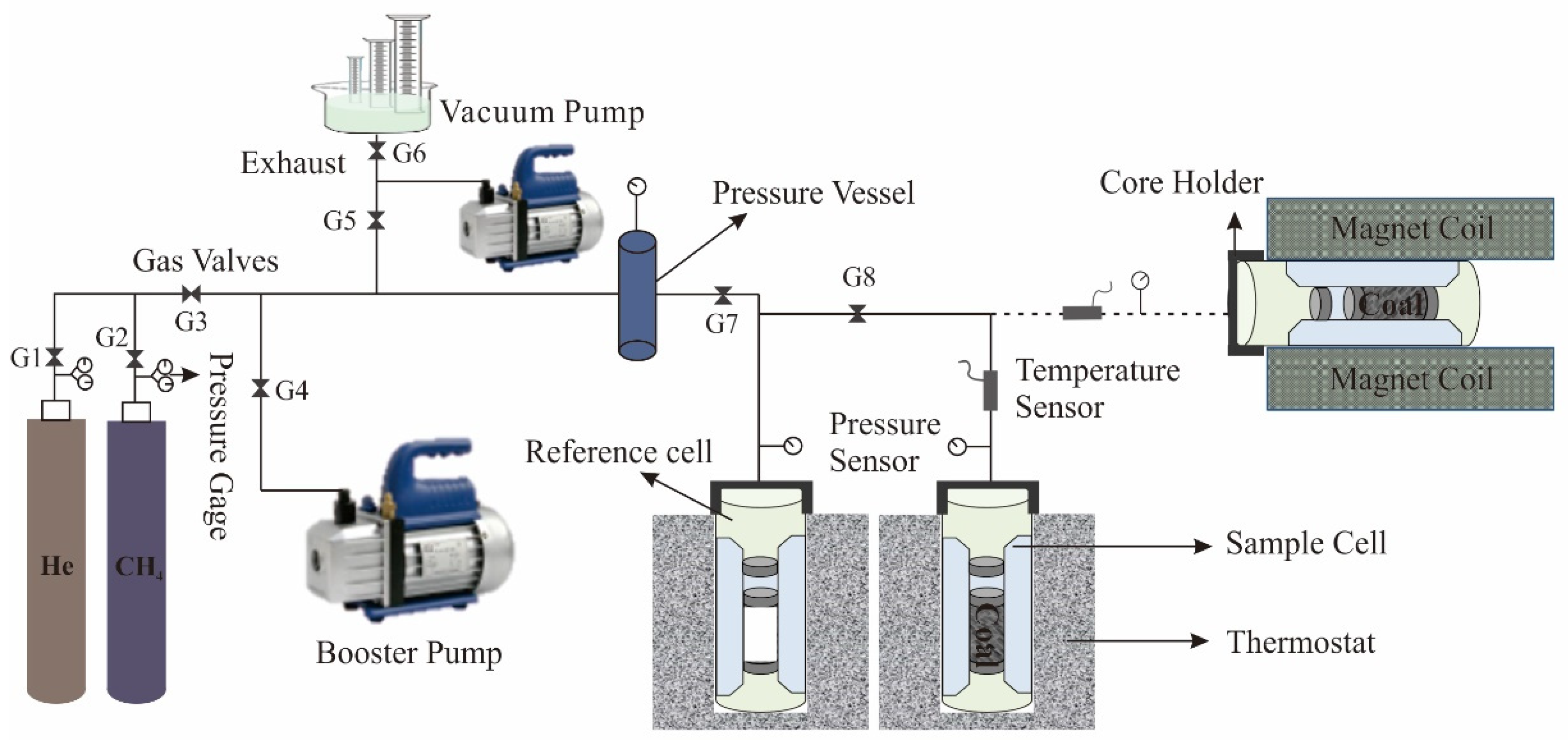
2.2. NMR Adsorption Measurements
3. Results and Discussions
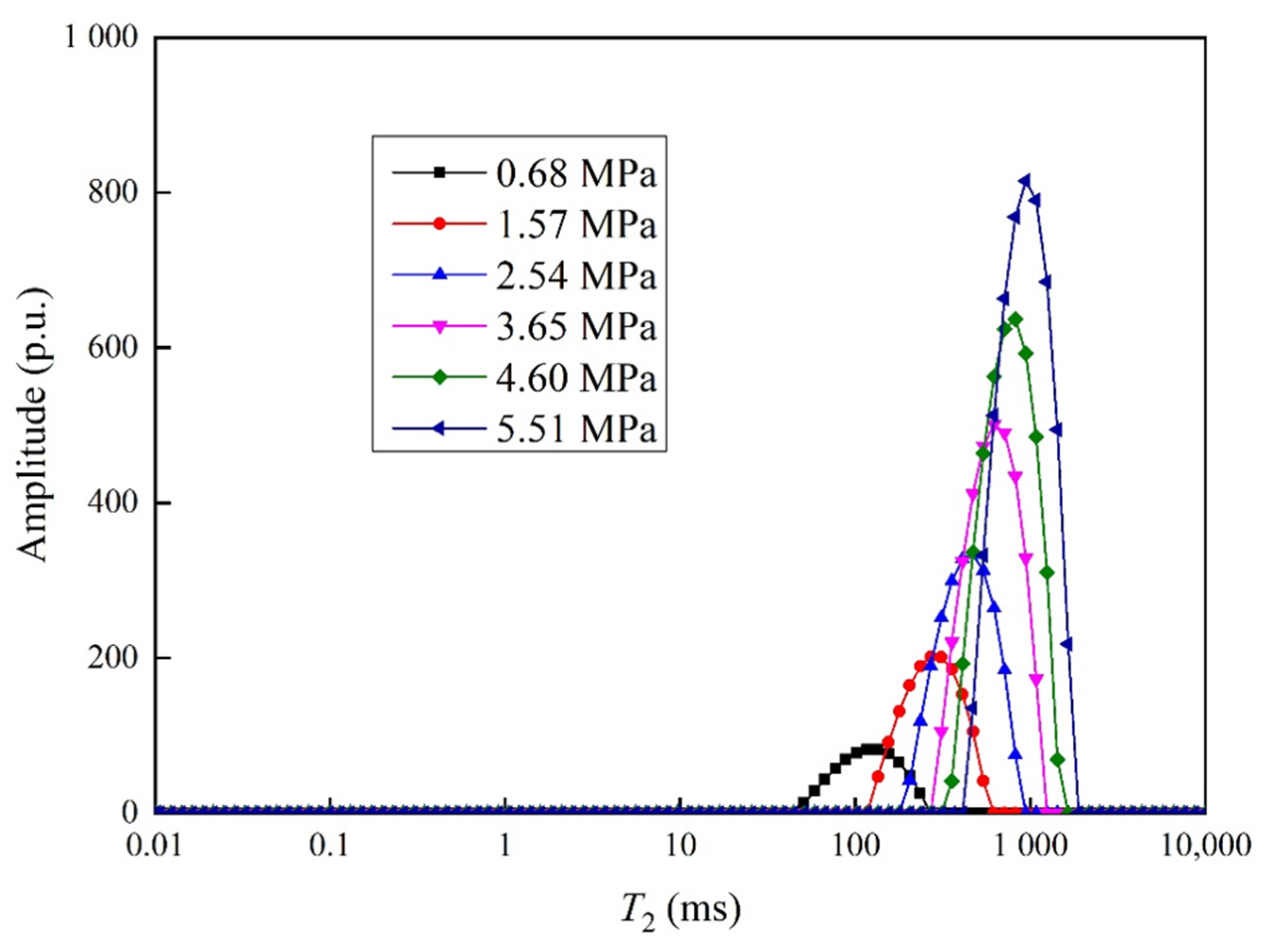
3.1. Free Methane T2 Distributions
3.2. Methane Adsorption T2 Relaxation Characteristics
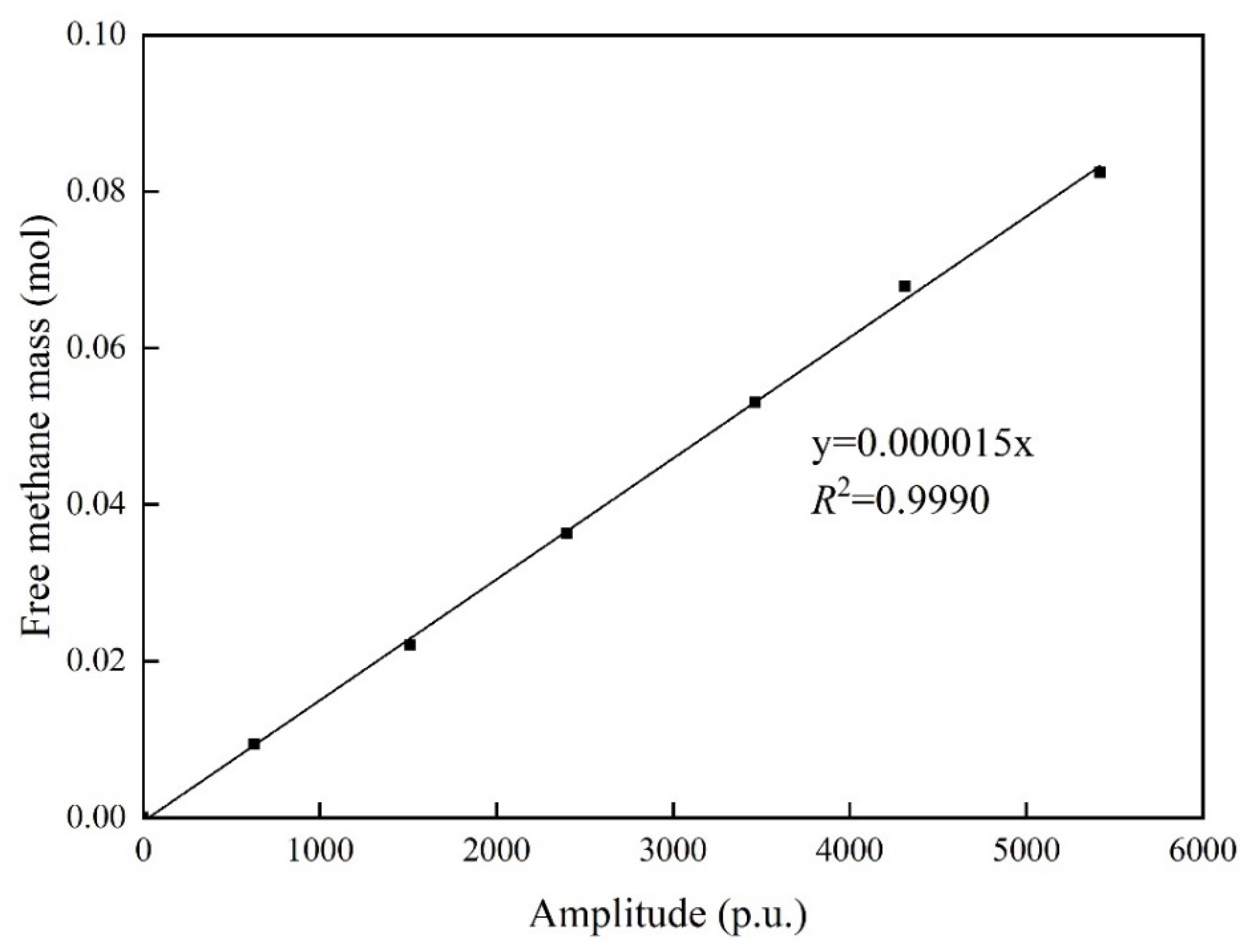
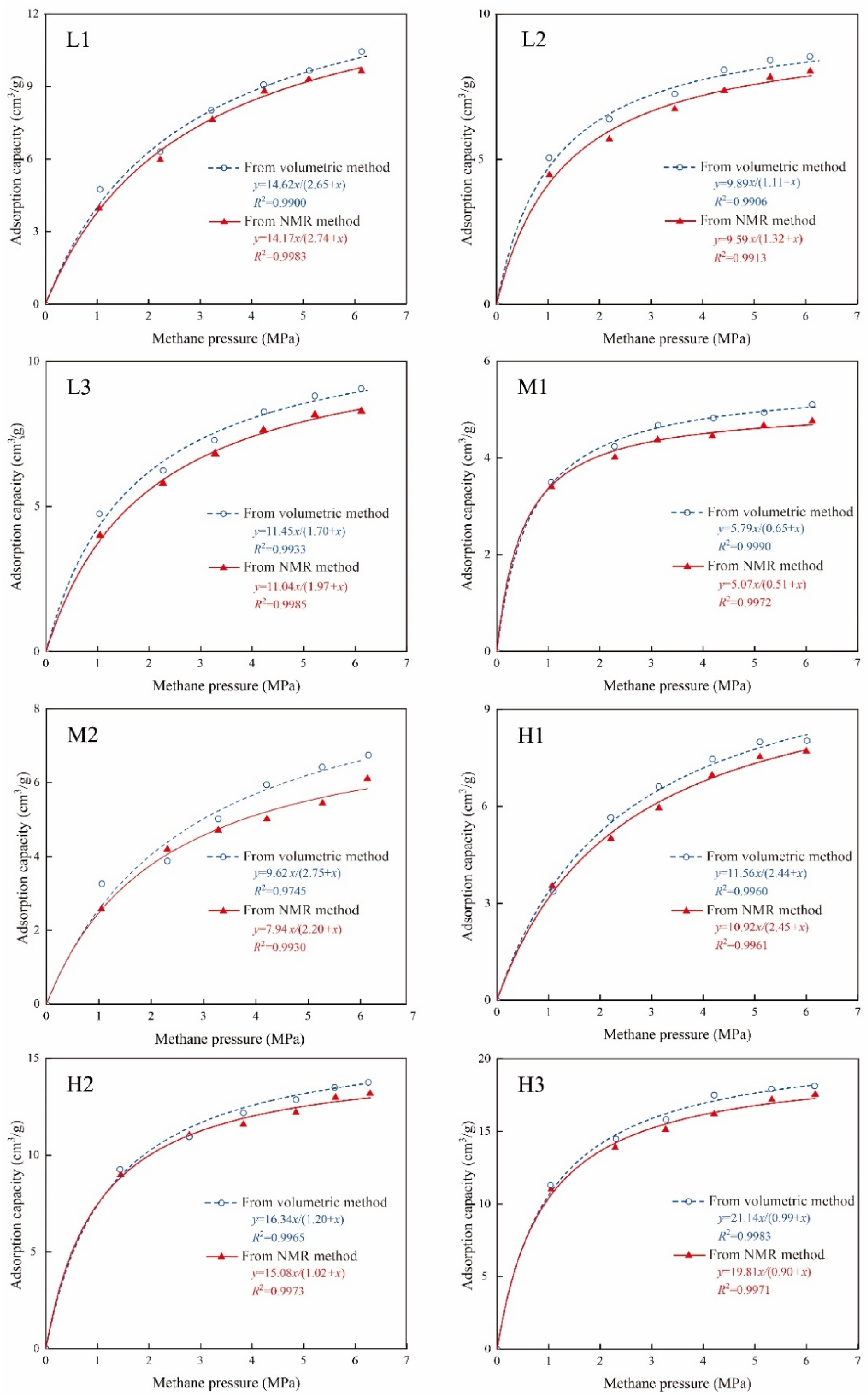
3.3. Methane Adsorption Capacity Determination
3.3.1. Volumetric-Based Method
3.3.2. NMR-Based Method
3.3.3. Validity Application of the NMR Method
3.4. Methane Adsorption Dynamic Process Characterization
4. Conclusions
- (1)
- The NMR T2 distributions of methane in coals exhibit three peaks, which are P1 (T2 < 8 ms), P2 (T2 = 20–300), and P3 (T2 > 300 ms), corresponding to adsorbed phase methane, free phase methane between pores, and free phase methane in the sample cell, respectively.
- (2)
- According to the results from the self-designed NMR isotherm adsorption measurements for eight coal samples, the NMR-based Langmuir volume ranges from 5.07 to 19.81 cm3/g. The adsorption isothermal curves evaluated from the NMR and volumetric methods show an excellent agreement, and the Langmuir volume relative deviation, of approximately ~6.0%, was within an allowable limit.
- (3)
- The NMR technique provides an alternative method for dynamic monitoring of multiphase methane in the methane adsorption process, which is difficult to implement using conventional methods, such as the volumetric- and gravimetric-based methods.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, X.H.; Qin, Y.; Wang, G.X.; Rudolph, V. Evaluation of coal structure and permeability with the aid of geophysical logging technology. Fuel 2009, 88, 2278–2285. [Google Scholar] [CrossRef]
- Karacan, C.Ö.; Ruiz, F.A.; Cotè, M.; Phipps, S. Coal mine methane: A review of capture and utilization practices with benefits to mining safety and to greenhouse gas reduction. Int. J. Coal Geol. 2011, 86, 121–156. [Google Scholar] [CrossRef]
- Moore, T.A. Coalbed methane: A review. Int. J. Coal Geol. 2012, 101, 36–81. [Google Scholar] [CrossRef]
- Cai, Y.D.; Liu, D.M.; Mathews, J.P.; Pan, Z.J.; Elsworth, D.; Yao, Y.B.; Li, J.Q.; Guo, X.Q. Permeability evolution in fractured coal—Combining triaxial confinement with X-ray computed tomography, acoustic emission and ultrasonic techniques. Int. J. Coal Geol. 2014, 122, 91–104. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Tang, D.Z.; Gan, Q.; Niu, X.L.; Wang, K.; Shen, R.Y. Coal pore size distributions controlled by the coalification process: An experimental study of coals from the Junggar, Ordos and Qinshui basins in China. Fuel 2017, 206, 352–363. [Google Scholar] [CrossRef]
- Li, Y.; Tang, D.Z.; Wu, P.; Niu, X.L.; Wang, K.; Qian, P.; Wang, Z.S. Continuous unconventional natural gas accumulations of Carboniferous-Permian coal-bearing strata in the Linxing area, northeastern Ordos basin, China. J. Nat. Gas Sci. Eng. 2016, 36, 317–324. [Google Scholar] [CrossRef]
- Cai, Y.D.; Liu, D.M.; Yao, Y.B.; Li, J.Q.; Qiu, Y.K. Geological controls on prediction of coalbed methane of No. 3 coal seam in Southern Qinshui Basin, North China. Int. J. Coal Geol. 2011, 88, 101–112. [Google Scholar] [CrossRef]
- Zheng, S.J.; Yao, Y.B.; Liu, D.M.; Cai, Y.D.; Liu, Y.; Li, X.W. Nuclear magnetic resonance T2 cutos of coals: A novel method by multifractal analysis. Fuel 2019, 241, 715–724. [Google Scholar] [CrossRef]
- Li, J.Q.; Lu, S.F.; Xie, L.J.; Zhang, J.; Xue, H.T.; Zhang, P.F.; Tian, S.S. Modeling of hydrocarbon adsorption on continental oil shale: A case study on n-alkane. Fuel 2021, 206, 603–613. [Google Scholar] [CrossRef]
- Li, J.Q.; Wang, S.Y.; Lu, S.F.; Zhang, P.F.; Cai, J.C.; Zhao, J.H.; Li, W.B. Microdistribution and mobility of water in gas shale: A theoretical and experimental study. Mar. Petrol. Geol. 2019, 102, 496–507. [Google Scholar] [CrossRef]
- Hou, H.H.; Shao, L.Y.; Li, Y.H.; Li, Z.; Wang, S.; Zhang, W.L.; Wang, X.T. Influence of coal petrology on methane adsorption capacity of the Middle Jurassic coal in the Yuqia Coalfield, northern Qaidam Basin, China. J. Pet. Sci. Eng. 2017, 149, 218–227. [Google Scholar] [CrossRef]
- Li, J.Q.; Lu, S.F.; Zhang, P.F.; Cai, J.C.; Li, W.B.; Wang, S.Y.; Feng, W.J. Estimation of gas-in-place content in coal and shale reservoirs: A process analysis method and its preliminary application. Fuel 2020, 259, 116266. [Google Scholar] [CrossRef]
- Song, W.H.; Jun, Y.; Wang, D.Y.; Li, Y.; Sun, H.; Yang, Y.F. Dynamic pore network modelling of real gas transport in shale nanopore structure. J. Pet. Sci. Eng. 2020, 184, 105506. [Google Scholar] [CrossRef]
- Pajdak, A.; Kudasik, M.; Skoczylas, N.; Wierzbicki, M.; Teixeira Palla Braga, L. Studies on the competitive sorption of CO2 and CH4 on hard coal. Int. J. Greenh. Gas Con. 2019, 90, 102798. [Google Scholar] [CrossRef]
- Kudasik, M.; Skoczylas, N.; Pajdak, A. The repeatability of sorption processes occurring in the coal-methane system during multiple measurement series. Energies 2017, 10, 661. [Google Scholar] [CrossRef] [Green Version]
- Genserblum, Y.; van Hemert, P.; Billemont, P.; Bush, A.; Charriére, D.; Li, D.; Krooss, B.M.; de Weireld, G.; Prinz, D.; Wolf, H.A.A. European inter-laboratory comparison of high-pressure CO2 sorption isotherms. I: Activated carbon. Carbon 2009, 47, 2958–2969. [Google Scholar]
- Ye, Z.H.; Chen, D.; Pan, Z.J.; Zhang, G.Q.; Xia, Y.; Ding, X. An improved Langmuir model for evaluating methane adsorption capacity in shale under various pressures and temperatures. J. Nat. Gas Sci. Eng. 2016, 31, 658–680. [Google Scholar] [CrossRef]
- Krooss, B.M.; van Bergen, F.; Gensterblum, Y.; Siemons, N.; Pagnier, H.J.M.; David, P. High-pressure methane and carbon dioxide adsorption on dry and moisture-equilibrated Pennsylvanian coals. Int. J. Coal Geol. 2002, 51, 69–92. [Google Scholar] [CrossRef]
- Coates, G.R.; Xiao, L.Z.; Primmer, M.G. NMR Logging Principles and Applications; Gulf Publishing Company: Houston, TX, USA, 1999. [Google Scholar]
- Akkurt, R.; Vinegar, H.J.; Tutunjian, P.N.; Guillory, A.J. NMR logging of natural gas reservoirs. Log. Anal. 1996, 37, 33–42. [Google Scholar]
- Howard, J.J. Quantitative estimates of porous media wettability from proton NMR. Magn. Reson. Imaging 1998, 16, 529–533. [Google Scholar] [CrossRef]
- Zheng, S.J.; Yao, Y.B.; Liu, D.M.; Cai, Y.D.; Liu, Y. Characterizations of full-scale pore size distribution, porosity and permeability of coals: A novel methodology by nuclear magnetic resonance and fractal analysis theory. Int. J. Coal Geol. 2018, 196, 148–158. [Google Scholar] [CrossRef]
- Zheng, S.J.; Yao, Y.B.; Liu, D.M.; Cai, Y.D.; Liu, Y. Nuclear magnetic resonance surface relaxivity of coals. Int. J. Coal Geol. 2019, 205, 1–13. [Google Scholar] [CrossRef]
- Hari, P.; Chang, C.T.P.; Kulkarni, R.; Lien, J.R.; Watson, A.T. NMR characterization of hydrocarbon gas in porous media. Magn. Reson. Imaging 1998, 16, 545–547. [Google Scholar] [CrossRef]
- Safiullin, K.; Kuzmin, V.; Bogaychuk, A.; Alakshin, E.; González, L.M.; Kondratyeva, E.; Dolgorukov, G.; Gafurov, M.; Klochkov, A.; Tagirov, M. Determination of pores properties in rocks by means of helium-3 NMR: A case study of oil-bearing arkosic conglomerate from North belt of crude oil, Republic of Cuba. J. Petrol. Sci. Eng. 2022, 210, 110010. [Google Scholar] [CrossRef]
- Guo, R.; Mannhardt, D.; Kantzas, A. Characterizing moisture and gas content of coal by low-field NMR. J. Can. Pet. Technol. 2007, 46, 49–54. [Google Scholar] [CrossRef]
- Vasilenko, T.A.; Kirillov, A.K.; Molchanov, A.N.; Pronskii, E.A. An NMR study of the ratio between free and sorbed methane in the pores of fossil coals. Solid Fuel Chem. 2018, 52, 361–369. [Google Scholar] [CrossRef]
- Liu, J.; Yao, Y.B.; Liu, D.M.; Elsworth, D. Experimental evaluation of CO2 enhanced recovery of adsorbed-gas from shale. Int. J. Coal Geol. 2017, 179, 211–218. [Google Scholar] [CrossRef]
- Yao, Y.B.; Liu, J.; Liu, D.M.; Chen, J.Y.; Pan, Z.J. A new application of NMR in characterization of multiphase methane and adsorption capacity of shale. Int. J. Coal Geol. 2019, 131, 76–85. [Google Scholar] [CrossRef]
- Yao, Y.B.; Liu, D.M.; Xie, S.B. Quantitative characterization of methane adsorption on coal using a low-field NMR relaxation method. Int. J. Coal Geol. 2014, 201, 32–40. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.B.; Wang, J.; Pan, Z.J. Variation in permeability during CO2–CH4 displacement in coal seams: Part 1—Experimental insights. Fuel 2020, 263, 116666. [Google Scholar] [CrossRef]
- Yao, Y.B.; Liu, D.M.; Che, Y.; Tang, D.Z.; Tang, S.H.; Huang, W.H. Petrophysical characterization of coals by low-field nuclear magnetic resonance (NMR). Fuel 2010, 89, 1371–1380. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Rezaee, R.; Verrall, M.; Hu, S.Y.; Zou, J.; Testmanti, N. Pore characterization and clay bound water assessment in shale with a combination of NMR and low-pressure nitrogen gas adsorption. Int. J. Coal Geol. 2018, 194, 11–21. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Zhu, G.P.; Dong, Y.H.; Danesh, N.N.; Chen, Z.W.; Zhang, T. Comparison of low-field NMR and microfocus X-ray computed tomography in fractal characterization of pores in artificial cores. Fuel 2017, 210, 217–226. [Google Scholar] [CrossRef]
- Sun, X.X.; Yao, Y.B.; Liu, D.M.; Zhou, Y. Investigations of CO2-water wettability of coal: NMR relaxation method. Int. J. Coal Geol. 2018, 188, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.J.; Yao, Y.B.; Liu, D.M.; Cai, Y.D.; Liu, Y. Quantitative characterization of multiphase methane in coals using the NMR relaxation method. J. Petrol. Sci. Eng. 2021, 198, 108148. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Choi, S.K.; Airey, D.; Weniger, P. Estimation of gas adsorption capacity in coal: A review and an analytical study. Int. J. Coal Prep. Util. 2012, 32, 25–55. [Google Scholar] [CrossRef]
- Siemons, N.; Wolf, K.A.A.; Bruining, J. Interpretation of carbon dioxide diffusion behavior in coals. Int. J. Coal Geol. 2007, 72, 315–324. [Google Scholar] [CrossRef]
- Weniger, P.; Franců, J.; Hemza, P.; Krooss, B.M. Investigations on the methane and carbon dioxide sorption capacity of coals from the SW upper Silesian Coal Basin Czech Republic. Int. J. Coal Geol. 2012, 93, 23–39. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, X.H. Characterization of pore structure and its impact on methane adsorption capacity for semi-anthracite in Shizhuangnan Block, Qinshui Basin. J. Nat. Gas Sci. Eng. 2018, 60, 49–62. [Google Scholar] [CrossRef]
- Laxminarayana, C.; Crosdale, P.J. Role of coal type and rank on methane sorption of Bowen Basin, Australia coals. Int. J. Coal Geol. 1999, 40, 309–325. [Google Scholar] [CrossRef]
- Sadian, M.; Prasad, M. Effect of mineralogy on nuclear magnetic resonance surface relaxivity: A case study of Middle Bakken and Three Forks formations. Fuel 2015, 161, 197–206. [Google Scholar] [CrossRef]
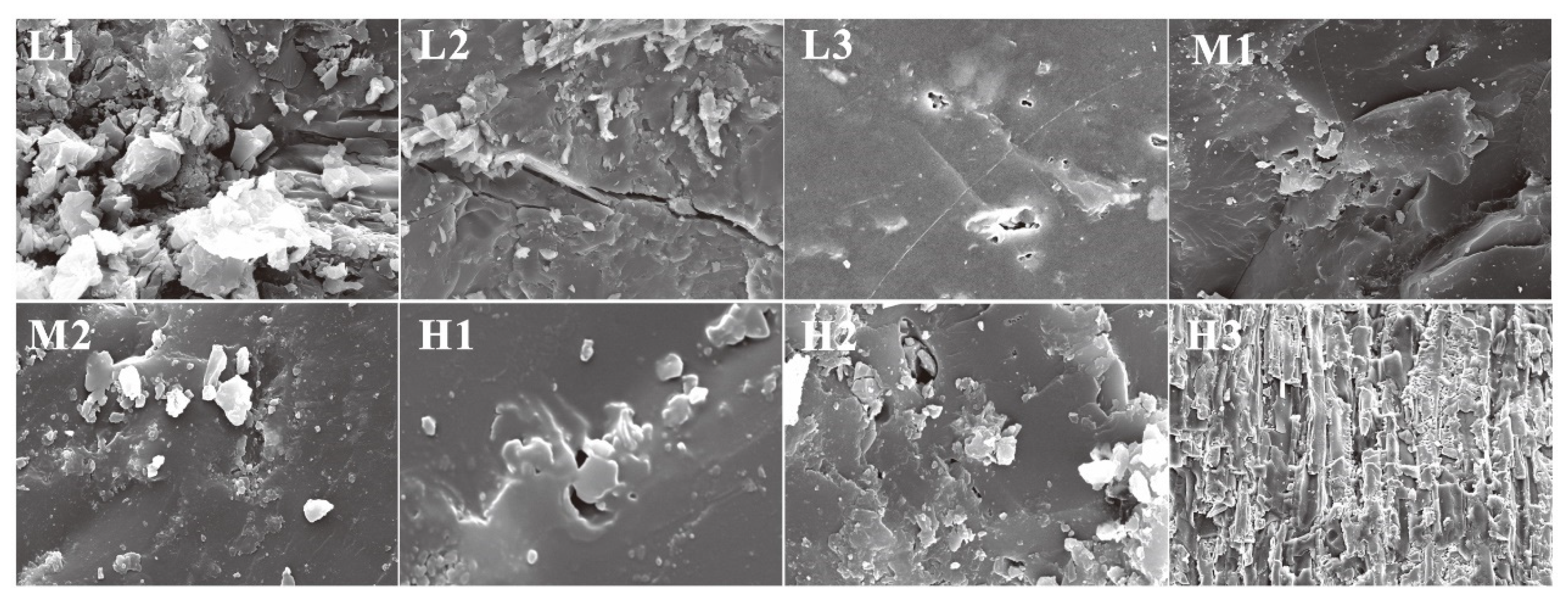







| Sample No. | Ro,m (%) | Maceral Composition (%) | Proximate Analysis (%) | |||||
|---|---|---|---|---|---|---|---|---|
| V | I | E | M | Mad | Ad | FCd | ||
| L1 | 0.52 | 64.3 | 30.7 | 4.7 | 0.3 | 7.67 | 21.34 | 44.95 |
| L2 | 0.60 | 64.8 | 16.1 | 16.4 | 2.7 | 5.82 | 11.15 | 45.83 |
| L3 | 0.70 | 50.2 | 37.2 | 4.7 | 7.9 | 2.55 | 15.49 | 56.42 |
| M1 | 1.52 | 50.9 | 20.3 | 19.1 | 9.7 | 5.43 | 33.54 | 33.77 |
| M2 | 1.68 | 58.6 | 22.1 | 14.3 | 5.0 | 6.30 | 29.19 | 36.51 |
| H1 | 2.36 | 80.3 | 10.7 | 1.0 | 8.0 | 0.88 | 17.8 | 87.21 |
| H2 | 2.54 | 83.4 | 15.9 | 0 | 0.7 | 0.74 | 12.06 | 75.05 |
| H3 | 3.03 | 86.2 | 10.1 | 0 | 3.7 | 1.46 | 1.62 | 95.94 |
| Sample No. | Volumetric Isotherm Adsorption Method | NMR Isotherm Adsorption Method | Powder Mass (g) | ||||
|---|---|---|---|---|---|---|---|
| VL-vol (cm3/g) | PL-vol (MPa) | Adjusted R-Square | VL-NMR (cm3/g) | PL-NMR (MPa) | Adjusted R-Square | ||
| L1 | 14.62 | 2.65 | 0.9900 | 14.17 | 2.74 | 0.9983 | 14.6 |
| L2 | 9.89 | 1.11 | 0.9906 | 9.59 | 1.32 | 0.9913 | 16.5 |
| L3 | 11.45 | 1.70 | 0.9933 | 11.04 | 1.97 | 0.9985 | 17.3 |
| M1 | 5.79 | 0.65 | 0.9990 | 5.07 | 0.51 | 0.9972 | 12.9 |
| M2 | 9.62 | 2.75 | 0.9745 | 7.97 | 2.20 | 0.9930 | 18.9 |
| H1 | 11.56 | 2.44 | 0.9960 | 10.92 | 2.45 | 0.9961 | 17.4 |
| H2 | 16.34 | 1.20 | 0.9965 | 15.08 | 1.02 | 0.9973 | 13.7 |
| H3 | 21.14 | 0.99 | 0.9983 | 19.81 | 0.90 | 0.9971 | 14.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Sang, S.; Liu, S.; Jin, X.; Wang, M.; Lu, S.; Feng, G.; Yang, Y.; Hou, J. An NMR-Based Method for Multiphase Methane Characterization in Coals. Energies 2022, 15, 1532. https://doi.org/10.3390/en15041532
Zheng S, Sang S, Liu S, Jin X, Wang M, Lu S, Feng G, Yang Y, Hou J. An NMR-Based Method for Multiphase Methane Characterization in Coals. Energies. 2022; 15(4):1532. https://doi.org/10.3390/en15041532
Chicago/Turabian StyleZheng, Sijian, Shuxun Sang, Shiqi Liu, Xin Jin, Meng Wang, Shijian Lu, Guangjun Feng, Yi Yang, and Jun Hou. 2022. "An NMR-Based Method for Multiphase Methane Characterization in Coals" Energies 15, no. 4: 1532. https://doi.org/10.3390/en15041532






