Optimal Discharge Parameters for Biomedical Surface Sterilization in Radiofrequency AR/O2 Plasma
Abstract
:1. Introduction
2. Description of the Simulation Model
2.1. Governing Equations
2.2. Chemical Model
2.3. Boundary Conditions and Computational Model
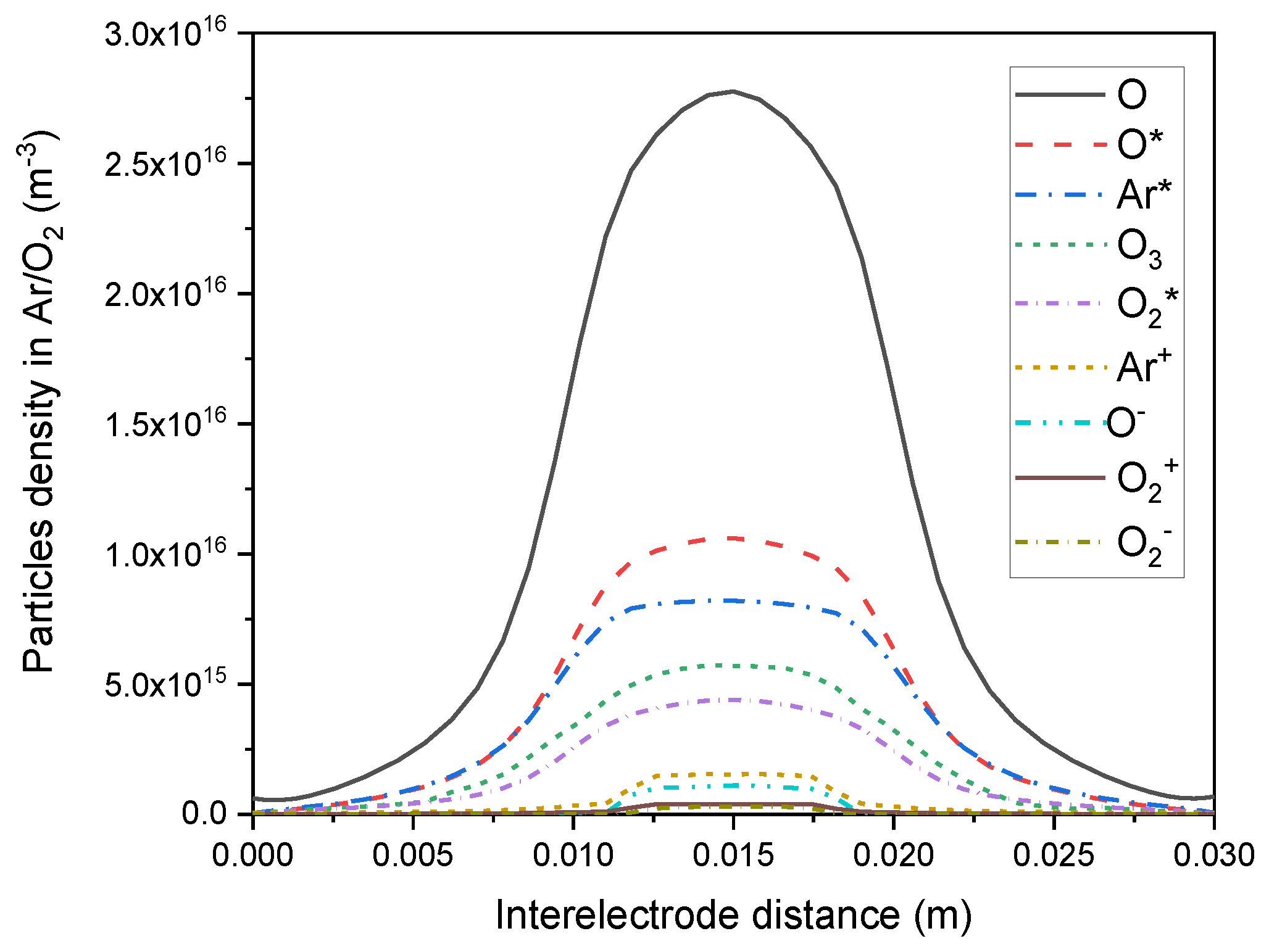
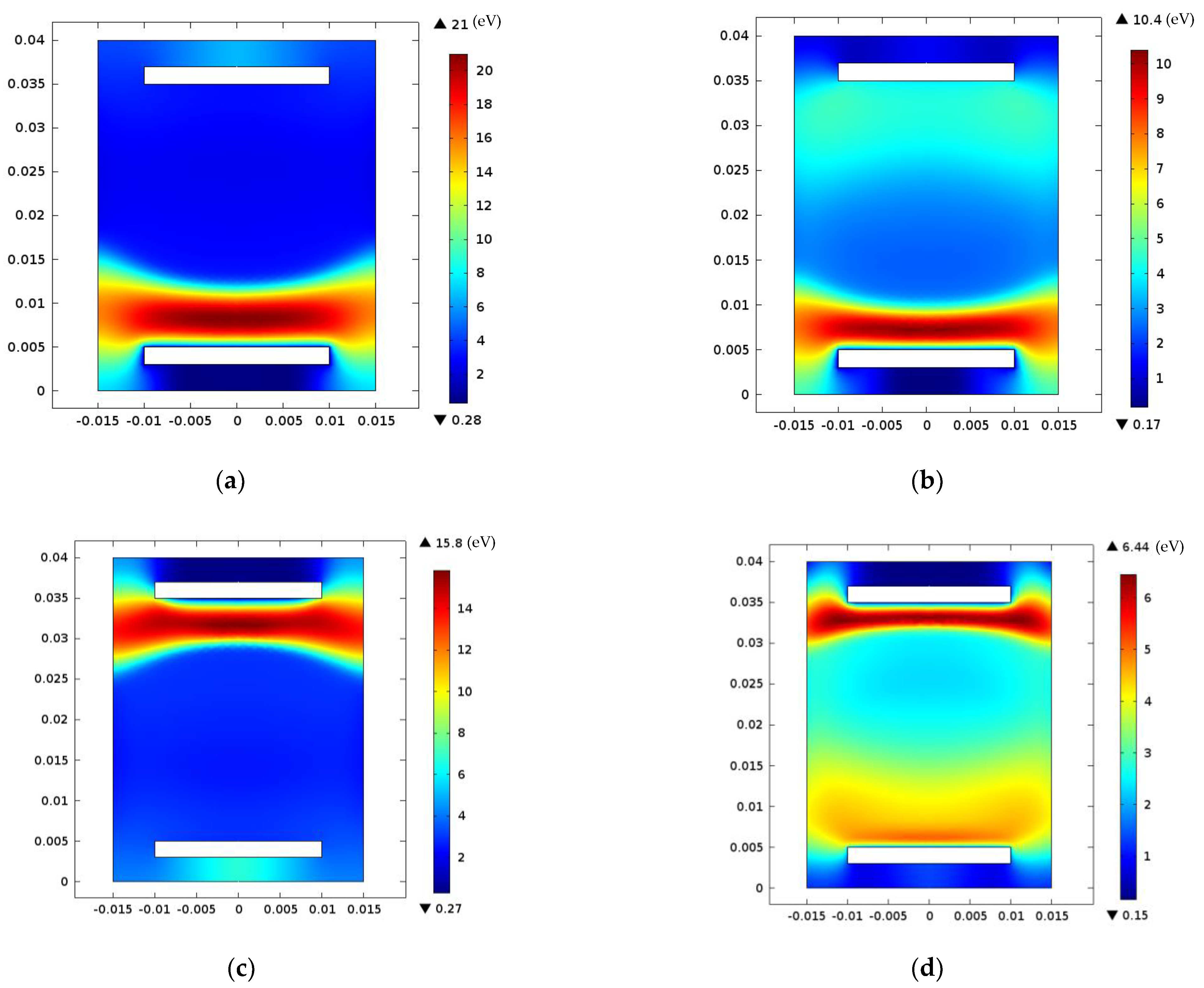
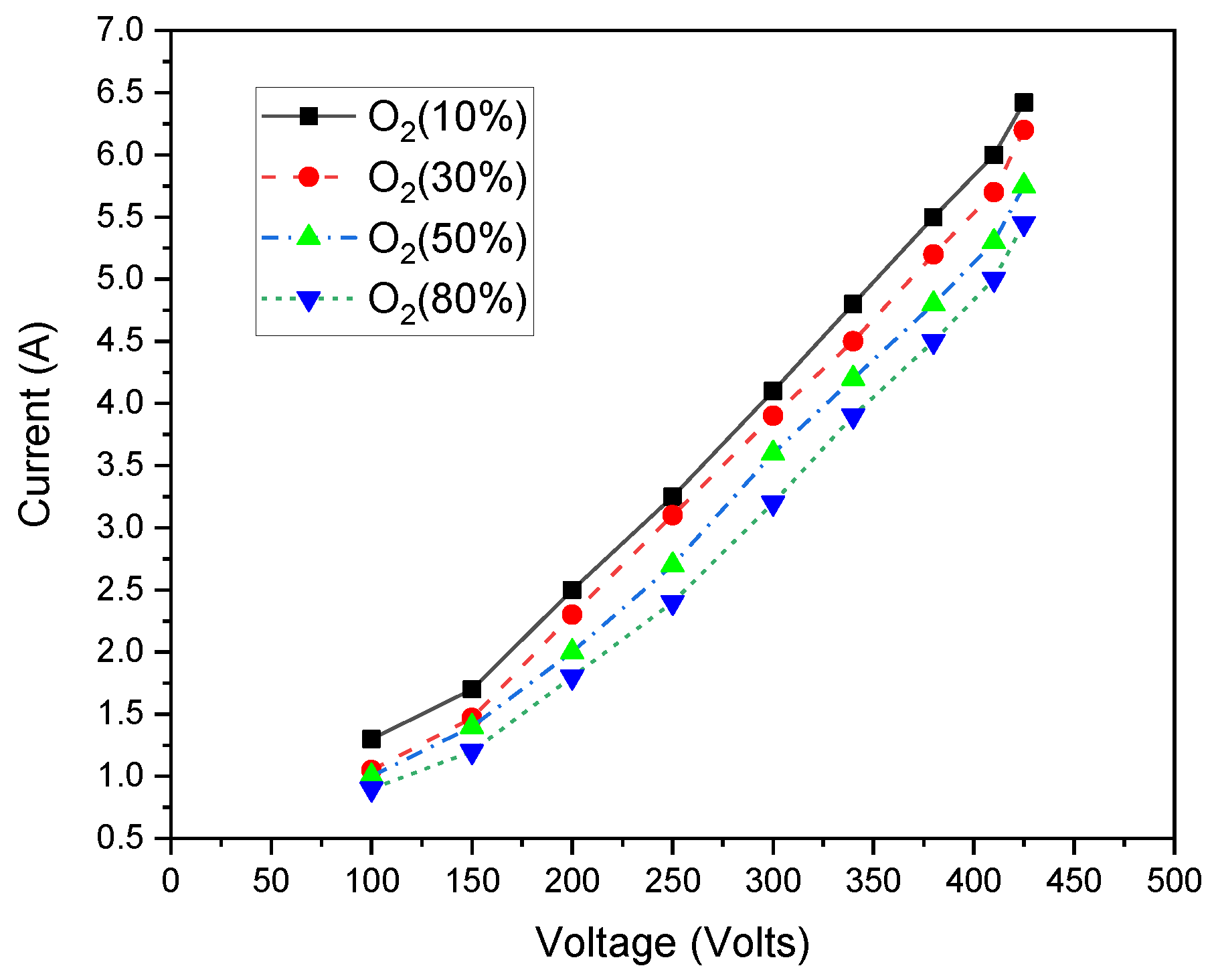
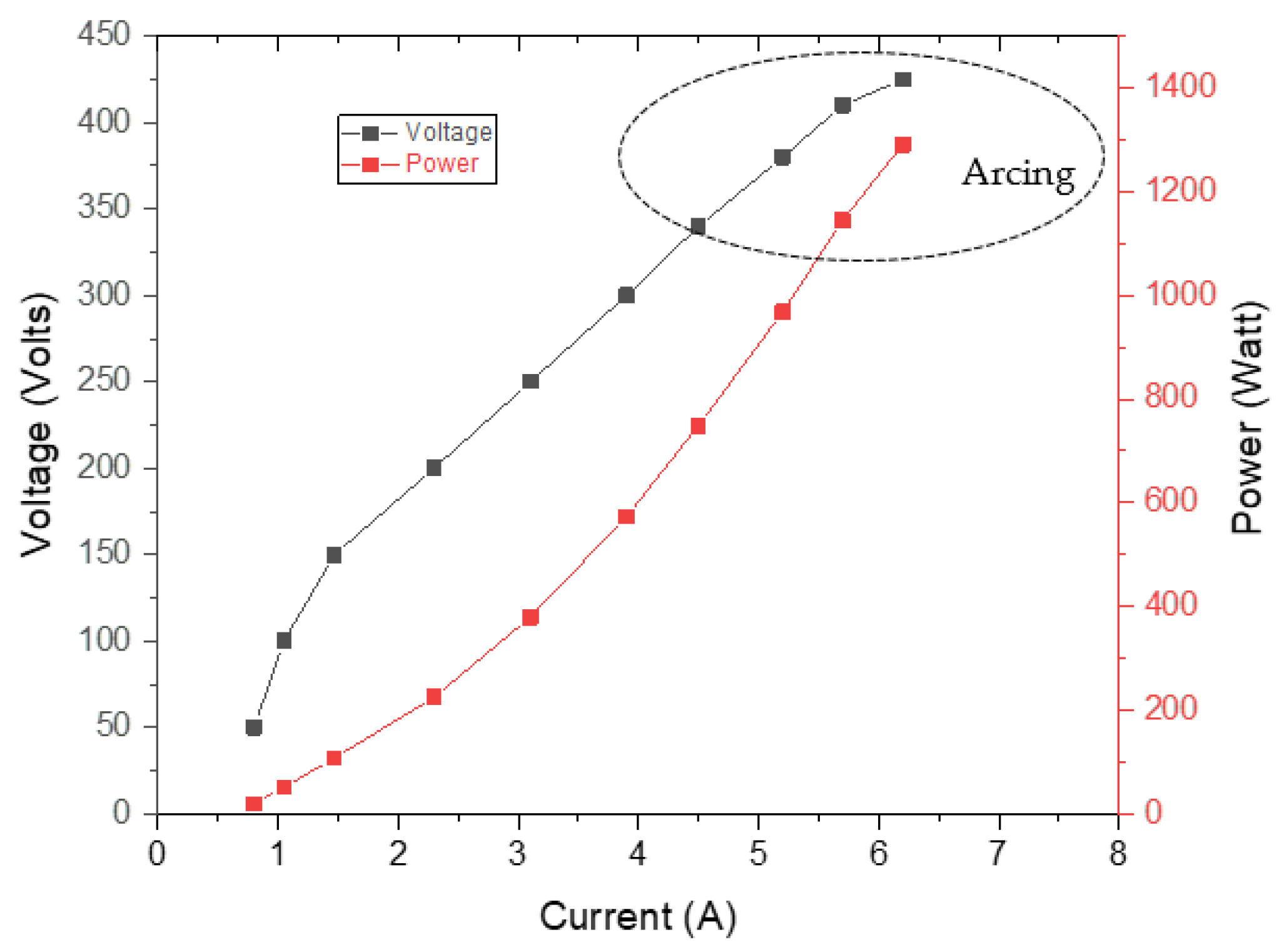
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rutala, W.A.; Weber, D.J. Disinfection, sterilization, and antisepsis: An overview. Am. J. Infect. Control 2019, 47, A3–A9. [Google Scholar] [CrossRef] [PubMed]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and Sterilization Using Plasma Technology: Fundamentals and Future Perspectives for Biological Applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barjasteh, A.; Dehghani, Z.; Lamichhane, P.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Recent Progress in Applications of Non-Thermal Plasma for Water Purification, Bio-Sterilization, and Decontamination. Appl. Sci. 2021, 11, 3372. [Google Scholar] [CrossRef]
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low-Temperature Plasma Applications. AIP Conf. Proc. 2020, 8, 74. [Google Scholar] [CrossRef]
- Chen, Z.; Garcia, G., Jr.; Arumugaswami, V.; Wirz, R.E. Cold atmospheric plasma for SARS-CoV-2 inactivation. Phys. Fluids 2020, 32, 111702. [Google Scholar] [CrossRef]
- Mravlje, J.; Regvar, M.; Vogel-Mikus, K. Development of Cold Plasma Technologies for Surface Decontamination of Seed Fungal Pathogens: Present Status and Perspectives. J. Fungi 2021, 7, 650. [Google Scholar] [CrossRef]
- Ben Belgacem, Z.; Carré, G.; Charpentier, E.; Le-Bras, F.; Maho, T.; Robert, E.; Pouvesle, J.-M.; Polidor, F.; Gangloff, S.C.; Boudifa, M.; et al. Innovative non-thermal plasma disinfection process inside sealed bags: Assessment of bactericidal and sporicidal effectiveness in regard to current sterilization norms. PLoS ONE 2017, 12, e0180183. [Google Scholar] [CrossRef] [Green Version]
- Khlyustova, A.; Cheng, Y.; Yang, R. Vapor-deposited functional polymer thin films in biological applications. J. Mater. Chem. B 2020, 8, 6588. [Google Scholar] [CrossRef]
- Rossi, F.; Kylian, O.; Rausher, H.; Gilliland, D.; Sirghi, L. Use of a low-pressure plasma discharge for the decontamination and sterilization of medical devices. Pure App. Chem. 2008, 80, 1939–1951. [Google Scholar] [CrossRef]
- Bolshakov, A.A.; Cruden, B.A.; Mogul, R.; Rao, M.V.V.S.; Sharma, S.P.; Khare, B.N.; Meyyappan, M. Radio-frequency oxygen plasma as a sterilization source. AIAA J. 2004, 42, 823–832. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Crevier, M.C.; Pelletier, J.; Phillips, N.; Saoudi, B. Plasma sterilization methods and mechanisms. Pure Appl. Chem. 2002, 74, 349–358. [Google Scholar] [CrossRef]
- Flores, O.; Castillo, F.; Martinez, H.; Villa, M.; Villalobos, S.; Reyes, P. Characterization of direct current He-N2 mixture plasma using optical emission spectroscopy and mass spectrometry. Phys. Plasmas 2014, 21, 053502. [Google Scholar] [CrossRef]
- Younus, M.; Rehman, N.; Shafiq, M.; Zakaullah, M.; Abrar, M. Evolution of plasma parameters in a He-N2/Ar magnetic pole enhanced inductive plasma source. Phys. Plasmas 2016, 23, 023512. [Google Scholar] [CrossRef]
- Goree, J.; Liu, B.; Drake, D. Gas flow dependence for plasma-needle disinfection of S. mutans bacteria. J. Phys. D Appl. Phys. 2006, 39, 3479. [Google Scholar] [CrossRef] [Green Version]
- Perni, S.; Shama, G.; Hobman, J.; Lund, P.; Kershaw, C.; Hidalgo-Arroyo, G.; Penn, C.; Deng, X.T.; Walsh, J.L.; Kong, M.G. Probing bactericidal mechanisms induced by cold atmospheric plasmas with Escherichia coli mutants. Appl. Phys. Lett. 2007, 90, 073902. [Google Scholar] [CrossRef]
- Jazbec, K.; Šala, M.; Mozetič, M.; Vesel, A.; Gorjanc, M. Functionalization of cellulose fibers with oxygen plasma and ZnO nanoparticles for achieving UV protective properties. J. Nanomater. 2015, 16, 25. [Google Scholar] [CrossRef] [Green Version]
- Ko, Y.M.; Myung, S.W.; Kim, B.H. O2/Ar Plasma Treatment for Enhancing the Biocompatibility of Hydroxyapatite Nanopowder and Polycaprolactone Composite Film. J. Nanosci. Nanotechnol. 2015, 15, 6048–6052. [Google Scholar] [CrossRef]
- Fiebrandt, M.; Lackmann, J.W.; Stapelmann, K. From patent to product? 50 years of low-pressure plasma sterilization. Plasma Process. Polym. 2018, 15, 1800139. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Cruden, B.; Rao, M.; Bolshakov, A. Analysis of emission data from O2 plasmas used for microbe sterilization. J. Appl. Phys. 2004, 95, 3324–3333. [Google Scholar] [CrossRef]
- Rossi, F.; Kylián, O.; Rauscher, H.; Hasiwa, M.; Gilliland, D. Low pressure plasma discharges for the sterilization and decontamination of surfaces. New J. Phys. 2009, 11, 115017. [Google Scholar] [CrossRef]
- Dai, H.; Li, L.; Ren, S.; Gong, X.; Wu, H.; Xiong, J.; Yu, B. Effects of Atmosphere on the Evolution Process of Graphite Electrodes under the Pulsed. In proceeding of the 2019 IEEE Pulsed Power & Plasma Science (PPPS), Orlando, FL, USA, 23–29 June 2019; IEEE: Orlando, FL, USA, 2019. [Google Scholar] [CrossRef]
- Khalaf, M.K.; Agool, I.R.; Abd Muslim, S.H. Electrical characteristics and plasma diagnostics of Ar/O2 gas mixture glow discharge. Int. J. Appl. Innov. Eng. Manag. 2014, 3, 113–119. [Google Scholar]
- Chen, X.; Tan, Z.; Liu, Y.; Wang, X.; Li, X. Effects of oxygen concentration on the electron energy distribution functions in atmospheric pressure helium/oxygen and argon/oxygen needle electrode plasmas. J. Phys. D Appl. Phys. 2018, 51, 375202. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Y.; Liu, D.; Sun, B.; Liu, Y.; Yang, A.; Wang, X. Effects of oxygen concentration on helium-oxygen dielectric barrier discharge: From multi-breakdowns to single breakdown per half-cycle. Phys. Plasmas 2018, 25, 103511. [Google Scholar] [CrossRef]
- Anjum, Z.; Rehman, N.U. Temporal evolution of plasma parameters in a pulse modulated capacitively coupled Ar/O2 mixture discharge. AIP Adv. 2020, 10, 115005. [Google Scholar] [CrossRef]
- Jabur, Y.K.; Hammed, M.G.; Khalaf, M. DC glow discharge plasma characteristics in Ar/O2 gas mixture. Iraqi J. Sci. 2021, 62, 475–482. [Google Scholar] [CrossRef]
- COMSOL Multiphysics® v. 5.4; COMSOL AB, Stockholm, Sweden. 2019. Available online: http://www.comsol.com (accessed on 1 November 2021).
- Li, S.Z.; Wu, Q.; Zhang, J.; Wang, D.; Uhm, H.S. Discharge characteristics of a radiofrequency capacitively coupled Ar/O2 glow discharge at atmospheric pressure. Thin Solid Film. 2011, 519, 6990–6993. [Google Scholar] [CrossRef]
- Rabah, T. Influence of Plasma Parameters and Circuit Connecting on Harmonics Generated in Ar/O2 13.56 MHz Plasma Discharge. Adv. Mater. Res. 2011, 227, 181–184. [Google Scholar]
- Loureiro, J.; Amorim, J. Kinetics and Spectroscopy of Low Temperature Plasmas; Springer: Berlin/Heidelberg, Germany, 2016; pp. 43–87. [Google Scholar] [CrossRef]
- Elaissi, S.; Yousfi, M.; Helali, H.; Kazziz, S.; Charrada, K.; Sassi, M. RF weakly electronegatif gas discharge behavior in parallel plate reactor for material processing. Plasma Devices Oper. 2006, 14, 1. [Google Scholar] [CrossRef]
- Park, G.; Lee, H.; Kim, G.; Lee, J.K. Global Model of He/O2 and Ar/O2 atmospheric pressure glow discharges. Plasma Process. Polym. 2008, 5, 569–576. [Google Scholar] [CrossRef]
- Pan, G.; Tan, Z.; Pan, J.; Wang, X.; Shan, C. A comparative study on the frequency effects of the electrical characteristics of the pulsed dielectric barrier discharge in He/O2 and in Ar/O2 at atmospheric pressure. Phys. Plasmas 2016, 23, 043508. [Google Scholar] [CrossRef]
- Rebiaï, S.; Bahouh, H.; Sahli, S. 2-D Simulation of Dual Frequency Capacitively Coupled Helium Plasma, using COMSOL Multiphysics. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 5. [Google Scholar] [CrossRef]
- COMSOL Multiphysics V5.4, COMSOL AB, COMSOL Multiphysics Reference Manual, Stockholm, Sweden. 2019. Available online: https://scholar.google.com/scholar?hl=fr&as_sdt=0%2C5&=35.%09COMSOL+Multiphysics+V5.4%2C+COMSOL+AB%2C+COMSOL+Multiphysics+Reference+Manual%2C+Stockholm%2C+Sweden%2C+2019&btnG= (accessed on 8 November 2021).
- COMSOL Multiphysics V5.4, Plasma Module, User’s Guide, COMSOL AB: Stockholm, Sweden. 2019. Available online: https://scholar.google.com/scholar_lookup?title=Multiphysics+V5.4.+Plasma+Module,+User%E2%80%99s+Guide&author=COMSOL&publication_year=2019 (accessed on 8 November 2021).
- Vanja, M.; Damir, V. Experimental study of Ar-O2 low-pressure discharge. Fiz. A 1998, 7, 49–63. Available online: http://fizika.hfd.hr/fizika_a/av98/a7p049.htm (accessed on 8 November 2021).
- Jorge, S.C.; Grace, V.; Laura, B.; Rawson-Acuña, F.E.; Asenjo, J.; Mora, J.; Ivan Vargas, V. Effectiveness and Efficiency Optimization Study of Oxygen and Argon DC Low-Pressure Plasma Sterilization. In Proceedings of the IEEE 16th Latin American Workshop on Plasma Physics (LAWPP), Mexico City, Mexico, 4–8 September 2017. [Google Scholar] [CrossRef]
- Wilczek, S.; Schulze, J.; Brinkmann, R.P.; Donkó, Z.; Trieschmann, J.; Mussenbrock, T. Electron dynamics in low pressure capacitively coupled radio frequency discharges. J. Appl. Phys. 2020, 127, 181101. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, B.; Zhu, W.; Zhang, C.; Tu, X.; Shao, T. Phase-Resolved Measurement of Atmospheric-Pressure Radio-Frequency Pulsed Discharges in Ar/CH4/CO2 Mixture. Plasma Chem. Plasma Process. 2020, 40, 937–953. [Google Scholar] [CrossRef]
- Merlino, R.L. Understanding Langmuir probe current-voltage characteristics. Am. J. Phys. 2007, 75, 12. [Google Scholar] [CrossRef] [Green Version]
- Rudd, M.E.; DuBois, R.D.; Toburen, L.H.; Ratcliffe, C.A.; Goff, T.V. Cross sections for ionization of gases by 5—4000-keV protons and for electron capture by 5—150-Kev protons. Phys. Rev. A 1983, 28, 6. [Google Scholar] [CrossRef]
- Park, J.; Henins, I.; Herrmann, H.W.; Selwyn, G.S. Discharge phenomena of an atmospheric pressure radio-frequency capacitive plasma source. J. Appl. Phys. 2001, 89, 20. [Google Scholar] [CrossRef] [Green Version]
- Stadnichenko, A.I.; Kibis, L.S.; Svintsitskiy, D.A.; Koshcheev, S.V.; Boronin, A.I. Application of RF discharge in oxygen to create highly oxidized metal layers. Surf. Eng. 2018, 34, 1. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Luo, H.X.; Guo, Z.; Zhen, X.J.; Chen, M.; Liu, J.N. Cleaning of carbon-contaminated optics using O2/Ar plasma. Nucl. Sci. Tech. 2017, 28, 127. [Google Scholar] [CrossRef]
- Legorreta, J.R.; Yousif, F.B.; Fuentes, B.E.; Vázquez, F.; Rivera, M.; Valencia, H.M. Characterization of Ar–O2 DC Discharge Employing Langmuir Probe in Conjunction with Photo detachment. IEEE Trans. Plasma Sci. 2016, 44, 1150–1154. [Google Scholar] [CrossRef]
- Gudmundsson, J.T.; Thorsteinsson, E.G. Oxygen discharges diluted with argon: Dissociation processes. Plasma Sources Sci. Technol. 2007, 16, 399–412. [Google Scholar] [CrossRef]
- Moreira, A.J.; Mansano, R.D.; Pinto, T.J.A.; Ruas, R.; Zambon, L.S.; Silva, M.V.; Verdonck, P.B. Sterilization by oxygen plasma. Appl. Surf. Sci. 2004, 235, 151–155. [Google Scholar] [CrossRef]
- Boscariol, M.R.; Moreira, A.J.; Mansano, R.D.; Kikuchi, I.S.; Pinto, T.J.A. Sterilization by pure oxygen plasma and by oxygen–hydrogen peroxide plasma: An efficacy study. Int. J. Pharm. 2008, 353, 170–1752. [Google Scholar] [CrossRef] [PubMed]
- Hassouba, M.A.; Galaly, A.R.; Rashed, U.M. Numerical Calculations of Some Plasma Parameters of the Capacitively Coupled RF Discharge. J. Mod. Phys. 2014, 5, 591–598. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.K.; Garg, M.K.; Ganesh Prasad, K.S.; Kumar, V.; Chowdhuri, M.B.; Prakah, R. Characterization of atmospheric pressure glow discharge in helium using Langmuir probe emission spectroscopy and discharge resistivity. IEEE Trans. Plasma Sci. 2007, 35, 4. [Google Scholar] [CrossRef]












| Neutral Species | Excited Species | Ions | Electrons |
|---|---|---|---|
| Ar | Ar* (in metastable level) | Ar+ | e− |
| O2 | O2*(1Δg) | O2+ | |
| O | O*(1D) | O+ | |
| O3 | O− |
| No. | Reaction | Rate Coefficient |
|---|---|---|
| Principal Reaction for Oxygen | ||
| R1 | e + O2 ➔ O2 + e | 4.7 × 10−14 Te0.5 (m3 s−1) |
| R2 | e + O2 ➔ O− + O | 8.8 × 10−17 exp (−4.4/Te) (m3 s−1) |
| R3 | e + O2 ➔ 2O + e | 4.2 × 10−15 exp (−5.6/Te) (m3 s−1) |
| R4 | e + O2 ➔ O2+ + 2e | 9 × 10−16 Te0.5 exp (−12.6/Te) (m3 s−1) |
| R5 | e + O− ➔ O + 2e | 2 × 10−13 exp (−5.5/Te) (m3 s−1) |
| R6 | e + O2+ ➔ O + O(1D) | 2.2 × 10−14 Te−0.5 (m3 s−1) |
| R7 | e + O2 ➔ O− + O+ + e | 7.1 × 10−17 Te0.5 exp (−17/Te) (m3 s−1) |
| R8 | e + O ➔ O + O+ + 2e | 5.3 × 10−16 Te0.9 exp (−20/Te) (m3 s−1) |
| R9 | e + O ➔ O+ + 2e | 9 × 10−15 Te0.7 exp (−13.6/Te) (m3 s−1) |
| R10 | e + O3 ➔ O2− + O | 1 × 10−15 (m3 s−1) |
| R11 | O− + O2+ ➔ O + O2 | 2 × 10−13 (200/Tg)0.5 (m3 s−1) |
| R12 | O− + O ➔ O2 + e | 5 × 10−16 (m3 s−1) |
| R13 | O− + O2+ ➔ 3O | 1 × 10−13 (m3 s−1) |
| R14 | O− + O− ➔ 2O | 2 × 10−13 (300/Tg)0.5 (m3 s−1) |
| R15 | O+ + O2 ➔ O + O2+ | 2 × 10−17 (300/Tg)0.5 (m3 s−1) |
| R16 | O− + O2 ➔ O3 + e | 5 × 10−21 (m3 s−1) |
| R17 | O3 + O2 ➔ 2O2 + O | 7.3 × 10−16 exp (−11400/Tg) (m3 s−1) |
| R18 | O3 + O ➔ 2O2 | 1.81 × 10−17 exp (−2300/Tg) (m3 s−1) |
| R19 | e + O2 + O2 ➔ O2− + O2 | 2.26 × 10−42 (300/Tg)0.5 (m3 s−1) |
| R20 | O + O2 + O2 ➔ O3 + O2 | 6.9 × 10−40 (300/Tg)1.25 (m3 s−1) |
| R21 | e + O2 ➔ O + O*(1D) + e | 5.0 × 10−14 Te0.22 exp (−8.4/Te) (m3 s−1) |
| R22 | e + O ➔ O*(1D) + e | 4.2 × 10−15 exp (−2.25/Te) (m3 s−1) |
| R23 | e + O*(1D) ➔ O + e | 8 × 10−15 (m3 s−1) |
| R24 | e + O*(1D) ➔ O+ + 2e | 9 × 10−15 Te0.7 exp (−11.6/Te) (m3 s−1) |
| R25 | O*(1D) + O➔ 2O | 8 × 10−18 (m3 s−1) |
| R26 | O*(1D) + O2 ➔ O + O2 | 7 × 10−18 exp (67/Tg) (m3 s−1) |
| R27 | O*(1D) + O2 ➔ O + O2*(1Δg) | 1 × 10−18 (m3 s−1) |
| R28 | e + O2 ➔ O2*(1Δg) + e | 1.7 × 10−15 exp (−3.1/Te) (m3 s−1) |
| R29 | e + O2*(1Δg) ➔ O2 + e | 5.6 × 10−15 exp (−2.2/Te) (m3 s−1) |
| R30 | O2*(1Δg) + O2 ➔ 2O2 | 2.2 × 10−24 (Tg/300)0.8 (m3 s−1) |
| R31 | O2*(1Δg) + O ➔ O + O2 | 7 × 10−22 (m3 s−1) |
| R32 | O− + O2*(1Δg) ➔ O3 + e | 3 × 10−16 (m3 s−1) |
| R33 | O− + O2*(1Δg) ➔ O2− + O | 1 × 10−16 (m3 s−1) |
| R34 | O2*(1Δg) + O3 ➔ 2O2 + O | 6.01 × 10−17 exp (−2853/Tg) (m3 s−1) |
| Tg (K) and Te (eV) | ||
| No. | Reaction | Rate Coefficient |
|---|---|---|
| Principal Reaction for Argon | ||
| R35 | e + Ar ➔ Ar + e | 3.9 × 10−19 exp (−4.6/Te) (m3 s−1) |
| R36 | e + Ar ➔ Ar* + e | 5.0 × 10−15 exp (−12.64/Te) (m3 s−1) |
| R37 | e + Ar ➔ Ar+ + 2e | 2.3 × 10−14 Te0.59 exp (−17.44/Te) (m3 s−1) |
| R38 | e + Ar* ➔ Ar+ + 2e | 6.8 × 10−15 Te0.67 exp (−4.2/Te) (m3 s−1) |
| R39 | e + Ar* ➔ Ar + e | 4.3 × 10−16 Te0.74 (m3 s−1) |
| Principal Reaction between Argon and Oxygen | ||
| R40 | O2 + Ar* ➔ O2 + Ar | 1.0 × 10−15 (m3 s−1) |
| R41 | O + Ar* ➔ O + Ar | 4.1 × 10−17 (m3 s−1) |
| R42 | O2 + Ar* ➔ 2O + Ar | 1.01 × 10−16 (m3 s−1) |
| R43 | O2 + Ar+ ➔ O2+ + Ar | 4.9 × 10−17 (300/Tg)0.78 (m3 s−1) |
| R44 | O + Ar+ ➔ O+ + Ar | 6.4 × 10−18 (m3 s−1) |
| Tg (K) and Te (eV) | ||
| No. | Reaction | Rate Coefficient |
|---|---|---|
| Principal Reaction for Helium | ||
| R1 | e + He ➔ He* + e | 4.2 × 10−15 Te0.31 exp (−19.8/Te) (m3 s−1) |
| R2 | e + He* ➔ He + e | 2 × 10−16 (m3 s−1) |
| R3 | e + He ➔ He+ + 2e | 1.5 × 10−15 Te0.68 exp (−24.6/Te) (m3 s−1) |
| R4 | e + He* ➔ He+ + 2e | 1.28 × 10−13 Te0.6 exp (−4.78/Te) (m3 s−1) |
| R5 | e + He2* ➔ He2+ + e | 9.75 × 10−16 Te0.71 exp (−3.4/Te) (m3 s−1) |
| R6 | e + He2+ ➔ He* + He | 5 × 10−15 Te−0.5 (m3 s−1) |
| R7 | e + He2+ ➔ He + He | 2 × 10−14 (m3 s−1) |
| R8 | e + He2+ + He➔ 3He | 2 × 10−39 (m3 s−1) |
| R9 | e + e + He2+ ➔ 2He + e | 5 × 10−29 Te−4.5 (m6 s−1) |
| R10 | He* + He* ➔ He+ + He + e | 2.7 × 10−16 (m3 s−1) |
| R11 | He* + 2He ➔ He2* + He | 1.3 × 10−45 (m6 s−1) |
| R12 | He+ + 2He ➔ He2+ + He | 1 × 10−43 (m6 s−1) |
| Principal Reaction between Helium and Oxygen | ||
| R13 | e + O2 + He ➔ O2− + He | 1 × 10−43 (m6 s−1) |
| R14 | O2 + He* ➔ O2+ + He + e | 2.54 × 10−16 (3000/Tg)−0.5 (m3 s−1) |
| R15 | O2 + He + O ➔ O3 + He | 6.27 × 10−46 (m6 s−1) |
| R16 | O + O + He ➔ O2 + He | 1.3 × 10−45 (m3 s−1) |
| R17 | O2*(1Δg) + He ➔ O2 + He | 8 × 10−27 (m3 s−1) |
| Tg (K) and Te (eV) | ||
| Reaction | Formula | Sticking Coefficient |
|---|---|---|
| 1 | O2*(1Δg) ➔ O2 | 1 |
| 2 | O2+ ➔ O2 | 1 |
| 3 | O*(1D) ➔ O | 1 |
| 4 | O+ ➔ O | 1 |
| 5 | O + O ➔ O2 | 1 × 10−3 |
| 6 | Ar+ ➔ Ar | 1 |
| 7 | Ar* ➔ Ar | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elaissi, S.; H. Alkallas, F.; Ben Gouider Trabelsi, A.; Abu El Maati, L.; Charrada, K. Optimal Discharge Parameters for Biomedical Surface Sterilization in Radiofrequency AR/O2 Plasma. Energies 2022, 15, 1589. https://doi.org/10.3390/en15041589
Elaissi S, H. Alkallas F, Ben Gouider Trabelsi A, Abu El Maati L, Charrada K. Optimal Discharge Parameters for Biomedical Surface Sterilization in Radiofrequency AR/O2 Plasma. Energies. 2022; 15(4):1589. https://doi.org/10.3390/en15041589
Chicago/Turabian StyleElaissi, Samira, Fatemah. H. Alkallas, Amira Ben Gouider Trabelsi, Lamia Abu El Maati, and Kamel Charrada. 2022. "Optimal Discharge Parameters for Biomedical Surface Sterilization in Radiofrequency AR/O2 Plasma" Energies 15, no. 4: 1589. https://doi.org/10.3390/en15041589
APA StyleElaissi, S., H. Alkallas, F., Ben Gouider Trabelsi, A., Abu El Maati, L., & Charrada, K. (2022). Optimal Discharge Parameters for Biomedical Surface Sterilization in Radiofrequency AR/O2 Plasma. Energies, 15(4), 1589. https://doi.org/10.3390/en15041589






