Photovoltaic Performance of Spherical TiO2 Nanoparticles Derived from Titanium Hydroxide Ti(OH)4: Role of Annealing Varying Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of TiO2NPs
2.2. Characterization of TiO2NPs
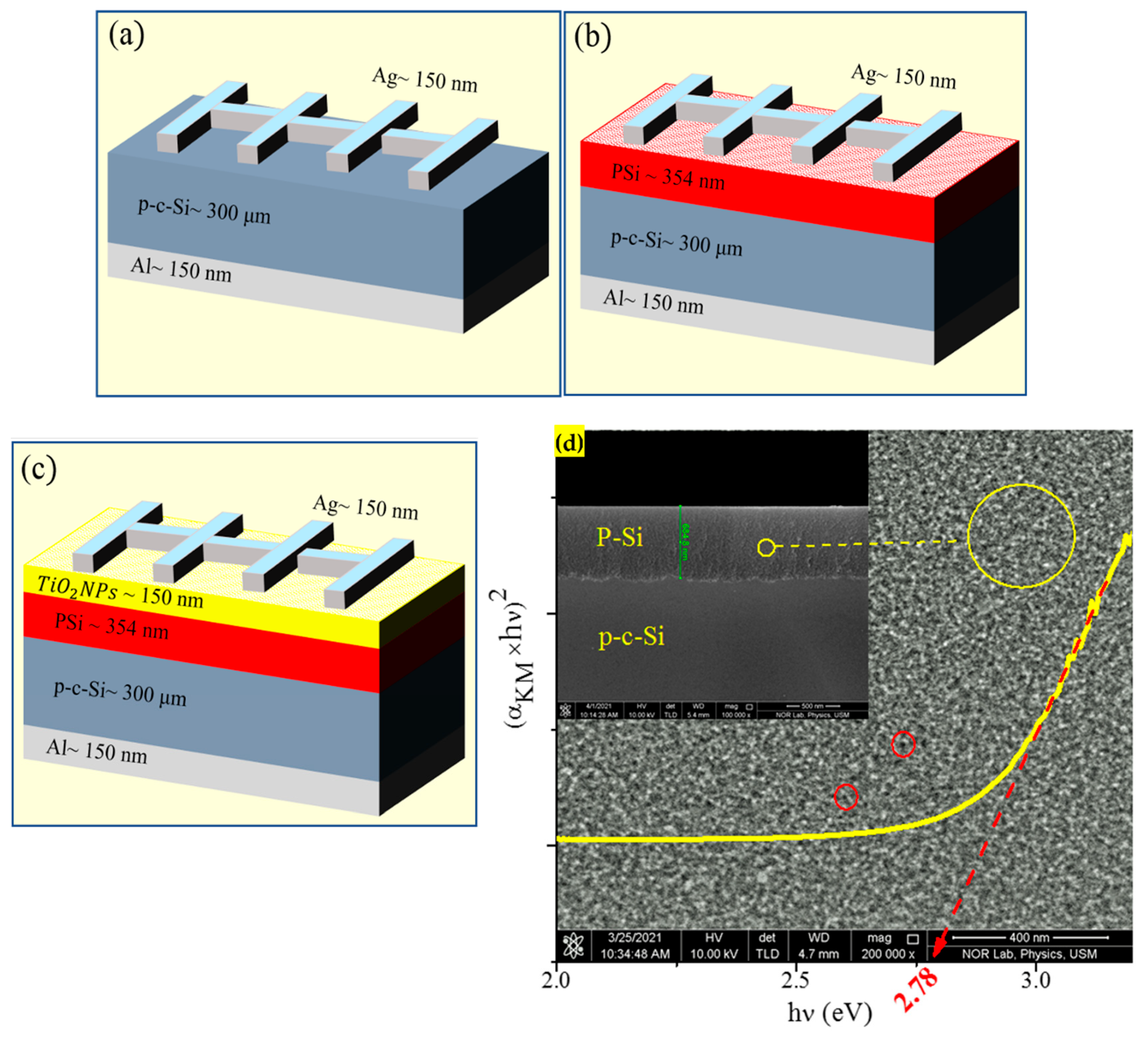
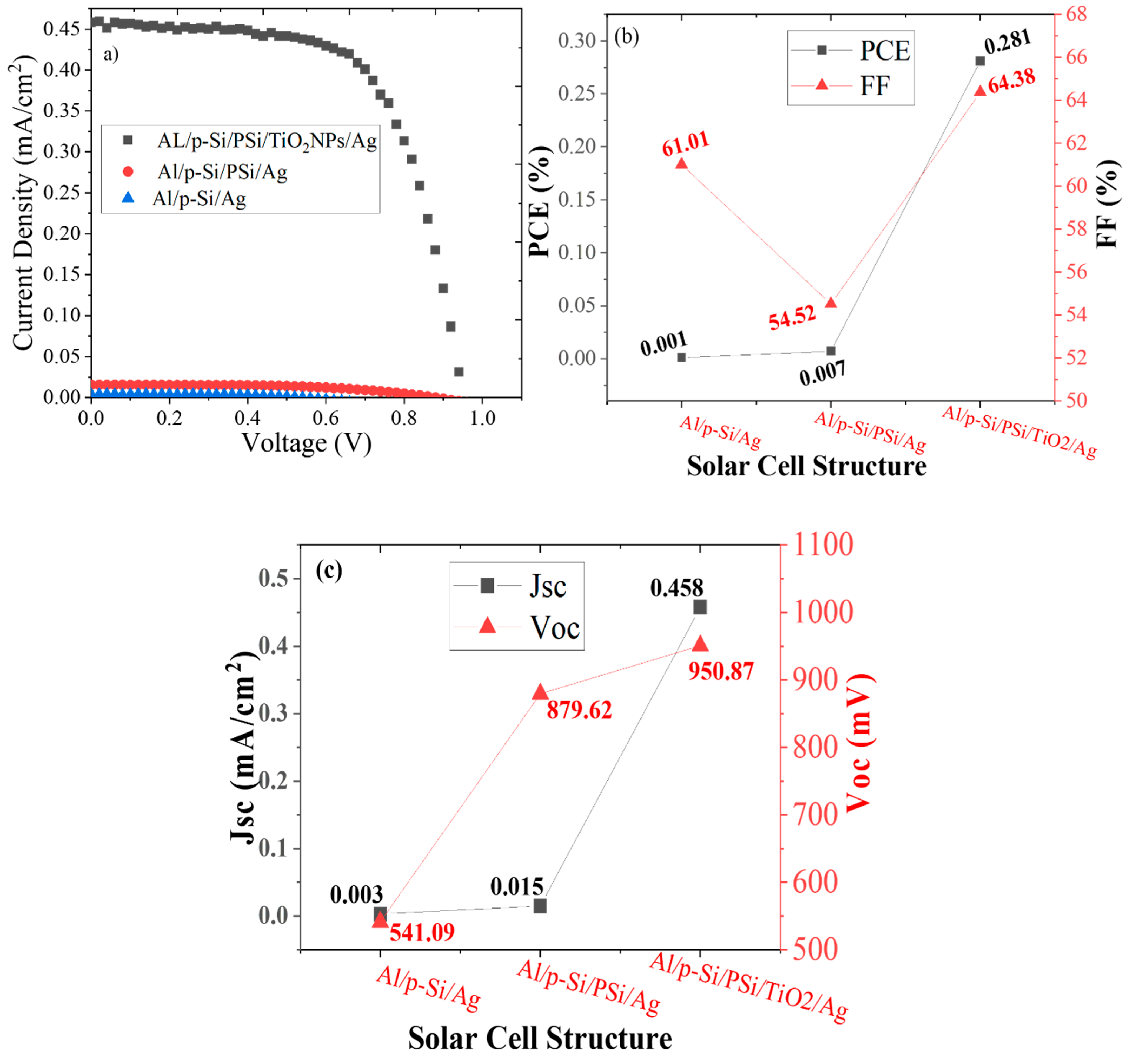
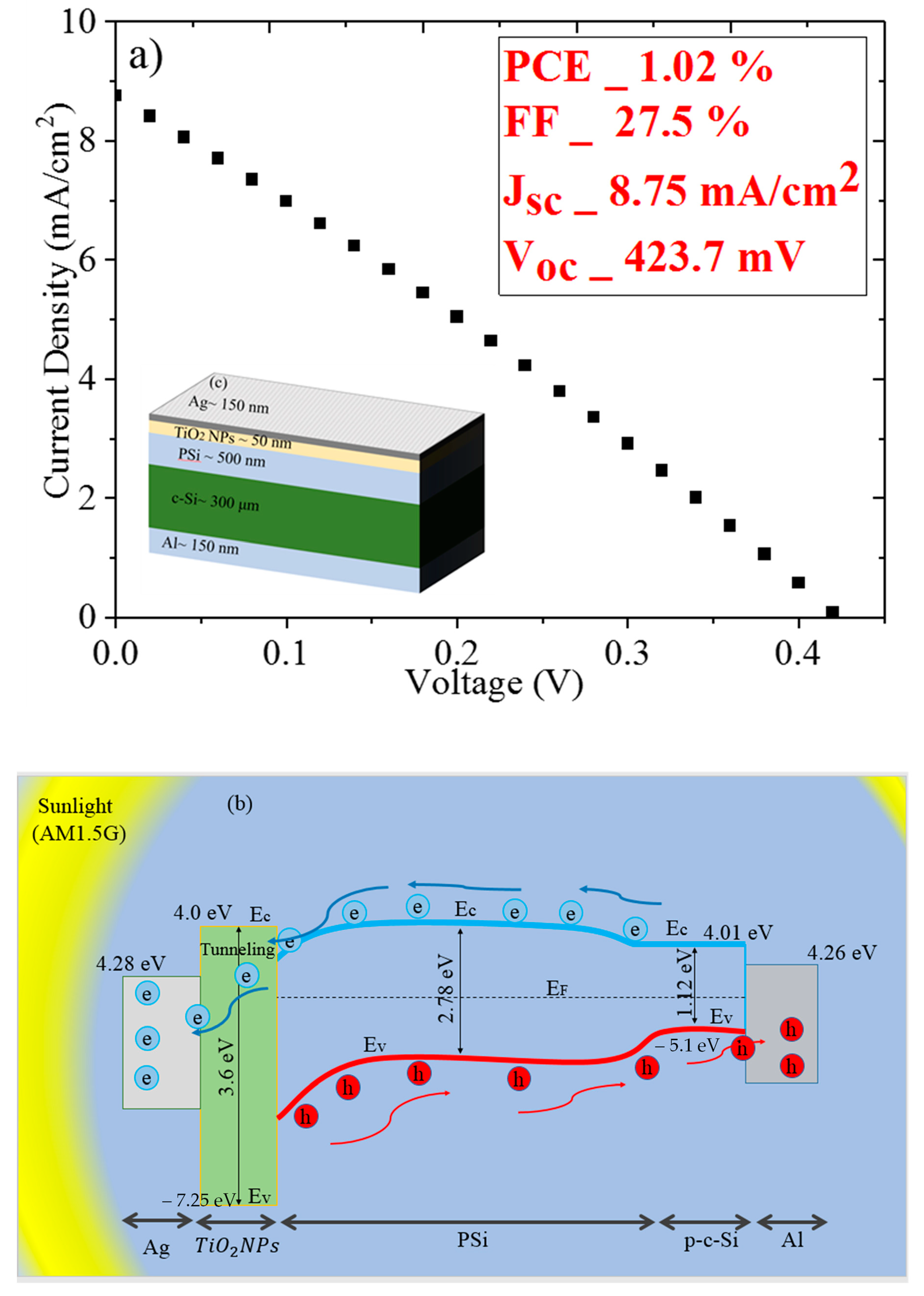
3. Results and Discussion
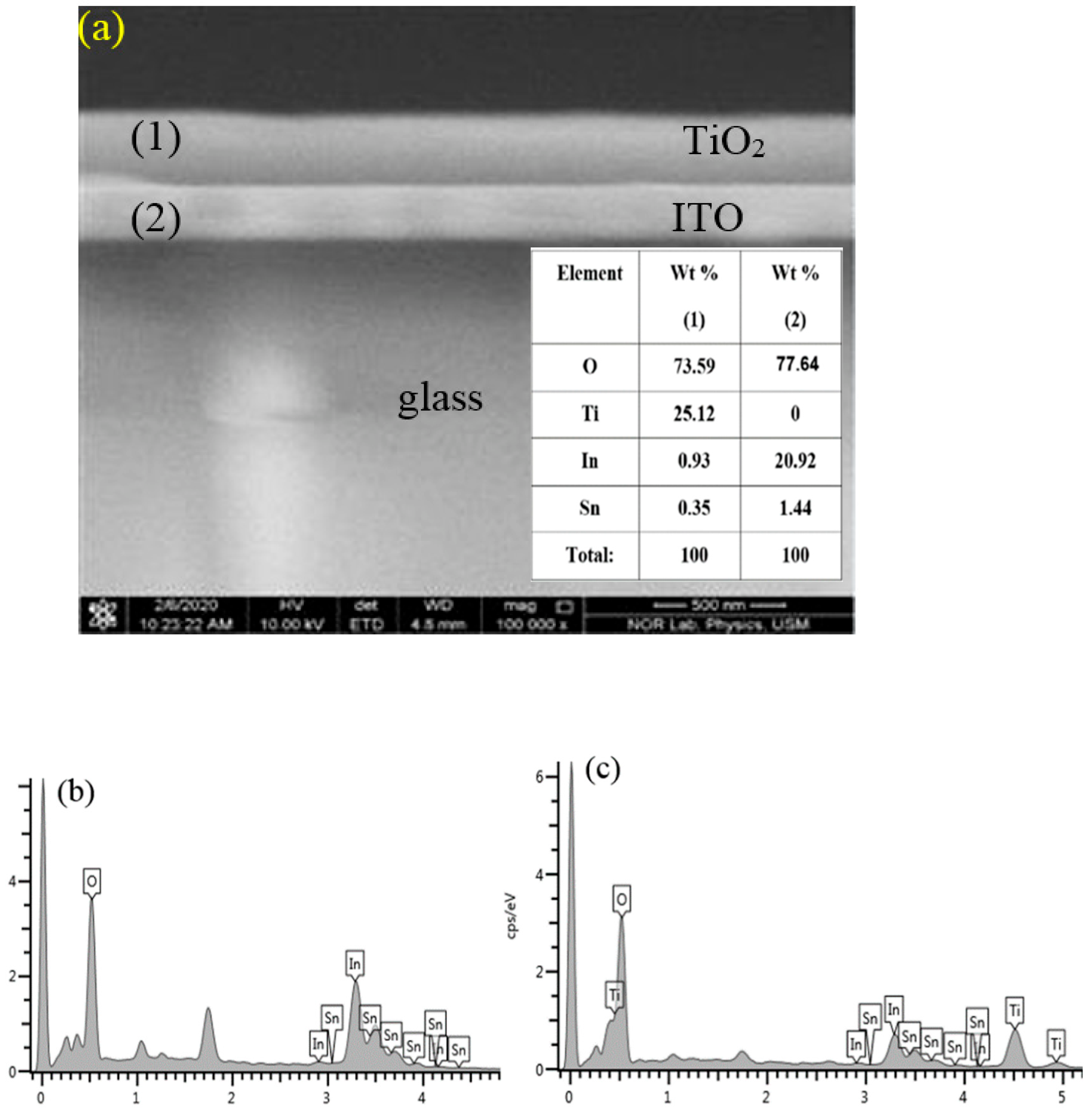
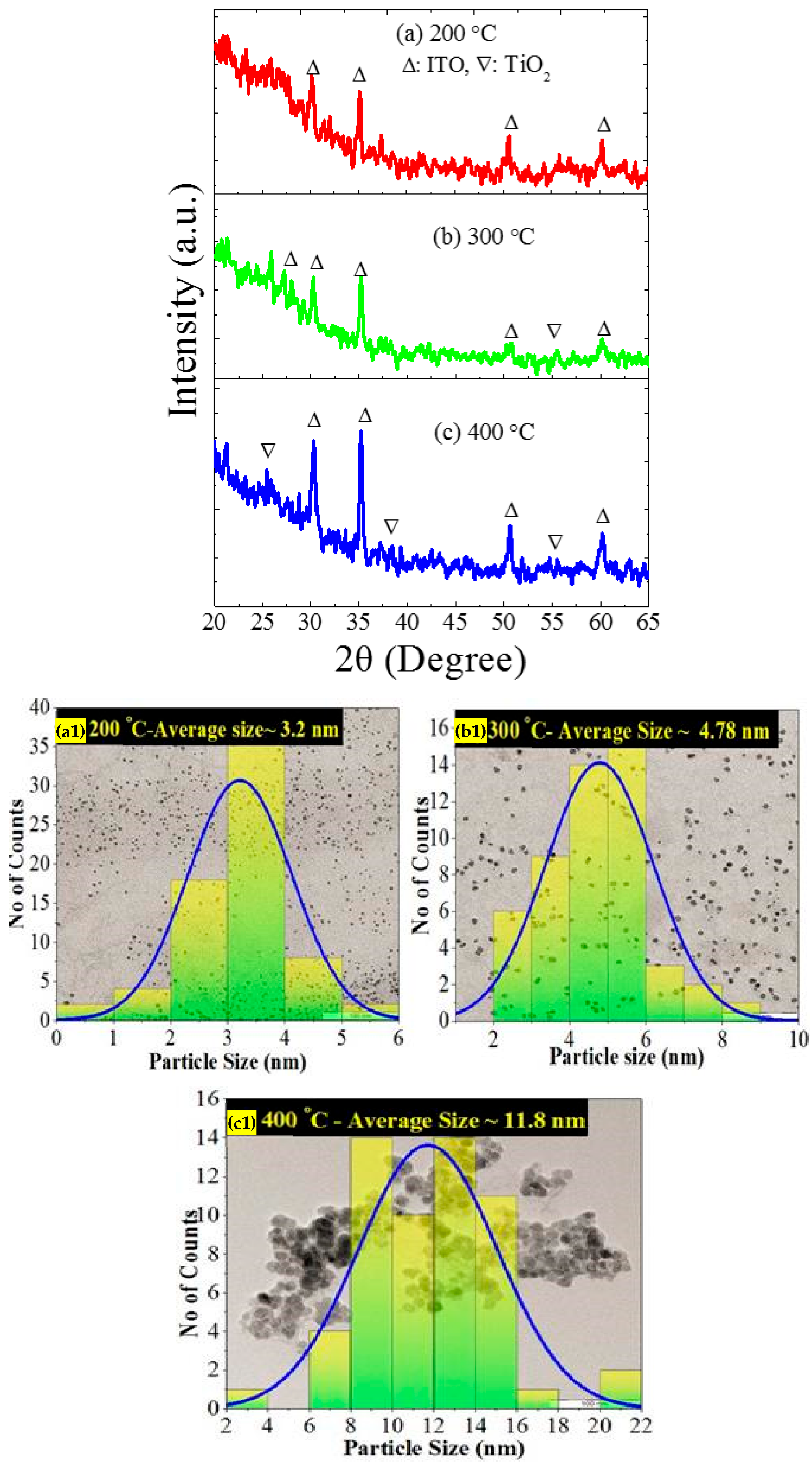
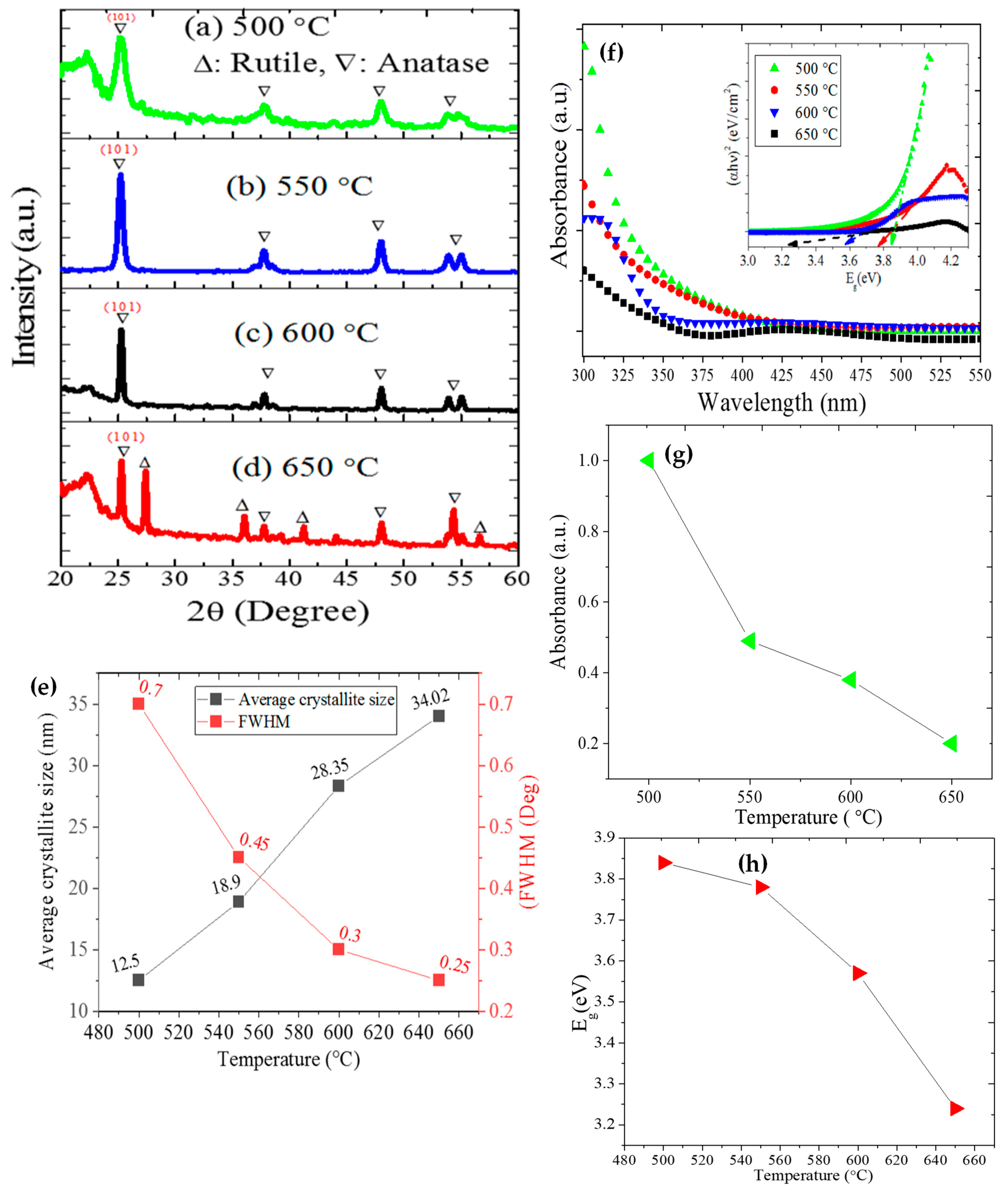
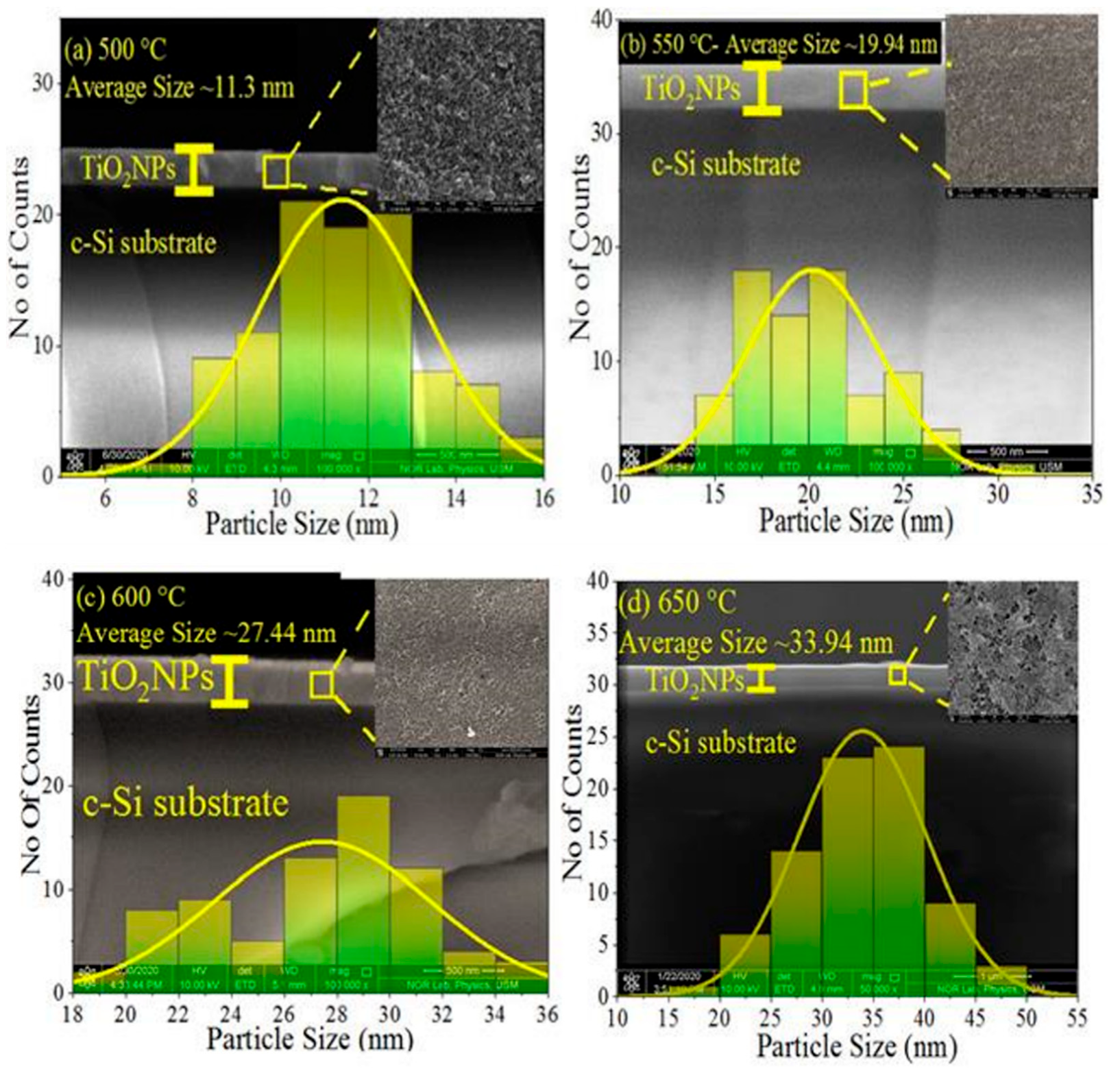
Morphology, Structure, and Optical Properties of Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, Y.; Sutton, N.B.; Rijnaarts, H.; Langenhoff, A. Degradation of pharmaceuticals in wastewater using immobilized TiO2 photocatalysis under simulated solar irradiation. Appl. Catal. B Environ. 2016, 182, 132–141. [Google Scholar] [CrossRef]
- Zatloukalová, K.; Obalová, L.; Koči, K.; Čapek, L.; Matěj, Z.; Šnajdhaufová, H.; Ryczkowski, J.; Słowik, G. Photocatalytic degradation of endocrine disruptor compounds in water over immobilized TiO2 photocatalysts. Iran. J. Chem. Chem. Eng. 2017, 36, 29–38. [Google Scholar]
- Dai, Y.; Cobley, C.M.; Zeng, J.; Sun, Y.; Xia, Y. Synthesis of anatase TiO2 nanocrystals with exposed {001} facets. Nano Lett. 2009, 9, 2455–2459. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-W.; Jang, J.-T.; Cheon, J.; Lee, H.-H.; Lee, D.R.; Lee, Y. Shape-Dependent Compressibility of TiO2 Anatase Nanoparticles. J. Phys. Chem. C 2008, 112, 9627–9631. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Kornowski, A.A.; Weller, H. Low-Temperature Synthesis of Soluble and Processable Organic-Capped Anatase TiO2 Nanorods. J. Am. Chem. Soc. 2003, 125, 14539–14548. [Google Scholar] [CrossRef]
- Elsaeedy, H.; Qasem, A.; Yakout, H.; Mahmoud, M. The pivotal role of TiO2 layer thickness in optimizing the performance of TiO2/P-Si solar cell. J. Alloys Compd. 2021, 867, 159150. [Google Scholar] [CrossRef]
- Saint-André, S.; Rodríguez, D.; Perillo, P.; Barrera, M. TiO2 nanotubes antireflection coating design for GaAs solar cells. Sol. Energy Mater. Sol. Cells 2021, 230, 111201. [Google Scholar] [CrossRef]
- Lv, Y.; Tong, H.; Cai, W.; Zhang, Z.; Chen, H.; Zhou, X. Boosting the efficiency of commercial available carbon-based perovskite solar cells using Zinc-doped TiO2 nanorod arrays as electron transport layer. J. Alloys Compd. 2021, 851, 156785. [Google Scholar] [CrossRef]
- Ojo, A.A.; Cranton, W.M.; Dharmadasa, I.M. Photovoltaic Solar Cells: Materials, Concepts and Devices. In Next Generation Multilayer Graded Bandgap Solar Cells; Springer Science and Business Media LLC: Berlin, Germany, 2018; pp. 17–40. [Google Scholar]
- Dharmadasa, I.M.; Thornton, J.M.; Williams, R.H. Effects of surface treatments on Schottky barrier formation at metal/n-type CdTe contacts. Appl. Phys. Lett. 1989, 54, 137–139. [Google Scholar] [CrossRef]
- Godfrey, R.B.; Green, M.A. Enhancement of MIS solar-cell’’efficiency’’by peripheral collection. Appl. Phys. Lett. 1977, 31, 705–707. [Google Scholar] [CrossRef]
- Nevin, W.; Chamberlain, G. Effect of oxide thickness on the properties of metal-insulator-organic semiconductor photovoltaic cells. IEEE Trans. Electron Devices 1993, 40, 75–81. [Google Scholar] [CrossRef]
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Kim, J.M.; Kim, S.; Shin, D.H.; Seo, S.W.; Lee, H.S.; Kim, J.H.; Jang, C.W.; Kang, S.S.; Choi, S.-H.; Kwak, G.Y.; et al. Si-quantum-dot heterojunction solar cells with 16.2% efficiency achieved by employing doped-graphene transparent conductive electrodes. Nano Energy 2018, 43, 124–129. [Google Scholar] [CrossRef]
- Sasirekha, N.; Rajesh, B.; Chen, Y.-W. Synthesis of TiO2 sol in a neutral solution using TiCl4 as a precursor and H2O2 as an oxidizing agent. Thin Solid Film. 2009, 518, 43–48. [Google Scholar] [CrossRef]
- Lee, D.-S.; Liu, T.-K. Preparation of TiO2 Sol Using TiCl4 as a Precursor. J. Sol-Gel Sci. Technol. 2002, 25, 121–136. [Google Scholar] [CrossRef]
- Al-Diabat, A.M.; Ahmed, N.M.; Hashim, M.R.; Chahrour, K. Influence of the spray distance to substrate on optical properties of chemically sprayed ZnS thin films. J. Mater. Sci. Mater. Electron. 2017, 28, 371–375. [Google Scholar] [CrossRef]
- Eze, M.C.; Ugwuanyi, G.; Li, M.; Eze, H.U.; Rodriguez, G.M.; Evans, A.; Rocha, V.G.; Li, Z.; Min, G. Optimum silver contact sputtering parameters for efficient perovskite solar cell fabrication. Sol. Energy Mater. Sol. Cells 2021, 230, 111185. [Google Scholar] [CrossRef]
- Balaji, P.; Dauksher, W.J.; Bowden, S.G.; Augusto, A. Improving surface passivation on very thin substrates for high efficiency silicon heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2020, 216, 110715. [Google Scholar] [CrossRef]
- Mechiakh, R.; Ben Sedrine, N.; Ben Naceur, J.; Chtourou, R. Elaboration and characterization of nanocrystalline TiO2 thin films prepared by sol–gel dip-coating. Surf. Coat. Technol. 2011, 206, 243–249. [Google Scholar] [CrossRef]
- Šutka, A.; Eglitis, R.; Kuzma, A.; Smits, K.; Zukuls, A.; Prades, J.D.; Fàbrega, C. Photodoping-Inspired Room-Temperature Gas Sensing by Anatase TiO2 Quantum Dots. ACS Appl. Nano Mater. 2021, 4, 2522–2527. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Hemamalini, R.; Ravichandran, K. Synthesis and characterization of TiO2 quantum dots for photocatalytic application. J. Saudi Chem. Soc. 2015, 19, 589–594. [Google Scholar] [CrossRef] [Green Version]
- Nomaan, A.T.; Ahmed, A.A.; Ahmed, N.M.; Idris, M.; Hashim, M.; Rashid, M. ZnO quantum dot based thin films as promising electron transport layer: Influence of surface-to-volume ratio on the photoelectric properties. Ceram. Int. 2021, 47, 12397–12409. [Google Scholar] [CrossRef]
- Bakri, A.S.; Sahdan, M.Z.; Adriyanto, F.; Raship, N.A.; Said, N.D.M.; Abdullah, S.A.; Rahim, M.S. Effect of annealing temperature of titanium dioxide thin films on structural and electrical properties. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2017; Volume 1788, p. 030030. [Google Scholar] [CrossRef] [Green Version]
- Viseu, T.M.; Ferreira, M.C. Morphological characterization of TiO2 thin films. Vacuum 1999, 52, 115–120. [Google Scholar] [CrossRef]
- Manickam, K.; Muthusamy, V.; Manickam, S.; Senthil, T.S.; Periyasamy, G.; Shanmugam, S. Effect of annealing temperature on structural, morphological and optical properties of nanocrystalline TiO2 thin films synthesized by sol–gel dip coating method. Mater. Today Proc. 2020, 23, 68–72. [Google Scholar]
- Nandani; Supriyanto, A.; Ramelan, A.H.; Nurosyid, F. Effect of annealing temperature on optical properties of TiO2 18 NR-T type thin film. J. Phys. Conf. Ser. 2018, 1011, 012016. [Google Scholar] [CrossRef]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar]
- Kheiri, F.; Soleimanian, V.; Ghasemi, M.; Mokhtari, A. The microstructure, optical and gas sensing properties of bilayer TiO2/ZnO systems in terms of annealing temperature. Mater. Sci. Semicond. Process. 2021, 121, 105462. [Google Scholar]
- Munguti, L.; Dejene, F. Influence of annealing temperature on structural, optical and photocatalytic properties of ZnO–TiO2 composites for application in dye removal in water. Nano-Struct. Nano-Objects 2020, 24, 100594. [Google Scholar] [CrossRef]
- Timoumi, A.; Albetran, H.M.; Alamri, H.R.; Alamri, S.N.; Low, I.M. Impact of annealing temperature on structural, morphological and optical properties of GO-TiO2 thin films prepared by spin coating technique. Superlattices Microstruct. 2020, 139, 106423. [Google Scholar]
- He, Y.; Yan, Q.; Liu, X.; Dong, M.; Yang, J. Effect of annealing on the structure, morphology and photocatalytic activity of surface-fluorinated TiO2 with dominant {001} facets. J. Photochem. Photobiol. A Chem. 2020, 393, 112400. [Google Scholar]
- Quintero, Y.; Mosquera, E.; Diosa, J.; García, A. Ultrasonic-assisted sol–gel synthesis of TiO2 nanostructures: Influence of synthesis parameters on morphology, crystallinity, and photocatalytic performance. J. Sol-Gel Sci. Technol. 2020, 94, 477–485. [Google Scholar] [CrossRef]
- Kubelka, P. New contributions to the optics of intensely light-scattering materials. Part I. Josa 1948, 38, 448–457. [Google Scholar] [CrossRef] [PubMed]
- López-rojas, O.; Guzmán, J.G. A review on quantum dot solar cells: Properties, materials, synthesis and devices. In Proceedings of the 2019 IEEE International Conference on Engineering Veracruz (ICEV), Boca del Rio, Mexico, 14–17 October 2019; Volume 1, pp. 1–5. [Google Scholar]
- Herbert, F.; Krishnamoorthy, A.; Van Vliet, K.; Yildiz, B. Quantification of electronic band gap and surface states on FeS2(100). Surf. Sci. 2013, 618, 53–61. [Google Scholar] [CrossRef]
- Almomani, M.S.; Ahmed, N.M.; Rashid, M.; Almessiere, M.; Altowyan, A.S. Broadband visible emission from photoelectrochemical etched porous silicon quantum dots containing zinc. Mater. Chem. Phys. 2021, 258, 123935. [Google Scholar] [CrossRef]
- Almomani, M.S.; Ahmed, N.M.; Rashid, M.; Almessiere, M.A.; Altowyan, A.S. White, blue and green emission from Si QDs derived from zinc incorporated porous silicon. J. Lumin. 2021, 232, 117845. [Google Scholar] [CrossRef]
- Almomani, M.S.; Ahmed, N.M.; Rashid, M.; Ali, M.K.M.; Akhdar, H.; Aldaghri, O.; Ibnaouf, K.H. Enhancement of Temperature Fluorescence Brightness of Zn@Si Core-Shell Quantum Dots Produced via a Unified Strategy. Nanomaterials 2021, 11, 3158. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Pavlenko, M.; Viter, R.; Jancelewicz, M.; Nowaczyk, G.; Baleviciute, I.; Załęski, K.; Jurga, S.; Ramanavicius, A.; Smyntyna, V. Tailoring the Structural, Optical, and Photoluminescence Properties of Porous Silicon/TiO2 Nanostructures. J. Phys. Chem. C 2015, 119, 7164–7171. [Google Scholar] [CrossRef]
- Hao, Y.; Kang, Y.; Mi, Y.; Wang, W.; Lei, Z. Highly ordered micro-meso-macroporous Co-N-doped carbon polyhedrons from bimetal-organic frameworks for rechargeable Zn-air batteries. J. Colloid Interface Sci. 2021, 598, 83–92. [Google Scholar] [CrossRef]
- Kaulgud, S.; Sharma, R.; Jolly, L.; Mishra, B.K. Enhanced surface passivation with TIPS pentacene and additional interfacial layer for MIS solar cells. Int. J. Inf. Technol. 2021, 13, 1323–1330. [Google Scholar] [CrossRef]
- Ramadan, R.; Manso-silva, M. Composites Hybrid porous silicon / silver nanostructures for the development of enhanced photovoltaic devices. J. Mater. Sci. 2020, 55, 5458–5470. [Google Scholar] [CrossRef]
- Shin, D.H.; Kim, J.H.; Kim, J.H.; Jang, C.W.; Seo, S.W.; Lee, H.S.; Kim, S.; Choi, S.-H. Graphene/porous silicon Schottky-junction solar cells. J. Alloys Compd. 2017, 715, 291–296. [Google Scholar] [CrossRef]
- Gallach-Pérez, D.; Muñoz-Noval, A.; García-Pelayo, L.; Manso-Silván, M.; Torres-Costa, V. Tunnel conduction regimes, white-light emission and band diagram of porous silicon–zinc oxide nanocomposites. J. Lumin. 2017, 191, 107–111. [Google Scholar] [CrossRef]
- Zha, R.; Nadimicherla, R.; Guo, X. Ultraviolet photocatalytic degradation of methyl orange by nanostructured TiO2/ZnO heterojunctions. J. Mater. Chem. A 2015, 3, 6565–6574. [Google Scholar] [CrossRef]
- Deb, P.; Dhar, J.C. Graphene oxide charge blocking layer with high K TiO2 nanowire for improved capacitive memory. J. Alloys Compd. 2021, 868, 159095. [Google Scholar] [CrossRef]
- Choudhury, B.D.; Lin, C.; Shawon, S.M.A.Z.; Soliz-Martinez, J.; Huq, H.; Uddin, M.J. A photoanode with hierarchical nanoforest TiO2 structure and silver plasmonic nanoparticles for flexible dye sensitized solar cell. Sci. Rep. 2021, 11, 7552. [Google Scholar] [CrossRef] [PubMed]
- Kanjana, N.; Maiaugree, W.; Poolcharuansin, P.; Laokul, P. Synthesis and characterization of Fe-doped TiO2 hollow spheres for dye-sensitized solar cell applications. Mater. Sci. Eng. B 2021, 271, 115311. [Google Scholar] [CrossRef]
- Mansfeldova, V.; Zlamalova, M.; Tarabkova, H.; Janda, P.; Vorokhta, M.; Piliai, L.; Kavan, L. Work Function of TiO2 (Anatase, Rutile, and Brookite) Single Crystals: Effects of the Environment. J. Phys. Chem. C 2021, 125, 1902–1912. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Hong, Y.-T.; Shyue, J.-J.; Hsueh, C.-H. Construction of Schottky junction solar cell using silicon nanowires and multi-layered graphene. Superlattices Microstruct. 2019, 126, 42–48. [Google Scholar] [CrossRef]
- Gnisci, A.; Faggio, G.; Lancellotti, L.; Messina, G.; Carotenuto, R.; Bobeico, E.; Veneri, P.D.; Capasso, A.; Dikonimos, T.; Lisi, N. The Role of Graphene-Based Derivative as Interfacial Layer in Graphene/n-Si Schottky Barrier Solar Cells. Phys. Status Solidi 2018, 216, 1800555. [Google Scholar] [CrossRef]
- Yi, Z.; Zeng, Y.; Wu, H.; Chen, X.; Fan, Y.; Yang, H.; Tang, Y.; Yi, Y.; Wang, J.; Wu, P. Synthesis, surface properties, crystal structure and dye-sensitized solar cell performance of TiO2 nanotube arrays anodized under different parameters. Results Phys. 2019, 15, 102609. [Google Scholar] [CrossRef]
- Tong, C.; Yun, J.; Song, H.; Gan, Q.; Anderson, W.A. Plasmonic-enhanced Si Schottky barrier solar cells. Sol. Energy Mater. Sol. Cells 2014, 120, 591–595. [Google Scholar] [CrossRef]
- Solaiyammal, T.; Murugakoothan, P. Green synthesis of Au and the impact of Au on the efficiency of TiO2 based dye sensitized solar cell. Mater. Sci. Energy Technol. 2019, 2, 171–180. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Muthukumarasamy, N.; Balraju, P.; Pitchaiya, S.; Velauthapillai, D.; Pugazhendhi, A. Transformation of TiO2 nanoparticles to nanotubes by simple solvothermal route and its performance as dye-sensitized solar cell (DSSC) photoanode. Int. J. Hydrogen Energy 2020, 45, 15441–15452. [Google Scholar] [CrossRef]
- Chong, S.W.; Lai, C.W.; Juan, J.C.; Leo, B.F. An investigation on surface modified TiO2 incorporated with graphene oxide for dye-sensitized solar cell. Sol. Energy 2019, 191, 663–671. [Google Scholar] [CrossRef]
- Neetu; Singh, S.; Srivastava, P.; Bahadur, L. Hydrothermal synthesized Nd-doped TiO2 with Anatase and Brookite phases as highly improved photoanode for dye-sensitized solar cell. Sol. Energy 2020, 208, 173–181. [Google Scholar] [CrossRef]
- Kathirvel, S.; Pedaballi, S.; Su, C.; Chen, B.-R.; Li, W.-R. Morphological control of TiO2 nanocrystals by solvothermal synthesis for dye-sensitized solar cell applications. Appl. Surf. Sci. 2020, 519, 146082. [Google Scholar] [CrossRef]
- Teimouri, R.; Heydari, Z.; Ghaziani, M.P.; Madani, M.; Abdy, H.; Kolahdouz, M.; Asl-Soleimani, E. Synthesizing Li doped TiO2 electron transport layers for highly efficient planar perovskite solar cell. Superlattices Microstruct. 2020, 145, 106627. [Google Scholar] [CrossRef]
- Wang, L.; Chen, K.; Tong, H.; Wang, K.; Tao, L.; Zhang, Y.; Zhou, X. Inverted pyramid Er3+ and Yb3+ Co-doped TiO2 nanorod arrays based perovskite solar cell: Infrared response and improved current density. Ceram. Int. 2020, 46, 12073–12079. [Google Scholar] [CrossRef]
- Kim, M.; Choi, I.-W.; Choi, S.J.; Song, J.W.; Mo, S.-I.; An, J.-H.; Jo, Y.; Ahn, S.; Ahn, S.K.; Kim, G.-H.; et al. Enhanced electrical properties of Li-salts doped mesoporous TiO2 in perovskite solar cells. Joule 2021, 5, 659–672. [Google Scholar] [CrossRef]

| Solar Cell Structure | Improvement by | Enhancement η (%) | Ref. |
|---|---|---|---|
| Glass/Cu/n-Si/Si NW + G/SiO2/Ag | Si NW + G | 100 | [51] |
| FLG/2GBD/n-Si | 2GBD | 56.2 | [52] |
| DSSC | 719dye + TiO2 nanotube | 87.5 | [53] |
| Al/p-c-Si/SiO2/Cr/Au/Ag | Ag nanoparticle + SiO2 | 80.4 | [54] |
| Al/p-c-Si/P-Si + Ag/TiO2/Au | P-Si + Ag | 111.2 | [43] |
| Au:TiO2 based DSSC | Au/TiO2 | 65.1 | [55] |
| TiO2 nanotube based DSSC | TiO2 nanotube | 53.6 | [56] |
| GO-TiO2 based DSSC | GO-TiO2 | 126.4 | [57] |
| Nd-TiO2 based DSSC | Nd-TiO2 | 34.3 | [58] |
| DSSC | TiO2 | 50.5 | [59] |
| FTO/Li-TiO2/perovskite/Spiro-OMeTAD/Au | Li-TiO2 | 1.97 | [60] |
| FTO/TiO2:Yb3+,Er3+/perovskite/Spiro-OMeTAD/Ag | TiO2: Yb3+, Er3+ | 16.5 | [61] |
| FTO/cTiO2/mpTiO2/perovskite/Spiro-OMeTAD/Ag | mpTiO2 | 9.2 | [62] |
| Al/p-c-Si/PSi/TiO2NPs/Ag | PSi | 264.3 | Present |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almomani, M.S.; Ahmed, N.M.; Rashid, M.; Suardi, N.; Almessiere, M.A.; Madkhali, N.; Aldaghri, O.A.; Ibnaouf, K.H. Photovoltaic Performance of Spherical TiO2 Nanoparticles Derived from Titanium Hydroxide Ti(OH)4: Role of Annealing Varying Temperature. Energies 2022, 15, 1648. https://doi.org/10.3390/en15051648
Almomani MS, Ahmed NM, Rashid M, Suardi N, Almessiere MA, Madkhali N, Aldaghri OA, Ibnaouf KH. Photovoltaic Performance of Spherical TiO2 Nanoparticles Derived from Titanium Hydroxide Ti(OH)4: Role of Annealing Varying Temperature. Energies. 2022; 15(5):1648. https://doi.org/10.3390/en15051648
Chicago/Turabian StyleAlmomani, Mohammad S., Naser M. Ahmed, Marzaini Rashid, Nursakinah Suardi, Munirah A. Almessiere, Nawal Madkhali, Osamah A. Aldaghri, and Khalid Hassan Ibnaouf. 2022. "Photovoltaic Performance of Spherical TiO2 Nanoparticles Derived from Titanium Hydroxide Ti(OH)4: Role of Annealing Varying Temperature" Energies 15, no. 5: 1648. https://doi.org/10.3390/en15051648
APA StyleAlmomani, M. S., Ahmed, N. M., Rashid, M., Suardi, N., Almessiere, M. A., Madkhali, N., Aldaghri, O. A., & Ibnaouf, K. H. (2022). Photovoltaic Performance of Spherical TiO2 Nanoparticles Derived from Titanium Hydroxide Ti(OH)4: Role of Annealing Varying Temperature. Energies, 15(5), 1648. https://doi.org/10.3390/en15051648









