Development and Characterization of Composite Desiccant Impregnated with LiCl for Thermoelectric Dehumidifier (TED)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Composite DCAS
2.2. Thermal Conductivity
2.3. Characterization Techniques
2.4. Sorption Kinetics
2.5. Moisture Sorption Isotherms
2.6. Moisture Sorption Isotherms Models
| BET | (1) | |
| GAB | (2) | |
| DLP | (3) |
3. Results
3.1. Thermal Conductivity
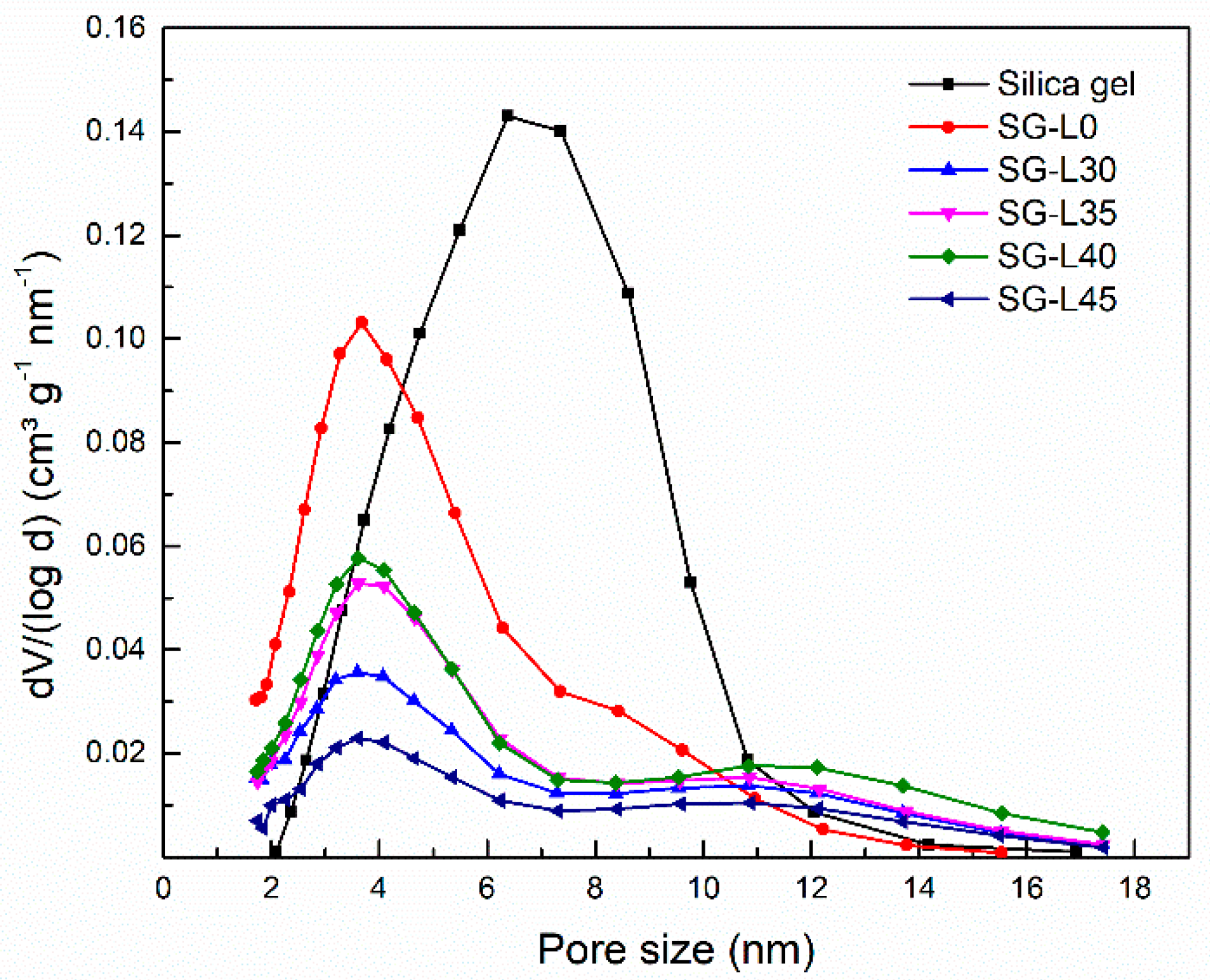
3.2. Textural and Morphological Characterization
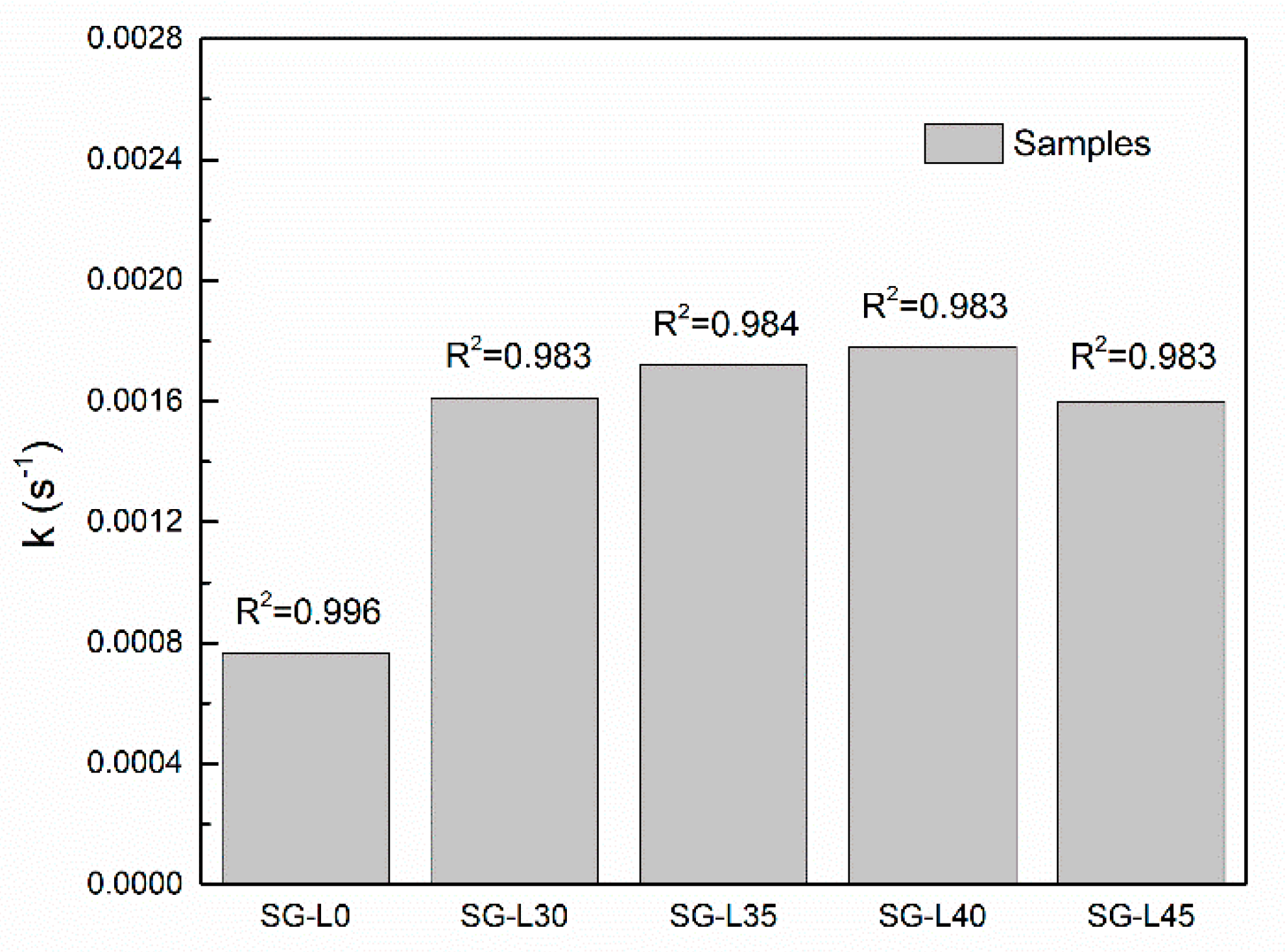
3.3. Sorption Kinetics
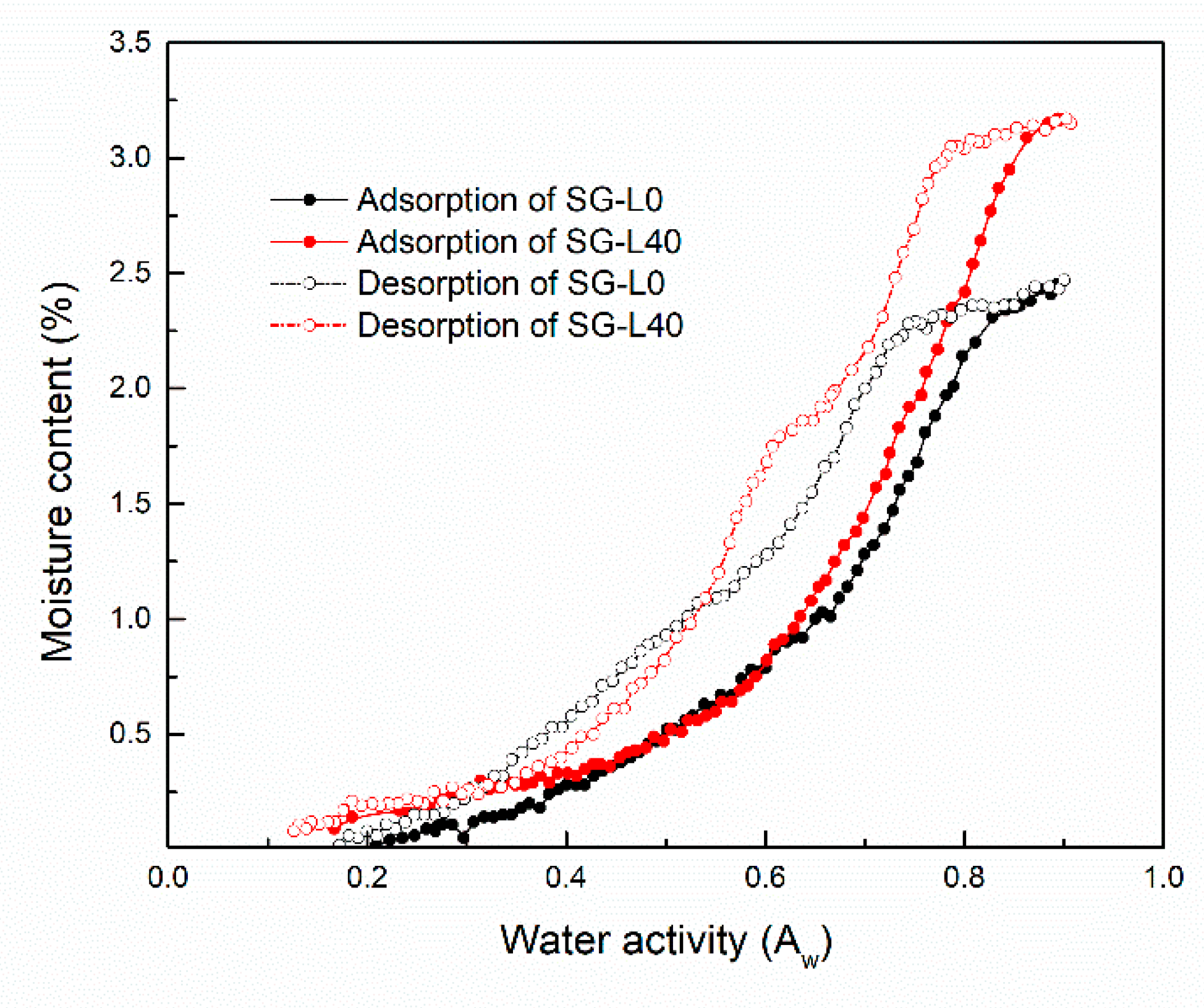
3.4. Moisture Sorption Isotherms
3.5. Moisture Sorption Isotherms Models
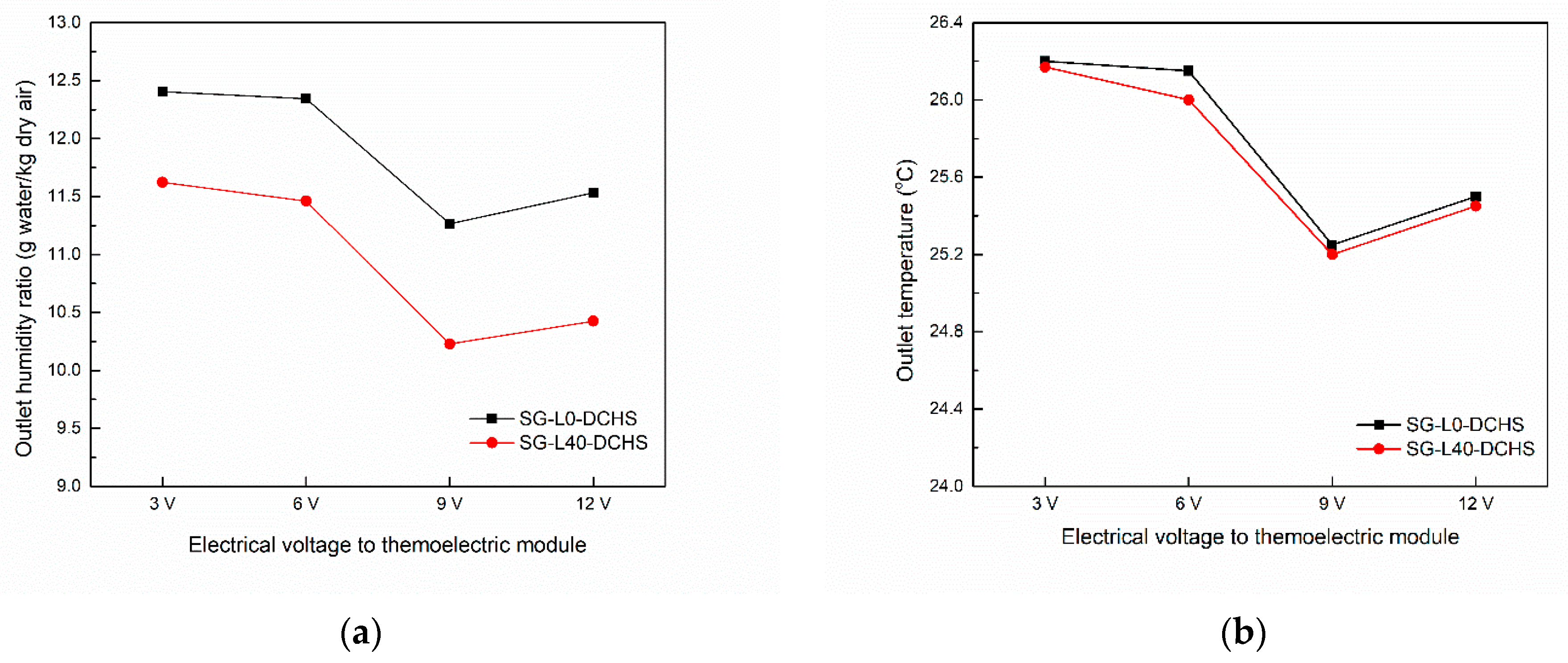
3.6. Dehumidification Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Aw | Water activity |
| Empirical constant | |
| Empirical constant | |
| Empirical constant | |
| Empirical constant | |
| A constant related to water binding energy to the primary binding sites | |
| A dimensionless measure of the strength of water binding to the primary binding sites | |
| Average pore size (nm) | |
| Sorption rate | |
| A constant related to the adsorption energies of multilayer adsorption sites | |
| Rate coefficient (s−1) | |
| Moisture content (%) | |
| The monolayer moisture content (%) | |
| The mass flow rate of process air (kg s−1) | |
| Dehumidification capacity (kg h−1) | |
| Root mean square error | |
| The coefficient of determination | |
| BET surface area (m2 g−1) | |
| BET surface area related to the mass of porous host matrix (silica gel) (m2 g−1) | |
| Dehumidification time (s) | |
| Total pore volume (cm3 g−1) | |
| Total pore volume related to the mass of porous host matrix (silica gel) (cm3 g−1) | |
| Inlet humidity ratios of the process air (g g−1) | |
| Outlet humidity ratios of the process air (g g−1) | |
| Mass concentration of impregnating salts (LiCl) (%) | |
| The equilibrium water sorption quantity (g g−1) | |
| Dynamic water sorption quantity (g g−1) |
Abbreviation
| DCAS | Desiccant-coated aluminum sheets |
| DCHS | Desiccant-coated heat sink |
| SGCAS | Silica-gel-coated aluminum sheets |
| SG-L0 | Silica gel |
| SG-L30 | Silica gel–LiCl composite prepared in 30 wt.% of LiCl aqueous solution |
| SG-L35 | Silica gel–LiCl composite prepared in 35 wt.% of LiCl aqueous solution |
| SG-L40 | Silica gel–LiCl composite prepared in 40 wt.% of LiCl aqueous solution |
| SG-L45 | Silica gel–LiCl composite prepared in 45 wt.% of LiCl aqueous solution |
References
- Tashiro, Y.; Kubo, M.; Katsumi, Y.; Meguro, T.; Komeya, K. Assessment of adsorption desorption characteristics of adsorbents for adsorptive desiccant cooling system. J. Mater. Sci. 2004, 39, 1315–1319. [Google Scholar] [CrossRef]
- Yu, N.; Wang, R.Z.; Lu, Z.S.; Wang, L.W. Sorption thermal storage for solar energy. Prog. Energy Combust. Sci. 2013, 39, 489–514. [Google Scholar] [CrossRef]
- Jia, C.X.; Dai, Y.J.; Wu, J.Y.; Wang, R.Z. Use of compound desiccant to develop high performance desiccant cooling system. Int. J. Refrig. 2007, 30, 345–353. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Restuccia, G.; Cacciola, G.; Parmon, V. A family of new working materials for solid sorption air conditioning systems. Appl. Therm. Eng. 2002, 22, 191–204. [Google Scholar] [CrossRef]
- Cortés, F.B.; Chejne, F.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Moreno-Castilla, C. Water sorption on silica-and zeolite-supported hygroscopic salts for cooling system applications. Energy Convers. Manag. 2012, 53, 219–223. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Huo, S.; Li, Z. Dynamics and isotherms of water vapor sorption on Mesoporous silica gels modified by different salts. Kinet. Catal. 2010, 51, 754–761. [Google Scholar] [CrossRef]
- Simonova, I.A.; Freni, A.; Restuccia, G.; Aristov, Y.I. Water sorption on composite “silica modified by calcium nitrate”. Microporous Mesoporous Mater. 2009, 122, 223–228. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Sapienza, A.; Ovoshchnikov, D.; Freni, A.; Restuccia, G. Reallocation of adsorption and desorption times for optimization of cooling cycles. Int. J. Refrig. 2012, 35, 525–531. [Google Scholar] [CrossRef]
- Ge, T.S.; Li, Y.; Wang, R.Z.; Dai, Y.J. Experimental study on a two-stage rotary desiccant cooling system. Int. J. Refrig. 2009, 32, 498–508. [Google Scholar] [CrossRef]
- Jeong, J.; Yamaguchi, S.; Saito, K.; Kawai, S. Performance analysis of desiccant dehumidification systems driven by low-grade heat source. Int. J. Refrig. 2011, 34, 928–945. [Google Scholar] [CrossRef]
- Hu, L.M.; Ge, T.S.; Jiang, Y.; Wang, R.Z. Performance study on composite desiccant material coated fin-tube heat exchangers. Int. J. Heat Mass Transfer. 2015, 90, 109–120. [Google Scholar] [CrossRef]
- Chua, K.J. Heat and mass transfer of composite desiccants for energy efficient air dehumidification: Modelling and experiment. Appl. Therm. Eng. 2015, 89, 703–716. [Google Scholar] [CrossRef]
- Hu, L.M.; Ge, T.S.; Jiang, Y.; Wang, Z. Hygroscopic Property of Metal Matrix Composite Desiccant (in Chinese). J. Refrigeration. 2014, 35, 69–75. [Google Scholar]
- Zheng, X.; Ge, T.S.; Jiang, Y.; Wang, R.Z. Experimental study on silica gel-LiCl composite desiccants for desiccant coated heat exchanger. Int. J. Refrig. 2015, 51, 24–32. [Google Scholar] [CrossRef]
- Mandegari, M.A.; Pahlavanzadeh, H. Introduction of a new definition for effectiveness of desiccant wheels. Energy 2009, 34, 797–803. [Google Scholar] [CrossRef]
- Stabat, P.; Marchio, D. Heat-and-mass transfers modelled for rotary desiccant dehumidifiers. Appl. Energy 2008, 85, 128–142. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Timmermann, E.O. Multilayer sorption parameters: BET or GAB values? Colloids Surf. A 2003, 220, 235–260. [Google Scholar] [CrossRef] [Green Version]
- Carter, B.P.; Galloway, M.T.; Campbell, G.S.; Carter, A.H. The critical water activity from dynamic dewpoint isotherms as an indicator of crispness in low moisture cookies. J. Food. Meas. Charact. 2015, 9, 463–470. [Google Scholar] [CrossRef]
- Arthur, E.; Tuller, M.; Moldrup, P.; de Jonge, L.W. Evaluation of theoretical and empirical water vapor sorption isotherm models for soils. Water Resour. Res. 2016, 52, 190–205. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Wang, R.Z.; Li, T.X. Thermochemical characterizations of high-stable activated alumina/LiCl composites with multistage sorption process for thermal storage. Energy 2018, 156, 240–249. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Glaznev, I.S.; Savchenko, E.V.; Malakhov, V.V.; Aristov, Y.I. Impact of phase composition on water adsorption on inorganic hybrids “salt/silica”. J. Colloid Interface Sci. 2006, 301, 685–691. [Google Scholar] [CrossRef]
- Gong, L.X.; Wang, R.Z.; Xia, Z.Z.; Chen, C.J. Adsorption equilibrium of water on a composite adsorbent employing lithium chloride in silica gel. J. Chem. Eng. Data 2010, 55, 2920–2923. [Google Scholar] [CrossRef]
- Zheng, X.; Ge, T.S.; Wang, R.Z.; Hu, L.M. Performance study of composite silica gels with different pore sizes and different impregnating hygroscopic salts. Chem. Eng. Sci. 2014, 120, 1–9. [Google Scholar] [CrossRef]
- Yu, N.; Wang, R.Z.; Lu, Z.S.; Wang, L.W. Development and characterization of silica gel-LiCl composite sorbents for thermal energy storage. Chem. Eng. Sci. 2014, 111, 73–84. [Google Scholar] [CrossRef]
- Frazzica, A.; Brancato, V.; Caprì, A.; Cannilla, C.; Gordeeva, L.G.; Aristov, Y.I. Development of “salt in porous matrix” composites based on LiCl for sorption thermal energy storage. Energy 2020, 208, 118338. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.; Everett, D.; Haynes, J.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (Technical report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Huo, Q.; Margolese, D.I.; Stucky, G.D. Surfactant Control of Phases in the Synthesis of Mesoporous Silica-Based Materials. Chem. Mater. 1996, 8, 1147–1160. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Application of large pore MCM-41 molecular sieves to improve pore size analysis using nitrogen adsorption measurements. Langmuir 1997, 13, 6267–6273. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, R.Z. Microstructure and sorption performance of consolidated composites impregnated with LiCl. Int. J. Refrig. 2019, 98, 452–458. [Google Scholar] [CrossRef]
- Glueckauf, E. Theory of chromatography. Part 10.-Formulæ for diffusion into spheres and their application to chromatography. Trans. Faraday Soc. 1955, 51, 1540–1551. [Google Scholar] [CrossRef]
- Oulkadi, D.; Desobry, S.; Mustin, C.; Desobry-Banon, S. Water sorption in hybrid silica gels containing colloidal nontronite. J Solgel. Sci. Technol. 2012, 64, 653–660. [Google Scholar] [CrossRef]


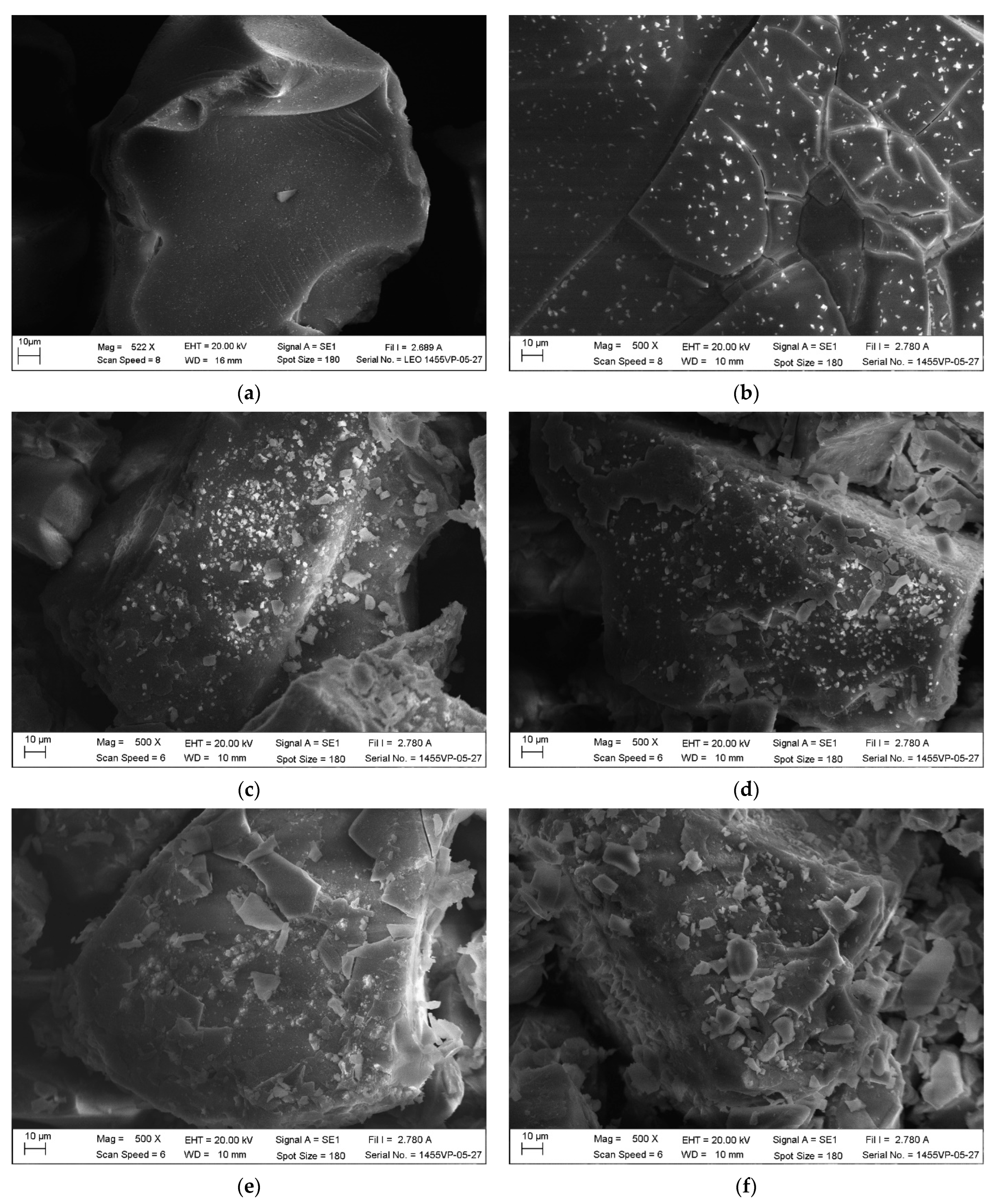




| Name | Description | Manufacturer |
|---|---|---|
| Aluminum sheet | 60 mm × 40 mm × 1 mm k = 230 Wm−1 K−1 | AIC Aluminum Profile Service Center (Bangkok, Thailand) |
| Silica gel | Mesoporous, the grain size of 70 to 230 mesh SBET = 500 m2 g−1; Vt = 0.75 cm3 g−1; dav = 6 nm k = 0.1 Wm−1 K−1 | Merck PTE Ltd. (Singapore) |
| Silica sol | 30 wt.% suspension in H2O, pH = 9.1 SBET = 230 m2 g−1; Vt = 0.21 cm3g−1; dav = 4.3 nm | Merck PTE Ltd. (Singapore) |
| Liquid glue | Polyurethane base (BT550) k = 0.26 Wm−1 K−1 | Bond Chemicals Co., Ltd. |
| Anhydrous LiCl | ACS reagent, assay ≥99% | Merck PTE Ltd. (Singapore) (Bangkok, Thailand) |
| Composition | Impregnating Concentration of LiCl (wt.%) | Mass of Desiccants (g) | Total Mass (g) | Salt Content (wt.%) | Coating Amount (kg m−2) |
|---|---|---|---|---|---|
| SG-L0 | 0 | 1.05 | 7.45 | 0.00 | 0.44 |
| SG-L30 | 30 | 1.18 | 7.58 | 12.38 | 0.49 |
| SG-L35 | 35 | 1.20 | 7.60 | 14.29 | 0.50 |
| SG-L40 | 40 | 1.23 | 7.63 | 17.14 | 0.51 |
| SG-L45 | 45 | 1.27 | 7.67 | 20.95 | 0.53 |
| Physical Parameter | Range or Deviation |
|---|---|
| accuracy | ) |
| Sample temperature control range (°C) | 15–60 at STP |
| Operating environment (°C) | 4–50 |
| Sample weight (mg) | 500–5000 |
| Mass resolution (mg) | ±0.5 |
| Composition | Impregnating Concentration of LiCl (wt.%) | Salt Content (wt.%) | SBET (m² g−1) | SBET * (m² g−1) | vt (cm³ g−1) | vt * (cm³ g−1) | dav (nm) |
|---|---|---|---|---|---|---|---|
| SG-L0 | 0 | 0.00 | 208.10 | - | 0.31 | - | 3.71 |
| SG-L30 | 30 | 12.38 | 128.55 | 154.31 | 0.21 | 0.25 | 3.74 |
| SG-L35 | 35 | 14.29 | 117.33 | 147.07 | 0.18 | 0.22 | 3.86 |
| SG-L40 | 40 | 17.14 | 89.59 | 125.26 | 0.14 | 0.19 | 3.69 |
| SG-L45 | 45 | 20.95 | 59.49 | 103.09 | 0.10 | 0.16 | 3.68 |
| Model | Para. | SG-L0 | SG-L40 | ||
|---|---|---|---|---|---|
| Adsorption | Desorption | Adsorption | Desorption | ||
| BET | c | 3.33187 | 16.1423 | 2.62282 | 8.41901 |
| m0 | 0.35426 | 0.40358 | 0.43488 | 0.49152 | |
| R2 | 0.91465 | 0.70074 | 0.92648 | 0.75911 | |
| RMSE | 0.2570 | 0.47098 | 0.26471 | 0.56232 | |
| GAB | c1 | 1.60935 | 1.13943 | 1.46069 | 0.9858 |
| K | 0.94432 | 0.70814 | 0.96112 | 0.7583 | |
| m0 | 0.50833 | 1.61023 | 0.56778 | 1.78218 | |
| R2 | 0.9516 | 0.93344 | 0.95263 | 0.93973 | |
| RMSE | 0.19354 | 0.22213 | 0.21248 | 0.28126 | |
| DLP | b0 | 0.36629 | 0.78821 | 0.40107 | 1.15945 |
| b1 | −2.49502 | −7.17614 | −1.05856 | −10.01023 | |
| b2 | 5.24140 | 21.08981 | −0.49086 | 26.20371 | |
| b3 | −2.49502 | −12.18092 | 6.04448 | −13.72783 | |
| R2 | 0.99198 | 0.98990 | 0.99255 | 0.98878 | |
| RMSE | 0.07878 | 0.08651 | 0.08428 | 0.12138 | |
| Composition | Sorption Direction | DLP Fitting Equations |
|---|---|---|
| SG-L0 | Adsorption | |
| Desorption | ||
| SG-L40 | Adsorption | |
| Desorption |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Channoy, C.; Maneewan, S.; Chirarattananon, S.; Punlek, C. Development and Characterization of Composite Desiccant Impregnated with LiCl for Thermoelectric Dehumidifier (TED). Energies 2022, 15, 1778. https://doi.org/10.3390/en15051778
Channoy C, Maneewan S, Chirarattananon S, Punlek C. Development and Characterization of Composite Desiccant Impregnated with LiCl for Thermoelectric Dehumidifier (TED). Energies. 2022; 15(5):1778. https://doi.org/10.3390/en15051778
Chicago/Turabian StyleChannoy, Chanchira, Somchai Maneewan, Surapong Chirarattananon, and Chantana Punlek. 2022. "Development and Characterization of Composite Desiccant Impregnated with LiCl for Thermoelectric Dehumidifier (TED)" Energies 15, no. 5: 1778. https://doi.org/10.3390/en15051778






