Catalytic Removal of NOx on Ceramic Foam-Supported ZnO and TiO2 Nanorods Ornamented with W and V Oxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TiO2 or ZnO Nanofilaments at Ceramic Foams
2.3. General Preparation of W and V Nanoparticles at Ceramic Foams and Other Carriers
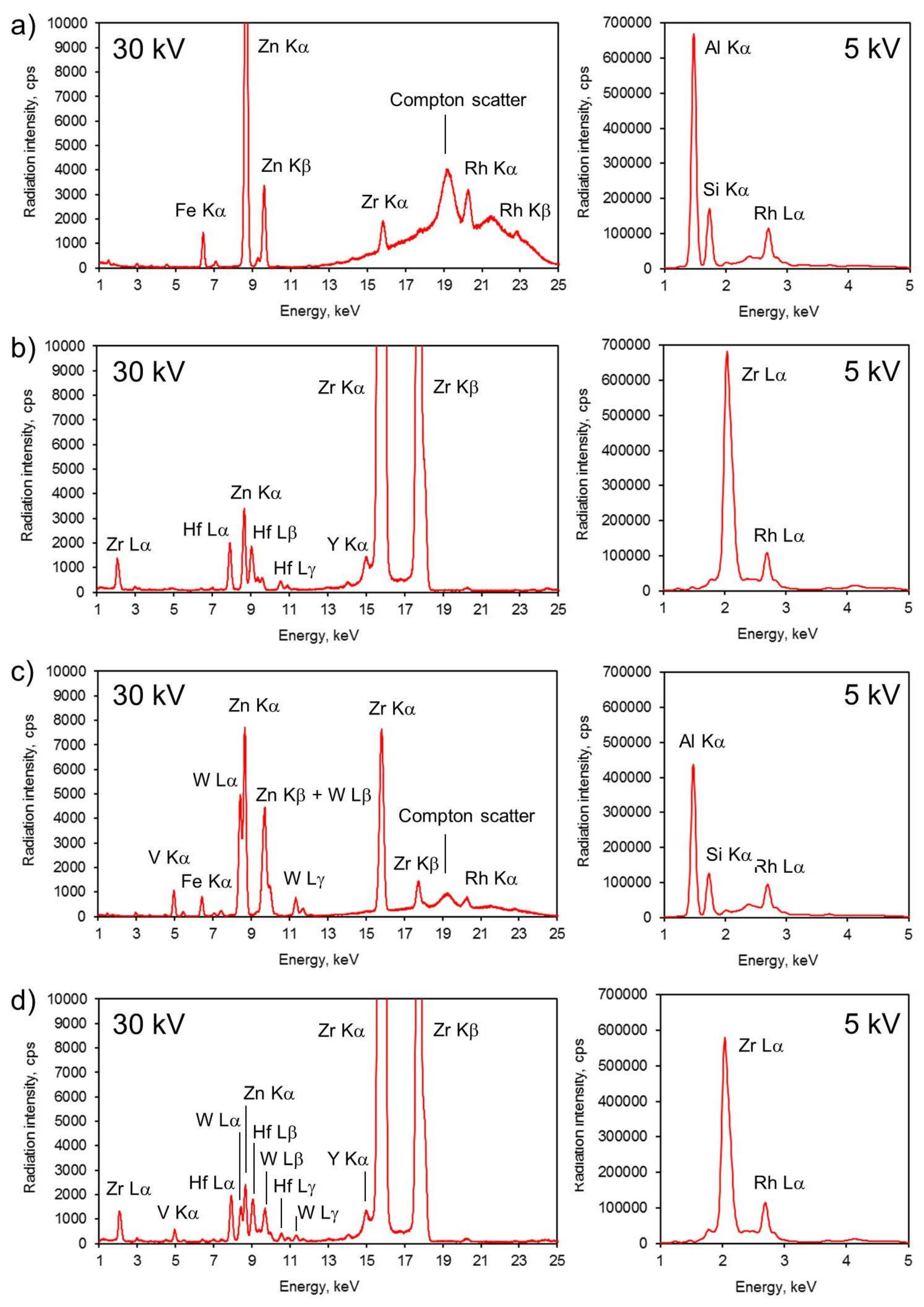
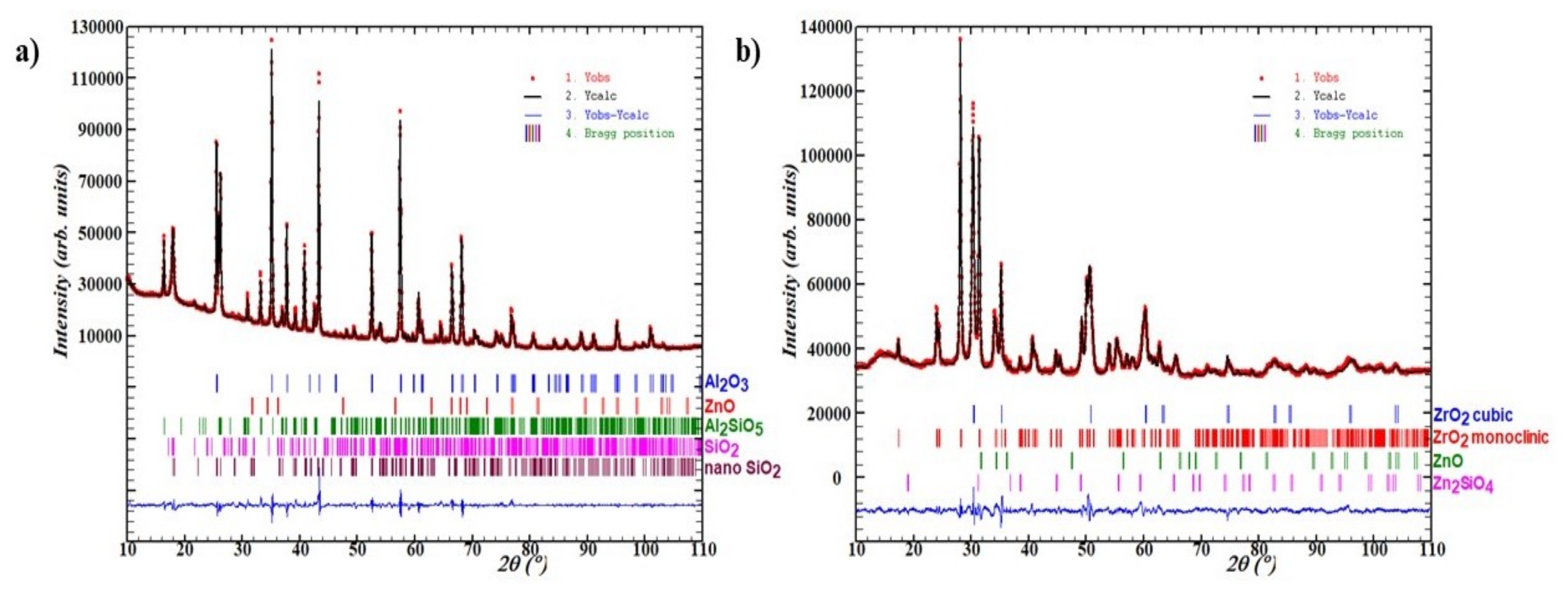
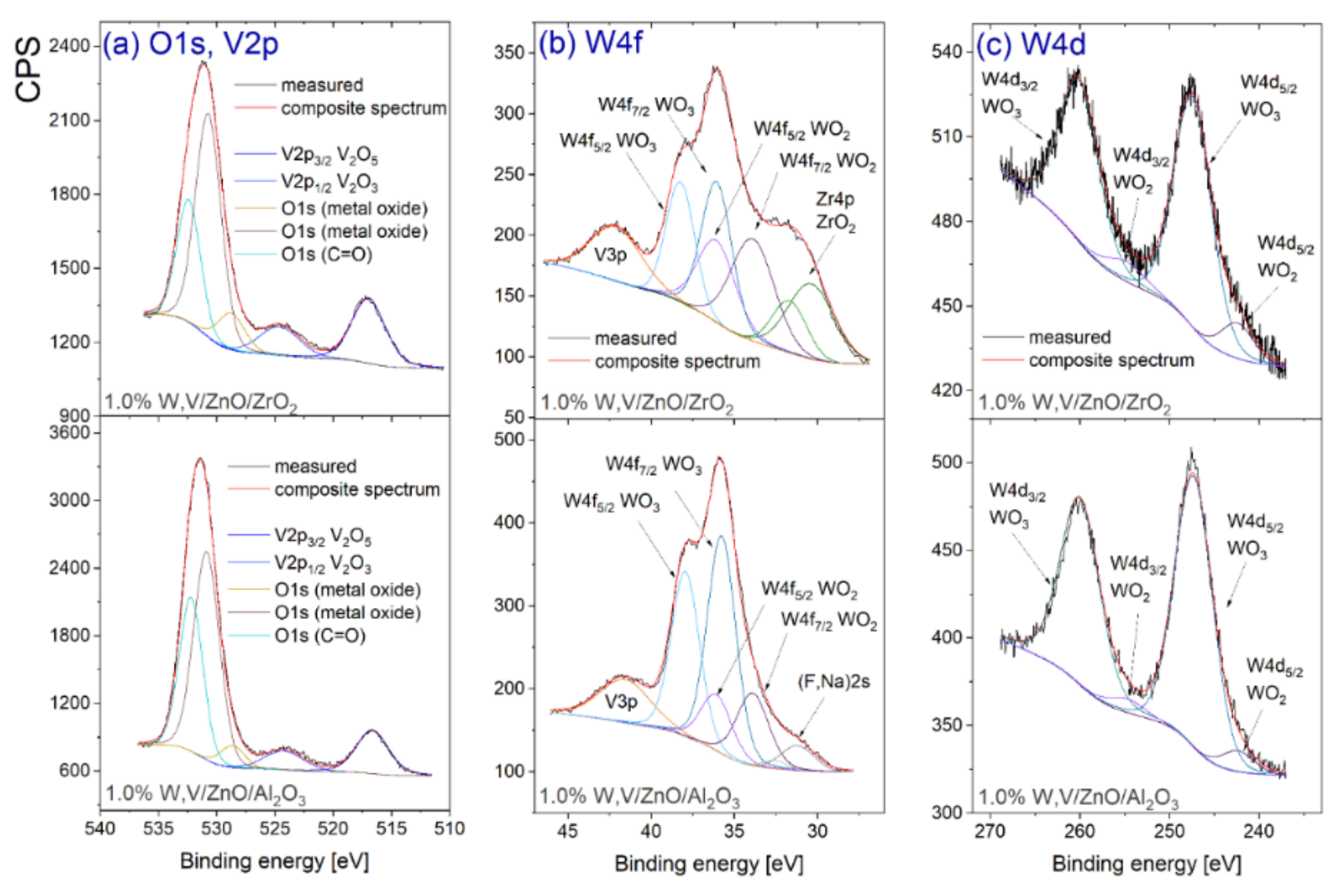
2.4. Methods of Catalyst Characterization
2.5. NOx Decomposition in a Flow Reactor
3. Results and Discussion
3.1. The Catalysts Design, Preparation, and Structure
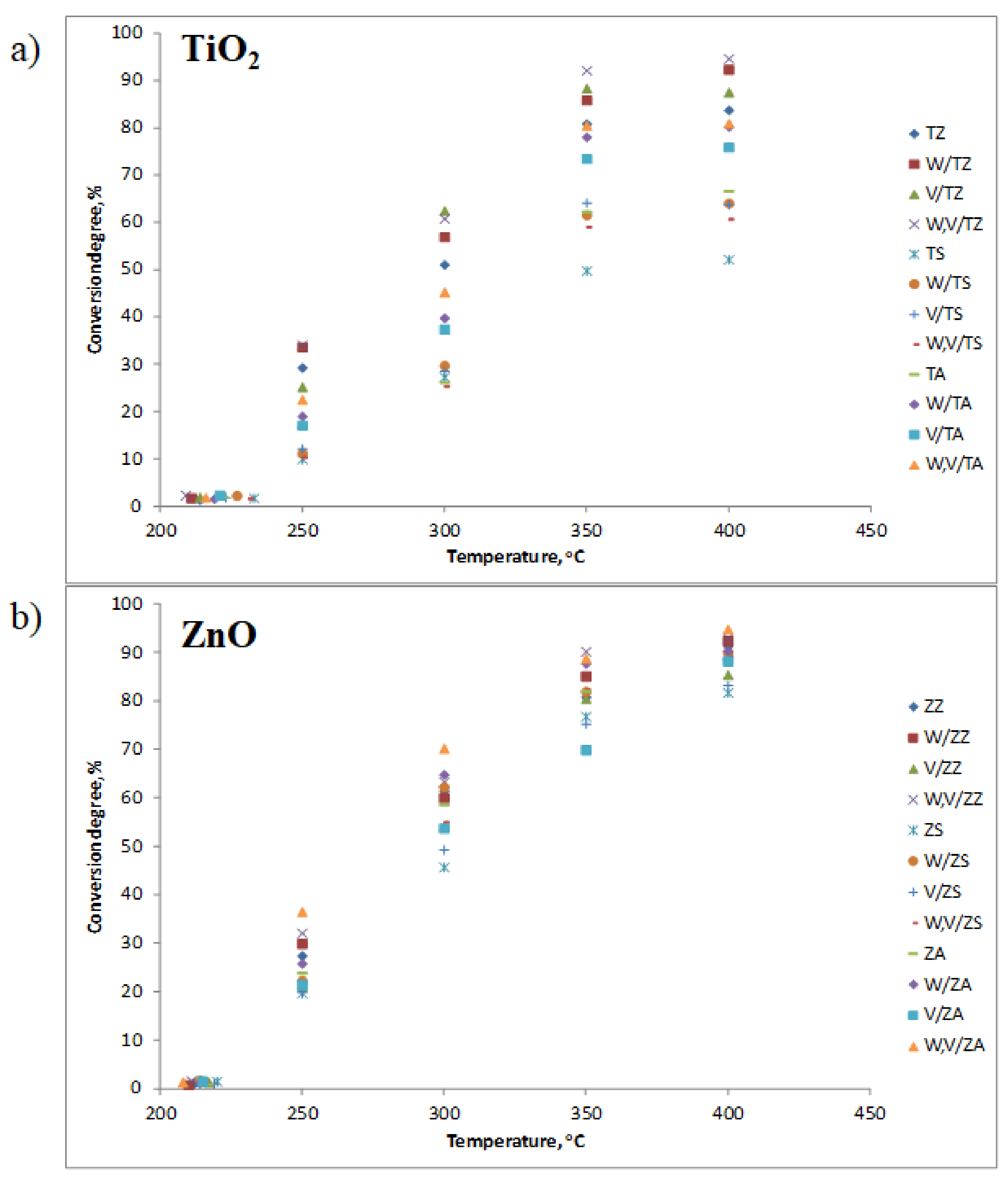
3.2. Catalyst Performance in SCR Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polanski, J.; Lach, D.; Kapkowski, M.; Bartczak, P.; Siudyga, T.; Smolinski, A. Ru and Ni—Privileged Metal Combination for Environmental Nanocatalysis. Catalysts 2020, 10, 992. [Google Scholar] [CrossRef]
- Van Noyen, J.; Mullens, S.; Snijkers, F.; Luyten, J. Catalyst design with porous functional structures. Sustain. Chem. WIT Trans. Ecol. Environ. 2011, 154, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Carty, W.M.; Lednor, P.W. Monolithic ceramics and heterogeneous catalysts: Honeycombs and foams. Curr. Opin. Solid State Mater. Sci. 1996, 1, 88–95. [Google Scholar] [CrossRef]
- Twigg, M.V.; Richardson, J.T. Theory and Applications of Ceramic Foam Catalysts. Chem. Eng. Res. Des. 2002, 90, 183–189. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Ke, L.; Peng, Y.; Liu, Y.; Wu, Q.; Tian, X.; Dai, L.; Ruan, R.; Jiang, L. Review on the catalytic pyrolysis of waste oil for the production of renewable hydrocarbon fuels. Fuel 2021, 283, 119170. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Cortese, M.; Martino, M. Recent Advances in Structured Catalysts Preparation and Use in Water-Gas Shift Reaction. Catalysts 2019, 9, 991. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, E.M.; Rey, A.; Mena, E.; Beltrán, F.J. Application of solar photocatalytic ozonation in water treatment using supported TiO2. Appl. Catal. B Environ. 2019, 254, 237–245. [Google Scholar] [CrossRef]
- Leonzio, G. ANOVA analysis of an integrated membrane reactor for hydrogen production by methane steam reforming. Int. J. Hydrog. Energy 2019, 44, 11535–11545. [Google Scholar] [CrossRef]
- Zuercher, S.; Pabst, K.; Schaub, G. Ceramic foams as structured catalyst inserts in gas–particle filters for gas reactions—Effect of backmixing. Appl. Catal. A Gen. 2009, 357, 85–92. [Google Scholar] [CrossRef]
- Huangfu, L.; Abubakar, A.; Li, C.; Li, Y.; Wang, C.; Gao, S.; Liu, Z.; Yu, J. Development of Red Mud Coated Catalytic Filter for NOx Removal in the High Temperature Range of 300–450 °C. Catal. Lett. 2020, 150, 702–712. [Google Scholar] [CrossRef]
- Seijger, G.B.F.; Oudshoorn, O.L.; Boekhorst, A.; van Bekkum, H.; van den Bleek, C.M.; Calis, H.P.A. Selective catalytic reduction of NO over zeolite-coated structured catalyst packings. Chem. Eng. Sci. 2001, 56, 849–857. [Google Scholar] [CrossRef]
- Seo, H.J.; Jeong, R.H.; Boo, J.-H.; Song, J.; Boo, J.-H. Study on Chemical Removal of Nitric Oxide (NO) as a Main Cause of Fine Dust (Air Pollution) and Acid Rain. Appl. Sci. Converg. Technol. 2017, 26, 218–222. [Google Scholar] [CrossRef]
- Irfan, M.F.; Goo, J.H.; Kim, S.D. Co3O4 based catalysts for NO oxidation and NOx reduction in fast SCR process. Appl. Catal. B Environ. 2008, 78, 267–274. [Google Scholar] [CrossRef]
- Lai, J.K.; Wachs, I.E. A Perspective on the Selective Catalytic Reduction (SCR) of NO with NH3 by Supported V2O5–WO3/TiO2 Catalysts. ACS Catal. 2018, 8, 6537–6551. [Google Scholar] [CrossRef]
- Kapkowski, M.; Siudyga, T.; Sitko, R.; Niemczyk-Wojdyla, A.; Zelenka, T.; Zelenkova, G.; Golba, S.; Smolinski, A.; Polanski, J. Toward a viable ecological method for regenerating a commercial SCR catalyst–Selectively leaching surface deposits and reconstructing a pore landscape. J. Clean. Prod. 2021, 316, 128291. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Yuan, H. Recycling strategies of spent V2O5-WO3/TiO2 catalyst: A review. Resour. Conserv. Recycl. 2020, 161, 104983. [Google Scholar] [CrossRef]
- Huo, Y.; Chang, Z.; Li, W.; Liu, S.; Dong, B. Reuse and valorization of vanadium and tungsten from waste V2O5–WO3/TiO2 SCR catalyst. Waste Biomass Valorization 2015, 6, 159–165. [Google Scholar] [CrossRef]
- Salonen, H.; Salthammer, T.; Morawska, L. Human exposure to NO2 in school and office indoor environments. Environ. Int. 2019, 130, 104887. [Google Scholar] [CrossRef]
- Koebel, M.; Madia, G.; Raimondi, F.; Wokaun, A. How NO2 Affects the Reaction Mechanism of the SCR Reaction. Available online: https://www.osti.gov/etdeweb/servlets/purl/20398744 (accessed on 10 February 2022).
- Zhu, X.; Zhang, L.; Dong, Y.; Ma, C. NO2–NH3 SCR over Activated Carbon: A Combination of NH4NO3 Formation and Consumption. Energy Fuels 2021, 35, 6167–6178. [Google Scholar] [CrossRef]
- Xu, S.; Han, X.; Ma, Y.; Duong, T.D.; Lin, L.; Gibson, E.K.; Sheveleva, A.; Chansai, S.; Walton, A.; Ngo, D.-T.; et al. Catalytic decomposition of NO2 over a copper-decorated metal–organic framework by non-thermal plasma. Cell Rep. Phys. Sci. 2021, 2, 100349. [Google Scholar] [CrossRef]
- Fernández-Pérez, A.; Rodríguez-Casado, V.; Valdés-Solís, T.; Marbán, G. Room temperature sintering of polar ZnO nanosheets: I-evidence. Phys. Chem. Chem. Phys. 2017, 19, 16406–16412. [Google Scholar] [CrossRef] [Green Version]
- Colón, G.; Hidalgo, M.C.; Navío, J.A.; Melián, E.P.; Díaz, O.G.; Rodríguez, J.M.D. Highly photoactive ZnO by amine capping-assisted hydrothermal treatment. Appl. Catal. B Environ. 2008, 83, 30–38. [Google Scholar] [CrossRef]
- Yermolayeva, Y.V.; Savin, Y.N.; Tolmachev, A.V. Controlled Growth of ZnO Nanocrystals on the Surface of SiO2 Spheres. Solid State Phenom. 2009, 151, 264–268. [Google Scholar] [CrossRef]
- Siwinska-Stefanska, K.; Kubiak, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO Binary Oxide Systems: Comprehensive Characterization and Tests of Photocatalytic Activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Q.; Wang, W.; Zhang, Z.; Duan, J. Enhanced photocatalytic performance of Cu2O/MoS2/ZnO composites on Cu mesh substrate for nitrogen reduction. Nanotechnology 2021, 32, 285706. [Google Scholar] [CrossRef]
- Fernández-Pérez, A.; Marbán, G. Room temperature sintering of polar ZnO nanosheets: III-Prevention. Microporous Mesoporous Mater. 2020, 294, 109836. [Google Scholar] [CrossRef]
- Laurenti, M.; Stassi, S.; Canavese, G.; Cauda, V. Surface Engineering of Nanostructured ZnO Surfaces. Adv. Mater. Interfaces 2017, 4, 1600758. [Google Scholar] [CrossRef]
- Witkowski, B.S.; Wachnicki, L.; Gieraltowska, S.; Dluzewski, P.; Szczepanska, A.; Kaszewski, J.; Godlewski, M. Ultra-fast growth of the monocrystalline zinc oxide nanorods from the aqueous solution. Int. J. Nanotechnol. 2014, 11, 758–772. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database, Version 4.1 (National Institute of Standards and Technology, Gaithersburg, 2012). Available online: http://srdata.nist.gov/xps/ (accessed on 10 February 2022).
- Kim, D.; Kim, M.; Yi, J.; Nam, S.H.; Boo, J.H.; Park, Y.S.; Lee, J. Growth and characterization of VO2 thin film by pulsed DC sputtering of optical switching applications. Sci. Adv. Mater. 2017, 9, 1415–1419. [Google Scholar] [CrossRef]
- Cheng, I.C.; Garcia-Sanchez, E.; Hodge, A.M. Note: A method for minimizing oxide formation during elevated temperature nanoindentation. Rev. Sci. Instrum. 2014, 85, 096106. [Google Scholar] [CrossRef]
- Korduban, A.M.; Shpak, A.P.; Medvedskij, M.M. Electronic structure exploration of active element surface for hydrogen sensor based on WO3-x nanoparticles. In Hydrogen Materials Science and Chemistry of Carbon Nanomaterials; Springer: Dordrecht, The Netherlands, 2007; pp. 59–64. [Google Scholar] [CrossRef]
- Mendialdua, J.; Casanova, R.; Rueda, F.; Rodríguez, A.; Quiñones, J.; Alarcón, L.; Escalante, E.; Hoffmann, P.; Taebi, I.; Jalowiecki, L. X-ray photoelectron spectroscopy studies of laterite standard reference material. J. Mol. Catal. A Chem. 2005, 228, 151–162. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, J.; Yu, P.; Zhang, B.; Ma, M.; Zhang, X.; Liu, R. Facile synthesis of a ZnO–BiOI p–n nano-heterojunction with excellent visible-light photocatalytic activity. Beilstein J. Nanotechnol. 2018, 9, 789–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archana, J.; Navaneethan, M.; Hayakawa, Y. Morphological transformation of ZnO nanoparticle to nanorods via solid-solid interaction at high temperature annealing and functional properties. Scr. Mater. 2016, 113, 163–166. [Google Scholar] [CrossRef]
- Li, H.; Rameshan, C.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I.; Rupprechter, G. CO2 activation on ultrathin ZrO2 film by H2O co-adsorption: In situ NAP-XPS and IRAS studies. Surf. Sci. 2019, 679, 139–146. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Asami, K.; Ito, S.; Yashima, M.; Sugimoto, T. XPS study of the phase transition in pure zirconium oxide nanocrystallites. Appl. Surf. Sci. 2005, 252, 1651–1656. [Google Scholar] [CrossRef]
- Burdeinaya, T.N.; Matyshak, V.A.; Tret’yakov, V.F.; Glebov, L.S.; Zakirova, A.G.; Garcia, M.C.; Villanueva, M.A. Design of catalysts for deNOx process using synergistic phenomenon. Appl. Catal. B Environ. 2007, 70, 128–137. [Google Scholar] [CrossRef]
- Misono, M. Chemistry and Catalysis of Mixed Oxides. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2013; Volume 176, pp. 25–65. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, L.; Dong, G. Mn-Ce-V-WOx/TiO2 SCR Catalysts: Catalytic Activity, Stability and Interaction among Catalytic Oxides. Catalysts 2018, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Weng, D.; Si, Z.; Wu, X. Synergistic effect between ceria and tungsten oxide on WO3–CeO2–TiO2 catalysts for NH3-SCR reaction. Prog. Nat. Sci. 2012, 22, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Selleri, T.; Gramigni, F.; Nova, I.; Tronconi, E. NO oxidation on Fe- and Cu-zeolites mixed with BaO/Al2O3: Free oxidation regime and relevance for the NH3-SCR chemistry at low temperature. Appl. Catal. B Environ. 2018, 225, 324–331. [Google Scholar] [CrossRef]
- Ganjkhanlou, Y.; Janssens, T.V.W.; Vennestrøm, P.N.R.; Mino, L.; Paganini, M.C.; Signorile, M.; Bordiga, S.; Berlier, G. Location and activity of VOx species on TiO2 particles for NH3-SCR catalysis. Appl. Catal. B Environ. 2020, 278, 119337. [Google Scholar] [CrossRef]
- Hendi, A.H.Y.; Al-Kuhaili, M.F.; Durrani, S.M.A.; Faiza, M.M.; Ul-Hamid, A.; Qurashi, A.; Khan, I. Modulation of the band gap of tungsten oxide thin films through mixing with cadmium telluride towards photovoltaic applications. Mater. Res. Bull. 2017, 87, 148–154. [Google Scholar] [CrossRef]
- Srilakshmi, P.; Maheswari, A.U.; Sajeev, V.; Sivakumar, M. Tuning the optical bandgap of V2O5 nanoparticles by doping transition metal ions. Mater. Today 2019, 18, 1375–1379. [Google Scholar] [CrossRef]
- Karuppasamy, K.M.; Subrahmanyam, A. Results on the electrochromic and photocatalytic properties of vanadium doped tungsten oxide thin films prepared by reactive dc magnetron sputtering technique. J. Phys. D Appl. Phys. 2008, 41, 035302. [Google Scholar] [CrossRef]
- Oliveira, J.B.; Morbec, J.M.; Miwa, R.H. Mechanical and electronic properties of SiC nanowires: An ab initio study. J. Appl. Phys. 2017, 121, 104302. [Google Scholar] [CrossRef] [Green Version]

| Entry | Catalyst | NOx Conversion [%] a | TON |
|---|---|---|---|
| 1 | TiO2/Al2O3 | 78.7 | 527 |
| 2 | 1% V/TiO2/Al2O3 | 85.5 | 573 |
| 3 | 1% V,W(7:3)/TiO2/Al2O3 | 89.4 | 599 |
| 4 | 1% V,W(1:1)/TiO2/Al2O3 | 88.3 | 591 |
| 5 | 1% V,W(3:7)/TiO2/Al2O3 | 90.2 | 604 |
| 6 | 1% W/TiO2/Al2O3 | 91.5 | 613 |
| 7 | ZnO/Al2O3 | 81.1 | 543 |
| 8 | 1% V/ZnO/Al2O3 | 82.9 | 555 |
| 9 | 1% V,W(7:3)/ZnO/Al2O3 | 87.6 | 587 |
| 10 | 1% V,W(1:1)/ZnO/Al2O3 | 90.8 | 608 |
| 11 | 1% V,W(3:7)/ZnO/Al2O3 | 93.0 | 623 |
| 12 | 1% W/ZnO/Al2O3 | 92.4 | 619 |
| Entry | Catalyst | Sample Code | NOx Conversion (%) a |
|---|---|---|---|
| 1 | ZnO/Al2O3 | ZA | 88.6 |
| 2 | 1% W/ZnO/Al2O3 | W/ZA | 90.3 |
| 3 | 1% V/ZnO/Al2O3 | V/ZA | 88.2 |
| 4 | 1% W,V/ZnO/Al2O3 | W,V/ZA | 94.8 |
| 5 | ZnO/SiC | ZS | 81.7 |
| 6 | 1% W/ZnO/SiC | W/ZS | 89.9 |
| 7 | 1% V/ZnO/SiC | V/ZS | 83.2 |
| 8 | 1% W,V/ZnO/SiC | W,V/ZS | 89.1 |
| 9 | ZnO/ZrO2 | ZZ | 91.3 |
| 10 | 1% W/ZnO/ZrO2 | W/ZZ | 92.4 |
| 11 | 1% V/ZnO/ZrO2 | V/ZZ | 85.4 |
| 12 | 1% W,V/ZnO/ZrO2 | W,V/ZZ | 93.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapkowski, M.; Siudyga, T.; Bartczak, P.; Zubko, M.; Sitko, R.; Szade, J.; Balin, K.; Witkowski, B.S.; Ożga, M.; Pietruszka, R.; et al. Catalytic Removal of NOx on Ceramic Foam-Supported ZnO and TiO2 Nanorods Ornamented with W and V Oxides. Energies 2022, 15, 1798. https://doi.org/10.3390/en15051798
Kapkowski M, Siudyga T, Bartczak P, Zubko M, Sitko R, Szade J, Balin K, Witkowski BS, Ożga M, Pietruszka R, et al. Catalytic Removal of NOx on Ceramic Foam-Supported ZnO and TiO2 Nanorods Ornamented with W and V Oxides. Energies. 2022; 15(5):1798. https://doi.org/10.3390/en15051798
Chicago/Turabian StyleKapkowski, Maciej, Tomasz Siudyga, Piotr Bartczak, Maciej Zubko, Rafal Sitko, Jacek Szade, Katarzyna Balin, Bartłomiej S. Witkowski, Monika Ożga, Rafał Pietruszka, and et al. 2022. "Catalytic Removal of NOx on Ceramic Foam-Supported ZnO and TiO2 Nanorods Ornamented with W and V Oxides" Energies 15, no. 5: 1798. https://doi.org/10.3390/en15051798






