A Review on the Enhancement of Mechanical and Tribological Properties of MCrAlY Coatings Reinforced by Dispersed Micro and Nanoparticles
Abstract
1. Introduction
2. Characterization of MCrAlY Alloys and Reinforcing Materials
- Pure MCrAlY
- Reinforced MCrAlY
3. Incorporation Mechanisms of Particles into MCrAlY Coatings
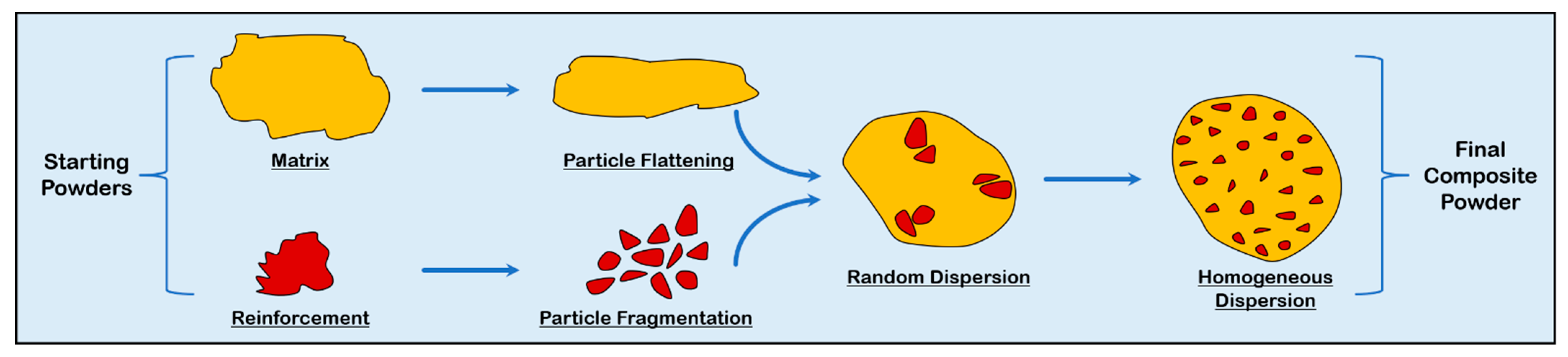
3.1. Feedstock Material Preparation
3.1.1. Mechanical Mixing
3.1.2. Mechanical Milling and Alloying
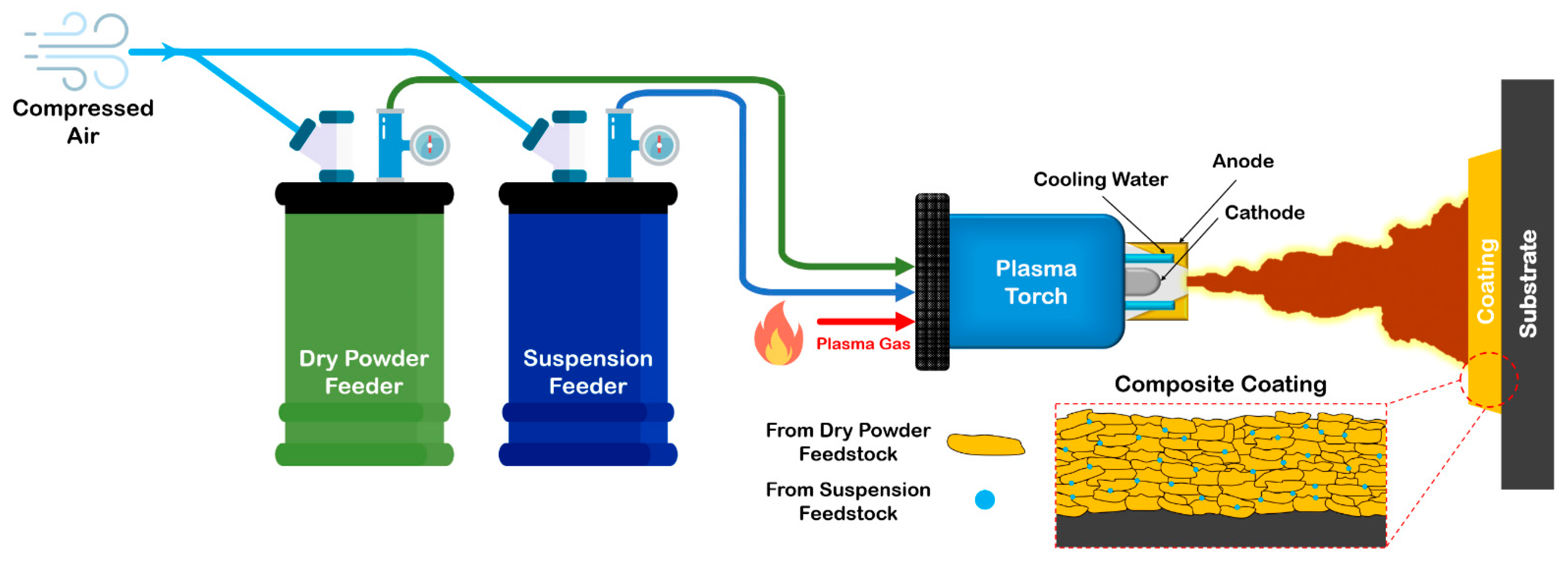
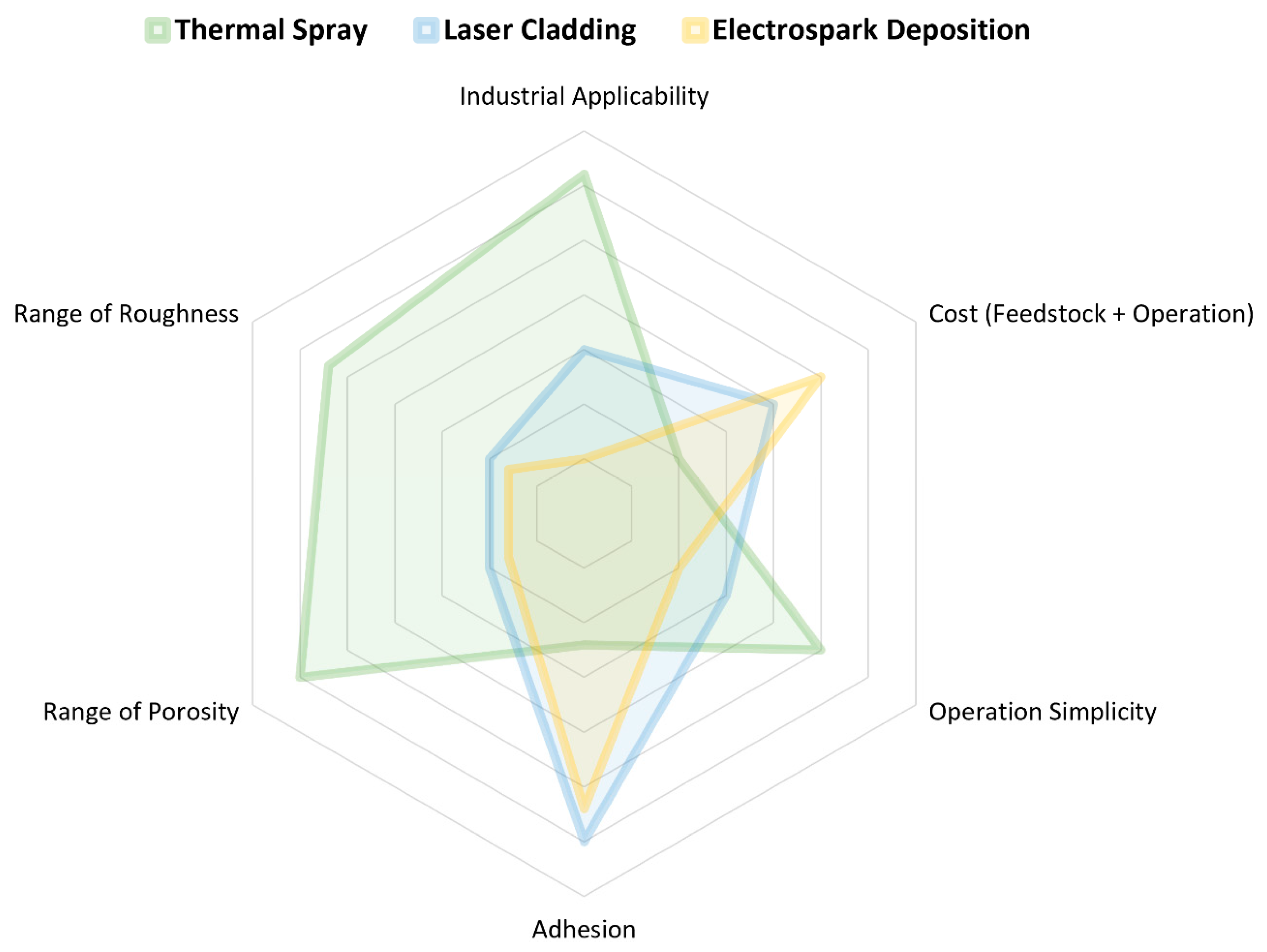
3.2. Feedstock Material Deposition
3.2.1. Thermal Spraying
- Spraying parameters (i.e., power and gas supply, material feeding rate and stand-off distance);
- Spray torch type;
- Coating material preparation (i.e., powder morphology and size distribution, flowability and deformability);
- Surrounding atmosphere (i.e., atmospheric air, controlled shielding atmosphere and vacuum);
- Substrate material, surface preparation, and its cooling.
- Plasma spraying
- High-velocity oxy/air-fuel (HVOF/HVAF) spraying
- Cold spraying
- Suspension and hybrid plasma spraying
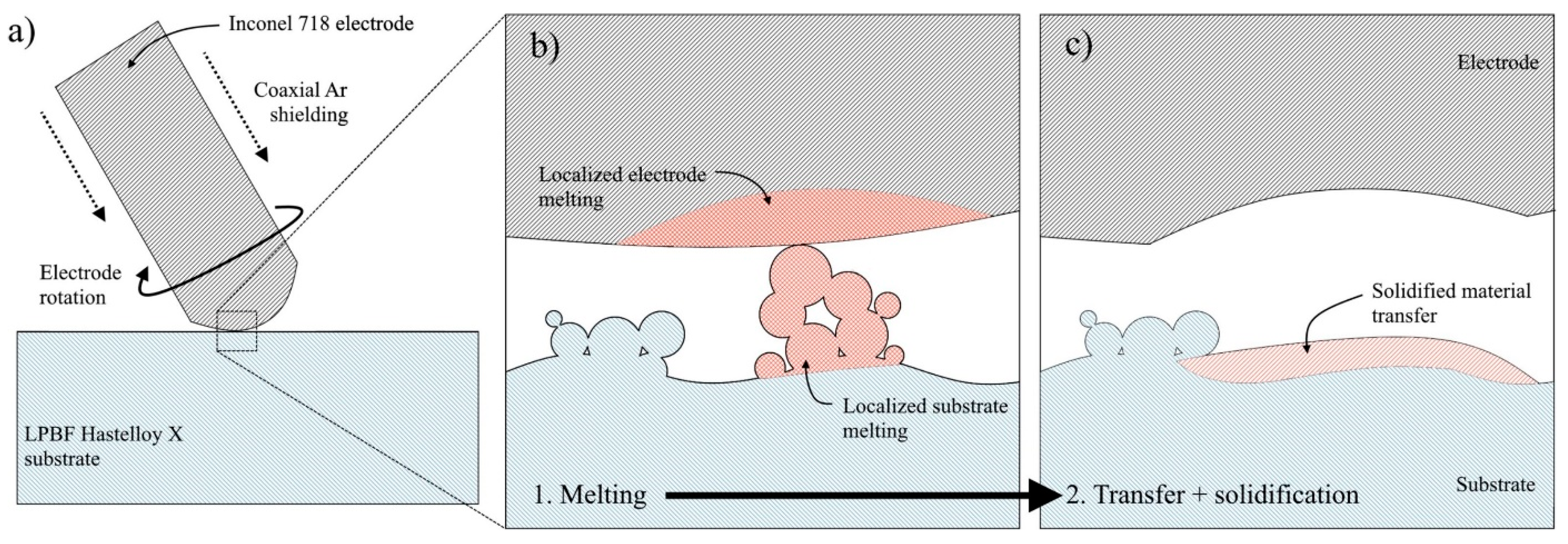
3.2.2. Electrospark Deposition
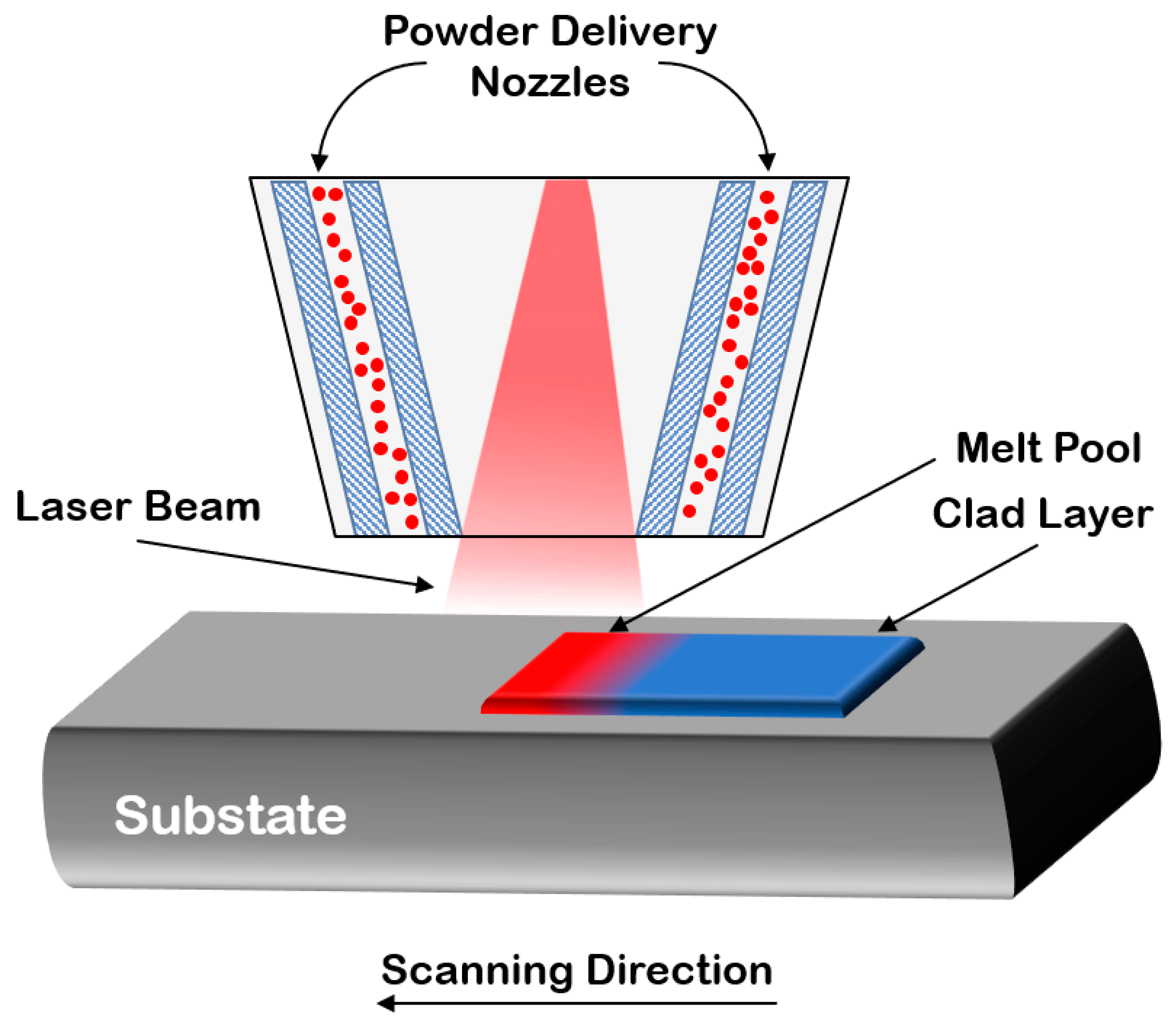
3.2.3. Laser Cladding
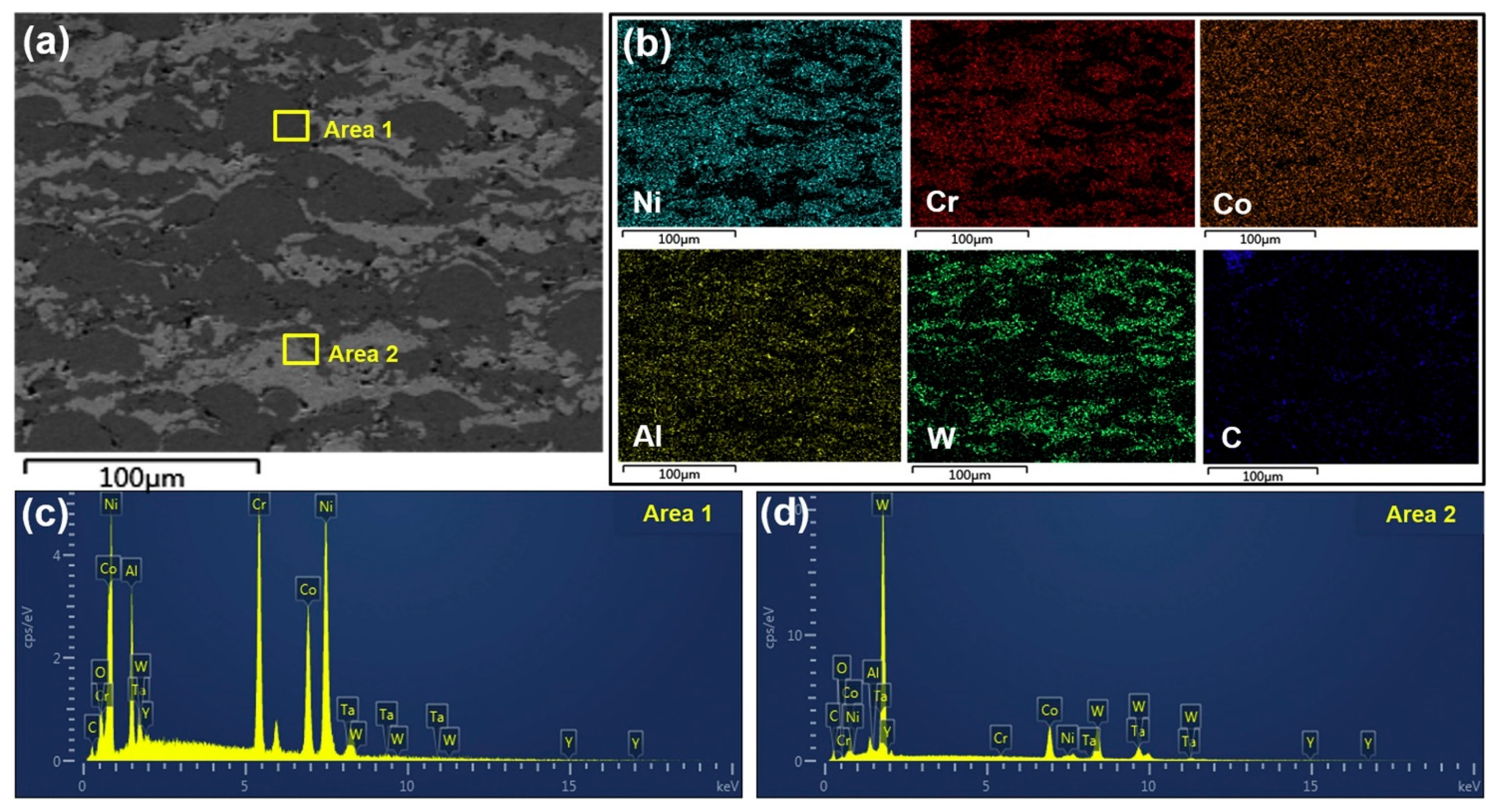
4. Microstructure and Phase Composition
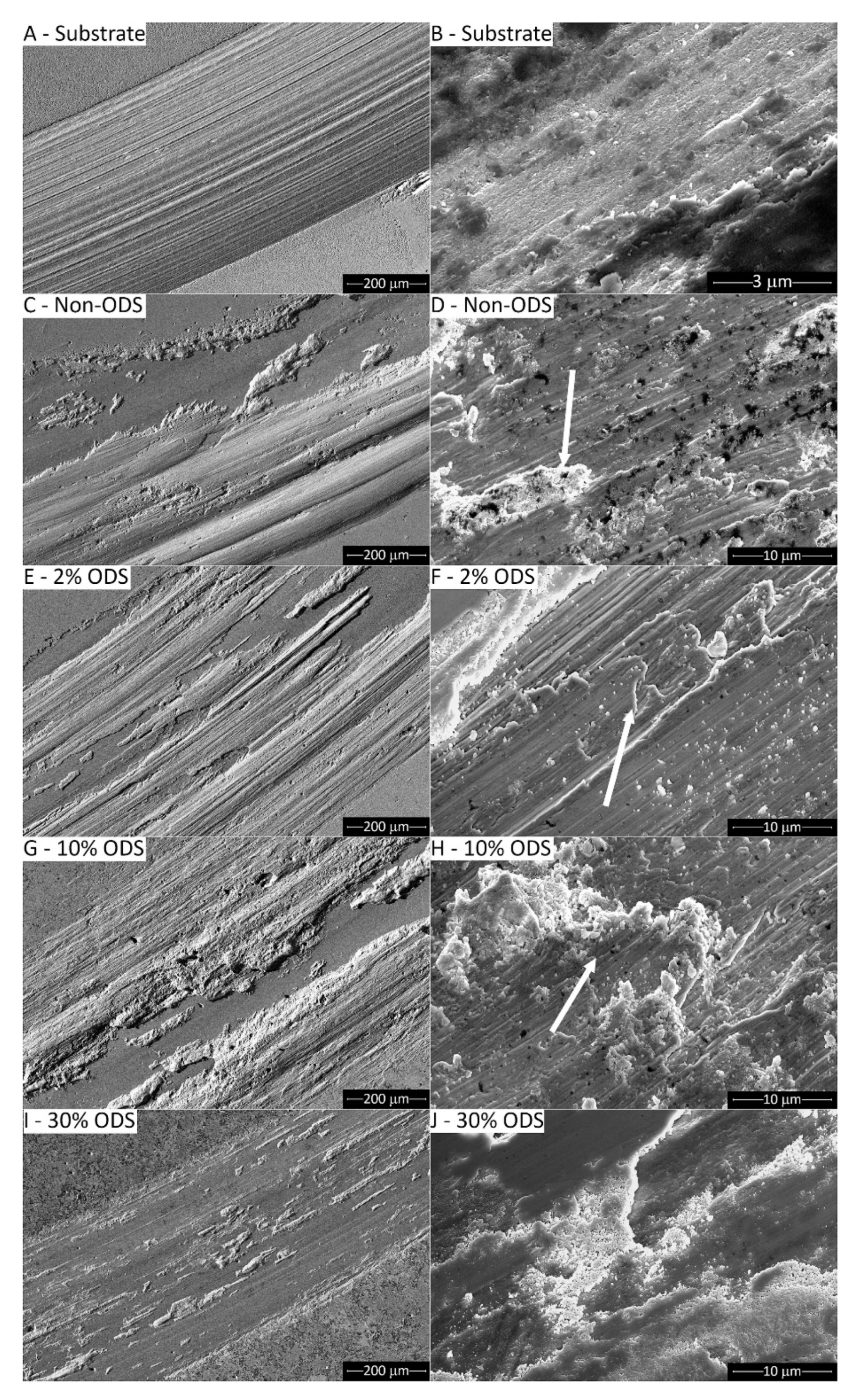
4.1. Thermally-Sprayed Coatings
4.2. Electrospark-Deposited Coatings
4.3. Laser-Cladded Coatings
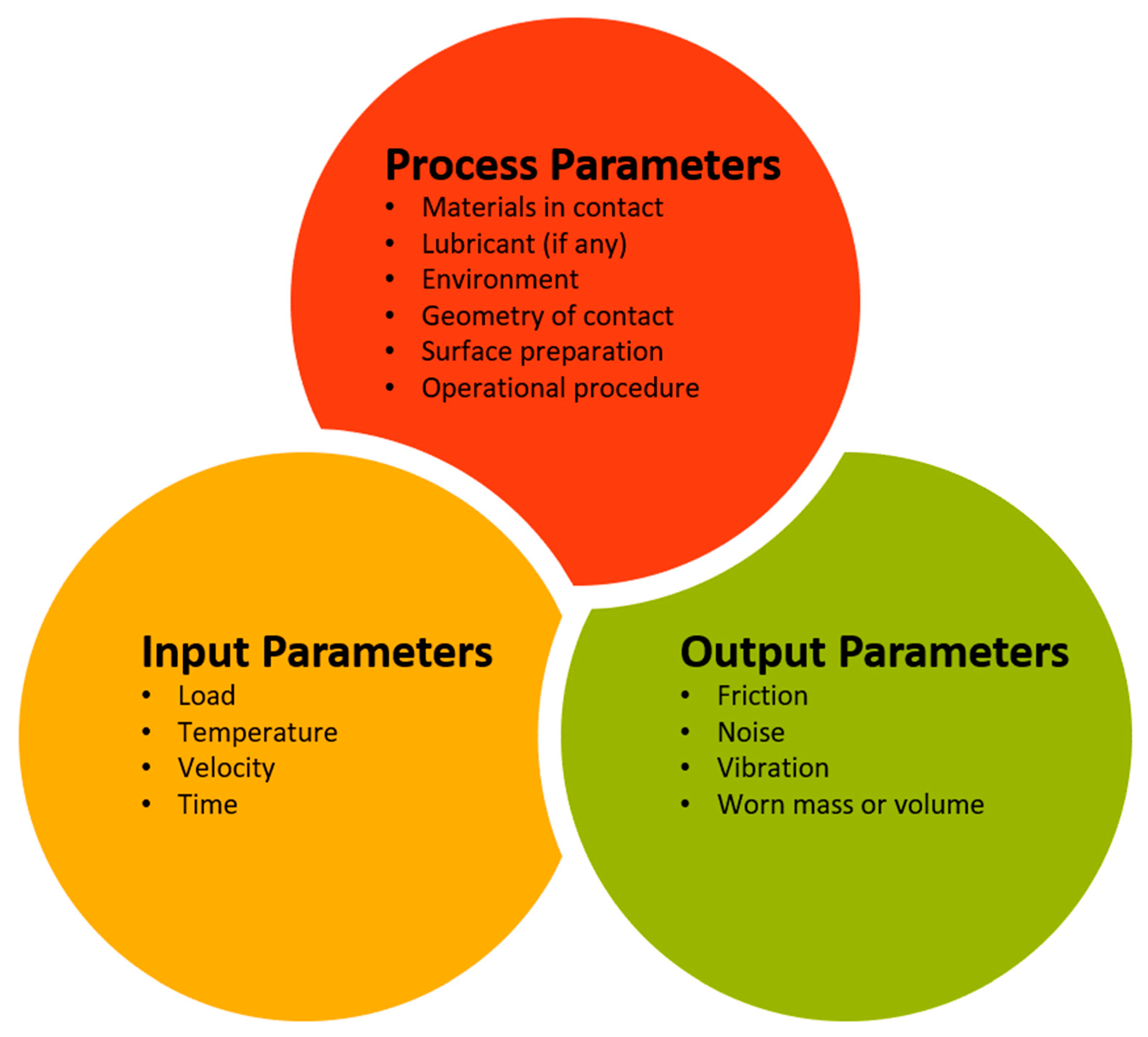
5. Experimentation and Instrumentation for Mechanical and Tribological Measurements
5.1. Evaluation of Mechanical Properties
- Adhesion
- Hardness
- Elastic modulus
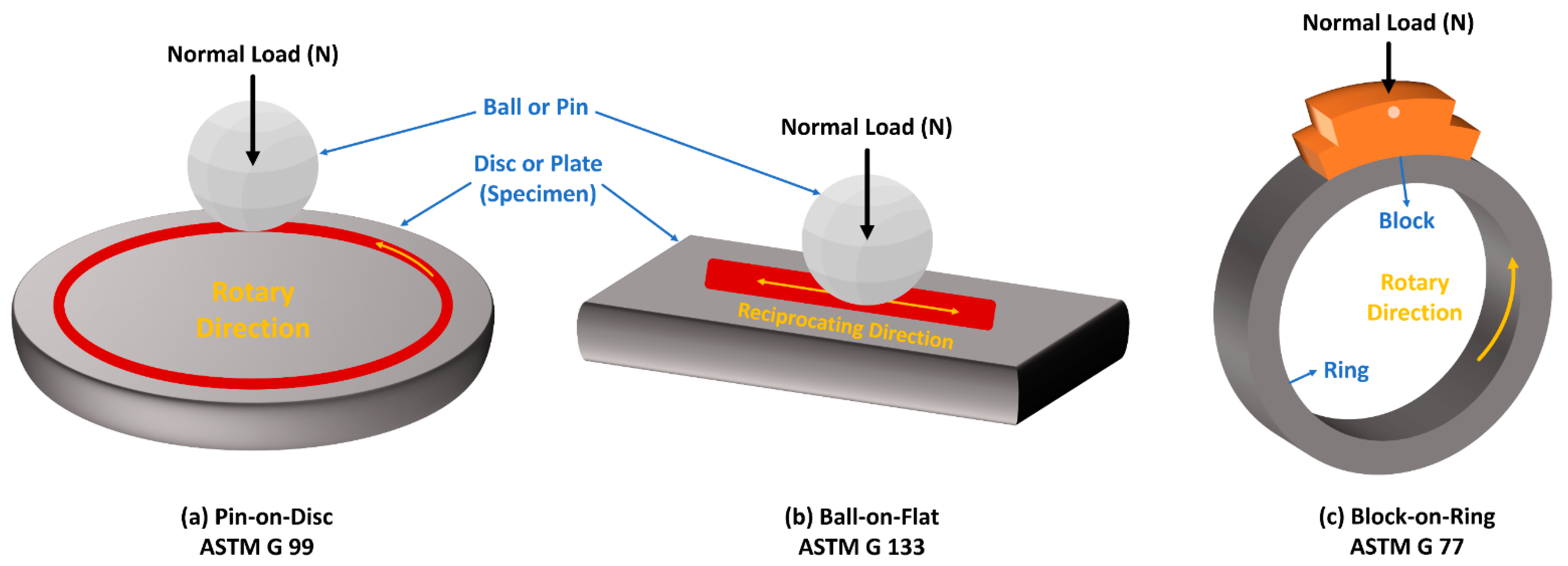
5.2. Evaluation of Tribological Properties
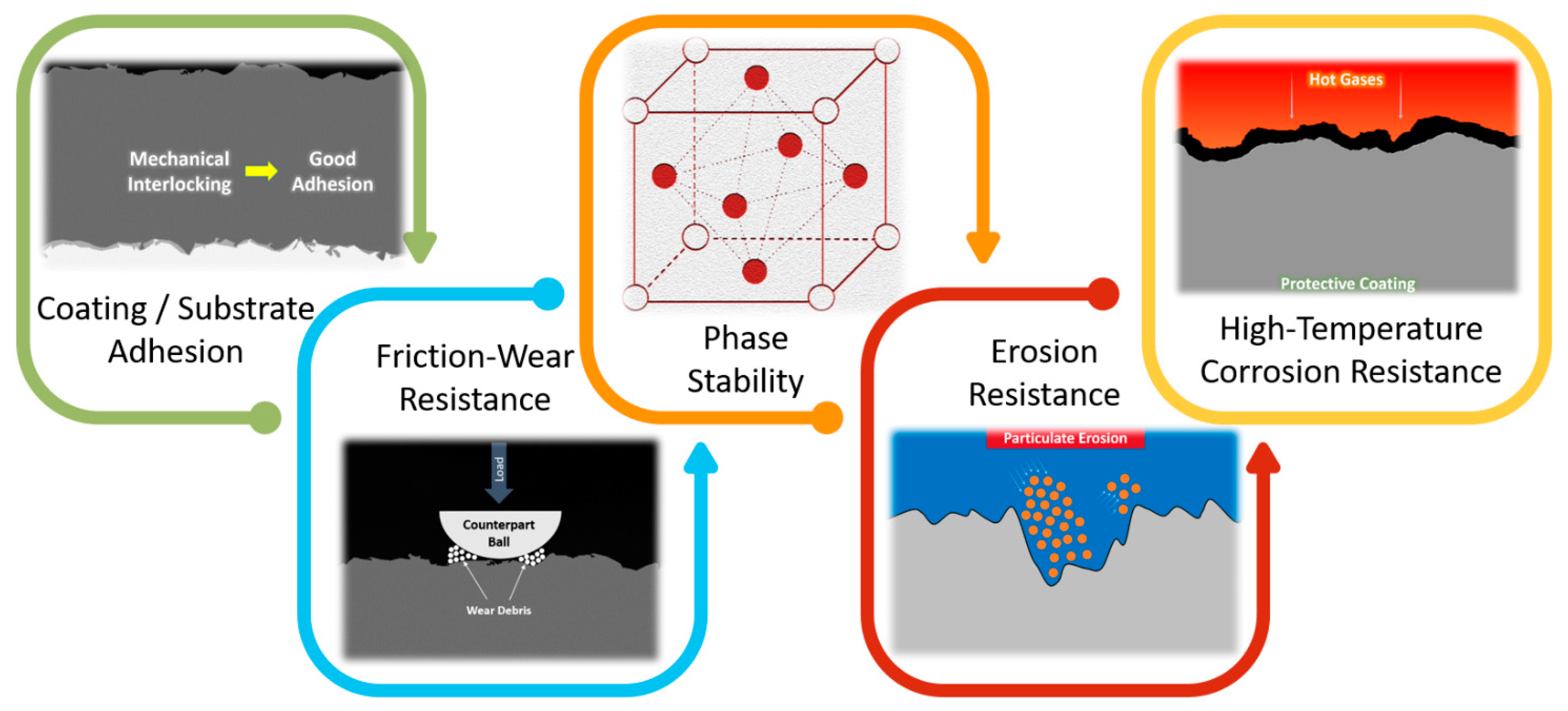
6. Mechanical and Tribological Properties
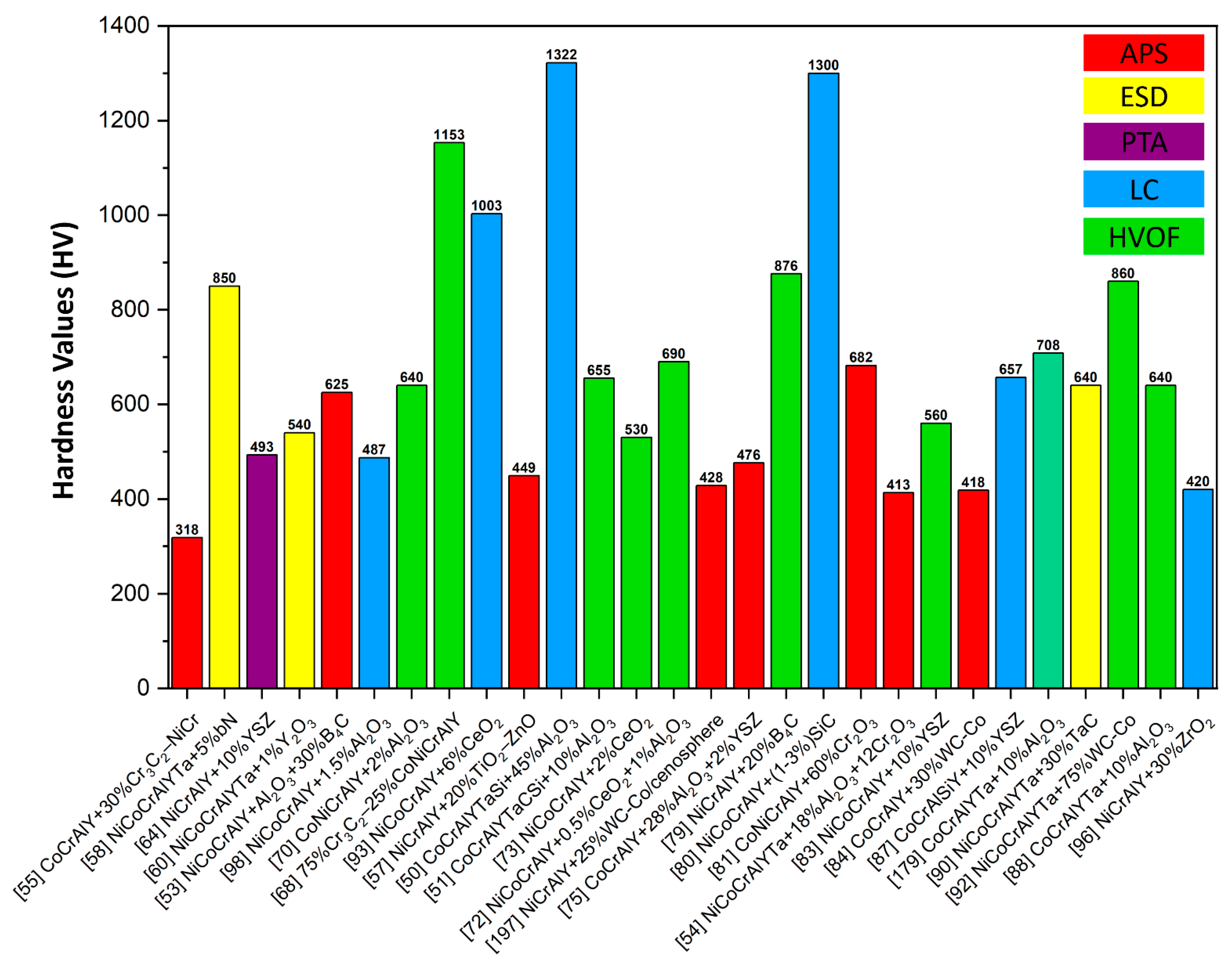
6.1. Hardness
6.2. Adhesion
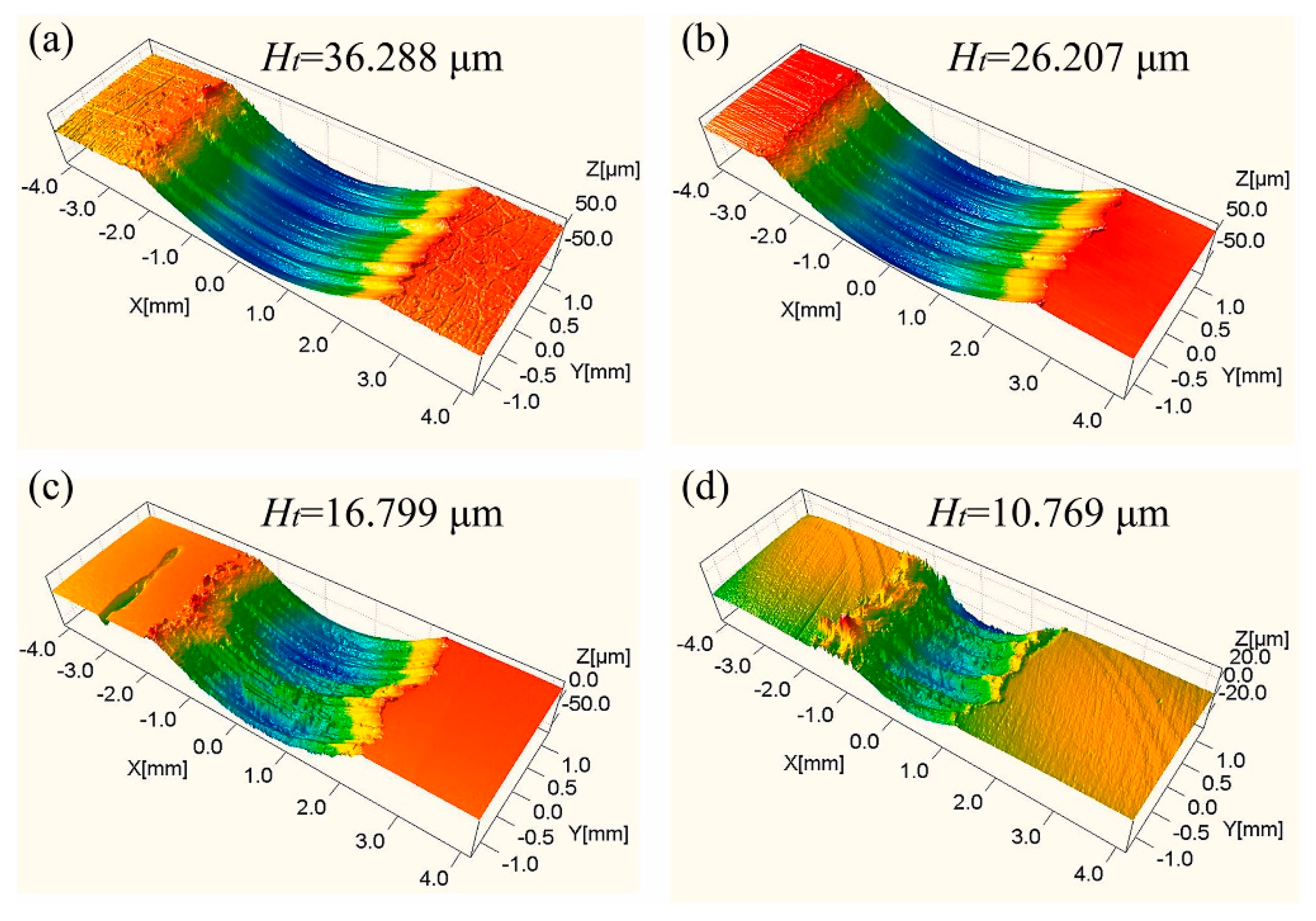
6.3. Friction-Wear Performance
6.3.1. Important Factors for Friction-Wear
6.3.2. Friction-Wear Behavior at Ambient Temperatures
6.4. Friction-Wear at Elevated Temperatures
6.4.1. Important Factors for Temperature-Affected Friction-Wear
- The wear process would not be altered, if the debris particles get removed during sliding of components.
- If the debris particles remain between the contacting surfaces, then they may act either as two- or three-body abradants. The former occurs when the particles become embedded in one surface, and the latter takes place when the particles get to move freely between the surfaces.
- In the nonmoving state, the debris particles promote the formation of solid layers consisting of sintered particles, which can provide wear protection.
- The intensification of the oxidation process due to the generation of high flash temperatures;
- The development of lattice defects as a result of plastic deformation at the surface adjacent to the contact point. The developed defects promote oxygen diffusion, by which it accelerates the oxidation kinetics.
6.4.2. Oxide Scale Formation on MCrAlY Alloys at High Temperatures
6.4.3. High-Temperature Friction-Wear Behavior
6.5. Erosion Performance
6.5.1. Important Factors for Erosion
6.5.2. Erosion Behavior at Ambient Temperatures
6.6. Erosion Performance at Elevated Temperatures
6.6.1. Important Factors for Temperature-Affected Erosion
6.6.2. High-Temperature Erosion Behavior
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, J.R. Surface Engineering for Corrosion and Wear Resistance; ASM International: Almere, The Netherlands, 2001. [Google Scholar]
- Pollock, T.M.; Tin, S. Nickel-Based Superalloys for Advanced Turbine Engines: Chemistry, Microstructure and Properties. J. Propuls. Power 2006, 22, 361–374. [Google Scholar] [CrossRef]
- Rhys-Jones, T. Coatings for blade and vane applications in gas turbines. Corros. Sci. 1989, 29, 623–646. [Google Scholar] [CrossRef]
- Rajendran, R. Gas turbine coatings–An overview. Eng. Fail. Anal. 2012, 26, 355–369. [Google Scholar] [CrossRef]
- Cernuschi, F.; Guardamagna, C.; Capelli, S.; Lorenzoni, L.; Mack, D.E.; Moscatelli, A. Solid particle erosion of standard and advanced thermal barrier coatings. Wear 2016, 348, 43–51. [Google Scholar] [CrossRef]
- Gohardani, O. Impact of erosion testing aspects on current and future flight conditions. Prog. Aerosp. Sci. 2011, 47, 280–303. [Google Scholar] [CrossRef]
- Miyoshi, K. Solid Lubricants and Coatings for Extreme Environments: State-of-the-Art Survey; NASA: Hanover, MD, USA, 2007.
- Radil, K.; DellaCorte, C. The Performance of PS400 Subjected to Sliding Contact at Temperatures from 260 to 927 °C. Tribol. Trans. 2017, 60, 957–964. [Google Scholar] [CrossRef]
- Bolelli, G.; Vorkötter, C.; Lusvarghi, L.; Morelli, S.; Testa, V.; Vaßen, R. Performance of wear resistant MCrAlY coatings with oxide dispersion strengthening. Wear 2020, 444–445, 203116. [Google Scholar] [CrossRef]
- Hong, S.; Wu, Y.; Wang, B.; Zhang, J.; Zheng, Y.; Qiao, L. The effect of temperature on the dry sliding wear behavior of HVOF sprayed nanostructured WC-CoCr coatings. Ceram. Int. 2017, 43, 458–462. [Google Scholar] [CrossRef]
- Wang, T.; Ye, F. The elevated-temperature wear behavior evolution of HVOF sprayed tungsten carbide coatings: Respond to heat treatment. Int. J. Refract. Met. Hard Mater. 2018, 71, 92–100. [Google Scholar] [CrossRef]
- Mi, P.; Zhao, H.; Wang, T.; Ye, F. Sliding wear behavior of HVOF sprayed WC-(nano-WC-Co) coating at elevated temperatures. Mater. Chem. Phys. 2018, 206, 1–6. [Google Scholar] [CrossRef]
- Mi, P.; Wang, T.; Ye, F. Influences of the compositions and mechanical properties of HVOF sprayed bimodal WC-Co coating on its high temperature wear performance. Int. J. Refract. Met. Hard Mater. 2017, 69, 158–163. [Google Scholar] [CrossRef]
- Bonache, V.; Salvador, M.D.; Garcia, J.C.; Sánchez, E.; Bannier, E. Influence of Plasma Intensity on Wear and Erosion Resistance of Conventional and Nanometric WC-Co Coatings Deposited by APS. J. Therm. Spray Technol. 2011, 20, 549–559. [Google Scholar] [CrossRef]
- Geng, Z.; Hou, S.; Shi, G.; Duan, D.; Li, S. Tribological behaviour at various temperatures of WC-Co coatings prepared using different thermal spraying techniques. Tribol. Int. 2016, 104, 36–44. [Google Scholar] [CrossRef]
- Szymański, K.; Hernas, A.; Moskal, G.; Myalska, H. Thermally sprayed coatings resistant to erosion and corrosion for power plant boilers—A review. Surf. Coat. Technol. 2015, 268, 153–164. [Google Scholar] [CrossRef]
- Berger, L.-M. Application of hardmetals as thermal spray coatings. Int. J. Refract. Met. Hard Mater. 2015, 49, 350–364. [Google Scholar] [CrossRef]
- García, J.; Ciprés, V.C.; Blomqvist, A.; Kaplan, B. Cemented carbide microstructures: A review. Int. J. Refract. Met. Hard Mater. 2019, 80, 40–68. [Google Scholar] [CrossRef]
- Kear, B.; Skandan, G.; Sadangi, R. Factors controlling decarburization in HVOF sprayed nano-WC/Co hardcoatings. Scr. Mater. 2001, 44, 1703–1707. [Google Scholar] [CrossRef]
- Shipway, P.; McCartney, D.; Sudaprasert, T. Sliding wear behaviour of conventional and nanostructured HVOF sprayed WC–Co coatings. Wear 2005, 259, 820–827. [Google Scholar] [CrossRef]
- Souza, V.; Neville, A. Corrosion and synergy in a WCCoCr HVOF thermal spray coating—Understanding their role in erosion–corrosion degradation. Wear 2005, 259, 171–180. [Google Scholar] [CrossRef]
- Esmaeili, Z.; Loghman-Estarki, M.; Ramezani, M.; Naderi, M.; Sharifi, E.M. Toward hardening of NiCrAlY alloy by spark plasma sintering of NiCrAlY-nanoSi3N4-graphite nanocomposite. J. Alloys Compd. 2020, 847, 155802. [Google Scholar] [CrossRef]
- Yanliang, Y.; Shaolei, L.; Rui, Z.; Changyi, W.; Shengfeng, Z. Influence of Al2O3 particles on the tribological properties of CoCrAlYTa coating produced by laser-induction hybrid cladding. Ceram. Int. 2021, 47, 19434–19442. [Google Scholar] [CrossRef]
- Kazempour-Liacy, H.; Abouali, S.; Akbari-Garakani, M. Failure analysis of a first stage gas turbine blade. Eng. Fail. Anal. 2011, 18, 517–522. [Google Scholar] [CrossRef]
- Feuerstein, A.; Knapp, J.; Taylor, T.; Ashary, A.; Bolcavage, A.; Hitchman, N. Technical and Economical Aspects of Current Thermal Barrier Coating Systems for Gas Turbine Engines by Thermal Spray and EBPVD: A Review. J. Therm. Spray Technol. 2008, 17, 199–213. [Google Scholar] [CrossRef]
- Zakeri, A.; Balashadehi, M.R.M.; Aghdam, A.S.R. Development of hybrid electrodeposition/slurry diffusion aluminide coatings on Ni-based superalloy with enhanced hot corrosion resistance. J. Compos. Compd. 2021, 2, 1–8. [Google Scholar] [CrossRef]
- Nicholls, J. Designing oxidation-resistant coatings. JOM 2000, 52, 28–35. [Google Scholar] [CrossRef]
- Sloof, W.G.; Nijdam, T.J. On the high-temperature oxidation of MCrAlY coatings. Int. J. Mater. Res. 2009, 100, 1318–1330. [Google Scholar] [CrossRef]
- Zhang, P. Performance of MCrAlX Coatings: Oxidation, Hot Corrosion and Interdiffusion; Linköping University Electronic Press: Linköping, Sweden, 2019. [Google Scholar]
- Giggins, C.S.; Pettit, F.S. Oxidation of Ni-Cr-Al Alloys Between 1000° and 1200 °C. J. Electrochem. Soc. 1971, 118, 1782–1790. [Google Scholar] [CrossRef]
- Pomeroy, M. Coatings for gas turbine materials and long term stability issues. Mater. Des. 2005, 26, 223–231. [Google Scholar] [CrossRef]
- Bose, S. High Temperature Coatings; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Brandl, W.; Toma, D.; Grabke, H. The characteristics of alumina scales formed on HVOF-sprayed MCrAlY coatings. Surf. Coat. Technol. 1998, 108–109, 10–15. [Google Scholar] [CrossRef]
- ASM International. Protective Coatings for Turbine Components; ASM International: Russell Township, OH, USA, 1990. [Google Scholar]
- Naumenko, D.; Shemet, V.; Singheiser, L.; Quadakkers, W.J. Failure mechanisms of thermal barrier coatings on MCrAlY-type bondcoats associated with the formation of the thermally grown oxide. J. Mater. Sci. 2009, 44, 1687–1703. [Google Scholar] [CrossRef]
- Zakeri, A.; Bahmani, E.; Aghdam, A.S.R.; Saeedi, B.; Bai, M. A study on the effect of nano-CeO2 dispersion on the characteristics of thermally-grown oxide (TGO) formed on NiCoCrAlY powders and coatings during isothermal oxidation. J. Alloys Compd. 2020, 835, 155319. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, D.; Fu, Y.; Du, H. Recent advances of superhard nanocomposite coatings: A review. Surf. Coat. Technol. 2003, 167, 113–119. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Al-Aqeeli, N. Mechanically alloyed nanocomposites. Prog. Mater. Sci. 2013, 58, 383–502. [Google Scholar] [CrossRef]
- He, L.; Hassani, M. A Review of the Mechanical and Tribological Behavior of Cold Spray Metal Matrix Composites. J. Therm. Spray Technol. 2020, 29, 1565–1608. [Google Scholar] [CrossRef]
- Hatami, M.; Naeimi, F.; Shamanian, M.; Tahari, M. High-Temperature Oxidation Behavior of Nano-structured CoNiCrAlY–YSZ Coatings Produced by HVOF Thermal Spray Technique. Oxid. Met. 2018, 90, 153–167. [Google Scholar] [CrossRef]
- American Society for Metals. Properties and Selection: Stainless Steels, Tool Materials and Special Purpose Metals; ASM International: Russell Township, OH, USA, 1980. [Google Scholar]
- Gale, W.F.; Totemeier, T.C. Smithells Metals Reference Book, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Fahrenholtz, W.; Hilmas, G.; Talmy, I.G.; Zaykoski, J.A. Refractory Diborides of Zirconium and Hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Liu, H.; Jazi, H.S.; Bussmann, M.; Mostaghimi, J. Experiments and modeling of rapid solidification of plasma-sprayed yttria-stabilized zirconia. Acta Mater. 2009, 57, 6013–6021. [Google Scholar] [CrossRef]
- Anselmi-Tamburini, U.; Woolman, J.N.; Munir, Z.A. Transparent Nanometric Cubic and Tetragonal Zirconia Obtained by High-Pressure Pulsed Electric Current Sintering. Adv. Funct. Mater. 2007, 17, 3267–3273. [Google Scholar] [CrossRef]
- Sciti, D.; Guicciardi, S.; Nygren, M. Densification and Mechanical Behavior of HfC and HfB2Fabricated by Spark Plasma Sintering. J. Am. Ceram. Soc. 2008, 91, 1433–1440. [Google Scholar] [CrossRef]
- Pawłowski, L. The Science and Engineering of Thermal Spray Coatings, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2008; ISBN 978-0-471-49049-4. [Google Scholar]
- Saeedi, B.; Aghdam, A.S.R.; Gholami, G. A study on nanostructured in-situ oxide dispersed NiAl coating and its high temperature oxidation behavior. Surf. Coat. Technol. 2015, 276, 704–713. [Google Scholar] [CrossRef]
- Zhao, L.; Parco, M.; Lugscheider, E. Wear behaviour of Al2O3 dispersion strengthened MCrAlY coating. Surf. Coat. Technol. 2004, 184, 298–306. [Google Scholar] [CrossRef]
- Weitong, Z.; Dejun, K. Influence of Al2O3 mass fractions on microstructure, oxidation resistance and friction–wear behaviors of CoCrAlYTaSi coatings. Surf. Coat. Technol. 2019, 379, 125058. [Google Scholar] [CrossRef]
- Hou, G.; An, Y.; Zhao, X.; Zhou, H.; Chen, J. Effect of alumina dispersion on oxidation behavior as well as friction and wear behavior of HVOF-sprayed CoCrAlYTaCSi coating at elevated temperature up to 1000 °C. Acta Mater. 2015, 95, 164–175. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Zuo, D.-W.; Wang, M.-D.; Sun, G.-F.; Miao, H.; Sun, Y.-L. High temperature frictional wear behaviors of nano-particle reinforced NiCoCrAlY cladded coatings. Trans. Nonferrous Met. Soc. China 2011, 21, 1322–1328. [Google Scholar] [CrossRef]
- Cao, Y.; Huang, C.; Liu, W.; Zhang, W.; Du, L. Effects of Boron Carbide Content on the Microstructure and Properties of Atmospheric Plasma-Sprayed NiCoCrAlY/Al2O3-B4C Composite Coatings. J. Therm. Spray Technol. 2014, 23, 716–724. [Google Scholar] [CrossRef]
- Tao, C.; Wang, L.; Song, X. High-temperature frictional wear behavior of MCrAlY-based coatings deposited by atmosphere plasma spraying. Int. J. Miner. Met. Mater. 2017, 24, 222–228. [Google Scholar] [CrossRef]
- Nithin, H.S.; Nishchitha, K.M.; Shamanth, V.; Hemanth, K.; Babu, K.A. High-Temperature Oxidation and Corrosion Behaviour of APS CoCrAlY + Cr3C2–NiCr Composite Coating. J. Bio-Tribo-Corros. 2020, 6, 28. [Google Scholar] [CrossRef]
- Nithin, H.S.; Desai, V.; Ramesh, M.R.; Hs, N. Elevated temperature solid particle erosion behaviour of carbide reinforced CoCrAlY composite coatings. Mater. Res. Express 2018, 5, 066529. [Google Scholar] [CrossRef]
- Shi, P.; Wang, W.; Wan, S.; Gao, Q.; Sun, H.; Feng, X.; Yi, G.; Xie, E.; Wang, Q. Tribological performance and high temperature oxidation behaviour of thermal sprayed Ni- and NiCrAlY-based composite coatings. Surf. Coat. Technol. 2021, 405, 126615. [Google Scholar] [CrossRef]
- Xie, Y.-J.; Wang, M.-C. A feasibility study of preparing MCrAlX/BN composite coatings by electrospark deposition process. J. Alloys Compd. 2009, 484, 21–24. [Google Scholar] [CrossRef]
- Tahari, M.; Shamanian, M.; Salehi, M. The effect of heat treatment and thermal spray processes on the grain growth of nanostructured composite CoNiCrAlY/YSZ powders. J. Alloys Compd. 2015, 646, 372–379. [Google Scholar] [CrossRef]
- Wang, D.; Wang, W.; Wang, M.; Li, Y.; Chen, X.; Chi, C.; Xie, Y. Effect of operating voltage on microstructure and microhardness of NiCoCrAlYTa-Y2O3 composite coatings on single crystal superalloy produced by electrospark deposition. Surf. Coat. Technol. 2019, 358, 628–636. [Google Scholar] [CrossRef]
- Peng, H.; Guo, H.; He, J.; Gong, S. Cyclic oxidation and diffusion barrier behaviors of oxides dispersed NiCoCrAlY coatings. J. Alloys Compd. 2010, 502, 411–416. [Google Scholar] [CrossRef]
- Wang, D.; Gao, J.; Zhang, R.; Deng, S.; Jiang, S.; Cheng, D.; Liu, P.; Xiong, Z.; Wang, W. Effect of TaC particles on the microstructure and oxidation behavior of NiCoCrAlYTa coating prepared by electrospark deposition on single crystal superalloy. Surf. Coat. Technol. 2021, 408, 126851. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Ning, X.; Wang, Q.; Wang, F.; Chen, D. The influence of milling parameters on the characteristics of milled and spray-dried NiCoCrAlY–Al2O3 composite powders. Powder Met. 2017, 60, 15–21. [Google Scholar] [CrossRef]
- Demian, C.; Denoirjean, A.; Pawłowski, L.; Denoirjean, P.; El Ouardi, R. Microstructural investigations of NiCrAlY + Y2O3 stabilized ZrO2 cermet coatings deposited by plasma transferred arc (PTA). Surf. Coat. Technol. 2016, 300, 104–109. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Zuo, D.-W.; Sun, Y.-L.; Xu, F.; Zhang, D. Microstructure of nanometer Al2O3 dispersion strengthened Ni-based high-temperature protective coatings by laser cladding. Trans. Nonferrous Met. Soc. China 2009, 19, 586–591. [Google Scholar] [CrossRef]
- Chun, G.; Jianmin, C.; Rungang, Y.; Jiansong, Z. Microstructure and High Temperature Wear Resistance of Laser Cladding NiCoCrAlY/ZrB2 Coating. Rare Met. Mater. Eng. 2013, 42, 1547–1551. [Google Scholar] [CrossRef]
- Hu, Z.-X.; Liu, J.-R.; Guo, C.; Zhou, J.-S.; Zhang, S.-T. Microstructure and Wear Resistance of Laser Cladding NiCoCrAlY/HfB (2) Coating at Elevated Temperature, Zhingguo Biaomian Gongcheng. China Surf. Eng. 2012, 25, 69–74. [Google Scholar]
- Picas, J.; Punset, M.; Menargues, S.; Martín, E.; Baile, M. Microstructural and tribological studies of as-sprayed and heat-treated HVOF Cr3C2–CoNiCrAlY coatings with a CoNiCrAlY bond coat. Surf. Coat. Technol. 2015, 268, 317–324. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Xin, B.; Yu, Y.; Ren, S.; Li, Z. Phase transformation and tribological properties of Ag-MoO3 contained NiCrAlY based composite coatings fabricated by laser cladding. Opt. Laser Technol. 2017, 93, 79–86. [Google Scholar] [CrossRef]
- Bergholz, J.; Pint, B.; Unocic, K.A.; Vaßen, R. Fabrication of Oxide Dispersion Strengthened Bond Coats with Low Al2O3 Content. J. Therm. Spray Technol. 2017, 26, 868–879. [Google Scholar] [CrossRef]
- Bolelli, G.; Candeli, A.; Lusvarghi, L.; Ravaux, A.; Cazes, K.; Denoirjean, A.; Valette, S.; Chazelas, C.; Meillot, E.; Bianchi, L. Tribology of NiCrAlY+Al2O3 composite coatings by plasma spraying with hybrid feeding of dry powder+suspension. Wear 2015, 344–345, 69–85. [Google Scholar] [CrossRef]
- Ghadami, F.; Aghdam, A.S.R.; Zakeri, A.; Saeedi, B.; Tahvili, P. Synergistic effect of CeO2 and Al2O3 nanoparticle dispersion on the oxidation behavior of MCrAlY coatings deposited by HVOF. Ceram. Int. 2020, 46, 4556–4567. [Google Scholar] [CrossRef]
- Ghadami, F.; Zakeri, A.; Aghdam, A.S.R.; Tahmasebi, R. Structural characteristics and high-temperature oxidation behavior of HVOF sprayed nano-CeO2 reinforced NiCoCrAlY nanocomposite coatings. Surf. Coat. Technol. 2019, 373, 7–16. [Google Scholar] [CrossRef]
- Bolelli, G.; Candeli, A.; Lusvarghi, L.; Manfredini, T.; Denoirjean, A.; Valette, S.; Ravaux, A.; Meillot, E. “Hybrid” plasma spraying of NiCrAlY+Al 2 O 3 + h -BN composite coatings for sliding wear applications. Wear 2017, 378–379, 68–81. [Google Scholar] [CrossRef]
- Nithin, H.S.; Desai, V.; Ramesh, M.R. Elevated Temperature Solid Particle Erosion Performance of Plasma-Sprayed Co-based Composite Coatings with Additions of Al2O3 and CeO. J. Mater. Eng. Perform. 2017, 26, 5251–5261. [Google Scholar] [CrossRef]
- Bai, M.; Song, B.; Reddy, L.; Hussain, T. Preparation of MCrAlY–Al2O3 Composite Coatings with Enhanced Oxidation Resistance through a Novel Powder Manufacturing Process. J. Therm. Spray Technol. 2019, 28, 433–443. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, G.; Feng, Z.; Zhang, B.; Liang, Y.; Liu, F. Oxidation behavior of laser remelted plasma sprayed NiCrAlY and NiCrAlY–Al2O3 coatings. Surf. Coat. Technol. 2001, 138, 56–60. [Google Scholar] [CrossRef]
- Singh, G.; Bala, N.; Chawla, V. Oxidation Behaviour of HVOF Sprayed NiCrAlY and NiCrAlY-20SiC Coatings on T-91 Boiler Tube Steel. Prot. Met. Phys. Chem. Surf. 2020, 56, 134–150. [Google Scholar] [CrossRef]
- Singh, G.; Bala, N.; Chawla, V. High temperature oxidation behaviour and characterization of NiCrAlY-B4C coatings deposited by HVOF. Mater. Res. Express 2019, 6, 086436. [Google Scholar] [CrossRef]
- Wang, D.S.; Tian, Z.J.; Yang, B.; Da Shen, L. Preparation and Characterization of Nano-SiCP Strengthened MCrAlY Graded Coating by Laser Multi-Layer Cladding. Adv. Mater. Res. 2012, 557–559, 1937–1940. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, C.; Lan, H.; Du, L.; Zhang, W. Oxidation and Hot Corrosion Behavior of Plasma-Sprayed MCrAlY–Cr2O3 Coatings. J. Therm. Spray Technol. 2016, 25, 1208–1216. [Google Scholar] [CrossRef]
- Jegadeeswaran, N.; Bhat, K.U.; Ramesh, M.R. Improving Hot Corrosion Resistance of Cobalt Based Superalloy (Superco-605) Using HVOF Sprayed Oxide Alloy Powder Coating. Trans. Indian Inst. Met. 2015, 68, 309–316. [Google Scholar] [CrossRef]
- Bobzin, K.; Schlafer, T.; Richardt, K.R.M.; Brühl, M. Development of Oxide Dispersion Strengthened MCrAlY Coatings. J. Therm. Spray Technol. 2008, 17, 853–857. [Google Scholar] [CrossRef]
- Nithin, H.S.; Vijay, D.; Ramesh, M.R. Cyclic Oxidation and Hot Corrosion Behavior of Plasma-Sprayed CoCrAlY + WC-Co Coating on Turbine Alloys. J. Fail. Anal. Prev. 2018, 18, 1133–1142. [Google Scholar] [CrossRef]
- Okada, M.; Vaßen, R.; Karger, M.; Sebold, D.; Mack, D.E.; Jarligo, M.O.; Bozza, F. Deposition and oxidation of ox-ide-dispersed CoNiCrAlY Bondcoats. Proc. Int. Therm. Spray Conf. 2013, 23, 16–21. [Google Scholar]
- An, Q.; Huang, L.; Wei, S.; Zhang, R.; Rong, X.; Wang, Y.; Geng, L. Enhanced interfacial bonding and superior oxidation resistance of CoCrAlY-TiB2 composite coating fabricated by air plasma spraying. Corros. Sci. 2019, 158. [Google Scholar] [CrossRef]
- Wang, T.; Liu, J.; Qin, L.; Tang, J.; Wu, J. Laser Direct Deposition of CoCrAlSiY/YSZ Composites: Densification, Microstructure and Mechanical Properties. J. Therm. Spray Technol. 2019, 28, 862–879. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, Y.; Hong, S.; Yang, W.; Shi, W. Effects of Temperature on Wear Properties and Mechanisms of HVOF Sprayed CoCrAlYTa-10%Al2O3 Coatings and H13 Steel. Metals 2019, 9, 1224. [Google Scholar] [CrossRef]
- Hu, L.; Song, X.; Zhao, X.; Guo, F.; Yang, F.; Xiao, P. A robust, hydrophobic CeO2/NiCoCrAlY composite coating with excellent thermal stability and corrosion resistance prepared by air plasma spray. J. Alloys Compd. 2021, 861, 158623. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, Y.; Wang, M.; Hou, J. MCrAlY/TaC metal matrix composite coatings produced by electrospark deposition. Acta Met. Sin. 2013, 26, 173–176. [Google Scholar] [CrossRef][Green Version]
- He, X.; Yuan, X.; Xu, H.; Song, P.; Yu, X.; Li, C.; Huang, T.; Li, Q.; Lü, K.; Feng, J.; et al. Analysis of structure and microhardness of Al2O3-40 wt.% TiO2/NiCoCrAl gradient coating with in-situ needle-like phase reinforcement after high-temperature treatment. Ceram. Int. 2019, 45, 14546–14554. [Google Scholar] [CrossRef]
- Hao, E.; Zhao, X.; An, Y.; Deng, W.; Ren, Y.; Zhou, H.; Chen, J. WC-Co reinforced NiCoCrAlYTa composite coating: Effect of the proportion on microstructure and tribological properties. Int. J. Refract. Met. Hard Mater. 2019, 84, 104978. [Google Scholar] [CrossRef]
- Jiaxing, L.; Jie, X.; Dejun, K. Microstructure and friction-wear performances of laser-cladded nano-CeO2–reinforced NiCoCrAlY coatings at high temperature. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2020, 234. [Google Scholar] [CrossRef]
- Houdková, Š.; Česánek, Z.; Smazalová, E.; Lukáč, F. The high temperature wear and oxidation behavior of CrC-based HVOF coatings. Proc. Int. Therm. Spray Conf. 2017, 2, 600–605. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, H.; Zhao, S.; Ding, Z.; Liu, B.; Li, W.; Xu, H.; Liu, H. Corrosion Behavior of the CoNiCrAlY-Al2O3 Composite Coating Based on Core-Shell Structured Powder Design. Materials 2021, 14, 7093. [Google Scholar] [CrossRef]
- Yi, Z.; Song, C.; Zhang, G.; Tong, T.; Ma, G.; Wu, D. Microstructure and Wear Property of ZrO2-Added NiCrAlY Prepared by Ultrasonic-Assisted Direct Laser Deposition. Materials 2021, 14, 5785. [Google Scholar] [CrossRef]
- Sun, X.; Chen, S.; Wang, Y.; Pan, Z.; Wang, L. Mechanical Properties and Thermal Shock Resistance of HVOF Sprayed NiCrAlY Coatings Without and With Nano Ceria. J. Therm. Spray Technol. 2012, 21, 818–824. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zuo, D.W.; Li, X.; Di Wang, M.; Zhao, Y. Effects of Nano-Al2O3p on High Temperature Frictional Wear Behaviors of NiCoCrAIY Cladded Coatings. Adv. Mater. Res. 2012, 426, 40–43. [Google Scholar] [CrossRef]
- Tjong, S.C. Novel Nanoparticle-Reinforced Metal Matrix Composites with Enhanced Mechanical Properties. Adv. Eng. Mater. 2007, 9, 639–652. [Google Scholar] [CrossRef]
- Matikainen, V.; Koivuluoto, H.; Vuoristo, P. A study of Cr3C2-based HVOF- and HVAF-sprayed coatings: Abrasion, dry particle erosion and cavitation erosion resistance. Wear 2020, 446–447, 203188. [Google Scholar] [CrossRef]
- Casati, R.; Vedani, M. Metal Matrix Composites Reinforced by Nano-Particles—A Review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Ashgriz, N. Handbook of Atomization and Sprays; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Fu, W.; Chen, Q.; Xu, M.; Du, D.; Zou, Y.; Zhan, Y.; Zheng, B. Microstructure and mechanical properties of plasma sprayed WC-Ni coatings. Jinshu Rechuli/Heat Treat. Met. 2019, 44, 211–215. [Google Scholar] [CrossRef]
- Zakeri, A.; Tahvili, P.; Bahmani, E.; Aghdam, A.S.R. Effect of powder manufacturing process on characteristics of nanostructured MCrAlY coatings: Dry vs. wet ball milling. J. Compos. Compd. 2021, 2, 9–17. [Google Scholar] [CrossRef]
- Zakeri, A.; Bahmani, E.; Aghdam, A.S.R.; Saeedi, B. A comparative study on the microstructure evolution of conventional and nanostructured MCrAlY powders at high-temperature. Surf. Coat. Technol. 2020, 389, 125629. [Google Scholar] [CrossRef]
- Kaplin, C.; Brochu, M. The effect of grain size on the oxidation of NiCoCrAlY. Appl. Surf. Sci. 2014, 301, 258–263. [Google Scholar] [CrossRef]
- Khodsiani, Z.; Mansuri, H.; Mirian, T. The effect of cryomilling on the morphology and particle size distribution of the NiCoCrAlYSi powders with and without nano-sized alumina. Powder Technol. 2013, 245, 7–12. [Google Scholar] [CrossRef]
- Tahari, M.; Shamanian, M.; Salehi, M. Microstructural and morphological evaluation of MCrAlY/YSZ composite produced by mechanical alloying method. J. Alloys Compd. 2012, 525, 44–52. [Google Scholar] [CrossRef]
- Todde, S.; Licheri, R.; Orrù, R.; Cao, G. Spark plasma sintering processing for the evaluation of cryomilled CoNiCrAlY alloys for high temperature applications in oxidizing environment. Chem. Eng. J. 2012, 200–202, 68–80. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Hardwicke, C.U.; Lau, Y.-C. Advances in Thermal Spray Coatings for Gas Turbines and Energy Generation: A Review. J. Therm. Spray Technol. 2013, 22, 564–576. [Google Scholar] [CrossRef]
- Fauchais, P.L.; Heberlein, J.V.; Boulos, M.I. Thermal Spray Fundamentals; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Sobolev, V.V.; Guilemany, J.M.; Nutting, J. High Velocity Oxy-Fuel Spraying: Theory, Structure-Property Relationships and Applications; Maney: London, UK, 2004. [Google Scholar]
- Singh, H.; Sidhu, B.S.; Puri, D.; Prakash, S. Use of plasma spray technology for deposition of high temperature oxidation/corrosion resistant coatings—A review. Mater. Corros. 2007, 58, 92–102. [Google Scholar] [CrossRef]
- Fauchais, P.; Vardelle, A. Modelling of plasma spraying of ceramic coatings at atmospheric pressure. Ceram. Int. 1991, 17, 367–379. [Google Scholar] [CrossRef]
- Fauchais, P.; Vardelle, A.; Dussoubs, B. Quo Vadis Thermal Spraying? J. Therm. Spray Technol. 2001, 10, 44–66. [Google Scholar] [CrossRef]
- Fauchais, P. Understanding plasma spraying. J. Phys. D Appl. Phys. 2004, 37, R86–R108. [Google Scholar] [CrossRef]
- Latka, L.; Pawlowski, L.; Winnicki, M.; Sokolowski, P.; Małachowska, A.; Kozerski, S. Review of functionally graded thermal sprayed coatings. Appl. Sci. 2020, 10, 5153. [Google Scholar] [CrossRef]
- Meghwal, A.; Anupam, A.; Murty, B.S.; Berndt, C.C.; Kottada, R.S.; Ang, A.S.M. Thermal spray high-entropy alloy coatings: A review. J. Therm. Spray Technol. 2020, 29, 857–893. [Google Scholar] [CrossRef]
- Korpiola, K.; Hirvonen, J.P.; Laas, L.; Rossi, F. The influence of nozzle design on HVOF exit gas velocity and coating microstructure. J. Therm. Spray Technol. 1997, 6, 469–474. [Google Scholar] [CrossRef]
- Joshi, S.; Nylen, P. Advanced Coatings by Thermal Spray Processes. Technologies 2019, 7, 79. [Google Scholar] [CrossRef]
- Gärtner, F.; Stoltenhoff, T.; Schmidt, T.; Kreye, H. The Cold Spray Process and Its Potential for Industrial Applications. J. Therm. Spray Technol. 2006, 15, 223–232. [Google Scholar] [CrossRef]
- Moridi, A.; Hassani-Gangaraj, S.M.; Guagliano, M.; Dao, M. Cold spray coating: Review of material systems and future perspectives. Surf. Eng. 2014, 30, 369–395. [Google Scholar] [CrossRef]
- Assadi, H.; Kreye, H.; Gärtner, F.; Klassen, T. Cold spraying—A materials perspective. Acta Mater. 2016, 116, 382–407. [Google Scholar] [CrossRef]
- Bonadei, A.; Marrocco, T. Cold sprayed MCrAlY+ X coating for gas turbine blades and vanes. Surf. Coat. Technol. 2014, 242, 200–206. [Google Scholar] [CrossRef]
- Chen, W.R.; Irissou, E.; Wu, X.; Legoux, J.-G.; Marple, B.R. The Oxidation Behavior of TBC with Cold Spray CoNiCrAlY Bond Coat. J. Therm. Spray Technol. 2010, 20, 132–138. [Google Scholar] [CrossRef]
- Khanna, A.; Rathod, W. Development of CoNiCrAlY oxidation resistant hard coatings using high velocity oxy fuel and cold spray techniques. Int. J. Refract. Met. Hard Mater. 2015, 49, 374–382. [Google Scholar] [CrossRef]
- Karaoglanli, A.C.; Ozgurluk, Y.; Doleker, K.M. Comparison of microstructure and oxidation behavior of CoNiCrAlY coatings produced by APS, SSAPS, D-gun, HVOF and CGDS techniques. Vacuum 2020, 180, 109609. [Google Scholar] [CrossRef]
- Guo, D.; Wang, Y.; Fernandez, R.; Zhao, L.; Jodoin, B. Cold spray for production of in-situ nanocrystalline MCrAlY coatings—Part I: Process analysis and microstructure characterization. Surf. Coat. Technol. 2021, 409, 126854. [Google Scholar] [CrossRef]
- Guo, D.; Zhao, L.; Jodoin, B. Cold spray for production of in-situ nanocrystalline MCrAlY coatings—Part II: Isothermal oxidation performance. Surf. Coat. Technol. 2021, 409, 126828. [Google Scholar] [CrossRef]
- Gitzhofer, F.; Bouyer, E.; Boulos, M.I. Suspension Plasma Spray. U.S. Patent 5,609,921,26, 11 March 1997. [Google Scholar]
- Xie, Y.-J.; Wang, D.; Wang, M.-S.; Ye, W. Evaluation of three kinds of MCrAlY coatings produced by electrospark deposition. Trans. Nonferrous Met. Soc. China 2016, 26, 1647–1654. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, M. Isothermal oxidation behavior of electrospark deposited MCrAlX-type coatings on a Ni-based superalloy. J. Alloys Compd. 2009, 480, 454–461. [Google Scholar] [CrossRef]
- Wang, M.-C.; Wang, W.-F.; Xie, Y.-J.; Zhang, J. Electro-spark epitaxial deposition of NiCoCrAlYTa alloy on directionally solidified nickel-based superalloy. Trans. Nonferrous Met. Soc. China 2010, 20, 795–802. [Google Scholar] [CrossRef]
- Enrique, P.D.; Keshavarzkermani, A.; Esmaeilizadeh, R.; Peterkin, S.; Jahed, H.; Toyserkani, E.; Zhou, N.Y. Enhancing fatigue life of additive manufactured parts with electrospark deposition post-processing. Addit. Manuf. 2020, 36, 101526. [Google Scholar] [CrossRef]
- Chang, D.U. Laser material processing. SAE Trans. 1985, 271–286. [Google Scholar] [CrossRef]
- Silvello, A.; Perrone, A. Laser Cladding of Metals; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Yakovlev, A.; Bertrand, P.; Smurov, I. Laser cladding of wear resistant metal matrix composite coatings. Thin Solid Films 2004, 453–454, 133–138. [Google Scholar] [CrossRef]
- Singh, S.; Goyal, D.K.; Kumar, P.; Bansal, A. Laser cladding technique for erosive wear applications: A review. Mater. Res. Express 2020, 7, 012007. [Google Scholar] [CrossRef]
- Partes, K.; Giolli, C.; Borgioli, F.; Bardi, U.; Seefeld, T.; Vollertsen, F. High temperature behaviour of NiCrAlY coatings made by laser cladding. Surf. Coat. Technol. 2008, 202, 2208–2213. [Google Scholar] [CrossRef]
- Luo, L.; Shan, X.; Zou, Z.; Zhao, C.; Wang, X.; Zhang, A.; Zhao, X.; Guo, F.; Xiao, P. A high performance NiCoCrAlY bond coat manufactured using laser powder deposition. Corros. Sci. 2017, 126, 356–365. [Google Scholar] [CrossRef]
- Pereira, J.; Zambrano, J.; Tobar, M.; Yáñez, A.; Amigó, V. High temperature oxidation behavior of laser cladding MCrAlY coatings on austenitic stainless steel. Surf. Coat. Technol. 2015, 270, 243–248. [Google Scholar] [CrossRef]
- Pereira, J.; Zambrano, J.; Rayón, E.; Yañez, A.; Amigó, V. Mechanical and microstructural characterization of MCrAlY coatings produced by laser cladding: The influence of the Ni, Co and Al content. Surf. Coat. Technol. 2018, 338, 22–31. [Google Scholar] [CrossRef]
- Texier, D.; Monceau, D.; Hervier, Z.; Andrieu, E. Effect of interdiffusion on mechanical and thermal expansion properties at high temperature of a MCrAlY coated Ni-based superalloy. Surf. Coat. Technol. 2016, 307, 81–90. [Google Scholar] [CrossRef]
- Chen, H.; McCartney, D. Some aspects on modelling of the β-phase depletion behaviour under different oxide growth kinetics in HVOF CoNiCrAlY coatings. Surf. Coat. Technol. 2017, 313, 107–114. [Google Scholar] [CrossRef]
- Zhou, S.; Xiong, Z.; Lei, J.; Dai, X.; Zhang, T.; Wang, C. Influence of milling time on the microstructure evolution and oxidation behavior of NiCrAlY coatings by laser induction hybrid cladding. Corros. Sci. 2016, 103, 105–116. [Google Scholar] [CrossRef]
- Xie, Y.-J.; Wang, M.-C. Epitaxial MCrAlY coating on a Ni-base superalloy produced by electrospark deposition. Surf. Coat. Technol. 2006, 201, 3564–3570. [Google Scholar] [CrossRef]
- Sobolev, V.V.; Guilemany, J.M.; Nutting, J.; Miquel, J.R. Development of substrate-coating adhesion in thermal spraying. Int. Mater. Rev. 1997, 42, 117–136. [Google Scholar] [CrossRef]
- Bobzin, K.; Ote, M.; Linke, T.F.; Sommer, J.; Liao, X. Influence of Process Parameter on Grit Blasting as a Pretreatment Process for Thermal Spraying. J. Therm. Spray Technol. 2015, 25, 3–11. [Google Scholar] [CrossRef]
- Oksa, M.; Turunen, E.; Suhonen, T.; Varis, T.; Hannula, S.-P. Optimization and Characterization of High Velocity Oxy-fuel Sprayed Coatings: Techniques, Materials, and Applications. Coatings 2011, 1, 17–52. [Google Scholar] [CrossRef]
- Rajasekaran, B.; Mauer, G.; Vaßen, R. Enhanced Characteristics of HVOF-sprayed MCrAlY Bond Coats for TBC Applications. J. Therm. Spray Technol. 2011, 20, 1209–1216. [Google Scholar] [CrossRef]
- Lugscheider, E.; Herbst, C.; Zhao, L. Parameter studies on high-velocity oxy-fuel spraying of MCrAlY coatings. Surf. Coat. Technol. 1998, 108–109, 16–23. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.A.; Gaona-Tiburcio, C.; Almeraya, F.; Estupiñan-Lopez, F.H.; Pedraza-Basulto, G.K.; A Poblano-Salas, C. Parameter Studies on High-Velocity Oxy-Fuel Spraying of CoNiCrAlY Coatings Used in the Aeronautical Industry. Int. J. Corros. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Dongmo, E.; Wenzelburger, M.; Gadow, R. Analysis and optimization of the HVOF process by combined experimental and numerical approaches. Surf. Coat. Technol. 2008, 202, 4470–4478. [Google Scholar] [CrossRef]
- Li, M.; Christofides, P.D. Modeling and Control of High-Velocity Oxygen-Fuel (HVOF) Thermal Spray: A Tutorial Review. J. Therm. Spray Technol. 2009, 18, 753–768. [Google Scholar] [CrossRef]
- Holmberg, K.; Laukkanen, A.; Turunen, E.; Laitinen, T. Wear resistance optimisation of composite coatings by computational microstructural modelling. Surf. Coat. Technol. 2014, 247, 1–13. [Google Scholar] [CrossRef]
- Li, M.; Christofides, P.D. Multiscale Modeling of Hvof Thermal Spray Process. IFAC Proc. Vol. 2005, 38, 327–332. [Google Scholar] [CrossRef]
- Li, M.; Christofides, P.D. Computational study of particle in-flight behavior in the HVOF thermal spray process. Chem. Eng. Sci. 2006, 61, 6540–6552. [Google Scholar] [CrossRef]
- Zakeri, A.; Bahmani, E.; Aghdam, A.S.R. Impact of MCrAlY feedstock powder modification by high-energy ball milling on the microstructure and high-temperature oxidation performance of HVOF-sprayed coatings. Surf. Coat. Technol. 2020, 395, 125935. [Google Scholar] [CrossRef]
- Zakeri, A.; Ghadami, F.; Rouhaghdam, A.S.; Saeedi, B. Study on production of modified MCrAlY powder with nano oxide dispersoids as HVOF thermal spray feedstock using mechanical milling. Mater. Res. Express 2019, 7, 015030. [Google Scholar] [CrossRef]
- Li, S.; Langlade, C.; Fayeulle, S.; Tréheux, D. Influence of the microstructure of plasma deposited MCrAlY coatings on their tribological behaviour. Surf. Coat. Technol. 1998, 100–101, 7–11. [Google Scholar] [CrossRef]
- Clyne, T.W.; Gill, S.C. Residual Stresses in Thermal Spray Coatings and Their Effect on Interfacial Adhesion: A Review of Recent Work. J. Therm. Spray Technol. 1996, 5, 401–418. [Google Scholar] [CrossRef]
- He, Y.; Pang, H.; Qi, H.; Wang, D.; Li, Z.; Gao, W. Micro-crystalline Fe–Cr–Ni–Al–Y2O3 ODS alloy coatings produced by high frequency electric-spark deposition. Mater. Sci. Eng. A 2002, 334, 179–186. [Google Scholar] [CrossRef]
- Tang, S.K.; Nguyen, T.C.; Zhou, Y. Materials transfer in electro-spark deposition of TiCp/Ni metal-matrix composite coating on Cu substrate. Weld. J. 2010, 89, 172–180. [Google Scholar]
- He, Y.; Huang, Z.; Qi, H.; Wang, D.; Li, Z.; Gao, W. Oxidation behaviour of micro-crystalline Ni–20Cr–Y2O3 ODS alloy coatings. Mater. Lett. 2000, 45, 79–85. [Google Scholar] [CrossRef]
- Wu, Q.; Zheng, H.; Zhang, Z.; Hu, P.; Fan, C.; Zhong, N.; Liu, Y. High-temperature wear and cyclic oxidation behavior of (Ti, W)C reinforced stainless steel coating deposited by PTA on a plain carbon steel. Surf. Coat. Technol. 2021, 425, 127736. [Google Scholar] [CrossRef]
- Chavoshi, S.Z.; Xu, S. A Review on Micro- and Nanoscratching/Tribology at High Temperatures: Instrumentation and Experimentation. J. Mater. Eng. Perform. 2018, 27, 3844–3858. [Google Scholar] [CrossRef]
- Steckelmacher, W. Coatings tribology: Properties, techniques and applications in surface engineering. Vacuum 1995, 46, 88. [Google Scholar] [CrossRef]
- Ang, A.S.; Berndt, C. A review of testing methods for thermal spray coatings. Int. Mater. Rev. 2014, 59, 179–223. [Google Scholar] [CrossRef]
- Łatka, L.; Chicot, D.; Cattini, A.; Pawłowski, L.; Ambroziak, A. Modeling of elastic modulus and hardness determination by indentation of porous yttria stabilized zirconia coatings. Surf. Coat. Technol. 2013, 220, 131–139. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Bai, M.; Yang, L.; Li, C.; Wang, L.; Carr, J.; Xiao, P. A mechanistic understanding on rumpling of a NiCoCrAlY bond coat for thermal barrier coating applications. Acta Mater. 2017, 128, 31–42. [Google Scholar] [CrossRef]
- Waki, H.; Kitamura, T.; Kobayashi, A. Effect of Thermal Treatment on High-Temperature Mechanical Properties Enhancement in LPPS, HVOF, and APS CoNiCrAlY Coatings. J. Therm. Spray Technol. 2009, 18, 500–509. [Google Scholar] [CrossRef]
- Deng, T.; Bingley, M.S.; Bradley, M.S.A. Understanding particle dynamics in erosion testers—A review of in-fluences of particle movement on erosion test conditions. Wear 2009, 267, 2132–2140. [Google Scholar] [CrossRef]
- Shipway, P.; Hutchings, I. Measurement of coating durability by solid particle erosion. Surf. Coat. Technol. 1995, 71, 1–8. [Google Scholar] [CrossRef]
- Szala, M.; Walczak, M.; Świetlicki, A. Effect of Microstructure and Hardness on Cavitation Erosion and Dry Sliding Wear of HVOF Deposited CoNiCrAlY, NiCoCrAlY and NiCrMoNbTa Coatings. Materials 2022, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; An, Y.; Zhao, X.; Zhou, H.; Chen, J. NiCoCrAlYTa coatings on nickel-base superalloy substrate: Deposition by high velocity oxy-fuel spraying as well as investigation of mechanical properties and wear resistance in relation to heat-treatment duration. Appl. Surf. Sci. 2018, 462, 194–206. [Google Scholar] [CrossRef]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Jojith, R.; Sam, M.; Radhika, N. Recent advances in tribological behavior of functionally graded composites: A review. Eng. Sci. Technol. Int. J. 2021, 25, 100999. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, Y.; Yang, W.; Hong, S.; Qiao, L.; Cheng, J. Effects of loads on high-temperature wear properties of HVOF sprayed CoCrAlYTa-10%Al2O3 and Cr3C2-NiCr coatings. Mater. Res. Express 2019, 6, 106541. [Google Scholar] [CrossRef]
- Blau, P.J. Elevated-temperature tribology of metallic materials. Tribol. Int. 2010, 43, 1203–1208. [Google Scholar] [CrossRef]
- Stott, F. High-temperature sliding wear of metals. Tribol. Int. 2002, 35, 489–495. [Google Scholar] [CrossRef]
- Stott, F.; Lin, D.; Wood, G. The structure and mechanism of formation of the ‘glaze’ oxide layers produced on nickel-based alloys during wear at high temperatures. Corros. Sci. 1973, 13, 449–469. [Google Scholar] [CrossRef]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals, 2nd ed.; Elsevier: Sydney, Australia, 2016; ISBN 9780081001011. [Google Scholar]
- Chen, Y.; Zhao, X.; Xiao, P. Effect of microstructure on early oxidation of MCrAlY coatings. Acta Mater. 2018, 159, 150–162. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, T.; Sun, B.; Wang, B.; Zhang, X.; Song, L. Isothermal oxidation and TGO growth behavior of NiCoCrAlY-YSZ thermal barrier coatings on a Ni-based superalloy. J. Alloys Compd. 2020, 844, 156093. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.M.; Kay, C.M.; Bakhsheshi-Rad, H.; Gupta, R.K.; Yusof, N.M.; Ghandvar, H.; Arshad, A.; Zulkifli, I.S.M. Effects of Al2O3 diffusion barrier layer (including Y-containing small oxide precipitates) and nanostructured YSZ top coat on the oxidation behavior of HVOF NiCoCrAlTaY/APS YSZ coatings at 1100 °C. Corros. Sci. 2018, 144, 13–34. [Google Scholar] [CrossRef]
- Roy, M. Approaches to enhance elevated temperature erosion resistance of Ni-base superalloys. Mater. High Temp. 2019, 36, 142–156. [Google Scholar] [CrossRef]
- Bousser, E.; Martinu, L.; Klemberg-Sapieha, J. Solid particle erosion mechanisms of protective coatings for aerospace applications. Surf. Coat. Technol. 2014, 257, 165–181. [Google Scholar] [CrossRef]
- Demirci, M.; Bagci, M. High Temperature Solid Particle Erosion Comparison of Atmospheric Plasma Sprayed MCrAlY Coatings. Surf. Topogr. Metrol. Prop. 2021. [Google Scholar] [CrossRef]
- Bousser, E.; Martinu, L.; Klemberg-Sapieha, J.E. Solid particle erosion mechanisms of hard protective coatings. Surf. Coat. Technol. 2013, 235, 383–393. [Google Scholar] [CrossRef]
- Lakshmi, S.G.; Swarup, K.T.; Das, D.; Roy, M. Erosion behaviour of platinum aluminide bond coat on directionally solidified CM247 and AM1 single crystal superalloys. Surf. Coat. Technol. 2022, 429, 127941. [Google Scholar] [CrossRef]
- Wood, R. The sand erosion performance of coatings. Mater. Des. 1999, 20, 179–191. [Google Scholar] [CrossRef]
- Mishra, S.; Prakash, S.; Chandra, K. Studies on erosion behaviour of plasma sprayed coatings on a Ni-based superalloy. Wear 2006, 260, 422–432. [Google Scholar] [CrossRef]
- Babu, K.A.; Jegadeeswaran, N.; Nithin, H.; Kapilan, N. Studies on solid particle erosion by HVOF sprayed 25% (Cr3C2-25 (Ni20Cr)) + 75% NiCrAlY on Ti-31. Mater. Today Proc. 2020, 45, 246–253. [Google Scholar] [CrossRef]
- Kablov, E.N.; Muboyadzhyan, S.A. Erosion-resistant coatings for gas turbine engine compressor blades. Russ. Met. 2017, 2017, 494–504. [Google Scholar] [CrossRef]
- Antonov, M.; Veinthal, R.; Huttunen-Saarivirta, E.; Hussainova, I.; Vallikivi, A.; Lelis, M.; Priss, J. Effect of oxidation on erosive wear behaviour of boiler steels. Tribol. Int. 2013, 68, 35–44. [Google Scholar] [CrossRef]
- Mathapati, M.; Ramesh, M.; Doddamani, M. High temperature erosion behavior of plasma sprayed NiCrAlY/WC-Co/cenosphere coating. Surf. Coat. Technol. 2017, 325, 98–106. [Google Scholar] [CrossRef]
- Lee, J.; Terner, M.; Copin, E.; Lours, P.; Hong, H.-U. A novel approach to the production of NiCrAlY bond coat onto IN625 superalloy by selective laser melting. Addit. Manuf. 2020, 31, 100998. [Google Scholar] [CrossRef]
- Luo, H.; Li, X.; Pan, C.; He, P.; Zeng, K.; Hu, K.; Li, H.; Yang, C. Microstructure and oxidation resistance of CoNiCrAlY coating manufactured by laser powder bed fusion. Surf. Coat. Technol. 2021, 427, 127846. [Google Scholar] [CrossRef]
- Derelizade, K.; Rincon, A.; Venturi, F.; Wellman, R.; Kholobystov, A.; Hussain, T. High temperature (900 °C) sliding wear of CrNiAlCY coatings deposited by high velocity oxy fuel thermal spray. Surf. Coat. Technol. 2022, 432, 128063. [Google Scholar] [CrossRef]
- Amer, M.; Hayat, Q.; Janik, V.; Jennett, N.; Nottingham, J.; Bai, M. A Review on In Situ Mechanical Testing of Coatings. Coatings 2022, 12, 299. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, J.; Jin, Z.; Prakash, B.; Hu, Y. A review of recent advances in tribology. Friction 2020, 8, 221–300. [Google Scholar] [CrossRef]




| Material | Density (kg/m3) | Hardness (GPa) |
|---|---|---|
| Al2O3 | 3970 | 21–30 |
| Cr2O3 | 5210 | 9–20 |
| ZrO2 | 5750 | 10.7 |
| CeO2 | 7220 | 5–7 |
| TiO2 | 4250 | 12–14 |
| ZrB2 | 6120 | 15–23 |
| SiC | 3210 | 24–32 |
| YSZ | 5890 | 13.3 |
| HfB2 | 11,212 | 28 |
| TaC | 14,500 | 18.9 |
| Substrate | Matrix | Reinforcement(s) | Materials Preparation | Coating Process | Ref. |
|---|---|---|---|---|---|
| Single crystal superalloy (ERBO 1) | CoNiCrAlY | Al2O3 | Mechanical milling | VPS | [9] |
| GH4169 superalloy | CoCrAlYTa | Al2O3 | Cryomilling | Laser-induction hybrid cladding | [23] |
| AISI 316 L | NiCoCrAlY | Al2O3 | Mechanical alloying | HVOF | [49] |
| Ti–6Al–4V | CoCrAlYTaSi | Al2O3 | Mechanical mixing | Laser cladding | [50] |
| Inconel 718 | CoCrAlYTaCSi | Al2O3 | Agglomeration and Sintering | HVOF | [51] |
| GH4033 | NiCoCrAlY | Al2O3, SiC, CeO2 | Mechanical milling | Laser cladding | [52] |
| Carbon steel | NiCoCrAlY | Al2O3, B4C | Solid-state alloying | APS | [53] |
| Stainless steel | NiCoCrAlYTa | Al2O3 + Cr2O3 | Mechanical mixing | APS | [54] |
| MDN321, Superni 76 | CoCrAlY | NiCr − Cr3C2 | Mechanical mixing | APS | [55] |
| Superni 76 | CoCrAlY | NiCr − Cr3C2, WC-Co | Mechanical mixing | APS | [56] |
| GH4169 | NiCrAlY | TiO2 − ZnO | Mechanical mixing | APS | [57] |
| IN-792 | NiCoCrAlYTa | BN | Mechanical mixing + Sintering into Electrode | Electrospark deposition | [58] |
| Inconel-617 | CoNiCrAlY | YSZ | Mechanical milling | HVOF | [59] |
| Single crystal superalloy | NiCoCrAlYTa | Y2O3 | Mechanical mixing + Sintering into Electrode | Electrospark deposition | [60] |
| DZ 125 | NiCoCrAlY | Al2O3 | In-situ oxide dispersion | EB-PVD | [61] |
| Single crystal superalloy | NiCoCrAlYTa | TaC | Mechanical mixing + Sintering into Electrode | Electrospark deposition | [62] |
| — | NiCoCrAlY | Al2O3 | Mechanical milling + Spray drying | APS | [63] |
| AISI 1035 | NiCrAlY | YSZ | Mechanical mixing | Plasma transferred arc (PTA) | [64] |
| GH4033 | NiCoCrAlY | Al2O3 | Suspension mixing | Laser cladding | [65] |
| Pure Ti | NiCoCrAlY | ZrB2 | Mechanical mixing | Laser cladding | [66] |
| Pure Ti | NiCoCrAlY | HfB2 | Mechanical mixing | Laser cladding | [67] |
| AISI H13 | CoNiCrAlY | Cr3C2 | Agglomeration and Sintering | HVOF | [68] |
| GH4169 | NiCrAlY | Ag-MoO3 | Mechanical milling | Laser cladding | [69] |
| Steel | CoNiCrAlY | Al2O3 | Mechanical milling | HVOF | [70] |
| Stainless steel | NiCrAlY | Al2O3 | Suspension mixing | Hybrid plasma spray | [71] |
| Low carbon steel | NiCoCrAlY | CeO2 + Al2O3 | Mechanical milling | HVOF | [72] |
| Low carbon steel | NiCoCrAlY | CeO2 | Mechanical milling | HVOF | [73] |
| — | NiCrAlY | Al2O3 + h-BN | Suspension mixing | Hybrid plasma spray | [74] |
| Superni 76 | CoCrAlY | Al2O3 + YSZ, CeO2 | Mechanical mixing | APS | [75] |
| AISI 304 | NiCrAlY | Al2O3 | Suspension mixing | HVOF | [76] |
| GH536 | NiCrAlY | Al2O3 | Mechanical milling | Argon shrouded plasma spray (ASPS) | [77] |
| T91 | NiCrAlY | SiC | Mechanical mixing | HVOF | [78] |
| T22 | NiCrAlY | B4C | Mechanical mixing | HVOF | [79] |
| TiAl | NiCoCrAlY | SiC | Suspension mixing | Laser cladding | [80] |
| GH4169 | NiCoCrAlY, CoNiCrAlY | Cr2O3 | Solid-state alloying | APS | [81] |
| Superco-605 | CoCrAlTaY | Al2O3 | Mechanical mixing | HVOF | [82] |
| Alloy 600 | NiCoCrAlY | YSZ | Mechanical milling | HVOF | [83] |
| Superni 76, MDN 321 | CoCrAlY | WC-Co | Mechanical mixing | APS | [84] |
| IN-738 | CoNiCrAlY | Al2O3 | Mechanical milling | HVOF | [85] |
| TiBw/Ti64 | CoCrAlY | TiB2 | Suspension mixing | APS | [86] |
| Ti-based superalloy | CoCrAlSiY | YSZ | Powder-mixing within the feeding system | Laser direct deposition (LDD) | [87] |
| AISI H13 | CoCrAlYTa | Al2O3 | Agglomeration and Sintering | HVOF | [88] |
| Steel | NiCoCrAlY | CeO2 | Mechanical mixing | APS | [89] |
| Ni-base superalloy | NiCoCrAlYTa | TaC | Electrode forming by laser casting wire | Electrospark deposition | [90] |
| Mild steel | NiCoCrAl | Al2O3-40%TiO2 | Powder-mixing within the feeding system | APS | [91] |
| 316L Stainless steel | NiCoCrAlYTa | WC-Co | Mechanical mixing | HVOF | [92] |
| Ti6Al4V | NiCoCrAlY | CeO2 | Mechanical milling | Laser cladding | [93] |
| Steel | CoNiCrAlY | Cr3C2 | Commercially available powders | HVOF | [94] |
| 304 Stainless steel | CoNiCrAlY | Al2O3 | Mechanical milling | HVOF | [95] |
| Inconel 718 | NiCrAlY | ZrO2 | Mechanical mixing | Ultrasonic-assisted direct laser deposition | [96] |
| Heat-resistant steel | NiCrAlY | CeO2 | Ultrasonic gas atomization | HVOF | [97] |
| Ni-based superalloy GH4033 | NiCoCrAlY | Al2O3 | Mechanical mixing | Laser cladding | [98] |
| Powder Composition | Milling Type | Observations | Ref. |
|---|---|---|---|
| CoNiCrAlY + Al2O3 | High-kinetic ball milling device (Simoloyer CM01) | Longer milling times caused the hardening of powder particles and an increase in the carbide contamination. Adding higher contents of stearic acid, as the process control agent (PCA), resulted in the elongated particles, which were present in the HVOF-sprayed coatings as well. Conducting the milling process in a low-volume chamber caused more collisions with higher energy input to the powder particles. | [70] |
| NiCoCrAlY + YSZ | High-kinetic ball milling device (Simoloyer CM08) | With a milling time not exceeding 5 h, powder particles with a near-spherical morphology and a homogenous dispersion of the added oxide phase were achieved. The incorporation of YSZ into the NiCoCrAlY matrix led to a drastic increase in the powder microhardness. The strengthened powders showed a microhardness value of 660 HV, in comparison with the 420 HV for the commercial powder. | [83] |
| Pure NiCoCrAlY | Cryomilling with an attrition mill (Union Process 1-D) | The SEM observations revealed a flattened and flaky morphology for the cryomilled powder. The XRD analysis showed a single broad peak corresponding to the γ phase, as opposed to the dual-phase (γ + β) structure of the unmilled powder. | [106] |
| NiCoCrAlYSi + Al2O3 | Cryomilling with an attrition mill | The spheroidal powder as received went under a series of morphological changes to rough oval-shaped particles (8 h-milled), irregular flake-shaped agglomerates (12 h-milled), and large disk-like particles (16 h-milled). The addition of nanosized Al2O3 to the powder mixture caused a number of changes such as lower tendency for cold-welding, which resulted in very small-sized particles, and an increase in the Fe content. | [107] |
| CoNiCrAlY + YSZ | High-energy planetary ball mill | It was suggested that the severe plastic deformation due to the milling process caused the dissolution of β phase into the γ phase of the matrix. An increase in the amount of YSZ resulted in the brittleness of the powder mixture and delayed the cold-welding phenomenon during the milling. Therefore, the final powder particles with higher reinforcement were finer. | [108] |
| Pure CoNiCrAlY | Cryomilling with an attrition mill (Union Process model 01-HD) | Cryomilling under the severe processing conditions (higher charge ratio and longer times) caused a significant increase in the average powder particle size above 100 µm. Particle coarsening with a flake-like morphology was observed in the case of cryomilled powders. The TEM study revealed the nanostructure of the cryomilled powders with a grain size of 20 ± 7 nm. Furthermore, the presence of Al oxides and oxynitrides were detected by this analysis. | [109] |
| Coating (Process) | Test Configuration | Abrasion Media | Applied Load | Test Frequency/Speed | Test Distance/Time | Test Temperature | Best Friction-Wear Resistance | Ref |
|---|---|---|---|---|---|---|---|---|
| CoNiCrAlY- Al2O3 (VPS) | Ball on disc | Al2O3 | 5 N | 0.20 m/s | 2500 m | 750 °C | ~3 × 10−6 (mm3 N−1 m−1) | [9] |
| CoCrAlYTa-Al2O3 (LC) | Block on ring | GCr15 steel | 196 N | 400 rpm | 600 m | RT | 5.58 × 10−3 (mg/m) | [23] |
| CoCrAlYTaSi- Al2O3 (LC) | Ball on disc | Si3N4 | 3 N | --- | 120 m | 800 °C | 0.3 × 10−4 (mm3 N−1 m−1) | [50] |
| NiCrAlY- TiO2–ZnO (APS) | Ball on disc | Al2O3 | 10 N | 0.105 m/s | 3600 s | 25, 400, 800 °C | 7.3 × 10−6 @800 °C (mm3 N−1 m−1) | [57] |
| CoNiCrAlY-Cr3C2 (HVOF) | Pin on disc | WC–6Co | 30 N | 0.10 m/s | 5 × 105 cycles | 25 °C | 2.8 × 10−16 (m3 N−1 m−1) | [68] |
| NiCrAlY-Al2O3 (Hybrid plasma spray) | Ball on disc | Sintered Al2O3 | 5 N | 0.10 m/s | 2000 m | RT, 400, 700 °C | ~5 × 10−6 @RT ~8 × 10−5 @400 °C ~2 × 10−5 @700 °C (mm3 N−1 m−1) | [71] |
| NiCrAlY-Al2O3-hBN (Hybrid plasma spray) | Ball on disc | Sintered Al2O3 | 5 N | 0.10 m/s | 2000 m | RT, 400, 700 °C | ~1 × 10−5 @RT ~3.5 × 10−5 @400 °C ~0.5 × 10−5 @700 °C (mm3 N−1 m−1) | [74] |
| CoCrAlYTa-Al2O3 (HVOF) | Ball on disc | Si3N4 | 10 N | 15.9 Hz | 60 min | 25–600 °C | ~3×10−5 (mm3 N−1 m−1) @600 °C | [88] |
| NiCoCrAlYTa-WC-Co (HVOF) | Ball on disc | GCr15 steel WC-Co | 5 N | 10 cm/s | 200 m | RT | 3.19 × 10−6 (GCr15) 3.13 × 10−6 (GCr15) (mm3 N−1 m−1) | [92] |
| NiCoCrAlY-CeO2 (LC) | Ball on disc | Si3N4 | 3 N | 500 r·min−1 | 30 min | 600 °C | 2.35 × 10−7 (mm3 N−1 m−1) | [93] |
| NiCoCrAlY- Al2O3 (LC) | Pin on block | Al2O3 | 20 N | 0.1 m/s | 120 m | 500 °C | 102.25 × 10−6 (mm3 N−1 m−1) | [98] |
| Oxide | Reaction | ΔG900°C (kJ/mol) | ΔG1000°C (kJ/mol) |
|---|---|---|---|
| NiO | Ni(s) + 0.5O2(g) = NiO(s) | −275.8 | −256.9 |
| CoO | Co(s) + 0.5O2(g) = CoO(s) | −353.8 | −343.4 |
| Cr2O3 | 2Cr(s) + 1.5O2(g) = Cr2O3(s) | −538.9 | −520.7 |
| Al2O3 | 2Al(s) + 1.5O2(g) = Al2O3(s) | −871.4 | −850.5 |
| NiCr2O4 | NiO(s) + Cr2O3(s) = NiCr2O4(s) | −43.7 | −42.9 |
| CoCr2O4 | CoO(s) + Cr2O3(s) = CoCr2O4(s) | −88.8 | −101.5 |
| NiAl2O4 | NiO(s) + Al2O3(s) = NiAl2O4(s) | −109.3 | −115.4 |
| CoAl2O4 | CoO(s) + Al2O3(s) = CoAl2O4(s) | −83.3 | −93.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakeri, A.; Bahmani, E.; Ramazani, A. A Review on the Enhancement of Mechanical and Tribological Properties of MCrAlY Coatings Reinforced by Dispersed Micro and Nanoparticles. Energies 2022, 15, 1914. https://doi.org/10.3390/en15051914
Zakeri A, Bahmani E, Ramazani A. A Review on the Enhancement of Mechanical and Tribological Properties of MCrAlY Coatings Reinforced by Dispersed Micro and Nanoparticles. Energies. 2022; 15(5):1914. https://doi.org/10.3390/en15051914
Chicago/Turabian StyleZakeri, Ali, Elnaz Bahmani, and Ali Ramazani. 2022. "A Review on the Enhancement of Mechanical and Tribological Properties of MCrAlY Coatings Reinforced by Dispersed Micro and Nanoparticles" Energies 15, no. 5: 1914. https://doi.org/10.3390/en15051914
APA StyleZakeri, A., Bahmani, E., & Ramazani, A. (2022). A Review on the Enhancement of Mechanical and Tribological Properties of MCrAlY Coatings Reinforced by Dispersed Micro and Nanoparticles. Energies, 15(5), 1914. https://doi.org/10.3390/en15051914







