1. Introduction
Due to the rapidly increasing global warming issues caused by the emission of greenhouse gases into the atmosphere from burning oil, gas and coal, there is a need for alternative technologies to satisfy the world’s energy demand, with the focus now being shifted to environmentally friendly technologies [
1]. One promising approach for the success of the energy change can be hydrogen, when it is produced by renewable energy sources (RES) [
2].
While hydrogen is already being used in the mobility sector [
3], it is as yet to be employed within the residential sector. This sector is responsible for 26.5% of the total energy demand in Germany which is predominantly covered by fossil energy sources [
4,
5]. Moreover, the highest energy demand in Germany occurs during winter which coincides with low photovoltaic (PV) generation. This leads to the necessity of long-term energy storage systems such as hydrogen, which are charged during the summer by usage of high excess energy produced, for example, via PV.
PV is already widely installed, as more than 1.7 million PV systems have been installed in Germany as of the end of 2020 [
6]. However, the degree of PV autoconsumption is around 35% on average due to the volatility of the energy production [
7]. The residual energy is fed into the external energy grid. Nonetheless, the high number of PV systems installed with grid connections leads to a burden of the grids during periods with high PV overproduction (e.g., on midday in summer), which becomes even more critical as the number of PV systems increases. This phenomenon contributes to the slow expansion of renewable energy systems in Germany: An increase in the share of renewable energy with grid connection implies further investment from grid operators in additional transmission and distribution capacity as well as in grid stabilising measures [
8]. Since the implementation of such measures is very time consuming, short-term solutions are preferable.
With the increase of renewable energy share, a transition from a predominantly centralised to a decentralised energy supply is performed [
9]. Not only decentralised production but also decentralised long-term energy storage should be considered to reduce the necessity of grid expansion. A degree of self-sufficiency of up to 100% by usage of decentralised long-term storages would lead to an expansion of PV systems which are not influenced by these constraints. However, the intermittent nature and the uncertainty of production of RES necessitates the usage of efficient and sufficient seasonal storage systems in order to store the overproduction for use during periods of low energy production [
10]. Hydrogen is especially well suited for long-term energy storage due to its inherent high energy density and its long lifetime as a storage device (compared to batteries) [
11].
In literature, some research has already been carried out on this topic but with a focus on specific partial aspects of hybrid, hydrogen-based energy systems. In [
12], the authors presented a hybrid energy system including RES as energy supply, an alkaline electrolyser, a proton exchange membrane fuel cell (PEMFC) and a hydrogen storage. They focused on the analysis of the system dynamics with the aim to improve self-sufficiency by reducing grid interaction. Within that energy system a Li-ion battery and a heating system have not been considered. Due to the lack of short-term energy storage, they used the hydrogen storage to store the amount of hydrogen produced by the electrolyser within one day and directly reconverted the hydrogen by usage of a fuel cell as soon as the energy production with RES was less than the energy demand. In [
13], a Simulink model of an energy system consisting of a PV system, a PEM electrolyser and a hydrogen storage system has been presented. The model was created for the analysis of system dynamics and was therefore used for short-term analysis. It also did not consider a fuel cell and real load profiles. In [
14], the authors compared different meta-heuristic methods to optimally design an isolated hydrogen-based microgrid. The model was good enough for energy evaluation. However, the model also did not consider short-term energy storage, which is important in terms of dealing with rapid load peaks for ensuring a high degree of self-sufficiency. In [
15], a hydrogen-based energy system model created in Simulink was presented. The authors used a time horizon of 24 h with focus on the power management system and the control system. They analysed the suitability of a hydrogen-based energy system consisting of hydrogen as the only energy storage type for stand-alone systems. However, the load profile did not contain rapid peaks which should not be covered by a fuel cell in terms of lifetime [
16]. In none of the literature found, with focus on hybrid, hydrogen-based energy systems, were the heating sector and thus the overall energy demand of a household considered. When dealing with the attempt to increase the self-sufficiency of a household, the heating system plays an important role, especially in terms of stand-alone systems which shall be fully supplied by RES. An energy system model including a hybrid energy storage consisting of a Li-ion battery and hydrogen has hardly been considered so far.
Within this paper, a model of a decentralised energy system whose energy demand is mainly covered via photovoltaics even during winter by including sufficient storage facilities is presented. The aim is to minimise the grid load by achieving low or even zero grid interaction, which is realised by the use of decentralised long-term energy storage. The model consists of an electrolyser, which produces hydrogen in case of PV overproduction (overproduction = present PV generation—autoconsumption), and the hydrogen is subsequently stored in a hydrogen tank via a compressor. If the energy demand exceeds the energy provided by the PV system, a fuel cell is used for reconversion of hydrogen. To guarantee fast reactions to rapid load changes and to avoid comparatively high energy losses that arise when only using hydrogen as a storage medium, the system has to be extended by a short-term lithium-ion battery. Such a system can provide autonomy for electricity over the whole year.
The model was created in Matlab/Simulink [
17] and was designed for the analysis of real data series over an entire year with a time resolution of 15 min. A representative dataset recorded in Switzerland in 2012 was used as the household load profile [
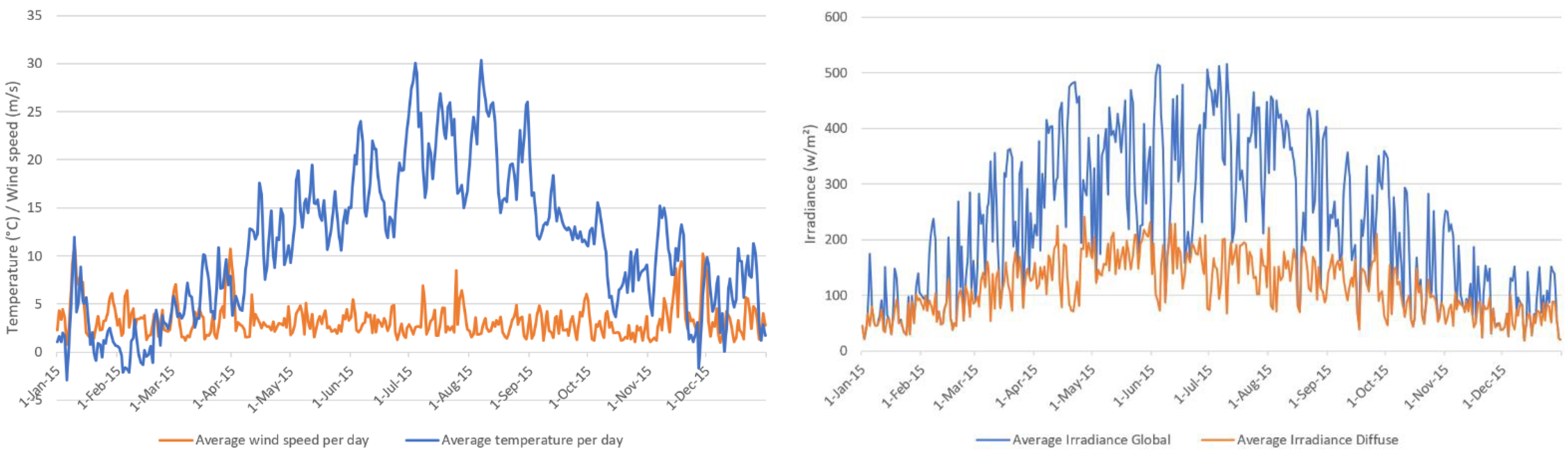
18]. The load profile originally had a time resolution of one second which was converted to a time resolution of 15 min by averaging the values. The irradiance and temperature profile have been granted by the Deutscher Wetterdienst (DWD) with a time resolution of one hour [
19]. The data used were recorded in 2015 in Wuerzburg (Germany). The given resolution was converted to a time resolution of 15 min using linear interpolation. As the heating system was also considered, a synthetic heat demand profile was generated using the reference load profiles from the VDI 4655 standard [
20]. Using the temperature profile for 2015 and an Excel tool created by Hessen [
21], which uses the reference load profiles from the VDI 6455 standard, a heating demand profile with a 15 min time resolution was generated.
This work shows the development of a model that has been designed to analyse the possibilities of a household system fully powered by a PV system considering hybrid energy storage composed of a lithium-ion battery and hydrogen. The focus of the research is based on the analysis of the kind and size of components required under different scenarios and an analysis as to what extent and under which conditions such a system is price-competitive with a conventional energy supply. The impact of different operating modes on the overall energy balance is also a subject of the investigation. Due to a time resolution of 15 min and a total timeframe of one year, a detailed analysis of the energy flows which is based on real data rather than synthetic profiles is possible.
The paper is organised as followed:
Section 2 introduces the architecture of the entire energy system,
Section 3 describes the model components mathematically and graphically and
Section 4 specifies the configuration of the components used for the test case. In
Section 5, the Simulink model is verified and compared to the results delivered by HOMER Energy Simulation [
22] for the same system dimensioning and finally
Section 6 concludes the findings.
3. System Architecture and Control System
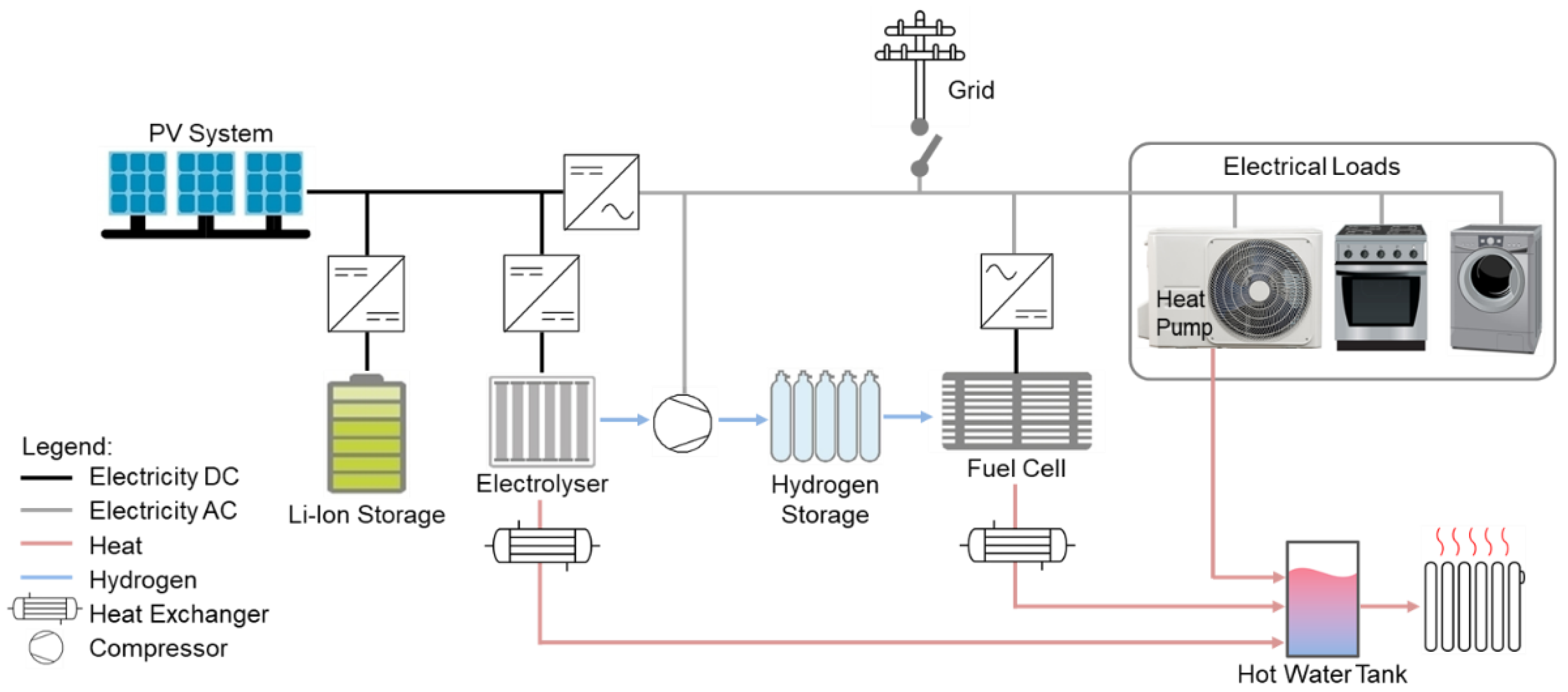
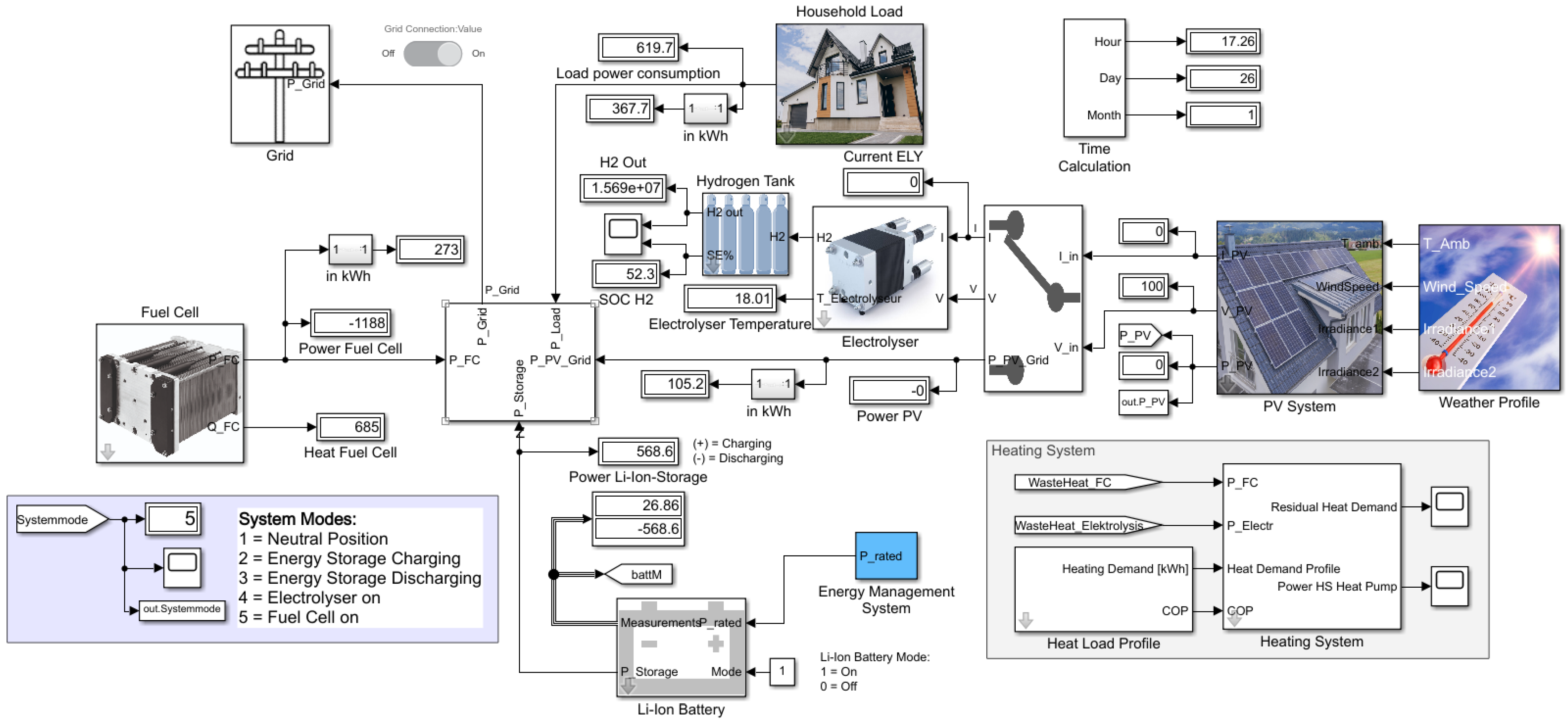
Within this section, the hybrid energy system, the functions of the individual components and the control procedure are qualitatively described. The core elements of the energy system model are a fuel cell (FC), an electrolyser, a lithium-ion battery, a hydrogen storage tank and a PV system.
Figure 3 shows the architecture of the proposed energy system. The PV system serves as the main energy source. With an inverter, the produced solar energy can directly be used for the electrical loads in the household (direct consumption). The surplus energy is stored in the lithium-ion battery by passing a DC/DC converter as a charge controller, and the energy is transferred back to the household grid via an inverter in case of less energy production than demand. If the lithium-ion battery is fully charged and the solar energy still exceeds the electricity demand, the electrolyser is switched on and hydrogen is produced by the surplus energy. After passing a compressor, the hydrogen is stored inside the hydrogen tank. In case of high electricity demand in combination with low energy production and an empty lithium-ion battery, the fuel cell is switched on. The fuel cell operates at constant power and acts as an electricity supplier for electrical loads. Since only the lithium-ion battery can deal with load peaks, the fuel cell switches on even before the lithium-ion battery is completely empty. So, the lithium-ion battery works as a buffer and is also charged by the residual energy delivered by the fuel cell in case of lower energy consumption than is delivered by the fuel cell and as long as the state of charge (SoC) of the lithium-ion battery does not reach a predefined state. A simple control algorithm via Simulink chart is used to control the energy flow.
Figure 4 shows the input and output parameters that are involved in the control algorithm, which has been qualitatively described before.
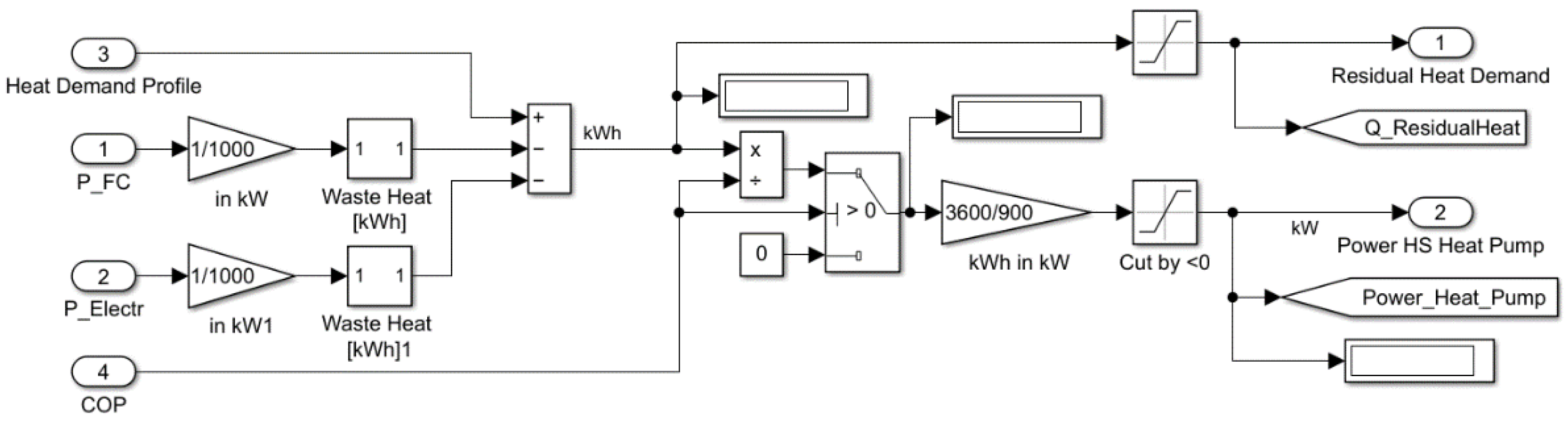
To profit from the waste heat produced by the fuel cell and the electrolyser, heat exchangers are employed which extract the heat and transfer it to the hot water tank. The residual heat demand is covered by a heat pump. The waste heat of the compressor has not been considered because the usable heat is comparatively small and real hydrogen compressors with waste heat utilisation have not been found.
6. Verification and Data Analysis
The validity of the energy system modelled in Simulink was analysed using a single-family house. Therefore, the energy system was rebuilt in HOMER Energy (HE) to be compared. HE provides a wide range of components for basic energy system modelling with focus on energy balance and profitability analysis. To evaluate the plausibility of the developed energy system model, the magnitudes of the energy and hydrogen generation and the consumption are compared, respectively.
For the initial verification of the data, a PV system with rated power of 6820
(22 modules of which 11 modules were in series connection 2 parallel strings) and the characteristics described in
Table 1 was used. Wuerzburg (Germany) was employed as the model location due to the availability of a one-year weather and irradiation profile in hourly time resolution provided by the DWD [
19]. The year 2015 was taken as the year of interest. The elevation angle was set to 45° with a direct orientation towards the south (azimuth angle = 0°). DWD provides global and diffuse irradiation profiles measured for a horizontal plane only. Due to this, the irradiation profile for an inclined plane had to be calculated first, using the formulas described by Quaschning [
52]. The positioning of the PV system was assumed as ideally, having no shadowing during the whole year. To generate realistic data, losses due to pollution, conduction losses, etc. have been considered by introducing an efficiency parameter, which was set to 90%.
For evaluation, the overall load of the household system for each month and a 1 h resolution for an average day within this month were recorded by running the simulation. This profile has been taken as input profile at HE for achieving an equal load within both models. In total, 3832 kWh has been calculated as the overall load during one year. A PV system, a electrolyser and a fuel cell have been integrated into the HE model. With these assumptions, the Simulink model calculated PV energy production of 7314.60 kWh for the whole year whereas HE calculated 7538 kWh.
Figure 14 shows the produced energy within each month (left bar) compared to the energy demand for every month (right bar). From October to March, the energy produced by the fuel cell is relatively high, while the energy required in these months is unneglectably higher than during the summer because of the higher heating demand. The green bar shows the surplus energy which is used in the electrolyser to produce hydrogen.
Simulink calculated that 4283.55 kWh energy was used inside the electrolyser for production of hydrogen, which leads to 80.50 kg (equal to 895.48 m3 at 1 bar, or 2.985 m³ at 300 bar) produced hydrogen within one year. In return, the fuel cell required 73.52 kg hydrogen for production of 1009.86 kWh energy.
The compression of hydrogen required a yearly energy demand of 268.14 kWh, while the heating demand was 1208.66 kWh. The heating demand was reduced by 643.69 kWh by using the waste heat produced within the electrolyser and the fuel cell. At the start of the simulation, which was set to the 1st of January, a hydrogen tank SoC of 45% by a tank volume of 4 m3 at 300 bar was assumed, which corresponds to 135 bar, as 300 bar was considered to be 100% SoC.
HE, in contrast, calculated an energy requirement of 684 kWh delivered by the fuel cell with a hydrogen requirement of 61.6 kg. This is much less energy than the value calculated by Simulink, which is caused by the averaging behaviour used by HE. Within HE, 3590 kWh energy has been used inside the electrolyser for production of 68.3 kg hydrogen. As calculated in HE, 52.6 kWh energy was required for the production of 1 kg hydrogen while Simulink calculated a necessity of 53.21 kWh, which is in good agreement.
Figure 15 shows that the energy produced and consumed (which has been calculated using the same dimensioning of components within Simulink as well as HE) coincides very well. Only the produced PV energy and fuel cell energy differ due to the fact that in Simulink real data instead of averaged data were used.
Figure 16 shows the energy flows over one year recorded by simulation. In the first diagram, the overall energy demand (yellow curve) and the energy produced by the PV system (blue curve) are shown. As can be seen, during the winter months the yellow curve is more dominant since the energy demand is higher than the energy provided by the PV system, while in summer the trend is reversed. In the second diagram, the SoC curves of hydrogen (blue curve) and the Li-ion battery (yellow curve) are depicted. The third diagram shows the moments of time when the electrolyser (yellow curve) and the fuel cell (blue curve) are switched on. The last diagram contains three curves: The orange curve represents the energy used inside the electrolyser for hydrogen production while the blue curve displays the energy provided by the fuel cell. Finally, the yellow curve represents the energy flow towards the Li-ion battery (negative value) and the energy supplied by the Li-ion battery (positive value), respectively.
Figure 16 shows that during summer the Li-ion battery with a capacity of 16 kWh has been sufficient to meet the energy demand (this also includes night demand). In contrast, in winter the Li-ion battery often has a low SoC and has been mainly charged by the fuel cell.
Within the Simulink simulation, the electrolyser has been switched on 303 times with an overall operation time of 1781.5 h while the fuel cell was switched on 168 times with an operation time of 801.5 h during the whole year. In contrast, HE calculated an operation time of the electrolyser of 1771 h and 43 starts of the fuel cell over one year with an operation time of 588 h.
Figure 17 illustrates how the control system in this simulation approach works. The figure shows a section of the week starting on 10 March and ending on 16 March. This week includes days providing sufficient PV generation to operate the electrolyser as well as days when the fuel cell has to be switched on to cover the remaining energy demand. The system mode (
Figure 17c) has five different stages:
Stage 1: Initial stage (only occurs at the beginning of the simulation);
Stage 2: Li-ion battery charging;
Stage 3: Li-ion battery discharging;
Stage 4: Electrolyser switched on;
Stage 5: Fuel cell switched on.
As can be seen in the first graph of the figure, during the sunshine hours of the first day of the week, the PV power is significantly higher than the power of the load. First, the Li-ion battery is fully charged up to the specified maximum SoC until late morning (
Figure 17b). The electrolyser is then switched on to produce hydrogen using surplus PV energy. The electrolyser operates until the load exceeds the PV generation (
Figure 17a). The power supplied to the electrolyser can be seen in
Figure 17d. During the evening and night hours, the load is covered by the Li-ion battery. On the second day, the procedure is the same. Due to high loads in the night from the second to the third day, the Li-ion battery is discharged down to the specified minimum SoC, which causes the fuel cell to start up at mid-morning on the third day. However, this is only in operation until the PV generation exceeds the actual load. On the fourth day, the Li-ion battery is discharged to the specified minimum SoC and the fuel cell is activated again. On this day, however, the FC is only activated until the Li-ion battery is recharged to a predefined SoC of around 50%. At the bottom part of
Figure 17, the power into and out of the Li-ion battery is shown. If the Li-ion battery is being discharged, the power
is positive, and during charging the Li-ion battery, the power
is negative.
is zero as long as the electrolyser is switched on. If the fuel cell is switched on, the power into the Li-ion battery is calculated by:
When the heat demand of a household is covered by electricity (heat pumps), a high impact on the load of the household is caused. Assuming future trends, the heat demand will be increasingly covered via electricity [
53], which would again lead to a burden on the electricity grid which should be reduced within the context of the energy system approach. When endeavouring for energy autonomy of households, the heat demand should be considered as is it has been done in this approach. However, taking the heat demand into account leads to a significant increase in hydrogen storage and production requirements. The hydrogen demand increases from 24.07 kg to 73.52 kg (205% increase) when the heat demand of 4000 kWh/a is considered. In return, 91.07 kg hydrogen instead of 80.50 kg hydrogen has been produced when neglecting the heat demand. In the example presented, a well-insulated house with a correspondingly low heat demand of 4000 kWh/a was selected. If the heat demand of the household has to be doubled to 8000 kWh/a, the hydrogen demand would increase to 161.23 kg, which corresponds to an increase of 119% (in comparison to a heat demand of 4000 kWh/a).
8. Discussion
The simulation based on the presented Simulink model allows a more detailed and meaningful analysis of energy systems due to its significantly higher resolution compared to HOMER Energy. Due to the usually strong fluctuating load, temperature and irradiation profiles, a simulation with a resolution of 15 min allows a more precise statement about the suitability and sizing of various components than is possible using monthly averaged values such as the commonly used modelling approach in HOMER Energy. The Simulink model is therefore preferable for analysing the system behaviour. Furthermore, Simulink enables the introduction of direct component dependencies such as the integration of the waste heat generated by the FC and the electrolyser into the heating system and the direct conjunction of the FC and the hydrogen storage, which are not considered in HOMER Energy. Moreover, with Simulink, a more precise and specifically tailored control algorithm for every component which has a significant impact on the energy balance is feasible.
By simulation using Simulink, it can be seen whether the chosen component dimensioning and key parameters are suitable for guaranteeing off-grid operation. Therefore, the input parameters can be changed according to their own energy usage preferences and the designated location.
The simulation was performed by usage of a 15 min resolution, whereby the input datasets had to be averaged, and, as a result, sudden rapid changes in the datasets have been neglected. Ramp-up and start-up times of the components thereby have not been considered, while they are neglectable in the considered time scale. A higher resolution of the entire input data failed due to the weather and solar irradiance datasets that were only available in hourly resolution. Therefore, input datasets with a much higher resolution recorded by us will be involved in future research to achieve more realistic solutions.