Exhaust Gas Temperature Pulsations of a Gasoline Engine and Its Stabilization Using Thermal Energy Storage System to Reduce Emissions
Abstract
:1. Introduction
- Value of lambda of the exhaust gas close to the stoichiometric mixture (λ = 1)
- The temperature of the exhaust gas being higher than the light-off temperature of the catalyst
2. Materials and Methods
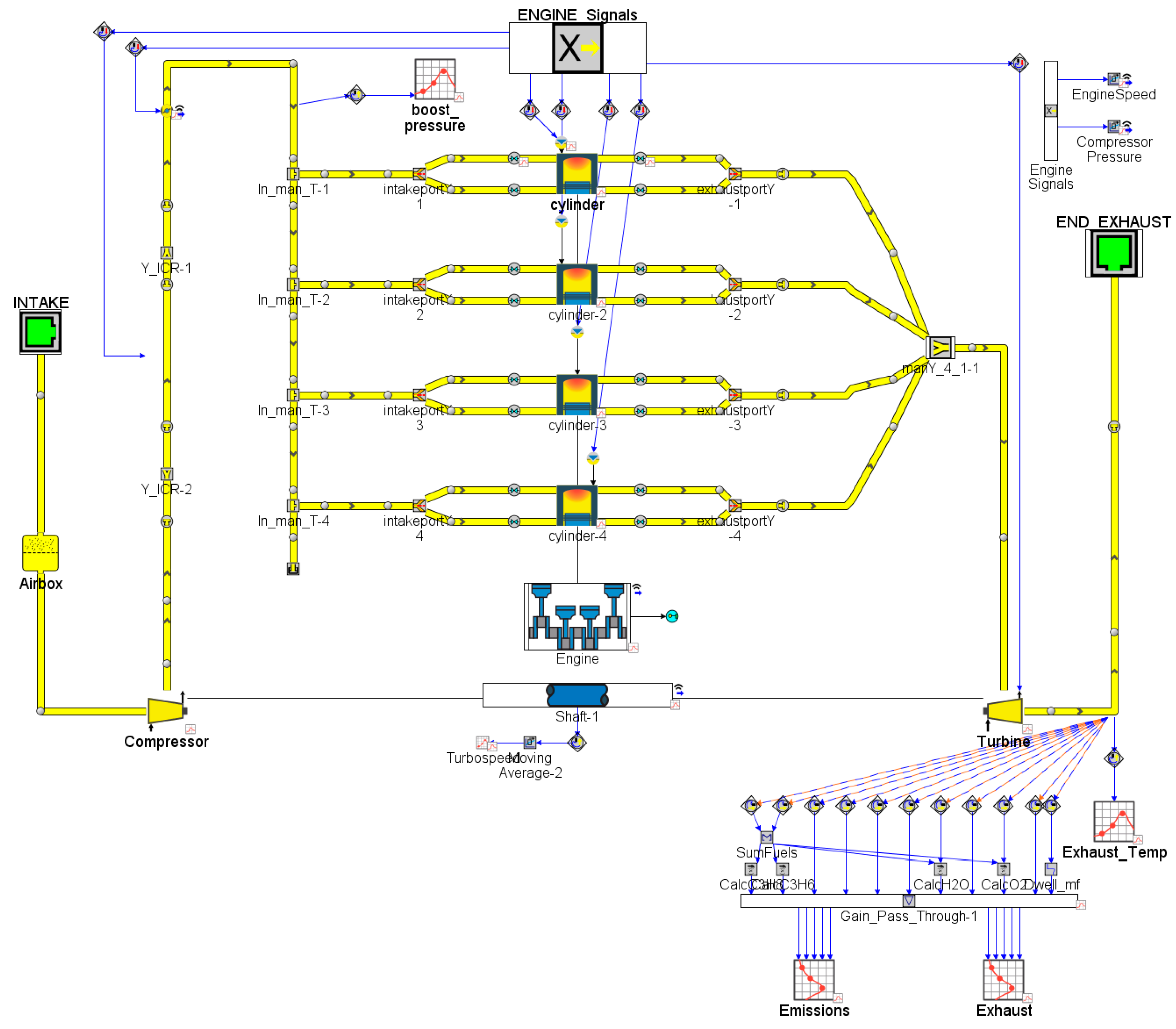
2.1. Engine Model
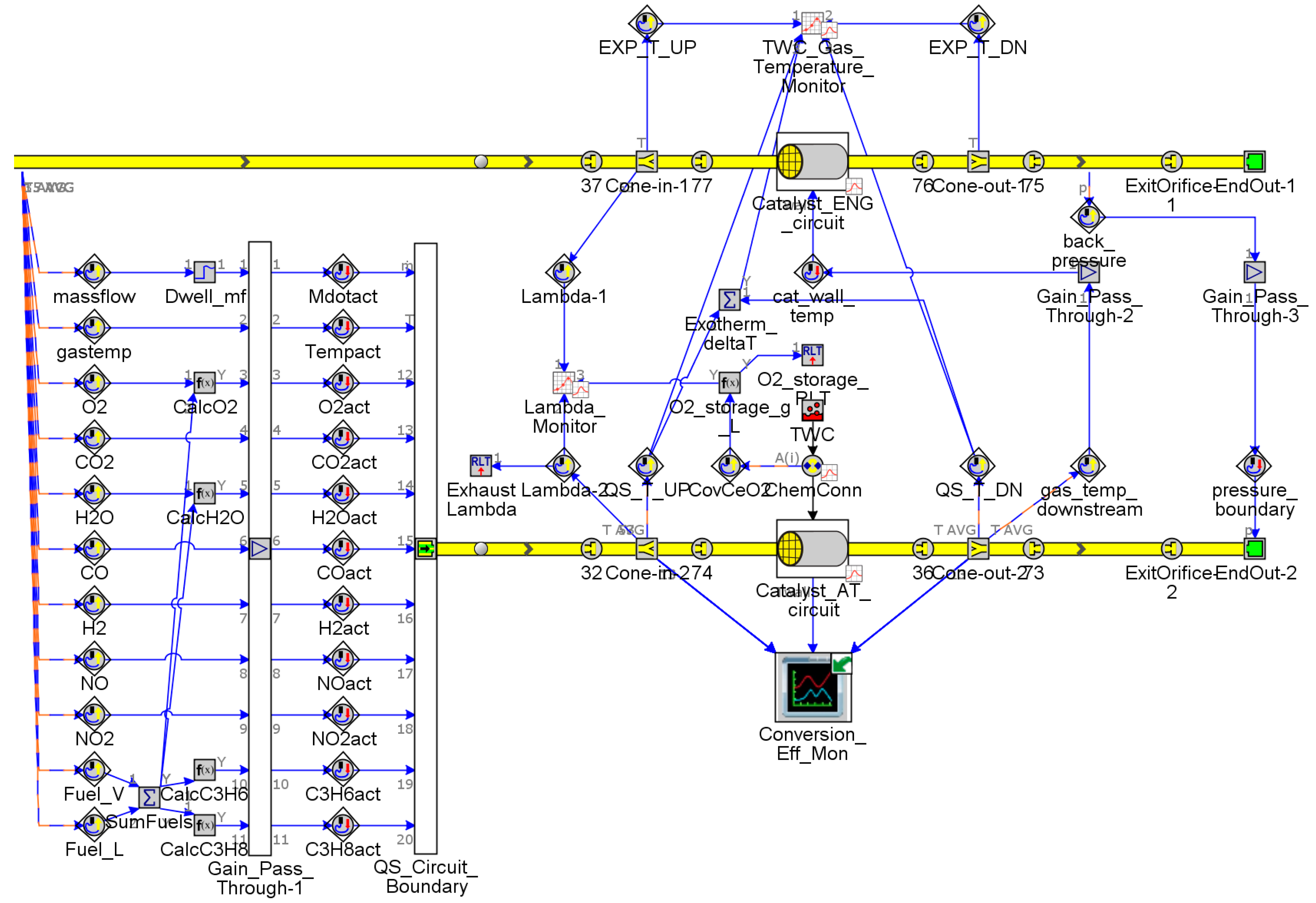
2.2. Aftertreatment System
- C3H6 (faster-oxidizing HC)
- C3H8 (slower-oxidizing HC)
2.3. Design of the Thermal Energy Storage System (TESS)
2.3.1. Problem Description
2.3.2. Geometry
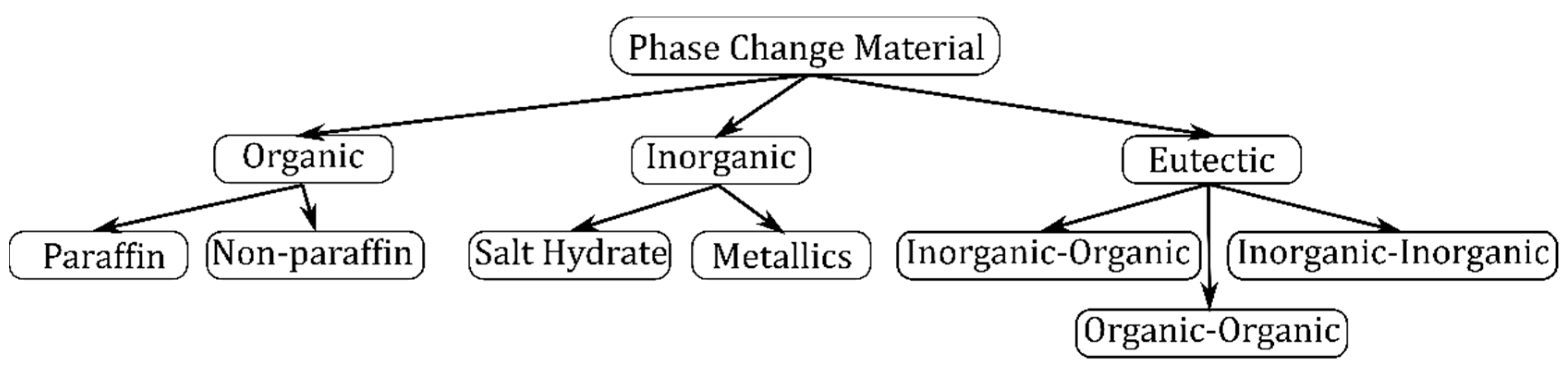
2.3.3. PCM Material Selection
- Chemical stability
- Non-corrosiveness, non-toxic, non-flammable and non-explosion
- No degradation after a large number of cycles
- High density, high specific heat and high thermal conductivity
- Melting in the desired temperature
- High latent heat of fusion
- Small volume change and low vapor pressure
- Abundant
- Available
- Cost-effective
3. Results and Discussion
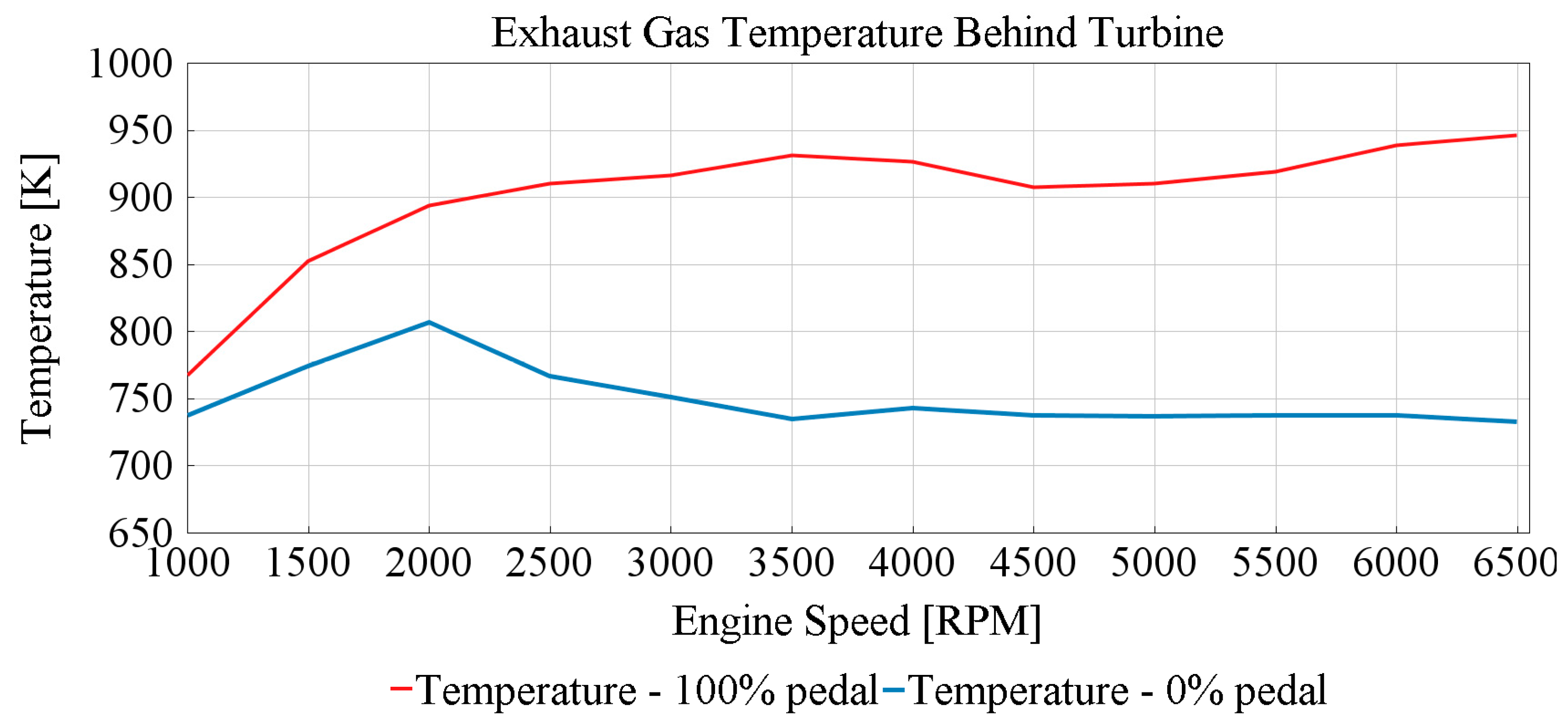
3.1. Exhaust Temperature Pulsations
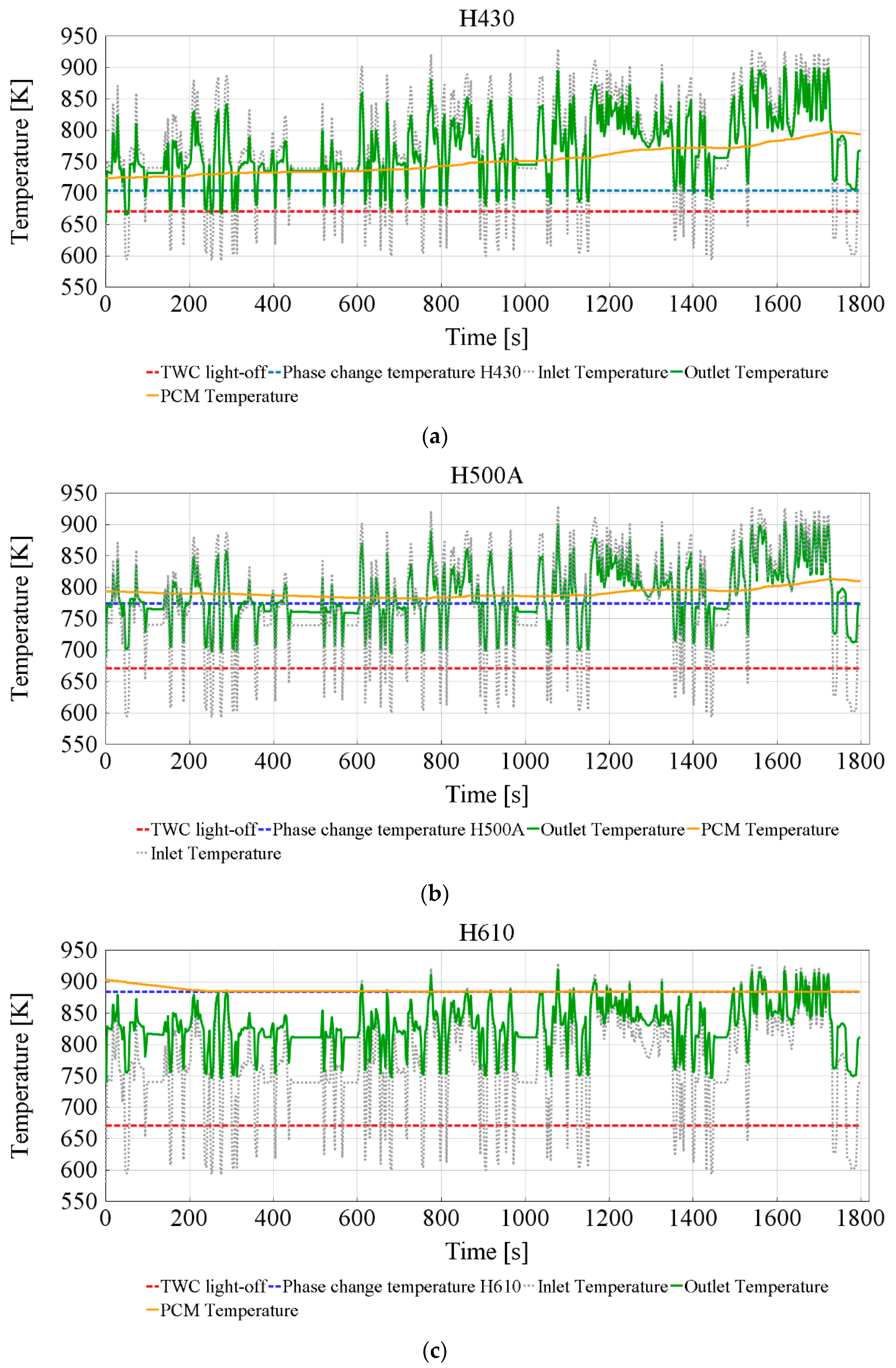
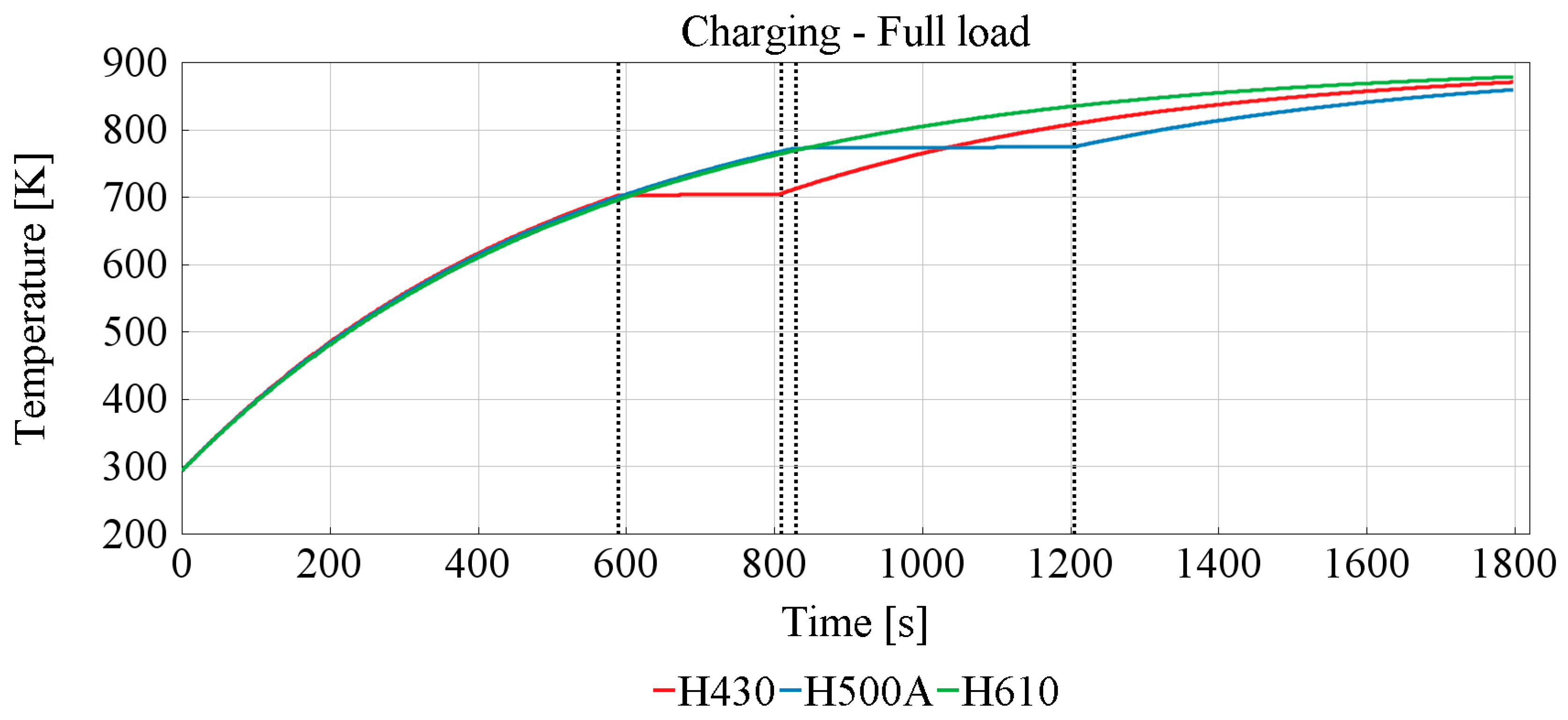
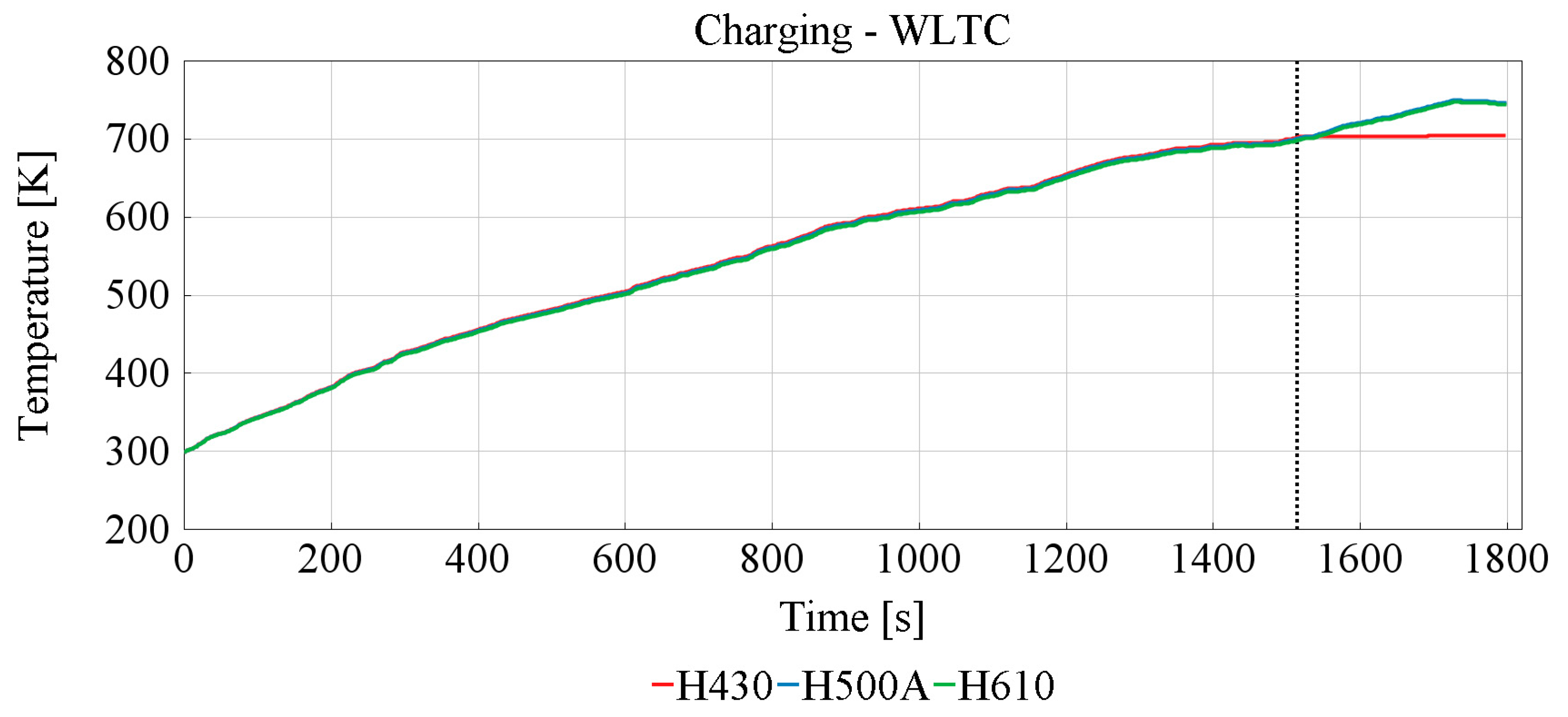
3.2. Effect of the TESS
Charging TESS
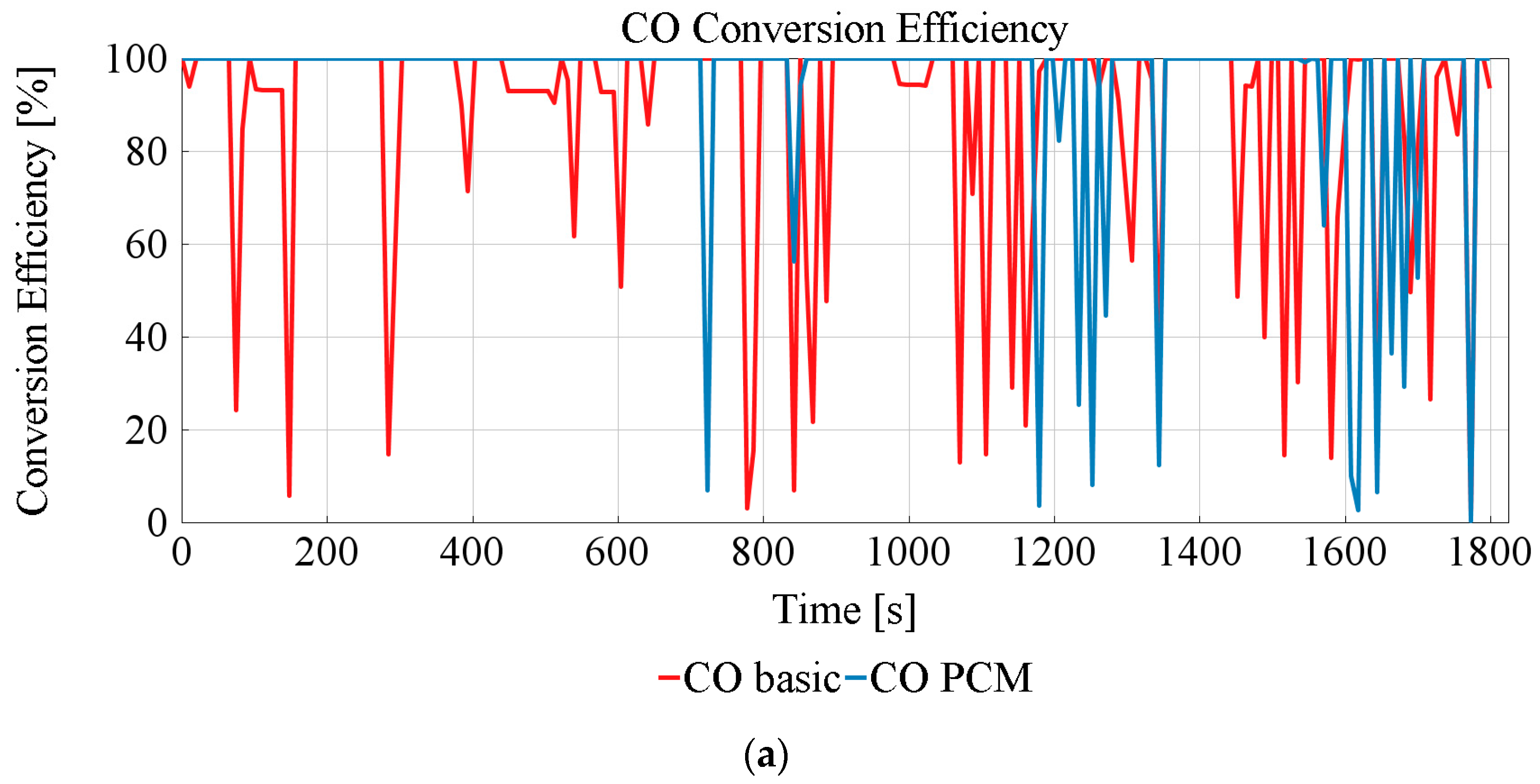
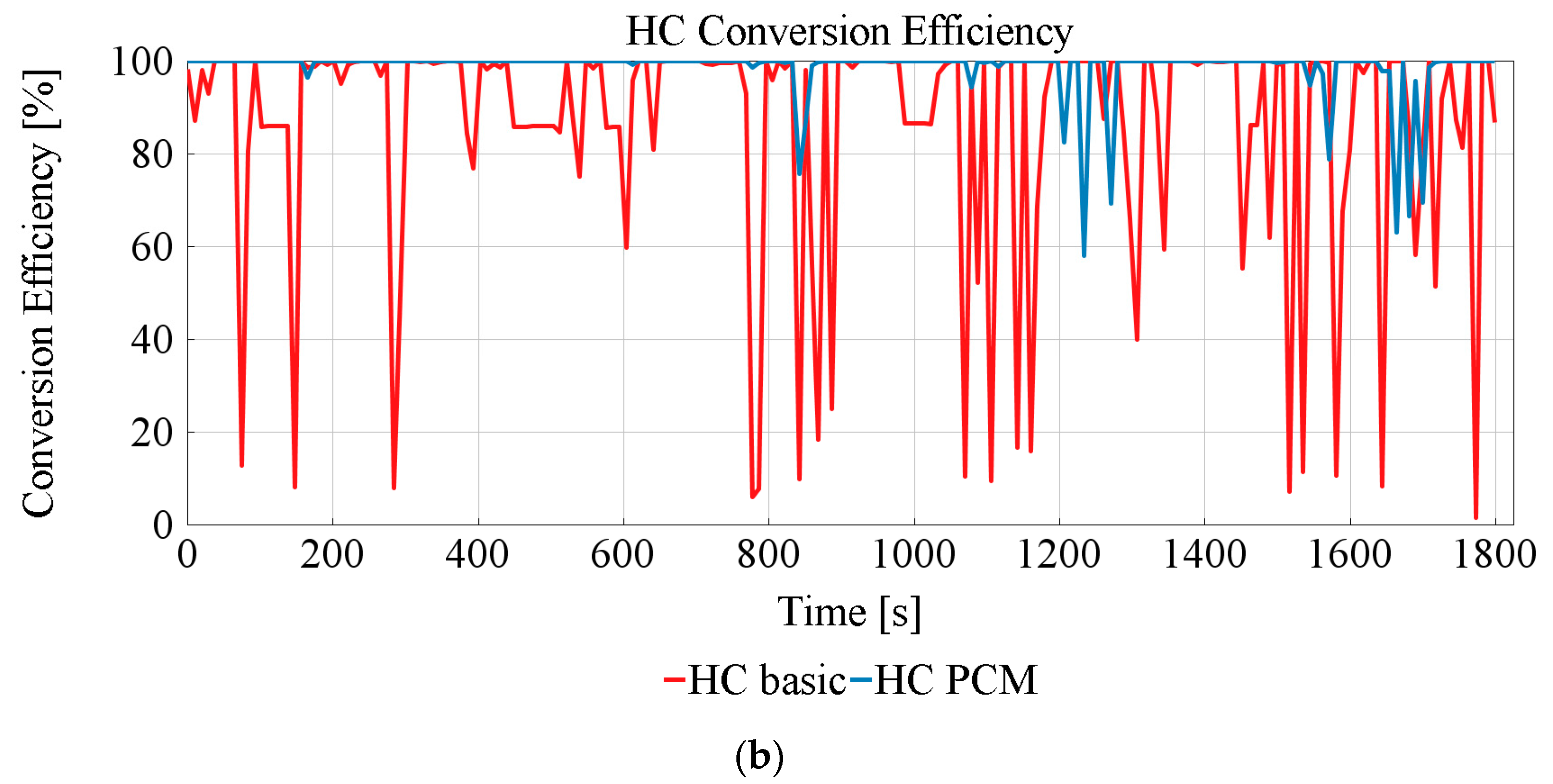
3.3. TWC Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C3H6 | Propene |
| C3H8 | Propane |
| CO | Carbon Monoxide |
| CO2 | Carbon Dioxide |
| H2O | Water |
| HC | Hydrocarbons |
| NOx | Nitrogen Oxides |
| O2 | Oxygen |
| PCM | Phase Change Material |
| TESS | Thermal Energy Storage System |
| TWC | Three-Way Catalyst |
| WLTC | Worldwide-harmonized Light duty vehicle Test Cycle |
References
- European Commission. The European Green Deal; European Commission: Brussels, Belgium, 2019; Volume 53, p. 24. [Google Scholar]
- European Commission. Combined Evaluation Roadmap/Inception Impact Assessment. 2020. Available online: https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/12313-European-vehicle-emissions-standards-Euro-7-for-cars-vans-lorries-and-buses (accessed on 19 February 2022).
- Guillen, N.; Rico-Pérez, V.; García, A.G.; Lozano-Castello, D.; Bueno-López, A. Three-way catalysts: Past, present and future. Edición Espec. 2012, 79, 114–121. Available online: https://www.researchgate.net/publication/260407090 (accessed on 19 February 2022).
- Koltsakis, G.C.; Stamatelos, A.M. Catalytic automotive exhaust aftertreatment. Prog. Energy Combust. Sci. 1997, 23, 1–39. [Google Scholar]
- Macián, V.; Monsalve-Serrano, J.; Villalta, D.; Fogué-Robles, Á. Extending the potential of the dual-mode dual-fuel combustion towards the prospective EURO VII emissions limits using gasoline and OMEx. Energy Convers. Manag. 2021, 233, 113927. [Google Scholar] [CrossRef]
- Duan, X.; Liu, Y.; Zhou, X.; Zou, P.; Liu, J. Experimental investigation energy balance and distribution of a turbocharged GDI engine fuelled with ethanol and gasoline blend under transient and steady-state operating conditions. Therm. Sci. 2020, 24, 243–257. [Google Scholar] [CrossRef]
- Pan, M.; Qian, W.; Wei, H.; Feng, D.; Pan, J. Effects on performance and emissions of gasoline compression ignition engine over a wide range of internal exhaust gas recirculation rates under lean conditions. Fuel 2019, 265, 116881. [Google Scholar] [CrossRef]
- Shahbakhti, M.; Ghazimirsaied, A.; Koch, C. Experimental study of exhaust temperature variation in a homogeneous charge compression ignition engine. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2010, 224, 1177–1197. [Google Scholar] [CrossRef]
- Twigg, M.V. Progress and future challenges in controlling automotive exhaust gas emissions. Appl. Catal. B Environ. 2007, 70, 2–15. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Inambao, F.L. Effect of cold start emissions from gasoline-fueled engines of light-duty vehicles at low and high ambient temperatures: Recent trends. Case Stud. Therm. Eng. 2019, 14, 100417. [Google Scholar] [CrossRef]
- Hamedi, M.; Doustdar, O.; Tsolakis, A.; Hartland, J. Thermal energy storage system for efficient diesel exhaust aftertreatment at low temperatures. Appl. Energy 2018, 235, 874–887. [Google Scholar] [CrossRef]
- Johar, D.K.; Sharma, D.; Soni, S.L.; Gupta, P.K.; Goyal, R. Experimental investigation on latent heat thermal energy storage system for stationary C.I. engine exhaust. Appl. Therm. Eng. 2016, 104, 64–73. [Google Scholar] [CrossRef]
- Gong, C.; Si, X.; Liu, F. Combustion and emissions behaviors of a stoichiometric GDI engine with simulated EGR (CO2) at low load and different spark timings. Fuel 2021, 295, 120614. [Google Scholar] [CrossRef]
- Tu, R.; Xu, J.; Wang, A.; Zhai, Z.; Hatzopoulou, M. Effects of ambient temperature and cold starts on excess NOx emissions in a gasoline direct injection vehicle. Sci. Total Environ. 2020, 760, 143402. [Google Scholar] [CrossRef] [PubMed]
- Pielecha, J.; Skobiej, K.; Kurtyka, K. Exhaust Emissions and Energy Consumption Analysis of Conventional, Hybrid, and Electric Vehicles in Real Driving Cycles. Energies 2020, 13, 6423. [Google Scholar] [CrossRef]
- Jaguemont, J.; Omar, N.; Van den Bossche, P.; Mierlo, J. Phase-change materials (PCM) for automotive applications: A review. Appl. Therm. Eng. 2018, 132, 308–320. [Google Scholar] [CrossRef]
- Charvat, P.; Stetina, J.; Pech, O.; Klimes, L.; Ostry, M. Experimental investigation of stabilization of flowing water temperature with a water-PCM heat exchanger. EPJ Web Conf. 2014, 67, 02046. [Google Scholar] [CrossRef]
- Korin, E.; Reshef, R.; Tshernichovesky, D.; Sher, E. Reducing cold-start emission from internal combustion engines by means of a catalytic converter embedded in a phase-change material. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 1999, 213, 575–583. [Google Scholar] [CrossRef]
- Kaltakkıran, G.; Ceviz, M.A. The performance improvement of direct injection engines in cold start conditions integrating with phase change material: Energy and exergy analysis. J. Energy Storage 2021, 42, 102895. [Google Scholar] [CrossRef]
- Pavlovic, J.; Marotta, A.; Ciuffo, B. CO2 emissions and energy demands of vehicles tested under the NEDC and the new WLTP type approval test procedures. Appl. Energy 2016, 177, 661–670. [Google Scholar] [CrossRef]
- Tsiakmakis, S.; Fontaras, G.; Cubito, C.; Pavlovic, J.; Anagnostopoulos, K.; Ciuffo, B. From NEDC to WLTP: Effect on the Type-Approval CO2 Emissions of Light-Duty Vehicles; Publications Office of the European Union: Luxembourg, 2017; p. 50. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Fu, J.; Liu, J.; Dong, H. Numerical research on the performance, combustion and energy flow characteristics of gasoline-powered vehicle under WLTC. Fuel 2020, 285, 119135. [Google Scholar] [CrossRef]
- Demmelbauer-Ebner, W.; Persigehl, K.; Görke, M.; Werstat, E. The New 1.5-l Four-cylinder TSI Engine from Volkswagen. MTZ Worldw. 2017, 78, 16–23. [Google Scholar] [CrossRef]
- Steiner, T.; Neurauter, D.; Moewius, P.; Pfeifer, C.; Schallhart, V.; Moeltner, L. Heat-Up Performance of Catalyst Carriers—A Parameter Study and Thermodynamic Analysis. Energies 2021, 14, 964. [Google Scholar] [CrossRef]
- Chatterjee, D.; Deutschmann, O.; Warnatz, J. Detailed surface reaction mechanism in a three-way catalyst. Faraday Discuss. 2001, 119, 371–384. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Zhang, M.; Xu, H.; Ma, X.; Shuai, S. Exhaust non-volatile particle filtration characteristics of three-way catalyst and influencing factors in a gasoline direct injection engine compared to gasoline particulate filter. Fuel 2021, 290, 120065. [Google Scholar] [CrossRef]
- Majumdar, S.S.; Pihl, J.A.; Toops, T.J. Reactivity of novel high-performance fuels on commercial three-way catalysts for control of emissions from spark-ignition engines. Appl. Energy 2019, 255, 113640. [Google Scholar] [CrossRef]
- Nandi, S.; Arango, P.; Chaillou, C.; Dujardin, C.; Granger, P.; Laigle, E.; Nicolle, A.; Norsic, C.; Richard, M. Relationship between design strategies of commercial three-way monolithic catalysts and their performances in realistic conditions. Catal. Today 2021, 384–386, 122–132. [Google Scholar] [CrossRef]
- Ramanathan, K.; Sharma, C.S. Kinetic Parameters Estimation for Three Way Catalyst Modeling. Ind. Eng. Chem. Res. 2011, 50, 9960–9979. [Google Scholar] [CrossRef]
- Gamma Technologies. GT-SUITE: Exhaust Aftertreatment Application Manual; Gamma Technologies: Westmont, IL, USA, 2020. [Google Scholar]
- Kim, K.-B.; Choi, K.-W.; Kim, Y.-J.; Lee, K.-H.; Lee, K.-S. Feasibility study on a novel cooling technique using a phase change material in an automotive engine. Energy 2010, 35, 478–484. [Google Scholar] [CrossRef]
- Dong, D.; Moriyoshi, Y.; Kuboyama, T. Effect of porous material as heat storage medium on fuel consumption in a turbocharged gasoline engine. Int. J. Engine Res. 2020, 22, 1551–1564. [Google Scholar] [CrossRef]
- Stritih, U. An experimental study of enhanced heat transfer in rectangular PCM thermal storage. Int. J. Heat Mass Transf. 2004, 47, 2841–2847. [Google Scholar] [CrossRef]
- Siddiqui, F.; Tso, C.Y.; Chan, K.C.; Fu, S.C.; Chao, C.Y. On trade-off for dispersion stability and thermal transport of Cu-Al2O3 hybrid nanofluid for various mixing ratios. Int. J. Heat Mass Transf. 2018, 132, 1200–1216. [Google Scholar] [CrossRef]
- Hosseinzadeh, K.; Moghaddam, M.E.; Asadi, A.; Mogharrebi, A.; Jafari, B.; Hasani, M.; Ganji, D. Effect of two different fins (longitudinal-tree like) and hybrid nano-particles (MoS2-TiO2) on solidification process in triplex latent heat thermal energy storage system. Alex. Eng. J. 2020, 60, 1967–1979. [Google Scholar] [CrossRef]
- Sarkar, J.; Ghosh, P.; Adil, A. A review on hybrid nanofluids: Recent research, development and applications. Renew. Sustain. Energy Rev. 2015, 43, 164–177. [Google Scholar] [CrossRef]
- Geete, P.; Kumar, N.; Somani, S.K.; Gangele, A. Simulation of Double Pipe Heat Exchanger with Implementation of PCM. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 658–663. [Google Scholar] [CrossRef]
- Hosseinzadeh, K.; Moghaddam, M.E.; Asadi, A.; Mogharrebi, A.; Ganji, D. Effect of internal fins along with Hybrid Nano-Particles on solid process in star shape triplex Latent Heat Thermal Energy Storage System by numerical simulation. Renew. Energy 2020, 154, 497–507. [Google Scholar] [CrossRef]
- Lone, M.I.; Jilte, R. A review on phase change materials for different applications. Mater. Today Proc. 2021, 46, 10980–10986. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]

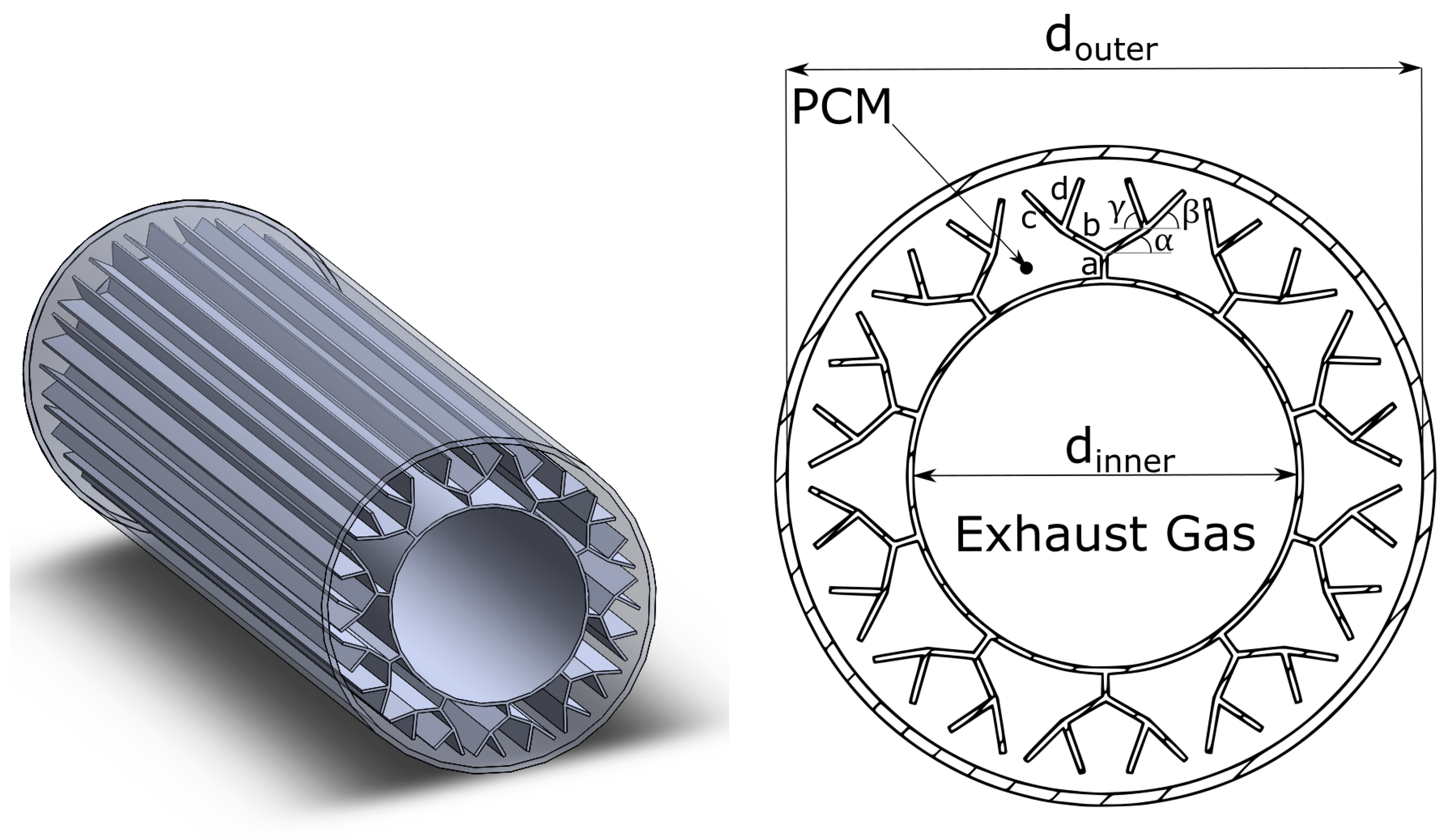


| Dimension | Unit | Value |
|---|---|---|
| [mm] | 64 | |
| [mm] | 108 | |
| [mm] | 5 | |
| [mm] | 8 | |
| [mm] | 9 | |
| [mm] | 9 | |
| [°] | 30 | |
| [°] | 45 | |
| [°] | 70 | |
| l | [mm] | 500 |
| Variable | H430 | H500A | H610 |
|---|---|---|---|
| Phase change temperature [°C] | 430 | 500 | 610 |
| Density [kg/m3] | 2160 | 2140 | 2070 |
| Latent Heat Capacity [kJ/kg] | 125 | 140 | 410 |
| Volumetric Heat Capacity [MJ/m3] | 270 | 300 | 849 |
| Specific Heat Capacity [J/kg K] | 1540 | 1555 | 1570 |
| Thermal Conductivity [W/m K] | 0.568 | 0.567 | 0.561 |
| Maximum Temperature [°C] | 1400 | 1400 | 1300 |
| Sample | Reaching Phase Change Temperature [s] | Phase Change Duration [s] |
|---|---|---|
| H430 | 600 | 200 |
| H500A | 830 | 380 |
| H610 | NaN | NaN |
| Sample | Reaching Phase Change Temperature [s] | Phase Change Duration [s] |
|---|---|---|
| H430 | 1509 | NaN |
| H500A | NaN | NaN |
| H610 | NaN | NaN |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohm, M.; Stetina, J.; Svida, D. Exhaust Gas Temperature Pulsations of a Gasoline Engine and Its Stabilization Using Thermal Energy Storage System to Reduce Emissions. Energies 2022, 15, 2365. https://doi.org/10.3390/en15072365
Bohm M, Stetina J, Svida D. Exhaust Gas Temperature Pulsations of a Gasoline Engine and Its Stabilization Using Thermal Energy Storage System to Reduce Emissions. Energies. 2022; 15(7):2365. https://doi.org/10.3390/en15072365
Chicago/Turabian StyleBohm, Michael, Josef Stetina, and David Svida. 2022. "Exhaust Gas Temperature Pulsations of a Gasoline Engine and Its Stabilization Using Thermal Energy Storage System to Reduce Emissions" Energies 15, no. 7: 2365. https://doi.org/10.3390/en15072365
APA StyleBohm, M., Stetina, J., & Svida, D. (2022). Exhaust Gas Temperature Pulsations of a Gasoline Engine and Its Stabilization Using Thermal Energy Storage System to Reduce Emissions. Energies, 15(7), 2365. https://doi.org/10.3390/en15072365






