Refueling of LH2 Aircraft—Assessment of Turnaround Procedures and Aircraft Design Implication
Abstract
:1. Introduction
2. State of the Art
2.1. Refueling of Jet A-1
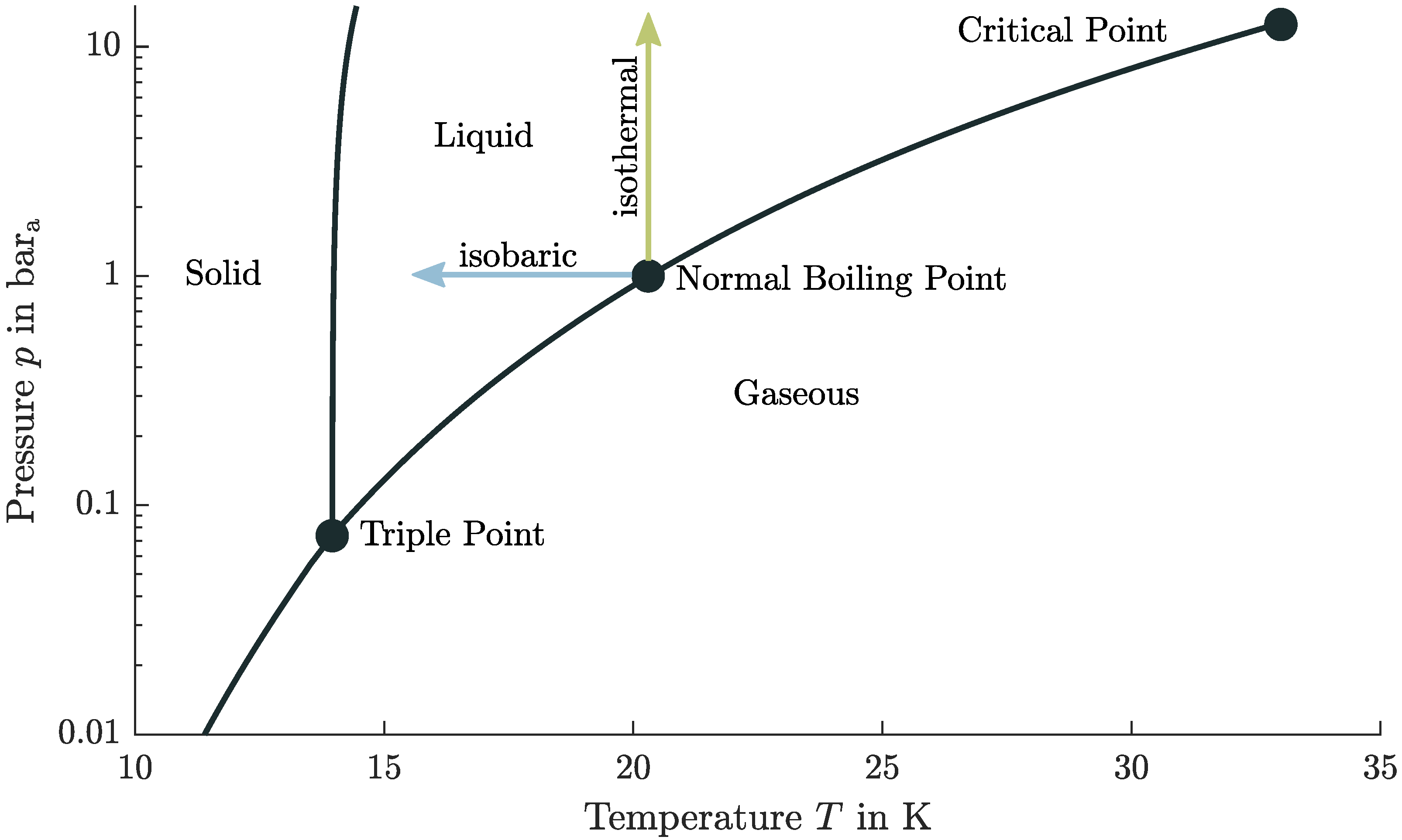
2.2. Properties of Liquid Hydrogen
2.3. Handling Options for Hydrogen
2.4. Non-Aircraft Liquid Hydrogen Refueling Applications
2.4.1. Automotive Industry
2.4.2. Space Shuttle Loading
2.4.3. Spacex Falcon 9 Loading
3. Methodology
- Docking maneuver.
- Connecting and purging.
- Chill-down and recovery line.
- Actual refueling mass flow.
- Steps afterwards.
3.1. Explosion Protection Regulations
3.2. Feed System
3.2.1. Cryogenic Pump System and Design Parameters
3.2.2. Considerations for a Pressurized Gas Feed System
3.3. Boiling Heat Transfer and Chill-Down Time
3.4. Evacuation and Pressurization Time for Purging
3.4.1. Calculation of Time Required for Evacuation
3.4.2. Calculation of Time Required for Pressurization
3.5. Calculation of GH2 Recovery Line
3.6. Mass and Loss of Vaporized Hydrogen through Chill-Down
3.7. Evaluation for Pressure Losses through Pipe Flow
3.8. First Law of Thermodynamics for Open System
3.9. Effects of Two-Phase Flow on Refueling
3.10. Liquid Hydrogen Aircraft Tank Volume
3.11. Performance Calculation Method for Subcooling of Liquid Hydrogen
3.12. Procedure of Refueling
3.12.1. Docking Maneuver
3.12.2. Connecting and Purging
3.12.3. Chill-Down of Hose and Reduced Mass Flow
3.13. Definition of GH2 Recovery Line
3.13.1. Fueling Mass Flow of Liquid Hydrogen
3.13.2. Procedure after the Refueling
4. Liquid Hydrogen Refueling Process and Implications on Turnaround Times
4.1. Results of the Refueling Procedure
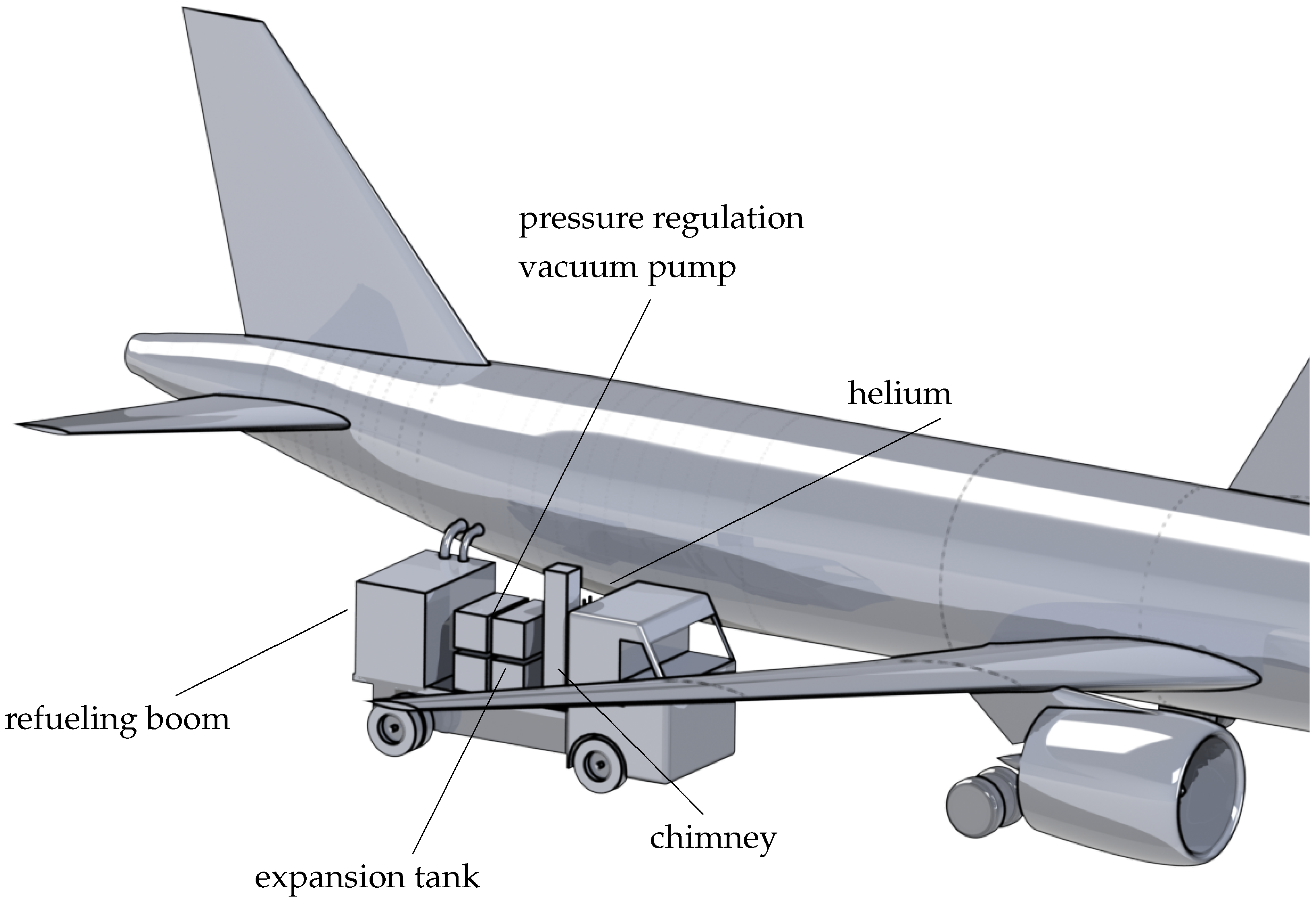
4.2. Airport Distribution System for Liquid Hydrogen
Refueling Tank Truck for Interim Phase
4.3. Pressurized Gas Feed System
4.4. Pump Feed System
4.4.1. Dispenser Truck and Pipeline Supply for Large Quantities
4.4.2. Airport Storage and Distribution Requirements
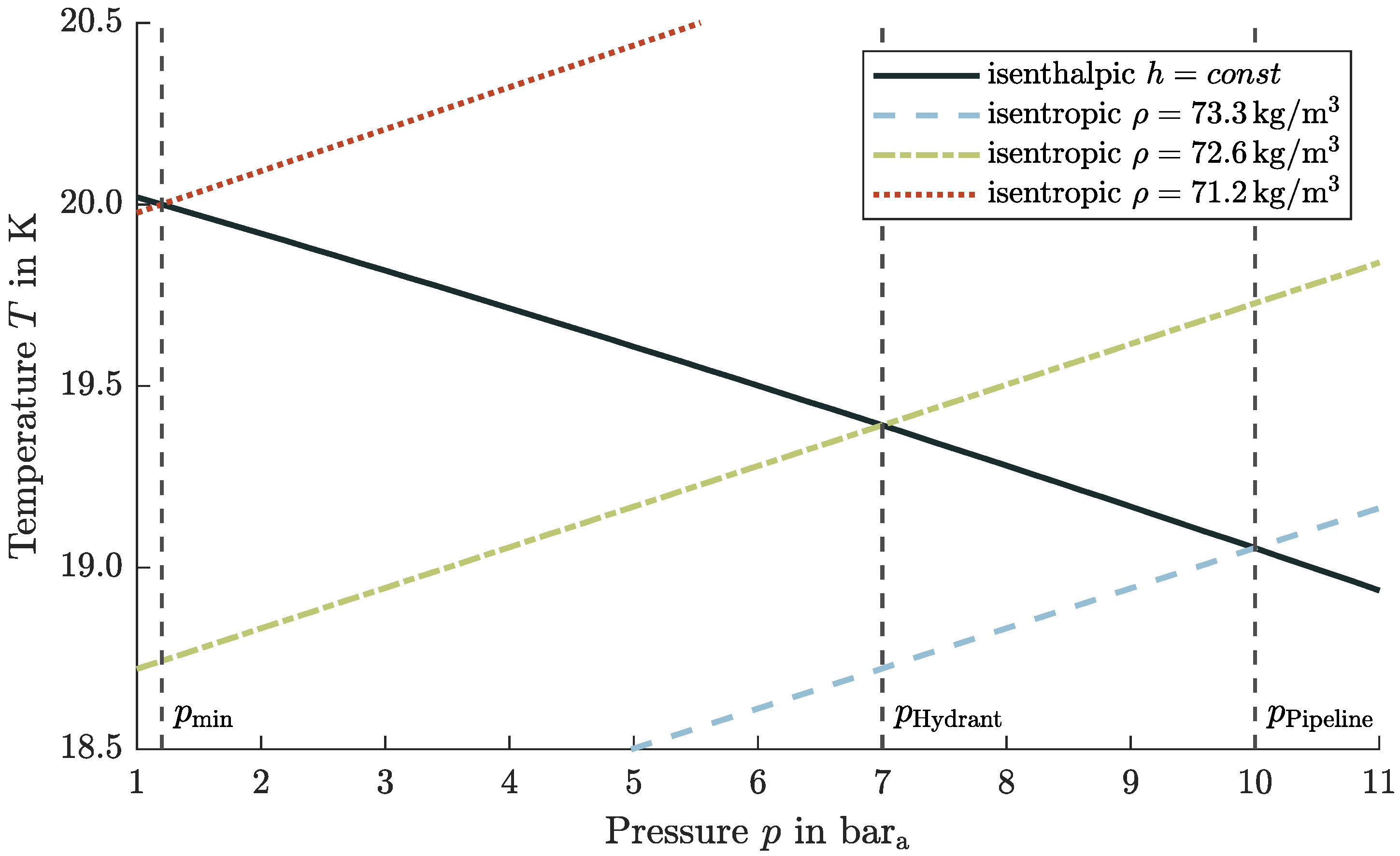
4.5. Subcooling Considerations for LH2 Refueling
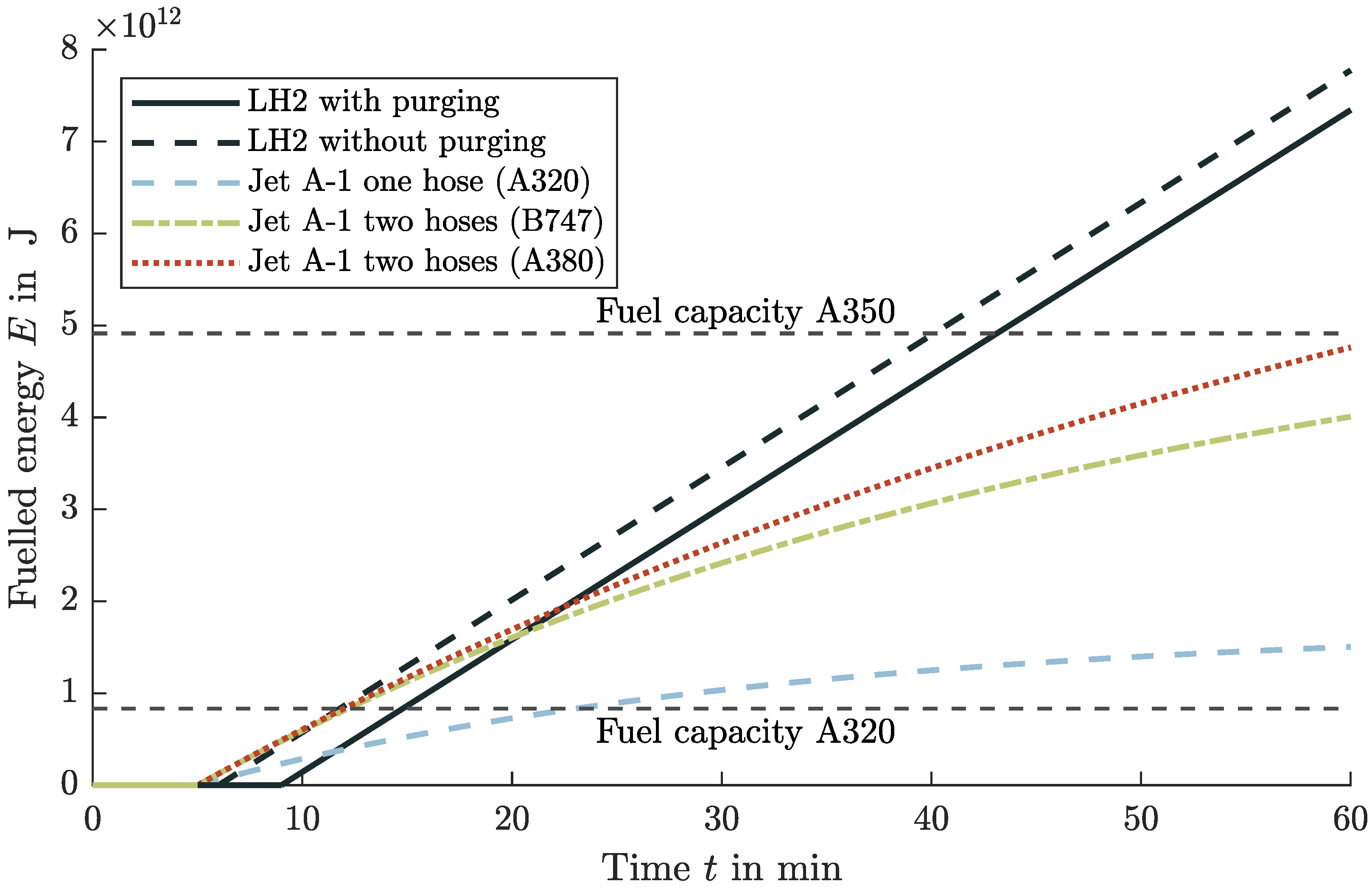
4.6. Comparison and Impacts of Liquid Hydrogen to Conventional Jet A-1 Turnarounds
4.7. Losses and Cost Adaption Due to Refueling with Liquid Hydrogen
5. Implications on Liquid Hydrogen Aircraft Design
5.1. Liquid Hydrogen Aircraft Tank Volume
5.2. Feeding Hydrogen from the Aircraft Tank to the Power Source
5.2.1. Fuel Cell
5.2.2. Combustion Engine
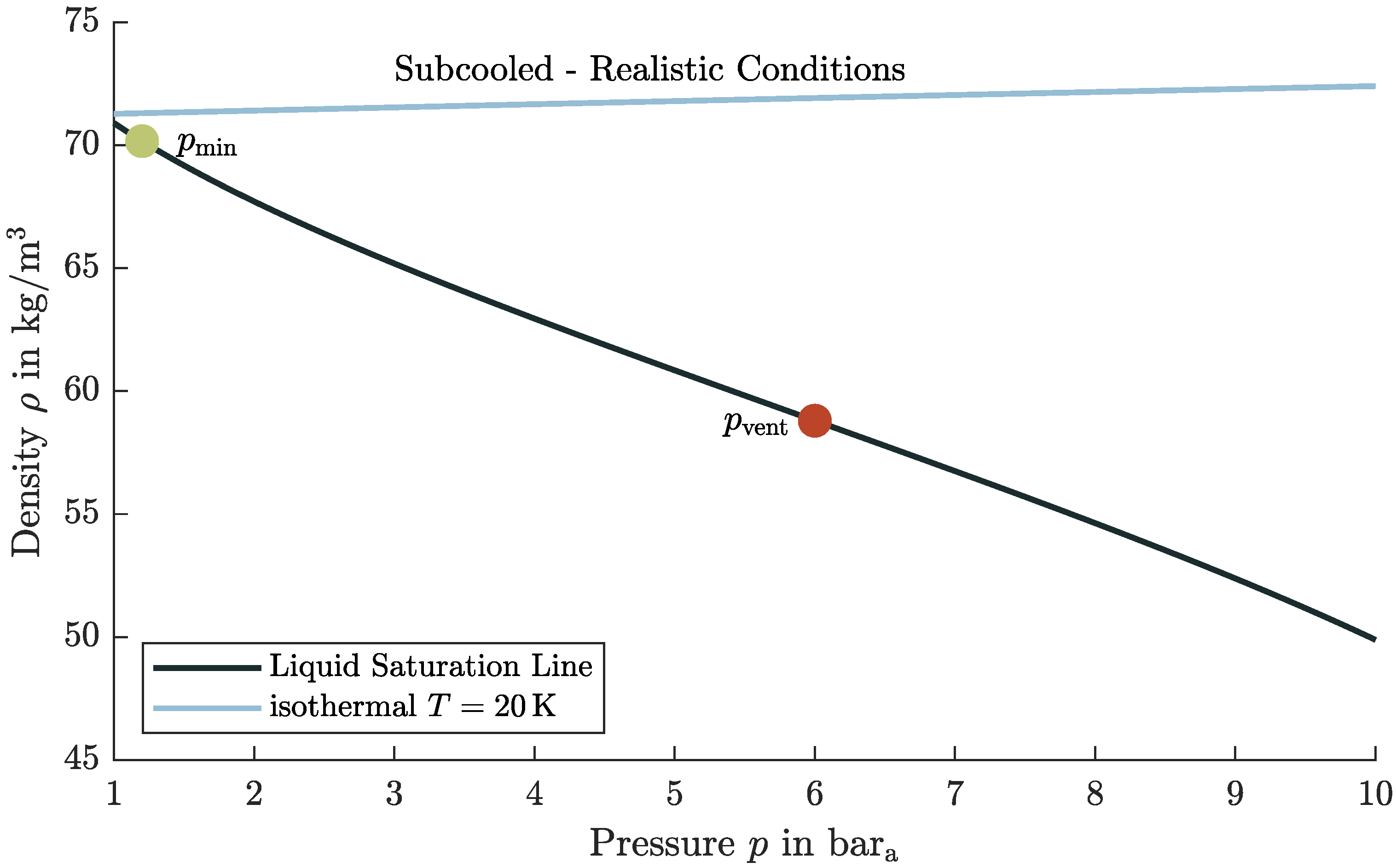
5.3. Realistic Tank Conditions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Abbreviations | ||
| CO | Carbon Dioxide | |
| COP | Coefficient of Performance | |
| DLR | German Aerospace Center | |
| DOC | Direct Operating Cost | |
| ET | External Tank | |
| EU | European Union | |
| GH2 | Gaseous Hydrogen | |
| H | Hydrogen (independent of the phase state) | |
| LFL | Lower Flammability Limit | |
| LH2 | Liquid Hydrogen | |
| LHV | Lower Heating Value | |
| LOX | Liquid Oxygen | |
| LPFTP | Low Pressure Fuel Turbo Pump | |
| NBP | Normal Boiling Point | |
| NPSH | Net Positive Suction Head | |
| NPSHa | Net Positive Suction Head available | |
| NPSHc | Net Positive Suction Head critical | |
| O | Oxygen | |
| ppm | parts per million | |
| PtL | Power to Liquid | |
| SSME | Space Shuttle Main Engine | |
| TRBS | Technical Rules for Operational Safety | |
| TRGS | Technical Rules for Hazardous Substances | |
| UFL | Upper Flammability Limit | |
| VIP | Vacuum Insulated Pipe | |
| Symbols | ||
| Refueling factor of Jet A-1 | / | |
| Heat of vaporization | / | |
| Heat capacity ratio | - | |
| Friction factor | - | |
| Dynamic viscosity | ||
| Kinematic viscosity | / | |
| ℜ | Universal gas constant | // |
| Density | / | |
| Surface tension | / | |
| A | Area | |
| Biot number | - | |
| c | Concentration | - |
| Heat capacity at constant pressure | // | |
| d | Diameter | |
| g | Gravitational acceleration | / |
| Effective Heat of vaporization | / | |
| H | Head | |
| h | Specific enthalpy | / |
| h | heat-transfer coefficient | // |
| i | Number of | - |
| k | Roughness height | |
| k | Thermal conductivity | // |
| Flow coefficient of the valve | / | |
| l | Length | |
| Laplace length | ||
| M | Mach number | - |
| m | Mass | |
| n | Amount of substance | |
| n | Rotational speed | / |
| P | Power | |
| p | Pressure | bar |
| Prandtl number | - | |
| Q | Heat | |
| Q | Volume flow | / |
| q | Heat flow per area | / |
| R | Gas constant | // |
| Reynolds number | - | |
| S | Pumping speed | / |
| s | Specific entropy | / |
| T | Temperature | |
| t | Time | |
| V | Volume | |
| v | Velocity | / |
| x | Vapor fraction | - |
| Subscripts | ||
| 0 | initial condition of gas tank | |
| a | absolute | |
| avg | average | |
| b | bulk | |
| boil | boiling | |
| c | characteristic | |
| conv | convective | |
| el | electrical | |
| g | gas | |
| g | gauge (difference to ambient) | |
| i | inner | |
| l | liquid | |
| min | minimum | |
| o | outer | |
| s | specific | |
| sat | saturated | |
| spl | single phase liquid | |
| spv | single phase vapor | |
| ss | suction specific | |
| v | vapor | |
| vap | vaporized | |
| vent | venting |
References
- McKinsey & Company for the Clean Sky 2 JU and Fuel Cells and Hydrogen 2 Joint Undertaking. Hydrogen-Powered Aviation: A Fact-Based Study of Hydrogen Technology, Economics, and Climate Impact by 2050; McKinsey Company: Redwood City, CA, USA, 2020. [Google Scholar]
- Verstraete, D.; Hendrick, P.; Pilidis, P.; Ramsden, K. Hydrogen fuel tanks for subsonic transport aircraft. Int. J. Hydrog. Energy 2010, 35, 11085–11098. [Google Scholar] [CrossRef]
- Klell, M.; Eichlseder, H.; Trattner, A. Wasserstoff in der Fahrzeugtechnik: Erzeugung, Speicherung, Anwendung, 4th ed.; Springer Vieweg: Wiesbaden, Germany, 2018. [Google Scholar]
- Silberhorn, D.; Hartmann, J.; Dzikus, N.M.; Atanasov, G.; Zill, T.; Brand, U.; Trillos, J.C.G.; Oswald, M.; Vogt, T.; Wilken, D.; et al. The Air-Vehicle as a Complex System of Air Transport Energy Systems. In Proceedings of the AIAA AVIATION 2020 FORUM, Vitual Event, 15–19 June 2020. [Google Scholar]
- Silberhorn, D.; Atanasov, G.; Walther, J.N.; Zill, T. Assessment of Hydrogen Fuel Tank Integration at Aircraft Level. In Proceedings of the Deutscher Luft- und Raumfahrtkongress 2019, Darmstadt, Germany, 30 September–2 October 2019. [Google Scholar]
- World Economic Forum. Clean Skies for Tomorrow: Sustainable Aviation Fuels as a Pathway to Net-Zero Aviation; World Economic Forum CH-1223: Cologny/Geneva, Switzerland, 2020. [Google Scholar]
- AIRBUS, S.A.S. A320 Aircraft Characteristics—Airport and Maintenance Planning; Customer Services, Technical Data Support and Services; AIRBUS S.A.S.: Blagnac, France, 2020. [Google Scholar]
- Ahd Lf/Hf Hydrant Dispenser. 2020. Available online: https://www.rohr-spezialfahrzeuge.com/fileadmin/user_upload/Produkte/03_Flugfeldtankfahrzeuge/Datenblaetter/Produktdatenblatt_AHD_EN.pdf (accessed on 15 November 2020).
- Simon, V.; Weigand, B.; Gomaa, H. Dimensional Analysis for Engineers, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- The Boeing Commercial Airplane Company. An Exploratory Study to Determine the Integrated Technological Air Tranportation Sytem Ground Requirements of Liquid-Hydrogen-Fueled Subsonic, Long-Haul Civil Air Transports; NASA CR 2699; National Aeronautics and Space Administration: Washington, DC, USA, 1976. [Google Scholar]
- Brewer, G.D. LH2 Airport Requirments Study; NASA CR 2700; National Aeronautics and Space Administration: Washington, DC, USA, 1976. [Google Scholar]
- Brewer, G.D.; Morris, R.E.; Lange, R.H.; Moore, J.W. Volume 2 Final Report: Study of the Application of Hydrogen Fuel to Long-Range Subsonic Transport Aircraft; NASA CR 132559; National Aeronautics and Space Administration: Washington, DC, USA, 1975. [Google Scholar]
- Sosounov, V.; Orlov, V. Experimental Turbofan Using Liquid Hydrogen and Liquid Natural Gas as Fuel. In Proceedings of the AIAA—26th Joint Propulsion Conference, Orlando, FL, USA, 16–18 July 1990. [Google Scholar]
- Airbus Deutschland GmbH. Liquid Hydrogen Fuelled Aircraft—System Analysis; Technical Report; Airbus Deutschland GmbH: Hamburg, Germany, 2013. [Google Scholar]
- Brewer, G.D. Hydrogen Aircraft Technology, 1st ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Brewer, G.D.; Morris, R.E.; Davis, G.W.; Versaw, E.F.; Cunnington, G.R., Jr.; Riple, J.C.; Baerst, C.F.; Baerst, C.F. Study of Fuel System for LH2-Fueled Subsonic Transport Aircraft; NASA CR 145369; NASA-Langley Research Center: Hampton, Virginia, 1978; Volume 1, pp. 1–194. [Google Scholar]
- Brewer, G.D.; Morris, R.E.; Davis, G.W.; Versaw, E.F.; Cunnington, G.R., Jr.; Riple, J.C.; Baerst, C.F.; Baerst, C.F. Study of Fuel System for LH2-Fueled Subsonic Transport Aircraft; NASA Contractor Report 145369; NASA-Langley Research Center: Hampton, Virginia, 1978; Volume 2, pp. 195–524. [Google Scholar]
- Mangold, J. Economical Assessment of Hydrogen Short-Range Aircraft with the Focus on the Turnaround Procedure. Master’s Thesis, University of Stuttgart, Stuttgart, Germany, 2021. [Google Scholar]
- Hoelzen, J.; Flohr, M.; Silberhorn, D.; Mangold, J.; Bensmann, A.; Hanke-Rauschenbach, R. H2-powered aviation at airports—Design and economics of LH2 refueling systems. Energy Convers. Manag. X 2022, 14, 100206. [Google Scholar] [CrossRef]
- American Petroleum Institure. Protection against Ignitions Arising Out of Static, Lightning, and Stray Currents; API RP 2003; Health and Environmental Affairs Department, American Petroleum Institute: Washington, DC, USA, 1998. [Google Scholar]
- Sera, A. Jet Fuel Pipelines And Storage Require Special Operation, Maintenance Considerations. Pipeline Gas J. 2009, 236. [Google Scholar]
- Kazda, A.; Caves, R.E. Airport Design and Operation, 3rd ed.; Emerald Group Publishing Limited: Bingley, UK, 2015. [Google Scholar]
- Coordinating Research Council. Handbook of Aviation Fuel Properties; CRC Report No. 530; Coordinating Research Council: Atlanta, Georgia, 1983. [Google Scholar]
- Parker Hannifin Corporation. Parker Industrial Hose Gold Label® Aircraft Fueling Hose; Parker Hannifin Corporation: Wickliffe, Ohio, 2013. [Google Scholar]
- Eaton Aerospace Group. Carter® Underwing Refueling Nozzle Model 60427; Eaton Aerospace Group: Irvine, CA, USA, 2013. [Google Scholar]
- Bundesanstalt für Arbeitsschutz und Arbeitsmedizin (BAuA). Gefährdungsbeurteilung Bei Physischer Belastung—Die neuen Leitmerkmalmethoden (LMM); Bundesanstalt für Arbeitsschutz und Arbeitsmedizin (BAuA): Dortmund, Germany, 2019. [Google Scholar]
- Horstmeier, T.; de Haan, F. Influence of ground handling on turn round time of new large aircraft. Aircr. Eng. Aerosp. Technol. 2001, 73, 266–271. [Google Scholar] [CrossRef]
- Schmidt, M. Ground-Operational Assessment of Novel Aircraft Cabin Configurations. Ph.D. Thesis, Technical University of Munich, Munich, Germany, 2018. [Google Scholar]
- Sanz de Vicente, S. Ground Handling Simulation with CAST. Master’s Thesis, Hamburg University of Applied Sciences, Hamburg, Germany, 2010. [Google Scholar]
- Office of Safety and Mission Assurance. Safety Standard for Hydrogen and Hydrogen Systems; NASA Technical Memorandum NSS 1740.16; Office of Safety and Mission Assurance: Washington, DC, USA, 1997. [Google Scholar]
- Sutton, G.P.; Biblarz, O. Rocket Propulsion Elements, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Huzel, D.K.; Huang, D.H. Design of Liquid Propellant Rocket Engines; NASA Special Publication 125; National Aeronautics and Space Administration: Washington, DC, USA, 1967. [Google Scholar]
- Leachman, J.W.; Jacobsen, R.T.; Lemmon, E.W.; Penoncello, S.G. Thermodynamic Properties of Cryogenic Fluids, 2nd ed.; International Cryogenics Monograph Series; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Peschka, W. Flüssiger Wasserstoff als Energieträger, 1st ed.; Springer: Vienna, Austria, 1984. [Google Scholar]
- Brechna, H.; Tuttle, W.A.; Stewart, R.G.; Jensen, J.E.; Commission, U.S.A.E.; Laboratory, B.N. Brookhaven National Laboratory Selected Cryogenic Data Notebook; Brookhaven National Laboratory: Upton, NY, USA, 1980. [Google Scholar]
- Lemmon, E.W.; Bell, I.H.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP; Version 10.0; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2018. [Google Scholar]
- Bostock, T.D.; Scurlock, R.G. Low-Loss Storage and Handling of Cryogenic Liquids: The Application of Cryogenic Fluid Dynamics, 2nd ed.; International Cryogenics Monograph Series; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Baehr, H.D.; Kabelac, S. Thermodynamik: Grundlagen und technische Anwendungen, 16th ed.; Springer Vieweg: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Ring, E. Rocket Propellant and Pressurization Systems, 1st ed.; Prentice-Hall, Inc.: Englwood Cliffs, NJ, USA, 1964. [Google Scholar]
- Stroman, R.O.; Schuette, M.W.; Swider-Lyons, K.; Rodgers, J.A.; Edwards, D.J. Liquid hydrogen fuel system design and demonstration in a small long endurance air vehicle. Int. J. Hydrog. Energy 2014, 39, 11279–11290. [Google Scholar] [CrossRef]
- SAE International. Fueling Protocols for Light Duty Gaseous Hydrogen Surface Vehicles; SAE International: Warrendale, PA, USA, 2020. [Google Scholar]
- SAE International. Compressed Hydrogen Surface Vehicle Fueling Connection Devices; SAE International: Warrendale, PA, USA, 2015. [Google Scholar]
- Bain, A. NASA space program experience in hydrogen transportation and handling. Int. J. Hydrog. Energy 1976, 1, 173–188. [Google Scholar] [CrossRef]
- Gerstl, P. Solutions for the LH2 Supply Chain. In Proceedings of the International Hydrogen Forum 2019, PyeongChang, Korea, 9 May 2019. [Google Scholar]
- Tamhankar, S. Terminal Operations for Tube Trailer and Liquid Tanker Filling: Status, Challenges and R & D Needs. In Proceedings of the DOE Hydrogen Transmission and Distribution Workshop, Golden, Colorado, 25–26 February 2014. [Google Scholar]
- Siegel, E. Humanity Is Thoughtlessly Wasting An Essential, Non-Renewable Resource: Helium. Available online: https://www.forbes.com/sites/startswithabang/2019/05/24/humanity-is-thoughtlessly-wasting-an-essential-non-renewable-resource-helium/?sh=3492c76829f8 (accessed on 1 October 2021).
- Stuck, D.E. Liquid Rocket Disconnects, Couplings, Fittings, Fixed Joints, and Seals; NASA Special Publication 8119; National Aeronautics and Space Administration: Cleveland, OH, USA, 1976. [Google Scholar]
- Stewart, W.F. Refueling Considerations For Liquid-Hydrogen Fueled Vehicles; Los Alamos National Lab.: Los Alamos, NM, USA, 1984. [Google Scholar]
- Hettinger, W.; Michel, F.; Ott, P.; Theissen, F. Refueling equipment for liquid hydrogen vehicles. Int. J. Hydrog. Energy 1998, 23, 943–947. [Google Scholar] [CrossRef]
- Linde, A.G. Linde Kupplung und Betankungssystem: Leistung und Meßergebnisse. 2003. Available online: http://www.eihp.org/public/Reports/Final_Report/Sub-Task_Reports/ST3.3/EIHP-Beitrag%20Linde%20Kuppl.pdf (accessed on 20 December 2020).
- NASA Lewis Research Center. Hydrogen Safety Manuel; NASA Technical Memorandum X-5245; National Aeronautics and Space Administration: Washingtion, DC, USA, 1968. [Google Scholar]
- Pehr, K.; Sauermann, P.; Traeger, O.; Bracha, M. Liquid hydrogen for motor vehicles—The world’s first public LH2 filling station. Int. J. Hydrog. Energy 2001, 26, 777–782. [Google Scholar] [CrossRef]
- Peschka, W. Liquid Hydrogen Fuel of the Future, 1st ed.; Springer: Vienna, Austra, 1992. [Google Scholar] [CrossRef]
- Wolf, J. Liquid hydrogen technology for vehicles. In Handbook of Fuel Cells; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Arnold, G.; Wolf, J. Liquid Hydrogen for Automotive Application Next Generation Fuel for FC and ICE Vehicles. TEION KOGAKU (J. Cryog. Supercond. Soc. Jpn.) 2005, 40, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Wetzel, F.J. Improved handling of liquid hydrogen at filling stations: Review of six years’ experience. Int. J. Hydrog. Energy 1998, 23, 339–348. [Google Scholar] [CrossRef]
- Osipov, V.V.; Daigle, M.J.; Muratov, C.B.; Foygel, M.; Smelyanskiy, V.N.; Watson, M.D. Dynamical Model of Rocket Propellant Loading with Liquid Hydrogen. J. Spacecr. Rocket. 2011, 48, 987–998. [Google Scholar] [CrossRef] [Green Version]
- Space Program Operations Contract. Shuttle Crew Operations Manuel. United Space Alliance. 2008. Available online: https://www.nasa.gov/centers/johnson/pdf/390651main_shuttle_crew_operations_manual.pdf (accessed on 20 January 2021).
- Nguyen, K.; Knowles, T.E.; Greene, W.D.; Tomsik, T.M. Propellant Densification for Launch Vehicles: Simulation and Testing. In Proceedings of the 38th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Indianapolis, Indiana, 7–10 July 2002. [Google Scholar]
- Foust, J. NASA Approves “Load-and-Go” Fueling for SpaceX Commercial Crew Launches. Available online: https://spacenews.com/nasa-approves-load-and-go-fueling-for-spacex-commercial-crew-launches/ (accessed on 11 January 2021).
- Notardonato, W.U.; Baik, J.H.; McIntosh, G.E. Operational Testing of Densified Hydrogen Using G-M Refrigeration. AIP Conf. Proc. 2004, 710, 64–74. [Google Scholar]
- Fernholz, T. The “Super Chill” Reason SpaceX Keeps Aborting Launches. Available online: https://qz.com/627430/the-super-chill-reason-spacex-keeps-aborting-launches/ (accessed on 11 January 2021).
- Wilken, J.; Scelzo, M.T.; Peveroni, L. System Study of Slush Propellants for Future European Launch Vehicles; Space Propulsion 2018. Available online: https://elib.dlr.de/120361/1/039_WILKEN_update.pdf (accessed on 15 February 2021).
- Baik, J.H.; T-Raissi, A. R & D processes for increasing density of cryogenic propellants at FSEC. Cryogenics 2004, 44, 451–458. [Google Scholar]
- Space Is Kind Of Cool. Why the Falcon 9 Uses Subcooled Liquid Oxygen. Available online: https://www.youtube.com/watch?v=gHwZk_qEeAY (accessed on 12 January 2021).
- Huzel, D.K.; Huang, D.H. Modern Engineering for Design of Liquid-Propellant Rocket Engines; AIAA: Washington, DC, USA, 1992. [Google Scholar]
- Richtlinie 2014/34/EU des Europäischen Parlaments und des Rates vom 26. Februar 2014 zur Harmonisierung der Rechtsvorschriften der Mitgliedstaaten für Geräte und Schutzsysteme zur bestimmungsgemäßen Verwendung in explosionsgefährdeten Bereichen (Neufassung). Available online: https://eur-lex.europa.eu/legal-content/DE/ALL/?uri=celex%3A32014L0034 (accessed on 12 November 2020).
- Richtlinie 1999/92/EG des Europäischen Parlaments und des Rates vom 16. Dezember 1999 über Mindestvorschriften zur Verbesserung des Gesundheitsschutzes und der Sicherheit der Arbeitnehmer, die Durch Explosionsfähige Atmosphären Gefährdet Werden Können (Fünfzehnte Einzelrichtlinie im Sinne von Artikel 16 Absatz 1 der Richtlinie 89/391/EWG). Available online: https://eur-lex.europa.eu/legal-content/DE/ALL/?uri=CELEX:31999L0092 (accessed on 12 November 2020).
- Verordnung über Sicherheit und Gesundheitsschutz bei der Verwendung von Arbeitsmitteln (Betriebssicherheitsverordnung—BetrSichV). 2015. Available online: https://www.gesetze-im-internet.de/betrsichv_2015/BetrSichV.pdf (accessed on 12 November 2020).
- Verordnung zum Schutz vor Gefahrstoffen (Gefahrstoffverordnung—GefStoffV). 2010. Available online: https://www.gesetze-im-internet.de/gefstoffv_2010/GefStoffV.pdf (accessed on 12 November 2020).
- Technische Regeln für Betriebssicherheit/Gefahrstoffe TRBS 2152 Teil 2/TRGS 722 Vermeidung oder Einschränkung Gefährlicher Explosionsfähiger Atmosphäre. 2012. Available online: https://www.baua.de/DE/Angebote/Rechtstexte-und-Technische-Regeln/Regelwerk/TRGS/pdf/TRGS-722.pdf?__blob=publicationFile&v=3 (accessed on 24 November 2020).
- Technische Regeln für Gefahrstoffe TRGS 720 Gefährliche explosionsfähige Gemische—Allgemeines. 2020. Available online: https://www.baua.de/DE/Angebote/Rechtstexte-und-Technische-Regeln/Regelwerk/TRGS/pdf/TRGS-720.pdf?__blob=publicationFile&v=5 (accessed on 14 October 2020).
- Technische Regeln für Betriebssicherheit/ Gefahrstoffe TRBS 2151 Teil 1/ TRGS 721 Gefährliche explosionsfähige Atmosphäre—Beurteilung der Explosionsgefährdung. 2006. Available online: https://www.baua.de/DE/Angebote/Rechtstexte-und-Technische-Regeln/Regelwerk/TRGS/pdf/TRGS-721.pdf?__blob=publicationFile&v=6 (accessed on 1 October 2020).
- Jallais, S. Pre-Normative Research for Safe use of Liquid HYdrogen (PRESLHY); Fuel Cells and Hydrogen Joint Undertaking. 2018. Available online: https://www.hysafe.info/wp-content/uploads/sites/3/2018/09/PRESLHY-D2.3-LH2-Installation-description.pdf (accessed on 23 January 2021).
- Gülich, J.F. Kreiselpumpen: Handbuch für Entwicklung, Anlagenplanung und Betrieb, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Sobin, A.J.; Bissell, W.R. Turbopump Systems For Liquid Rocket Engines; NASA Special Publication 8107; National Aeronautics and Space Administration: Cleveland, OH, USA, 1974. [Google Scholar]
- Rocketdyne. Space Shuttle Main Engine Orientation. 1998. Available online: http://large.stanford.edu/courses/2011/ph240/nguyen1/docs/SSME_PRESENTATION.pdf (accessed on 5 December 2020).
- DiStefano, J.F.; Caine, G.H. Cavitation Characteristics of Tank-Mounted Cryogenuc Pumps and Their Predicted Performance Unter Reduced Gravity; Advances in Cryogenic Engineering; Timmerhaus, D.K., Ed.; Springer: Boston, MA, USA, 1961; Volume 7. [Google Scholar]
- Caine, G.; Schafer, L.; Burgeson, D. Pumping of Liquid Hydrogen; Advances in Cryogenic Engineering; Timmerhaus, D.K., Ed.; Springer: Boston, MA, USA, 1958; Volume 4. [Google Scholar]
- Stinson, H.P.; Strickland, R.J. Experimental Findings from Zero-Tank Net Positive Suction Head Operation of the J-2 Hydrogen Pump; NASA Technical Note D-6824; National Aeronautics and Space Administration: Washington, DC, USA, 1972. [Google Scholar]
- Lockheed-Georgia Company. Main Propellant Tank Pressurization System Study and Test Program—Design Handbook; Final Report ER-5296; Contract AF 04(611)-6087; Lockheed-Georgia Company: Marietta, Georgia, USA, 1961; Volume 3. [Google Scholar]
- Barron, R.F.; Nellis, G.F. Cryogenic Heat Transfer, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Wang, L.; Li, Y.; Zhang, F.; Xie, F.; Ma, Y. Correlations for calculating heat transfer of hydrogen pool boiling. Int. J. Hydrog. Energy 2016, 41, 17118–17131. [Google Scholar] [CrossRef]
- Brentari, E.G.; Giarratano, P.J.; Smith, R.V. Boiling Heat Transfer for Oxygen, Nitrogen, Hydrogen, and Helium; National Bureau of Standards Technical Note 316; US National Bureau of Standards: Gaithersburg, MD, USA, 1965. [Google Scholar]
- Giarratano, P.J.; Smith, R.V. Comparative study of forced convection boiling heat transfer correlations for cryogenic fluids. In Proceedings of the Cryogenic Engineering Conference, Cryogenic Engineering Conference at Rice University, Houston, TX, USA, 23–25 August 1965. [Google Scholar]
- Steiner, D.; Taborek, J. Flow Boiling Heat Transfer in Vertical Tubes Correlated by an Asymptotic Model. Heat Transf. Eng. 1992, 13, 43–69. [Google Scholar] [CrossRef]
- Brennan, J.A.; Brentari, E.G.; Smith, R.V.; Steward, W.G. Cooldown of Cryogenic Transfer Lines; NASA Contractor Report 81338; Cryogenics Division, NBS Institute for Materials Research: Boulder, CO, USA, 1966. [Google Scholar]
- Hendricks, R.C.; Graham, R.W.; Hsu, Y.Y.; Friedman, R. Experimental Heat Transfer and Pressure Drop of Liquid Hydrogen Flowing through a Heated Tube; NASA Technical Note D-765; US National Bureau of Standards: Gaithersburg, MD, USA, 1961. [Google Scholar]
- Zuber, N. Hydrodynamic Aspects of Boiling Heat Transfer. Ph.D. Thesis, University of California, Los Angeles, CA, USA, 1959. [Google Scholar]
- Verein Deutscher Ingenieure. VDI-Wärmeatlas, 11th ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Lienhard, J.; Witte, L. An Historical Review of the Hydrodynamic Theory of Boiling. Rev. Chem. Eng. 1985, 3, 187–280. [Google Scholar] [CrossRef]
- Kida, M.; Kikuchci, Y.; Takahashi, O.; Michiyoshi, I. Pool-Boiling Heat Transfer in Liquid Nitrogen. J. Nucl. Sci. Technol. 1981, 18, 501–513. [Google Scholar] [CrossRef]
- Klell, M. Storage of Hydrogen in the Pure Form. In Handbook of Hydrogen Storage; Hirscher, M., Ed.; John Wiley & Sons: Weinheim, Germany, 2010. [Google Scholar]
- Marquardt, E.D.; Le, J.P.; Radebaugh, R. Cryogenic Material Properties Database. In Proceedings of the 11th International Cryocooler Conference, Keystone, CO, USA, 20–22 June 2000. [Google Scholar]
- Steward, W.G.; Smith, R.V.; Brennan, J.A. Cooldown transients in cryogenic transfer lines. In Advances in Cryogenic Engineering; Timmerhaus, K.D., Ed.; Springer: Berlin/Heidelberg, Germany, 1969; Volume 15. [Google Scholar]
- Rame, E.; Hartwig, J.W.; McQuillen, J.B. Flow Visualization of Liquid Hydrogen Line Chill Down Tests. In Proceedings of the 52nd Aerospace Sciences Meeting, National Harbor, MD, USA, 13–17 January 2014. [Google Scholar]
- Arbeitsgemeinschaft Druckbehälter. Zylinder- und Kugelschalen unter Innerem Überdruck AD 2000-Merkblatt B 1:2000-10. 2000. Available online: https://www.beuth.de/de/technische-regel/ad-2000-merkblatt-b-1/39490160 (accessed on 22 March 2021).
- Jousten, K. Handbuch Vakuumtechnik, 12th ed.; Springer: Wiesbaden, Germany, 2018. [Google Scholar]
- Pfeiffer Vacuum GmbH. HEPTADRY™, Die Trockene Schraubenpumpe. 2016. Available online: https://www.pfeiffer-vacuum.com/filepool/file/literature/broschuere-schraubenpumpen-heptadry.pdf?referer=2686&request_locale=de_DE (accessed on 14 December 2021).
- Oertel, H.; Böhle, M.; Reviol, T. Strömungsmechanik: Für Ingenieure und Naturwissenschaftler; Springer: Wiesbaden, Germany, 2015. [Google Scholar]
- Surek, D.; Stempin, S. Angewandte Strömungsmechanik, 1st ed.; Vieweg+Teubner: Wiesbaden, Germany, 2007. [Google Scholar]
- Erhard GmbH & Co. KG. Datenblatt ERHARD RKV Ringkolbenventile; Erhard GmbH & Co. KG: Heidenheim, Germany, 2019; Available online: https://www.erhard.de/produkte/kategorien/sicherheits-und-regelventile/ringkolbenventile/rkv-ringkolbenventil-dn100-300-pn40-160/?no_cache=1&tx_comatecdownloads_pi1%5Bid%5D=914&tx_comatecdownloads_pi1%5Baction%5D=downloadFile&tx_comatecdownloads_pi1%5Bcontroller%5D=Download (accessed on 22 March 2021).
- Technische Regeln für Betriebssicherheit/Gefahrstoffe TRBS 3151/TRGS 751 Vermeidung von Brand, Explosions- und Druckgefährdungen an Tankstellen und Gasfüllanlagen zur Befüllung von Landfahrzeugen. 2020. Available online: https://www.baua.de/DE/Angebote/Rechtstexte-und-Technische-Regeln/Regelwerk/TRBS/pdf/TRBS-3151.pdf?__blob=publicationFile (accessed on 1 October 2020).
- Linde GmbH. Helium 5.0 Produktdatenblatt. 2019. Available online: https://produkte.linde-gase.de/db_neu/helium_5.0.pdf (accessed on 28 November 2020).
- Novak, J.K. Cooldown flow rate limits imposed by thermal stresses in liquid hydrogen or nitrogen pipelines. In Advances in Cryogenic Engineering; Timmerhaus, K.D., Ed.; Springer: Berlin/Heidelberg, Germany, 1969; Volume 15. [Google Scholar]
- Yuan, K.; Ji, Y.; Chung, J. Cryogenic chilldown process under low flow rates. Int. J. Heat Mass Transf. 2007, 50, 4011–4022. [Google Scholar] [CrossRef]
- Hartwig, J.; Hu, H.; Styborski, J.; Chung, J. Comparison of cryogenic flow boiling in liquid nitrogen and liquid hydrogen chilldown experiments. Int. J. Heat Mass Transf. 2015, 88, 662–673. [Google Scholar] [CrossRef]
- Demaco Holland, B.V. Product Sheet: Vacuum Insulated Piping. 2021. Available online: https://d86wdzef8iurf.cloudfront.net/wp-content/uploads/2021/01/EN-VI-Piping_hq-02-2018-1.pdf (accessed on 13 January 2021).
- Demaco Holland, B.V. Product Sheet: Vacuum Insulated Flexible. 2021. Available online: https://d86wdzef8iurf.cloudfront.net/wp-content/uploads/2021/02/VI-Flex-English-28-07-2016-2-1.pdf (accessed on 13 January 2021).
- Fesmire, J.; Augustnynowicz, S. Thermal Performance of Cryogenic Piping Multilayer Insulation in Actual Field Installations. Cryogenics 2002, 2022. Available online: https://ntrs.nasa.gov/citations/20020039045 (accessed on 25 January 2021).
- Jones, L.; Wuschke, C.; Fahidy, T. Model of a cryogenic liquid-hydrogen pipeline for an airport ground distribution system. Int. J. Hydrog. Energy 1983, 8, 623–630. [Google Scholar] [CrossRef]
- Millis, M.G.; Tornabene, R.T.; Jurns, J.M.; Guynn, M.D.; Tomsik, T.M.; Overbe, T.J.V. Hydrogen Fuel System Design Trades for High-Altitude Long-Endurance Remotely-Operated Aircraft; NASA Technical Memorandum 2009-215521; National Aeronautics and Space Administration: Cleveland, OH, USA, 2009. [Google Scholar]
- ISO/PAS 15594:2004(E); Airport Hydrogen Fuelling Facility Operations. International Organization for Standardization: Geneva, Switzerland, 2004.
- Edeskuty, F.J.; Stewart, W.F. Safety in the Handling of Cryogenic Fluid; Springer: New York, NY, USA, 1996. [Google Scholar]
- Lin, C.S.; Van Dresar, N.T.; Hasan, M.M. Pressure Control Analysis of Cryogenic Storage Systems. J. Propuls. Power 2004, 20, 480–485. [Google Scholar] [CrossRef] [Green Version]
- Stolzenburg, K.; Berstad, D.; Decker, L.; Elliott, A.; Haberstroh, C.; Hatto, C.; Klaus, M.; Mortimer, N.; Mubbala, R.; Mwabonje, O.; et al. Efficient Liquefaction of Hydrogen: Results of the IDEALHY Project. In Proceedings of the XXth Energie—Symposium, Stralsund, Germany, 7–9 November 2013. [Google Scholar]
- Cardella, U.; Decker, L.; Klein, H. Roadmap to economically viable hydrogen liquefaction. Int. J. Hydrog. Energy 2017, 42, 13329–13338. [Google Scholar] [CrossRef]
- Burgunder, A.; Martinez, A.; Tamhankar, S. Current Status of Hydrogen Liquefaction Costs. In DOE Hydrogen and Fuel Cells Program Record; Department of Energy: Washington, DC, USA, 2019. Available online: https://www.hydrogen.energy.gov/pdfs/19001_hydrogen_liquefaction_costs.pdf (accessed on 4 January 2021).
- Hord, J. Selected Topics on Hydrogen Fuel; National Bureau of Standards NBSIR 75-803; National Bureau of Standards: Boulder, CO, USA, 1975. [Google Scholar]
- Tränkler, H.R.; Reindl, L.M. Sensortechnik: Handbuch für Praxis und Wissenschaft, 2nd ed.; Springer Vieweg: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Evler, J.; Asadi, E.; Preis, H.; Fricke, H. Stochastic Control of Turnarounds at HUB-Airports. In Proceedings of the Eighth SESAR Innovation Days, Salzburg, Austria, 3–7 December 2018. [Google Scholar]
- Fricke, H.; Schultz, M. Improving Aircraft Turn Around Reliability. In Proceedings of the ICRAT 3rd International Conference on Research and Air Transportation, Fairfax, CA, USA, 1–4 June 2008. [Google Scholar]
- Collins, L. A Wake-Up Call on Green Hydrogen: The Amount of Wind and Solar Needed is Immense. Available online: https://www.rechargenews.com/transition/a-wake-up-call-on-green-hydrogen-the-amount-of-wind-and-solar-needed-is-immense/2-1-776481 (accessed on 16 March 2021).
- U.S. Geological Survey. Mineral Commodity Summaries 2020; U.S. Geological Survey: Reston, VA, USA, 2020. [Google Scholar]
- Verstraete, D. The Potential of Liquid Hydrogen for Long Range Aircraft Propulsion. Ph.D. Thesis, Cranfield Unversity, Cranfield, UK, 2009. [Google Scholar]
- Winnefeld, C.; Kadyk, T.; Bensmann, B.; Krewer, U.; Hanke-Rauschenbach, R. Modelling and Designing Cryogenic Hydrogen Tanks for Future Aircraft Applications. Energies 2018, 11, 105. [Google Scholar] [CrossRef] [Green Version]
- Daigle, M.J.; Smelyanskiy, V.N.; Boschee, J.; Foygel, M. Temperature Stratification in a Cryogenic Fuel Tank. J. Thermophys. Heat Transf. 2013, 27, 116–126. [Google Scholar] [CrossRef]
- Vietze, M.B. Gekoppelte Aerothermodynamische und Strukturmechanische Optimierung Kryogener Raketenoberstufen. Ph.D. Thesis, Universität der Bundeswehr München, Munich, Germany, 2018. [Google Scholar]
- Migliardini, F.; Capasso, C.; Corbo, P. Optimization of hydrogen feeding procedure in PEM fuel cell systems for transportation. Int. J. Hydrog. Energy 2014, 39, 21746–21752. [Google Scholar] [CrossRef]
- Hoeflinger, J.; Hofmann, P. Air mass flow and pressure optimisation of a PEM fuel cell range extender system. Int. J. Hydrog. Energy 2020, 45, 29246–29258. [Google Scholar] [CrossRef]
- Mustafi, S.; Canavan, E.; Johnson, W.; Kutter, B.; Shull, J. Subcooling Cryogenic Propellants for Long Duration Space Exploration. In Proceedings of the AIAA SPACE 2009 Conference & Exposition, Pasadena, CA, USA, 14–17 September 2009. [Google Scholar]


| Source | kg/s | m3/min | d mm | m2/s | v m/s |
|---|---|---|---|---|---|
| Brewer [15] | 20 | 17.0 | 124.5 | 2.90 | 23.39 |
| Boeing [10] | 15 | 12.7 | 177.8 | 1.52 | 8.51 |
| Brewer et al. [11] | 13 | 11.0 | 203.2 | 1.15 | 5.67 |
| ISO/PAS 15594 [113] | 20 | 17.0 | 139.7 | 2.57 | 18.35 |
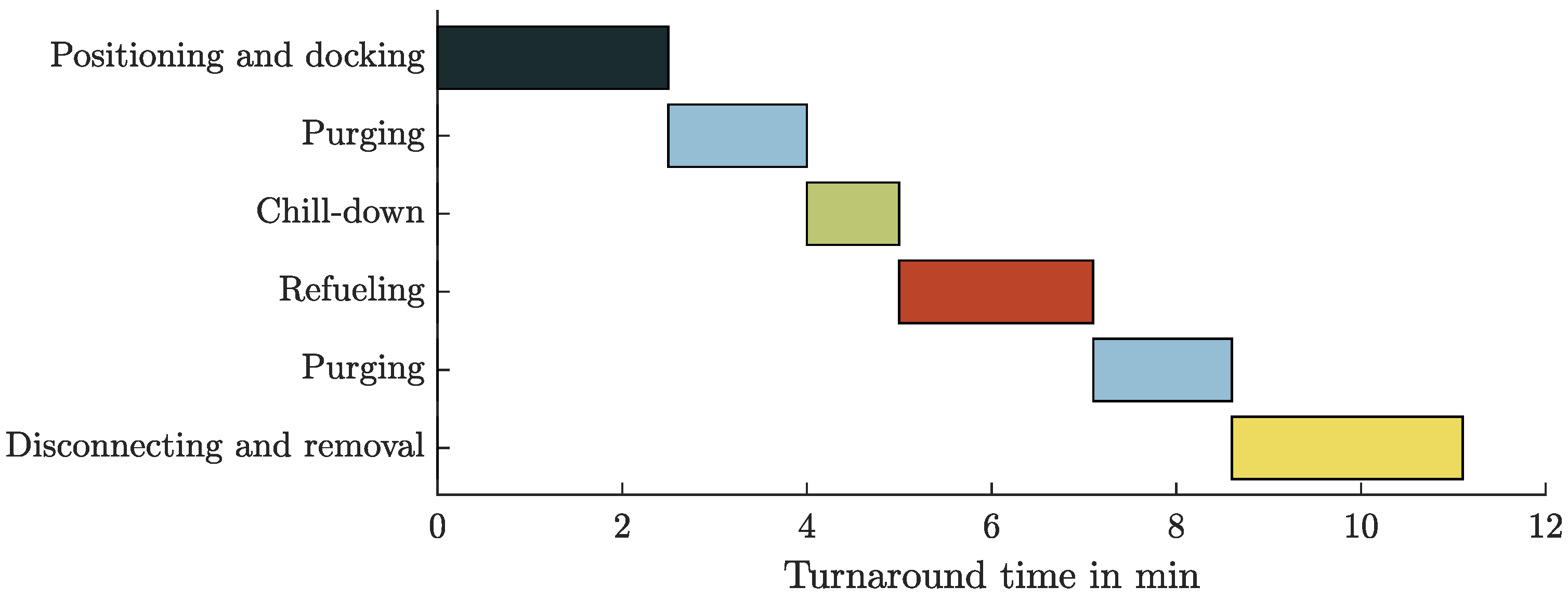
| Refueling Step | Time (min) |
|---|---|
| Positioning and connecting | 2.5 |
| Purging | 1.5 |
| Chill-down | 1.0 |
| Refueling | |
| Purging | 1.5 |
| Disconnecting and removal | 2.5 |
| Process | bara | K | bara | K | kJ/kg | P kWel | kWheat |
|---|---|---|---|---|---|---|---|
| isentropic | 1.2 | 20.00 | 5.0 | 20.17 | 4.91 | 0.98 | - |
| isentropic | 5.0 | 20.17 | 50.0 | 22.06 | 61.58 | 12.32 | - |
| isobaric | 50.0 | 22.06 | 50.0 | 260.00 | 3806.87 | - | 761.37 |
| Process | bara | K | bara | K | kJ/kg | P kWel | kWheat |
|---|---|---|---|---|---|---|---|
| isentropic | 1.2 | 20.00 | 5.0 | 20.17 | 4.91 | 0.98 | - |
| isobaric | 5.0 | 20.17 | 5.0 | 143.19 | 1989.05 | - | 397.81 |
| isentropic | 5.0 | 143.19 | 50.0 | 260.00 | 1879.40 | 375.88 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangold, J.; Silberhorn, D.; Moebs, N.; Dzikus, N.; Hoelzen, J.; Zill, T.; Strohmayer, A. Refueling of LH2 Aircraft—Assessment of Turnaround Procedures and Aircraft Design Implication. Energies 2022, 15, 2475. https://doi.org/10.3390/en15072475
Mangold J, Silberhorn D, Moebs N, Dzikus N, Hoelzen J, Zill T, Strohmayer A. Refueling of LH2 Aircraft—Assessment of Turnaround Procedures and Aircraft Design Implication. Energies. 2022; 15(7):2475. https://doi.org/10.3390/en15072475
Chicago/Turabian StyleMangold, Jonas, Daniel Silberhorn, Nicolas Moebs, Niclas Dzikus, Julian Hoelzen, Thomas Zill, and Andreas Strohmayer. 2022. "Refueling of LH2 Aircraft—Assessment of Turnaround Procedures and Aircraft Design Implication" Energies 15, no. 7: 2475. https://doi.org/10.3390/en15072475






