Impact of Preparation Method and Y2O3 Content on the Properties of the YSZ Electrolyte
Abstract
1. Introduction
- A number of researchers have investigated the properties of YSZ with very low dopant contents (i.e., 3 mol.%), confirming the very poor conductivity of such electrolytes [16,44,45,46]. On the other hand, a slight Y2O3 overdoping may be beneficial for solid oxide cell applications operating at lower temperatures [47,48,49]. Therefore, a lower limit of the range was chosen at a generally accepted optimum Y2O3 content to investigate potential property improvements with increasing the dopant content;
- On the other hand, excessive dopant content results in a decline in the YSZ conductivity. Y2O3 content of 18 mol.% was chosen as an upper dopant limit, because it clearly exceeds the optimum and falls into the declining conductivity range [47].
2. Experimental Section
2.1. Reference Materials
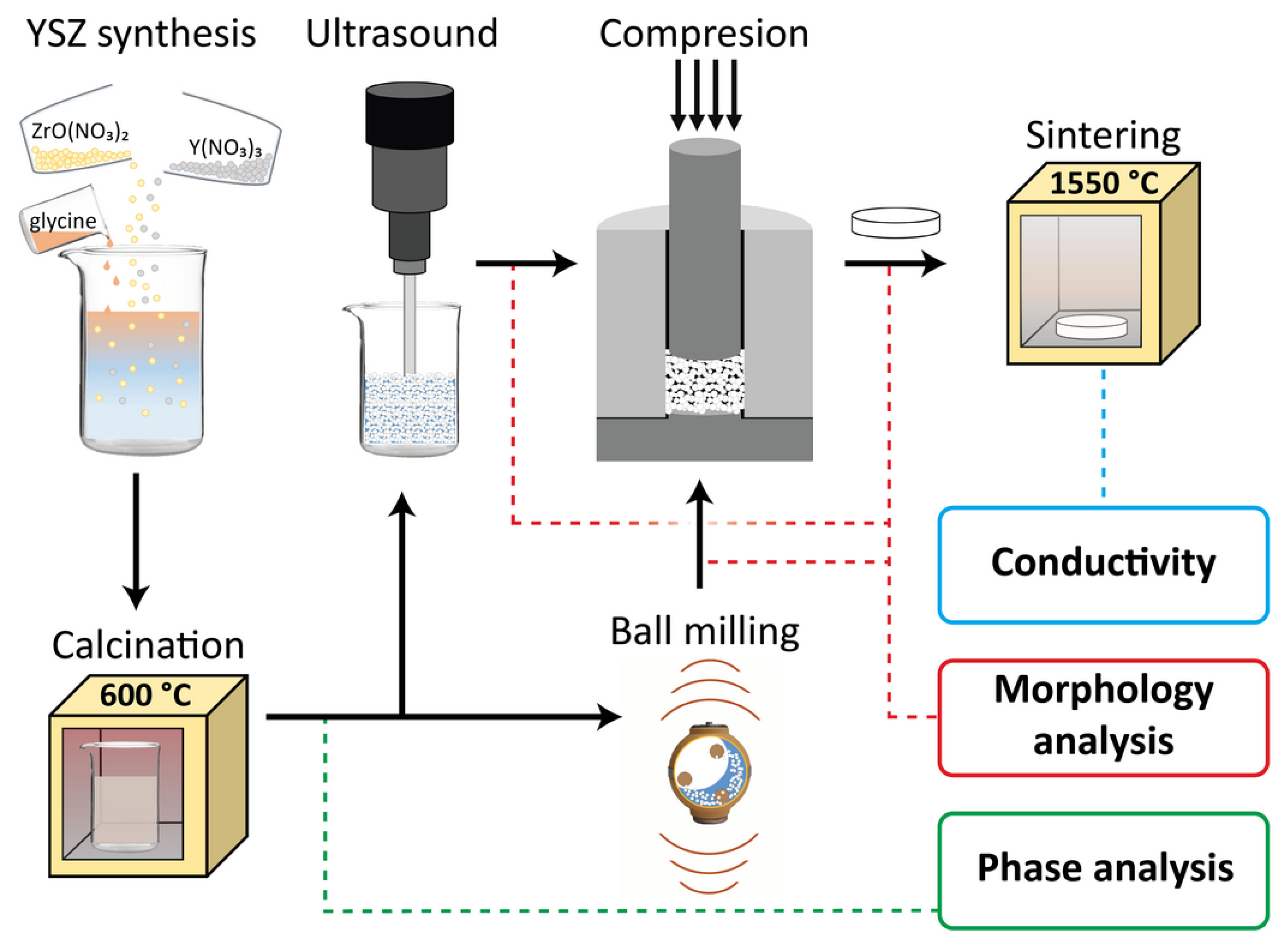
2.2. YSZ Powder Synthesis
2.3. Solid Electrolyte Shape Molding from YSZ Powders
2.4. Sintering Procedure
2.5. Determination of the Structure and Conductivity of the Samples
3. Results and Discussion
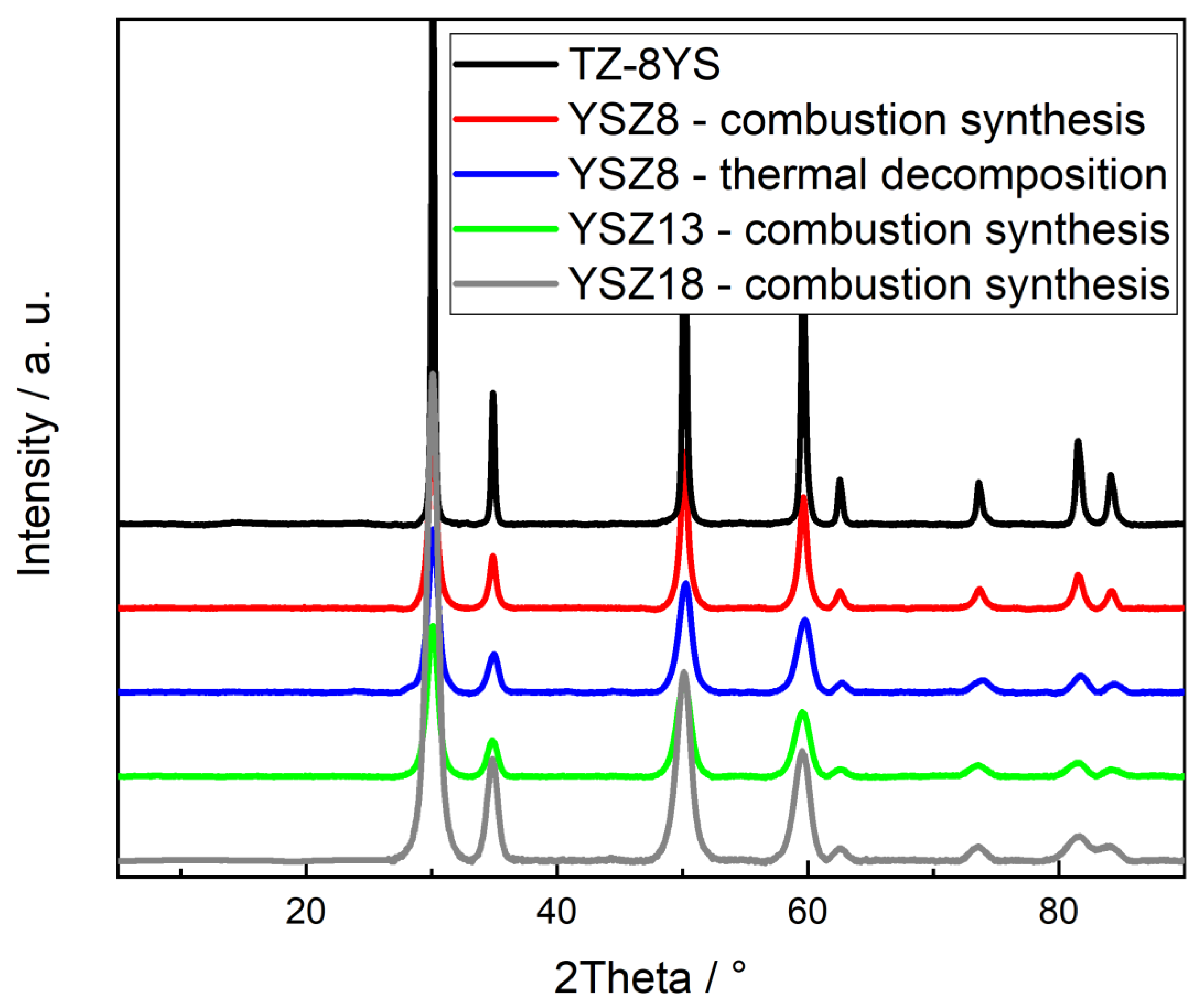
3.1. Determination of Structure and Composition of YSZ Powder
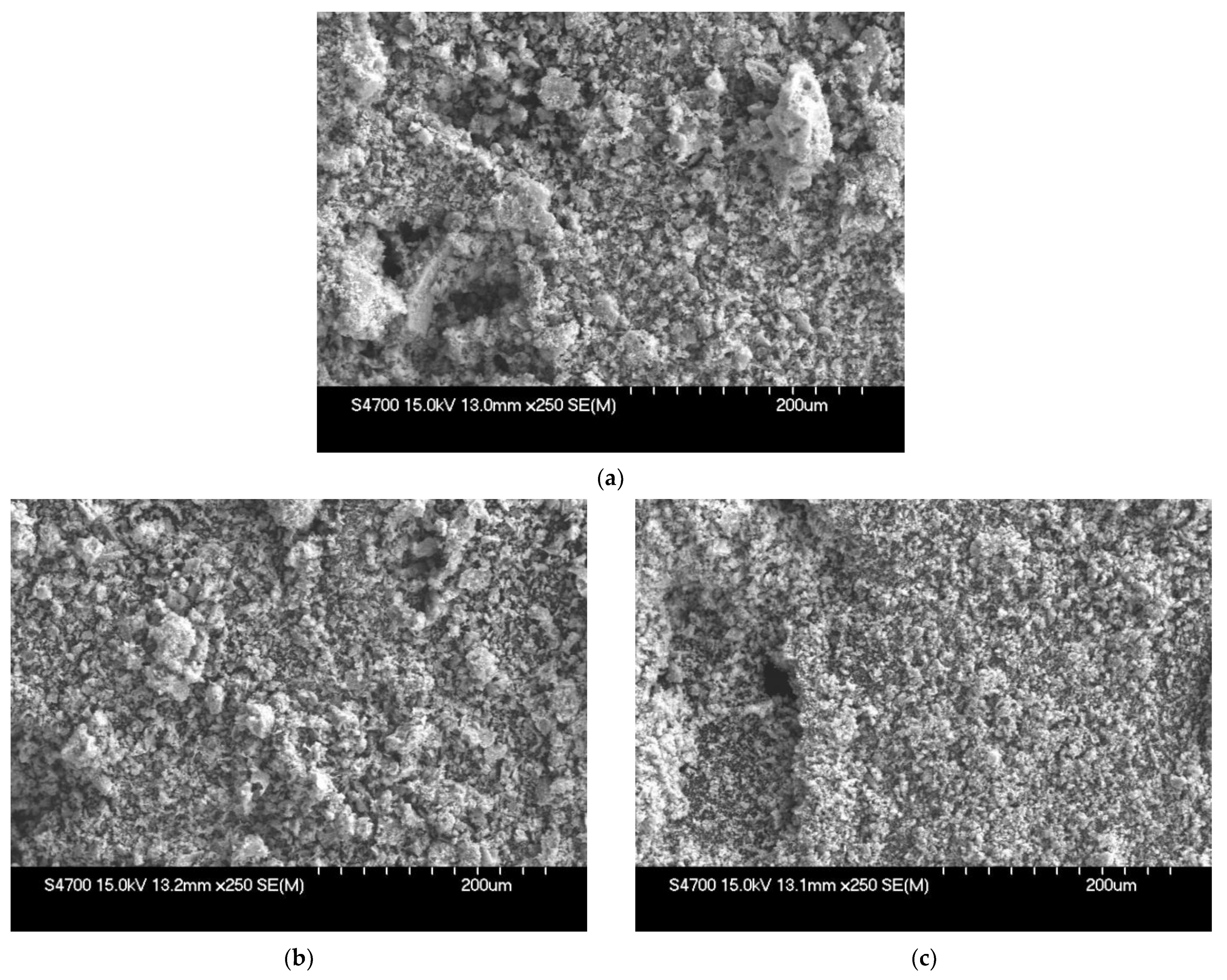
3.2. Synthesis and Post-Synthesis Treatment of YSZ Powder
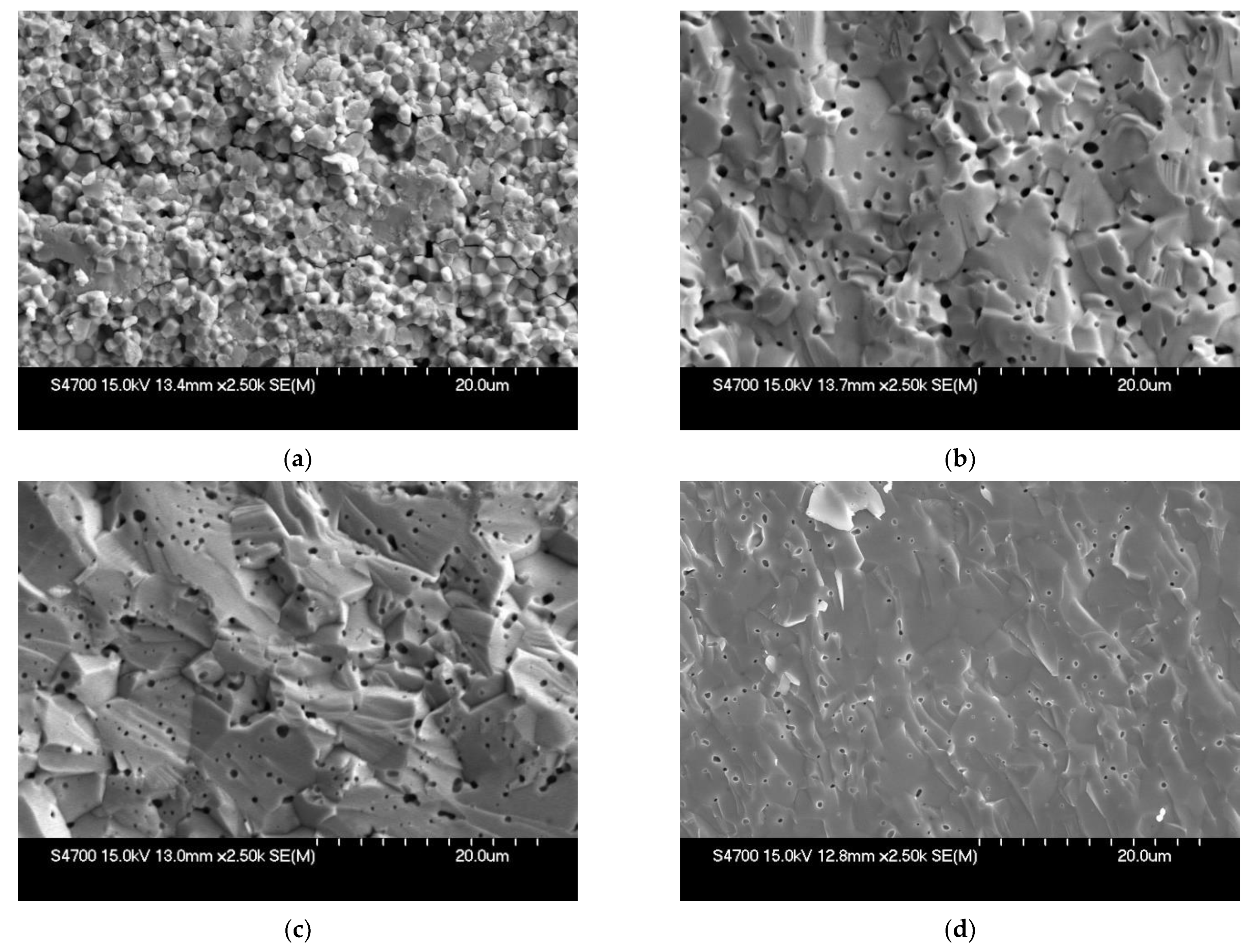
3.3. Shape Molding and Sintering Process
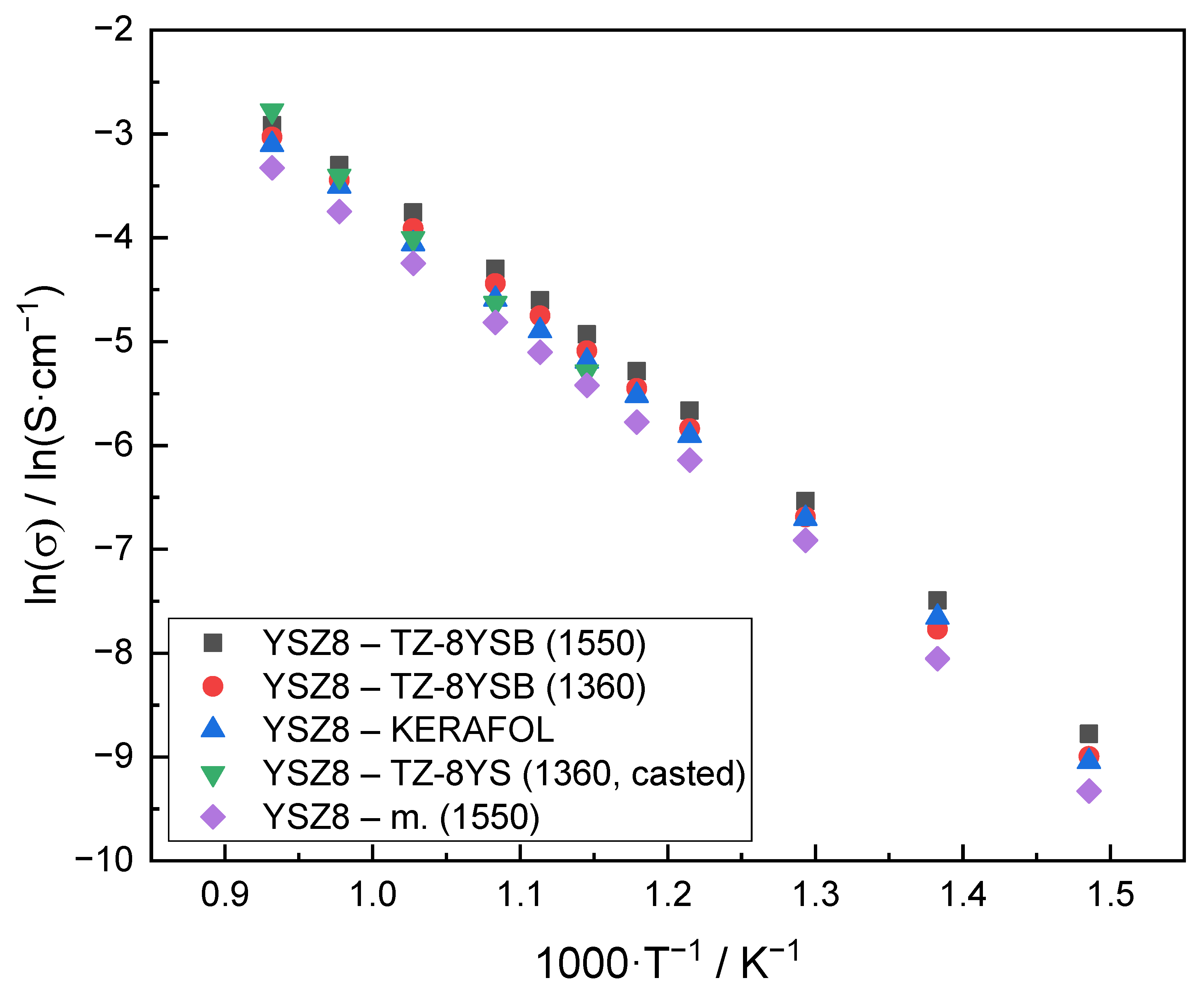
3.4. Conductivity Dependence of the YSZ Electrolyte on Temperature and Degree of Y2O3 Doping
4. Conclusions
- The combustion synthesis of precursors produced powders more suited for further processing than the thermal decomposition of the precursors;
- The combustion synthesis resulted in powder agglomerates that can be easily disintegrated during post-synthesis treatment to small particles of 3 µm in diameter and with a narrow size distribution;
- Small powder particles with uniform size distribution were essential in fabricating a dense YSZ electrolyte with minimal porosity, regardless of the molding technique used;
- The obtained conductivity values confirmed the assumed dependence of dopant content showing the maximum for the sample with 8 mol.% of Y2O3;
- All the studied samples displayed Arrhenius-like behavior in the whole range of temperatures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liu, Y.; Lao, L.E. Structural and electrical properties of ZnO-doped 8 mol% yttria-stabilized zirconia. Solid State Ion. 2006, 177, 159–163. [Google Scholar] [CrossRef]
- Fergus, J.W. Electrolytes for solid oxide fuel cells. J. Power Sources 2006, 162, 30–40. [Google Scholar] [CrossRef]
- Brodnikovska, I.; Korsunska, N.; Khomenkova, L.; Polishchuk, Y.; Lavoryk, S.; Brychevskyi, M.; Brodnikovskyi, Y.; Vasylyev, O. Grains, grain boundaries and total ionic conductivity of 10Sc1CeSZ and 8YSZ solid electrolytes affected by crystalline structure and dopant content. Mater. Today-Proc. 2019, 6, 79–85. [Google Scholar] [CrossRef]
- Ren, C.; He, Y.D.; Wang, D.R. Fabrication and Characteristics of YSZ–YSZ/Al2O3 Double-Layer TBC. Oxid. Met. 2011, 75, 325–335. [Google Scholar] [CrossRef]
- Jena, P.; Patro, P.K.; Sinha, A.; Lenka, R.K.; Singh, A.K.; Mahata, T.; Sinha, P.K. Hydrothermal Synthesis and Characterization of an Apatite-Type Lanthanum Silicate Ceramic for Solid Oxide Fuel Cell Electrolyte Applications. Energy Technol. 2018, 6, 1739–1746. [Google Scholar] [CrossRef]
- Tao, Z.; Fu, M.; Liu, Y.; Gao, Y.; Tong, H.; Hu, W.; Lei, L.; Bi, L. High-performing proton-conducting solid oxide fuel cells with triple-conducting cathode of Pr0.5Ba0.5(Co0.7Fe0.3)O3-δ tailored with W. Int. J. Hydrogen Energy 2022, 47, 1947–1953. [Google Scholar] [CrossRef]
- Tao, Z.; Fu, M.; Liu, Y. A mini-review of carbon-resistant anode materials for solid oxide fuel cells. Sustain. Energy Fuels 2021, 5, 5420–5430. [Google Scholar] [CrossRef]
- Ma, S.; Lin, M.; Lin, T.E.; Lan, T.; Liao, X.; Maréchal, F.; Van Herle, J.; Yang, Y.; Dong, C.; Wang, L. Fuel cell-battery hybrid systems for mobility and off-grid applications: A review. Renew. Sustain. Energy Rev. 2021, 135, 110119. [Google Scholar] [CrossRef]
- Ye, L.; Xie, K. High-temperature electrocatalysis and key materials in solid oxide electrolysis cells. J. Energy Chem. 2021, 54, 736–745. [Google Scholar] [CrossRef]
- Khan, M.Z.; Song, R.H.; Mehran, M.T.; Lee, S.B.; Lim, T.H. Controlling cation migration and inter-diffusion across cathode/interlayer/electrolyte interfaces of solid oxide fuel cells: A review. Ceram. Int. 2021, 47, 5839–5869. [Google Scholar] [CrossRef]
- Sengupta, P.; Bhattacharjee, A.; Maiti, H.S. Zirconia: A Unique Multifunctional Ceramic Material. Trans. Indian Inst. Met. 2019, 72, 1981–1998. [Google Scholar] [CrossRef]
- Carda, M.; Budáč, D.; Paidar, M.; Bouzek, K. Current trends in the description of lanthanum strontium manganite oxygen electrode reaction mechanism in a high-temperature solid oxide cell. Curr. Opin. Electrochem. 2022, 31, 100852. [Google Scholar] [CrossRef]
- Petot-Ervas, G.; Rizea, A.; Petot, C. Electrode materials, interface processes and transport properties of yttria-doped zirconia. Ionics 1997, 3, 405–411. [Google Scholar] [CrossRef]
- Wu, S.; Xu, X.; Li, X.; Bi, L. High-performance proton-conducting solid oxide fuel cells using the first-generation Sr-doped LaMnO3 cathode tailored with Zn ions. Sci. China Mater. 2022, 65, 675–682. [Google Scholar] [CrossRef]
- Ahamer, C.; Opitz, A.K.; Rupp, G.M.; Fleig, J. Revisiting the temperature dependent ionic conductivity of yttria stabilized zirconia (YSZ). J. Electrochem. Soc. 2017, 164, F790–F803. [Google Scholar] [CrossRef]
- Badwal, S.P.S. Zirconia-based solid electrolytes: Microstructure, stability and ionic conductivity. Solid State Ion. 1992, 52, 23–32. [Google Scholar] [CrossRef]
- Kharton, V.; Marques, F.; Atkinson, A. Transport properties of solid oxide electrolyte ceramics: A brief review. Solid State Ion. 2004, 174, 135–149. [Google Scholar] [CrossRef]
- Strickler, D.W.; Carlson, W.G. Ionic Conductivity of Cubic Solid Solutions in the System CaO—Y2O3—ZrO2. J. Am. Ceram. Soc. 1964, 47, 122–127. [Google Scholar] [CrossRef]
- Xue, Q.N.; Huang, X.W.; Wang, L.G.; Zhang, H.; Zhang, J.X. Computational and Experimental Investigations of Defect Interaction and Ionic Conductivity in Doped Zirconia. Phys. Rev. Appl. 2018, 10, 9. [Google Scholar] [CrossRef]
- Tsipis, E.V.; Kharton, V.V. Electrode materials and reaction mechanisms in solid oxide fuel cells: A brief review: I Performance-determining factors. J. Solid State Electrochem. 2008, 12, 1039–1060. [Google Scholar] [CrossRef]
- Tan, Z.H.; Guo, X. Synthesis and characterization of highly dispersed YSZ particles with diameter ≤ 5 nm. Ceram. Int. 2015, 41, 4953–4958. [Google Scholar] [CrossRef]
- Kaus, I.; Dahl, P.I.; Mastin, J.; Grande, T.; Einarsrud, M.A. Synthesis and characterization of nanocrystalline YSZ powder by smoldering combustion synthesis. J. Nanomater. 2006, 2006, 049283. [Google Scholar] [CrossRef]
- Syed, A.A.; Ilhan, Z.; Arnold, J.; Schiller, G.; Weckmann, H. Improving plasma-sprayed yttria-stabilized zirconia coatings for solid oxide fuel cell electrolytes. J. Therm. Spray Technol. 2006, 15, 617–622. [Google Scholar] [CrossRef]
- Reddy, B.S.B.; Mal, I.; Tewari, S.; Das, K.; Das, S. Aqueous combustion synthesis and characterization of nanosized tetragonal zirconia single crystals. Metall. Mater. Trans. A 2007, 38, 1786–1793. [Google Scholar] [CrossRef]
- Bitterlich, B.; Lutz, C.; Roosen, A. Rheological characterization of water-based slurries for the tape casting process. Ceram. Int. 2002, 28, 675–683. [Google Scholar] [CrossRef]
- Boaro, M.; Vohs, J.M.; Gorte, R.J. Synthesis of highly porous yttria-stabilized zirconia by tape-casting methods. J. Am. Ceram. Soc. 2003, 86, 395–400. [Google Scholar] [CrossRef]
- Xin, X.; Lü, Z.; Zhu, Q.; Huang, X.; Su, W. Fabrication of dense YSZ electrolyte membranes by a modified dry-pressing using nanocrystalline powders. J. Mater. Chem. 2007, 17, 1627–1630. [Google Scholar] [CrossRef]
- Hu, L.; Wang, C.A.; Huang, Y.; Sun, C.; Lu, S.; Hu, Z. Control of pore channel size during freeze casting of porous YSZ ceramics with unidirectionally aligned channels using different freezing temperatures. J. Eur. Ceram. Soc. 2010, 30, 3389–3396. [Google Scholar] [CrossRef]
- Van Herle, J.; McEvoy, A.J.; Thampi, K.R. Conductivity measurements of various yttria-stabilized zirconia samples. J. Mater. Sci. 1994, 29, 3691–3701. [Google Scholar] [CrossRef]
- Sasongko, M.N.; Perdana, F.; Wijayanti, W. Analysis of the Effect of Ionic Conductivity of Electrolyte Materials on the Solid Oxide Fuel Cell Performance. East.-Eur. J. Enterp. Technol. 2021, 3, 41–52. [Google Scholar] [CrossRef]
- Jiang, J.; Hertz, J.L. On the variability of reported ionic conductivity in nanoscale YSZ thin films. J. Electroceram. 2014, 32, 37–46. [Google Scholar] [CrossRef]
- Li, K. Preparation of Ceramic Membranes. In Ceramic Membranes for Separation and Reaction; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 21–57. [Google Scholar] [CrossRef]
- Enríquez-Martínez, A.; Mosqueda-Laffita, Y.; Aguilar-Frutis, M.; Alarcón-Flores, G.; Grima-Gallardo, P.; León-Ramírez, H.; Pérez-Cappe, E. YSZ nanoparticles and thin films prepared in a single crystallization step at low temperature. Mater. Res. Express 2019, 6, 126412. [Google Scholar] [CrossRef]
- Han, M.; Tang, X.; Yin, H.; Peng, S. Fabrication, microstructure and properties of a YSZ electrolyte for SOFCs. J. Power Sources 2007, 165, 757–763. [Google Scholar] [CrossRef]
- Cho, G.Y.; Noh, S.; Lee, Y.H.; Ji, S.; Hong, S.W.; Koo, B.; An, J.; Kim, Y.B.; Cha, S.W. Properties of nanostructured undoped ZrO2 thin film electrolytes by plasma enhanced atomic layer deposition for thin film solid oxide fuel cells. J. Vac. Sci. Technol. A Vac. Surf. Film. 2016, 34, 01A151. [Google Scholar] [CrossRef]
- Kim, S.-G.; Yoon, S.P.; Nam, S.W.; Hyun, S.-H.; Hong, S.-A. Fabrication and characterization of a YSZ/YDC composite electrolyte by a sol–gel coating method. J. Power Sources 2002, 110, 222–228. [Google Scholar] [CrossRef]
- Menzler, N.H.; Tietz, F.; Uhlenbruck, S.; Buchkremer, H.P.; Stöver, D. Materials and manufacturing technologies for solid oxide fuel cells. J. Mater. Sci. 2010, 45, 3109–3135. [Google Scholar] [CrossRef]
- Izumi, M.; Sasahara, N. Effect of pre-sintering of raw material powder on properties of solid oxide fuel cell electrolyte prepared by dip-coating method. J. Ceram. Soc. Jpn. 2010, 118, 944–947. [Google Scholar] [CrossRef][Green Version]
- Park, J.; Paek, J.Y.; Chang, I.; Ji, S.; Cha, S.W.; Oh, S.I. Pulsed laser deposition of Y-doped BaZrO3 thin film as electrolyte for low temperature solid oxide fuel cells. CIRP Ann.-Manuf. Technol. 2013, 62, 563–566. [Google Scholar] [CrossRef]
- Sherikar, B.N.; Sahoo, B.; Umarji, A.M. Effect of fuel and fuel to oxidizer ratio in solution combustion synthesis of nanoceramic powders: MgO, CaO and ZnO. Solid State Sci. 2020, 109, 106426. [Google Scholar] [CrossRef]
- Singh, K.L.; Singh, G.; Sharma, P.; Mago, S.; Singh, A.P. Effect of fuel/nitrate molar ratio on the properties of nio-YSZ nanocomposites as anode material for solid oxide fuel cell synthesised by combustion method. Int. J. Nanopart. 2018, 10, 298–311. [Google Scholar] [CrossRef]
- Hao, S.J.; Wang, C.; Liu, T.L.; Mao, Z.M.; Mao, Z.Q.; Wang, J.L. Fabrication of nanoscale yttria stabilized zirconia for solid oxide fuel cell. Int. J. Hydrogen Energy 2017, 42, 29949–29959. [Google Scholar] [CrossRef]
- Sanson, A.; Pinasco, P.; Roncari, E. Influence of pore formers on slurry composition and microstructure of tape cast supporting anodes for SOFCs. J. Eur. Ceram. Soc. 2008, 28, 1221–1226. [Google Scholar] [CrossRef]
- Filal, M.; Petot, C.; Mokchah, M.; Chateau, C.; Carpentier, J.L. Ionic conductivity of yttrium-doped zirconia and the “composite effect”. Solid State Ion. 1995, 80, 27–35. [Google Scholar] [CrossRef]
- Uchida, H.; Yoshida, M.; Watanabe, M. Effects of ionic conductivities of zirconia electrolytes on polarization properties of platinum anodes in solid oxide fuel cells. J. Phys. Chem. 1995, 99, 3282–3287. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Ciacchi, F.T. Oxygen-ion conducting electrolyte materials for solid oxide fuel cells. Ionics 2000, 6, 1–21. [Google Scholar] [CrossRef]
- Cho, G.Y.; Lee, Y.H.; Yu, W.; An, J.; Cha, S.W. Optimization of Y2O3 dopant concentration of yttria stabilized zirconia thin film electrolyte prepared by plasma enhanced atomic layer deposition for high performance thin film solid oxide fuel cells. Energy 2019, 173, 436–442. [Google Scholar] [CrossRef]
- Sik Son, K.; Bae, K.; Woo Kim, J.; Suk Ha, J.; Hyung Shim, J. Ion conduction in nanoscale yttria-stabilized zirconia fabricated by atomic layer deposition with various doping rates. J. Vac. Sci. Technol. A Vac. Surf. Film. 2013, 31, 01A107. [Google Scholar] [CrossRef]
- Chao, C.C.; Kim, Y.B.; Prinz, F.B. Surface modification of yttria-stabilized zirconia electrolyte by atomic layer deposition. Nano Lett. 2009, 9, 3626–3628. [Google Scholar] [CrossRef]
- Carda, M.; Adamová, N.; Budáč, D.; Paidar, M.; Bouzek, K. Preparation Protocol and Properties of YSZ Ceramic Electrolytes for Solid Oxide Cells. ECS Trans. 2021, 105, 97–105. [Google Scholar] [CrossRef]
- Konysheva, E.Y. The Effect of Chromium Oxide on the Conductivity of Ce0.9Gd0.1O2, a Solid-Oxide Fuel Cell Electrolyte. Russ. J. Electrochem. 2018, 54, 471–480. [Google Scholar] [CrossRef]
- Tu, H.; Stimming, U. Advances, aging mechanisms and lifetime in solid-oxide fuel cells. Eight Ulm. Electrochem. Tage 2004, 127, 284–293. [Google Scholar] [CrossRef]
- Schuler, J.A.; Yokokawa, H.; Calderone, C.F.; Jeangros, Q.; Wuillemin, Z.; Hessler-Wyser, A.; Van Herle, J. Combined Cr and S poisoning in solid oxide fuel cell cathodes. J. Power Sources 2012, 201, 112–120. [Google Scholar] [CrossRef]
- Lieberzeit, J. Preparation of Electrolyte for High Temperature Water Electrolysis. Bachelor’s Thesis, University of Chemistry and Technology Prague, Prague, Czech Republic, 2015. [Google Scholar]
- Atkinson, A. Solid Oxide Fuel Cell Electrolytes-Factors Influencing Lifetime. In Solid Oxide Fuel Cell Lifetime and Reliability: Critical Challenges in Fuel Cells; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 19–35. [Google Scholar] [CrossRef]
- Vendrell, X.; West, A.R. Electrical properties of yttria-stabilized zirconia, YSZ single crystal: Local AC and long range DC conduction. J. Electrochem. Soc. 2018, 165, F966–F975. [Google Scholar] [CrossRef]





| Sample Name | Supplier | Y2O3 Content [mol.%] | Mono-/Poly-Crystalline | Synthesis Method | Preparation Method | Sintering Procedure |
|---|---|---|---|---|---|---|
| YSZ8—KERAFOL | KERAFOL | 8 | Poly | - | - | - |
| YSZ8—TZ-8YS (1360, casted) | TOSOH | 8 | Poly | - | slurry casting | 1360 °C/6 h |
| YSZ8—TZ-8YSB (1360) | TOSOH | 8 | Poly | - | uniaxial compression | 1360 °C/6 h |
| YSZ8—TZ-8YSB (1550) | TOSOH | 8 | Poly | - | uniaxial compression | 1550 °C/24 h |
| YSZ8—m. (1550) | in-house | 8 | Poly | combustion + milled | uniaxial compression | 1550 °C/24 h |
| YSZ13—(1360) | in-house | 13 | Poly | combustion | uniaxial compression | 1360 °C/6 h |
| YSZ13—(1550) | in-house | 13 | Poly | combustion | uniaxial compression | 1550 °C/24 h |
| YSZ13—m. (1550) | in-house | 13 | Poly | combustion + milled | uniaxial compression | 1550 °C/24 h |
| YSZ13—US (1550) | in-house | 13 | Poly | combustion + ultrasonic horn | uniaxial compression | 1550 °C/24 h |
| YSZ18—m. (1550) | in-house | 18 | Poly | combustion + milled | uniaxial compression | 1550 °C/24 h |
| Sample Name | σ 103/S·cm−1 @ 800 °C | EA/kJ·mol−1 |
|---|---|---|
| ‘Y2O3 mol.%’—commercial name (sintering temperature) | ||
| YSZ8—TZ-8YSB (1550) | 54.12 | 87.9 |
| YSZ8—TZ-8YSB (1360) | 48.35 | 89.6 |
| YSZ8—KERAFOL | 44.91 | 87.4 |
| YSZ8—TZ-8YS (1360, casted) | 62.28 | 97.3 |
| ‘Y2O3 mol.%’—post-synthesis method (sintering temperature) | ||
| YSZ8—m. (1550) | 35.88 | 89.1 |
| YSZ13—m. (1550) | 9.10 | 103.5 |
| YSZ13—US (1550) | 10.58 | 106.9 |
| YSZ13—(1550) | 5.22 | 108.2 |
| YSZ13—(1360) | 2.42 | 113.1 |
| YSZ18—m. (1550) | 2.10 | 122.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carda, M.; Adamová, N.; Budáč, D.; Rečková, V.; Paidar, M.; Bouzek, K. Impact of Preparation Method and Y2O3 Content on the Properties of the YSZ Electrolyte. Energies 2022, 15, 2565. https://doi.org/10.3390/en15072565
Carda M, Adamová N, Budáč D, Rečková V, Paidar M, Bouzek K. Impact of Preparation Method and Y2O3 Content on the Properties of the YSZ Electrolyte. Energies. 2022; 15(7):2565. https://doi.org/10.3390/en15072565
Chicago/Turabian StyleCarda, Michal, Nela Adamová, Daniel Budáč, Veronika Rečková, Martin Paidar, and Karel Bouzek. 2022. "Impact of Preparation Method and Y2O3 Content on the Properties of the YSZ Electrolyte" Energies 15, no. 7: 2565. https://doi.org/10.3390/en15072565
APA StyleCarda, M., Adamová, N., Budáč, D., Rečková, V., Paidar, M., & Bouzek, K. (2022). Impact of Preparation Method and Y2O3 Content on the Properties of the YSZ Electrolyte. Energies, 15(7), 2565. https://doi.org/10.3390/en15072565







