Compact High Efficiency and Zero-Emission Gas-Fired Power Plant with Oxy-Combustion and Carbon Capture
Abstract
1. Introduction
1.1. Literature Survey Connected to Oxy-Combustion Thermodynamic Cycles
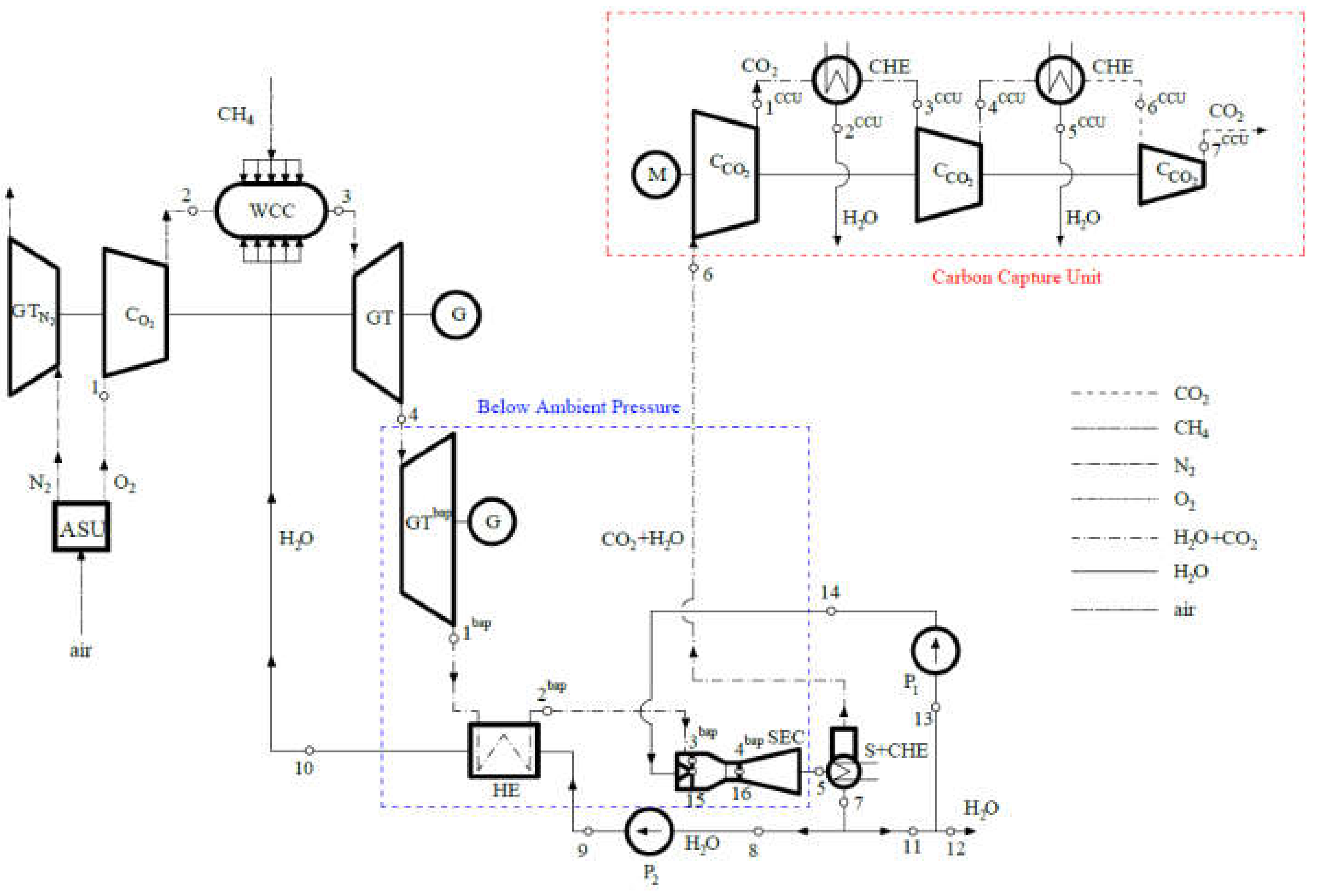
1.2. Elements of Compact Steam-Gas Power Plant with Oxy-Combustion and Carbon Capture
1.3. Purpose and Scope of the Article
2. Thermodynamic Cycle of a Compact, High-Efficiency, Zero-Emission Power Plant
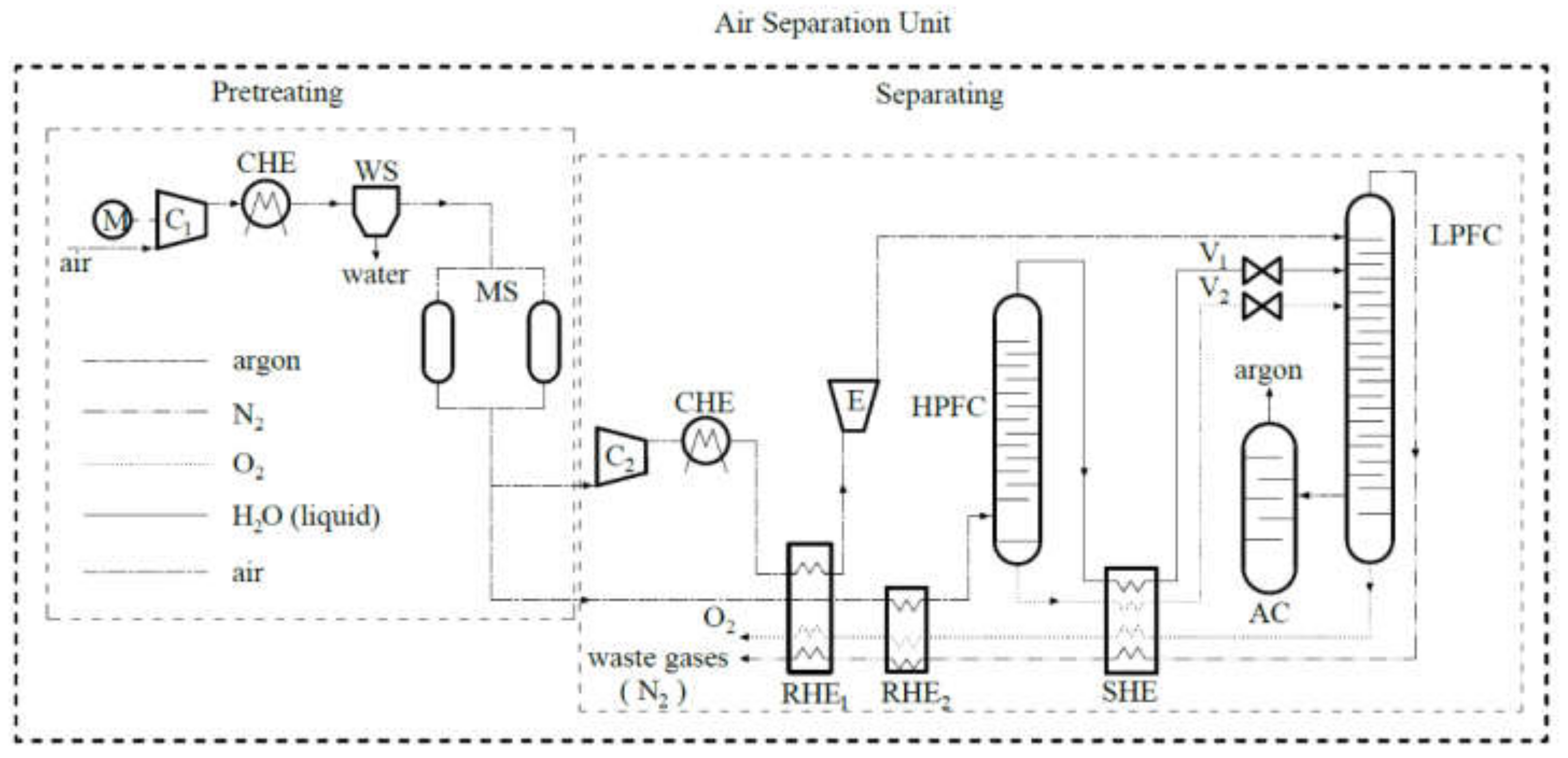
2.1. The Mechanism of ASU
- -
- main compressor (C1);
- -
- pre-cooling heat exchanger (CHE);
- -
- cleaning by molecular sieve (MS) and compressed air drying in water separator (WS) systems;
- -
- second compressor (C2), a cooler (CHE), and an expander (E);
- -
- high-pressure (HPFC) and low-pressure (LPFC) fractionating columns;
- -
- exchangers, namely, regenerative heat exchanger (RHE1, RHE2); subcooler (SHE);
- -
- valves (V1, V2).
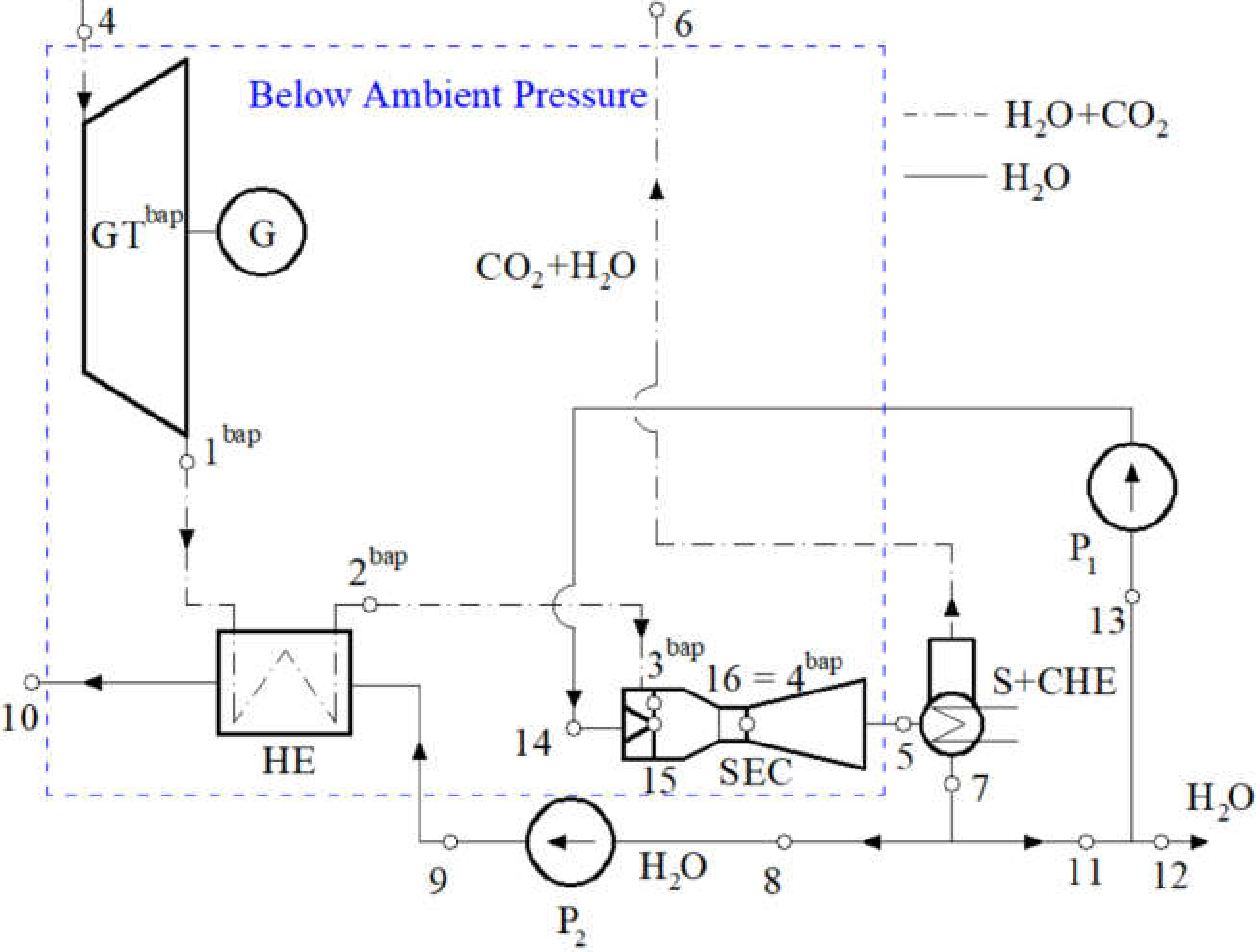
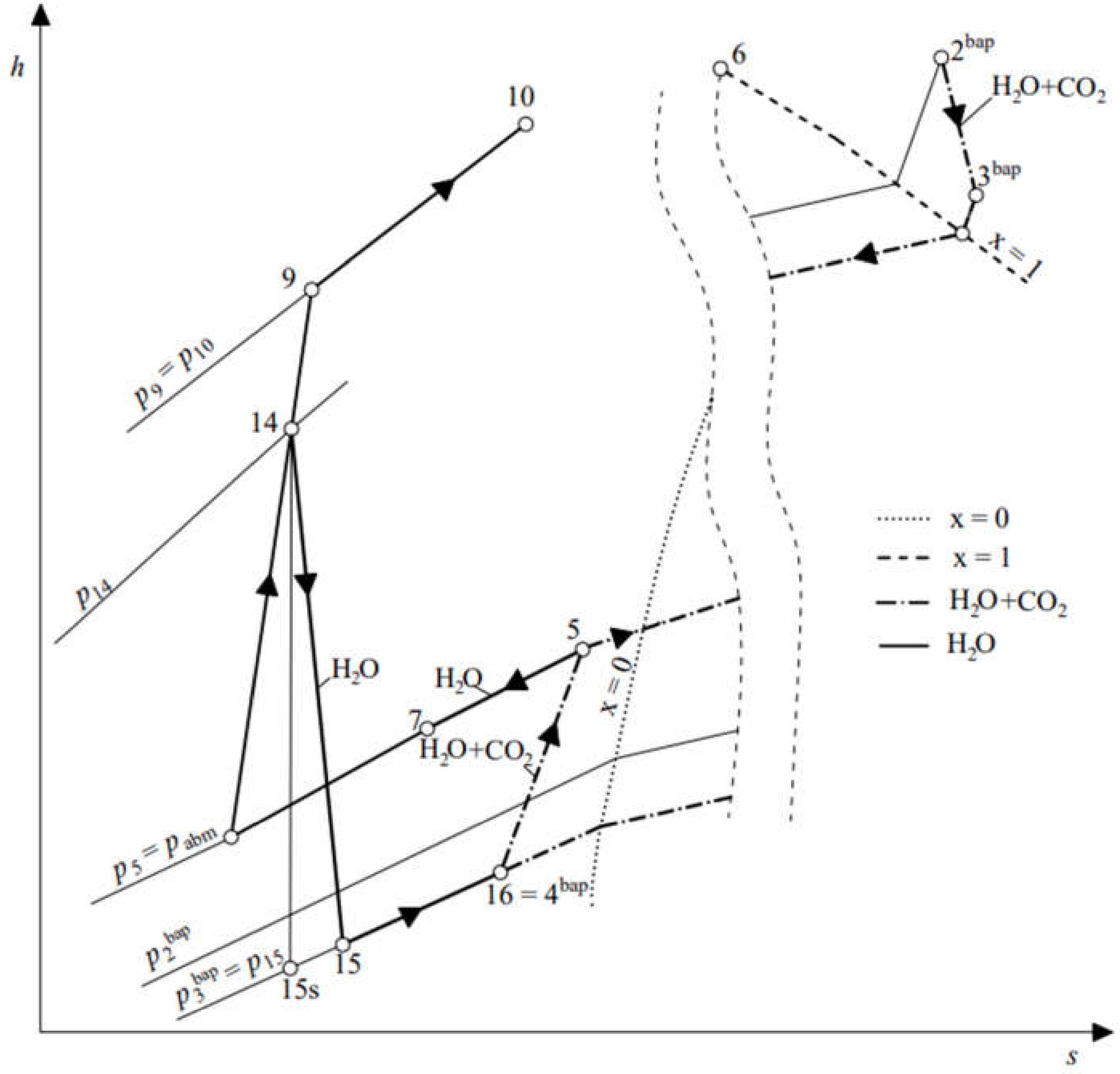
2.2. Sub-Cycle of the Spray-Ejector Condenser
2.3. COM-GAS Code
3. Governing Equations
3.1. Integral Parameters
- -
- pressure ratio with regard to suction pressure:
- -
- pressure ratio with regard to pressure at the outlet from the spray-ejector condenser:where pressures () are presented in Figure 8. Pressure ratios are correlated with the measurements . A more complex definition is characterised by coefficients taking into account the differential pressure, namely:
- -
- the dimensionless suction ratio:
- -
- the dimensionless compression ratio:
- -
- the dimensionless cavitation ratio:where —the saturation pressure of the liquid at a given temperature; —total pressure at the inlet to the ejector; and —total pressure in the suction area assuming that the velocity at the inlet to the suction chamber .
- -
- the dimensionless suction ratio as a function of the entrainment ratio:
- -
- the dimensionless compression ratio as a function of the entrainment ratio:
- -
- the dimensionless cavitation ratio as a function of the entrainment ratio:
- -
- the efficiency characteristics:
3.2. Mixing and Condensation Process for the Spray-Ejector Condenser
3.3. Mass, Momentum, and Energy Balance in the Spray-Ejector Condenser
3.4. Velocity Relations with Respect to Momentum and Energy Balances
3.5. Closures for the Spray-Ejector Condenser
3.6. Efficiency and Power Output
3.7. Simplifications and Assumptions Adopted for the Model
4. Results of Thermodynamic Analysis
5. Discussion—Compactness of the Steam Generator Condenser and Gas-Steam Turbine
5.1. Spray-Ejector Condenser
5.2. Wet Combustion Chamber
5.3. Gas-Steam Turbine
6. Conclusions and Perspectives
- The use of a spray-ejector condenser leads to an efficiency decrease of approximately 5.91 percentage points, with the simultaneous energy conversion enhancement due to the device’s size being reduced by 32 times in the lower heat source area.
- Furthermore, for the wet combustion chamber to achieve compactness, new cooling design concepts should be developed to bring it into line with the latest transpiration technologies. Additionally, the impact of a wet combustion chamber on the overall operation of the cycle has been estimated, yielding a 30-fold enhancement of energy conversion in the upper heat source area.
- The total efficiency of the system is = 37.78%. However, the installation of such a system provides a perspective on meeting the needs of small and large cities to produce heat and electricity with minimal negative—or even a positive—impact on the environment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| A | surface area, m2 |
| velocity in CFD approach, m/s | |
| velocity in CFM approach, m/s | |
| diffusive stress tensor, Pa | |
| available energy rate, kW | |
| unit vector in radius direction | |
| unit vector in axial direction | |
| e = u + p/ρ + zg + (c2)/2—specific total energy, J/kg | |
| force, N | |
| forces comes from the surface mechanism, N/m2 | |
| g | gravitation acceleration, m/s2 |
| h | specific enthalpy, kJ/kg |
| Gibbs unit tensor, - | |
| unit vector normal to section | |
| N | power, kW |
| mass flow rate, kg/s | |
| stress tensor associated with external and internal configuration forces, Pa | |
| p | pressure, MPa |
| R | gas constant, kJ/(kgK) |
| friction force, N | |
| Reynolds stress tensor, Pa | |
| heat transfer rate, kW | |
| chemical energy transfer rate, kW | |
| heat flux density, kW/m3 | |
| s | specific etropy, kJ/(kgK) |
| total momentum flux, Pa | |
| T | temperature, °C |
| specific internal energy, kJ/kg | |
| velocity vector, m/s | |
| specific volume, m3/kg | |
| volume, m3 | |
| volume flow rate, m3/s | |
| vapour quality, - | |
| volumetric fraction or mole fraction, - | |
| mass fraction, - | |
| height, m | |
| the contact area of the solid structure with the working medium | |
| 0D | zero-dimensional algebraic model of flow based on integral balances of mass, momentum and energy |
| 3D | three-dimensional model based on differential equations, which requires complete geometry of a flow channel |
| dyadic multiplicator | |
| pitch diameter of the stage, m | |
| isentropic static enthalpy drop at the stage; | |
| h | specific enthalpy; |
| mass flow rate; | |
| mobility coefficients | |
| Mach number | |
| power; MW | |
| s | specific entropy; |
| T | temperature in centigrade (Celsius scale); °C |
| v | specific volume; |
| V | volume; m3 |
| specific work, kJ/kg | |
| 0D | zero-dimensional algebraic model of flow based on integral balances of mass, momentum and energy |
| 3D | three-dimensional model based on differential equations, which requires complete geometry of a flow channel |
| CFD | Computational Fluid Dynamics, so-called three-dimensional description of unknowns parameters of the power plant devices |
| Greek symbols | |
| Δp | pressure drop, MPa |
| ΔT | the temperature difference in the heat exchanger, K |
| η | efficiency |
| flow losses for changing diameter channel | |
| surface friction coefficients | |
| π | pressure ratio |
| dimensionless compression ratio | |
| dimensionless suction ratio | |
| dimensionless cavitation ratio | |
| μ | mass flow capacity coefficient |
| density, kg/m3 | |
| viscous stress tensor, Pa | |
| τ | narrowing coefficient |
| velocity coefficient | |
| volumetric entrainment ratio | |
| mass entrainment ratio | |
| Subscripts and superscripts: | |
| air | air |
| bap | below ambient pressure |
| C | compressor |
| c | compression in spray-ejector condenser |
| cav | cavitation |
| CC | combustion chamber |
| CCU | carbon dioxide capture unit |
| CHE | cooling heat exchanger |
| d | diffuser |
| e | effective |
| ex | exhaust |
| el | electrical |
| f | fuel |
| G | electric generator |
| g | gaseous |
| GT | gas turbine |
| HE | heat exchanger |
| i | isentropic, ideal |
| lq | liquid |
| m | mechanical |
| M | motor |
| MC | mixing chamber |
| n | nozzle |
| P | pump |
| s | isentropic |
| sc | suction chamber |
| t | technical |
| t | total |
| 1s, 2s, … | isentropic points of process |
| 1, 2, … | real points of process |
| Abbreviations: | |
| ASU | air separation unit |
| bap | below ambient pressure |
| B | boiler |
| BC | Brayton cycle |
| C | compressor |
| CC | combustion chamber |
| CCS | carbon dioxide capture and storage systems |
| CCU | carbon capture unit |
| CES | clean energy systems |
| CFD | computational fluid dynamics, so-called three-dimensional description of unknown parameters of the power plant devices |
| CFM | computational flow mechanics, so-called zero-dimensional description of unknown parameters of the power plant apparatus |
| CHE | cooling heat exchanger |
| CSE | spray-ejector condenser |
| DBC | double Brayton cycle |
| DBCOCC | double Brayton cycle with oxy-combustion and CO2 capture |
| EC | energy consumption |
| G | electric generator |
| GT | gas turbine |
| HE | heat exchanger |
| HPFC | high-pressure fractionating column |
| HRSG | heat recovery steam generator |
| HTC | heat transfer coefficient |
| LHV | low heating value, kJ/kg |
| lq | liquid |
| LPFC | low-pressure fractionating column |
| M | motor |
| MC | mixing chamber |
| MS | molecular sieve |
| P | pump |
| RHE | regenerative heat exchanger |
| sat | saturation |
| S + CHE | condensate-cooler heat exchanger and separator |
| SHE | subcooler |
| TIT | turbine inlet temperature |
| V | valve |
| WCC | wet combustion chamber |
| WS | water separator |
References
- Yantovsky, E.; Górski, J.; Shokotov, M. Zero Emissions Power Cycles; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Kotowicz, J.; Brzęczek, M.; Job, M. The influence of carbon capture and compression unit on the characteristics of ultramodern combined cycle power plant. Int. J. Glob. Warn. 2017, 12, 164–187. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Badur, J. A study of a compact high-efficiency zero-emission power plant with oxy-fuel combustion. In Proceedings of the 32nd International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems ECOS 2019, Wroclaw, Poland, 23–28 June 2019; Stanek, W., Gładysz, P., Werle, S., Adamczyk, W., Eds.; Institute of Thermal Technology, Silesian University of Technology: Gliwice, Poland, 2019; pp. 1557–1568. [Google Scholar]
- Pawlak-Kruczek, H.; Arora, A.; Mościcki, K.; Krochmalny, K.; Sharma, S.; Niedzwiecki, L. A transition of a domestic boiler from coal to biomass—Emissions from combustion of raw and torrefied Palm Kernel shells (PKS). Fuel 2020, 263, 116718. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Badur, J.; Pawlak-Kruczek, H.; Nedźwiecki, Ł.; Kowal, M.; Krochmalny, K. A novel concept of negative CO2 emission power plant, based on combustion the gas from sewage sludge gasification in a gas turbine with spray-ejector condenser. In Proceedings of the XXIV International Symposium on Combustion Processes, Wrocław, Poland, 23–26 September 2019; pp. 103–104. [Google Scholar]
- Badur, J.; Lemański, M.; Kowalczyk, T.; Ziółkowski, P.; Kornet, S. Zero-dimensional robust model of an SOFC with internal re-forming for hybrid energy cycles. Energy 2018, 158, 128–138. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Hyrzyński, R.; Lemański, M.D.; Kraszewski, B.; Bykuć, S.; Głuch, S.; Sowiżdżał, A.; Pająk, L.; Wachowicz-Pyzik, A.; Badur, J. Different design aspects of an Organic Rankine Cycle turbine for electricity production using a geothermal binary power plant. Energy Convers. Manag. 2021, 246, 114672. [Google Scholar] [CrossRef]
- Ziółkowski, P. A thermodynamic analysis of a gas-steam turbine incorporating a full model of a spray—Ejector condenser. Trans. IFFM 2018, 139, 63–96. [Google Scholar]
- Ziółkowski, P. A Thermodynamic Analysis of Low Emission Gas-Steam Cycles with Oxy-Combustion. Ph.D. Thesis, Institute Fluid Flow Machinery, Polish Academy of Sciences, Gdańsk, Poland, 2018. (In Polish). [Google Scholar]
- Ziółkowski, P.; Madejski, P.; Amiri, M.; Kuś, T.; Stasiak, K.; Subramanian, N.; Pawlak-Kruczek, H.; Badur, J.; Niedźwiecki, Ł.; Mikielewicz, D. Thermodynamic analysis of negative CO2 emission power plant using Aspen Plus, Aspen Hysys, and Ebsilon software. Energies 2021, 14, 6304. [Google Scholar] [CrossRef]
- Anderson, R.; Viteri, F.; Hollis, R.; Hebbar, M.; Downs, J.; Davies, D.; Harris, M. Application of Existing Turbomachinery for Zero Emissions Oxy-Fuel Power Systems. In Proceedings of the ASME Turbo Expo 2009: Power for Land, Sea, and Air, Orlando, FL, USA, 8–12 June 2009; pp. 469–479. [Google Scholar] [CrossRef]
- Chodkiewicz, R.; Porochnicki, J.; Kaczan, B. Steam–Gas Condensing Turbine System for Power and Heat Generation. In Proceedings of the ASME Turbo Expo 2001: Power for Land, Sea, and Air, New Orleans, LA, USA, 4–7 June 2001. [Google Scholar] [CrossRef]
- Hollis, R.; Skutley, P.; Ortíz, C.; Varkey, V.; Lepage, D.; Brown, B.; Davies, D.; Harris, M. Oxy-Fuel Turbomachinery Development for Energy Intensive Industrial Applications. In Proceedings of the ASME Turbo Expo 2012: Turbine Technical Conference and Exposition, Copenhagen, Denmark, 11–15 June 2012; pp. 431–439. [Google Scholar] [CrossRef]
- Hustad, C.W.; Tronstad, I.; Anderson, R.; Pronske, K.; Veteri, F. Optimization of thermodynamically efficient nominal 40 MW zero emission pilot and demonstration power plant in Norway. In Proceedings of the ASME Turbo Expo 2005: Power for Land, Sea and Air, Reno, NV, USA, 6–9 June 2005; pp. 271–278. [Google Scholar]
- Pronske, K.; Trowsdale, L.; Macadam, S.; Viteri, F.; Bevc, F.; Horazak, D. An overview of turbine and combustor develompent for coal-based oxy-syngas systems. In Proceedings of the ASME Turbo Expo 2006: Power for Land, Sea and Air, Barcelona, Spain, 8–11 May 2006. [Google Scholar]
- Anderson, R.; Hustad, C.; Skutley, P.; Hollis, R. Oxy-fuel turbo machinery development for energy intensive industrial applica-tions. Energy Procedia 2014, 63, 511–523. [Google Scholar] [CrossRef]
- Marin, O.; Bourhis, Y.; Di Zanno, P.; Viteri, F.; Anderson, R. High Efficiency, Zero Emission Power Generation Based on a High-Temperature Steam Cycle. In Proceedings of the 28th International Technical Conference on Coal Utilization & Fuel Systems, Clearwater, FL, USA, 9–13 March 2003. [Google Scholar]
- Ziółkowski, P. Porous structures in aspects of transpirating cooling of oxycombustion chamber walls. AIP Conf. Proc. 2019, 2077, 020065. [Google Scholar] [CrossRef]
- Jericha, H.; Feshaaki. The GRAZ cycle—1500 °C max temperature potential H2-O2 fired CO2 capture with CH4-O2 firing. In Proceedings of the 1995 ASME Cogen-Turbo Power Conference, Houston, TX, USA, 5–8 June 1995. [Google Scholar]
- Sanz, W.; Hustad, C.-W.; Jericha, H. First Generation Graz Cycle Power Plant for Near-Term Deployment. In Proceedings of the ASME Turbo Expo: Turbine Technical Conference and Exposition, Vancouver, BC, Canada, 6–10 June 2011; pp. 969–979. [Google Scholar] [CrossRef]
- Miller, A.; Lewandowski, J.; Badyda, K.; Kiryk, S.; Milewski, J.; Hama, J.; Iki, N. Off-Design analysis of the GRAZ cycle performance. In Proceedings of the International Gas Turbine Congress, Tokyo, Japan, 2–7 November 2003. [Google Scholar]
- Kotowicz, J.; Job, M. Thermodynamic and economic analysis of a gas turbine combined cycle plant with oxy-combustion. Arch. Thermodyn. 2013, 34, 215–233. [Google Scholar] [CrossRef][Green Version]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and techniques for CO2 capture: Review of potential solutions and applications in modern energy technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Kanniche, M.; Gros-Bonnivard, R.; Jaud, P.; Valle-Marcos, J.; Amann, J.M.; Bouallou, C. Pre-combustion, post-combustion and oxy-combustion in thermal power plant for CO2 capture. Appl. Therm. Eng. 2010, 30, 53–62. [Google Scholar] [CrossRef]
- Yang, H.J.; Kang, D.W.; Ahn, J.H.; Kim, T.S. Evaluation of design performance of the semi-closed oxy-fuel combustion combined cycle. In Proceedings of the ASME Turbo Expo: Power for Land, Sea and Air 2012, Copenhagen, Denmark, 11–15 June 2012. [Google Scholar]
- Yantovski, E.; Zvagolsky, K.; Gavrilenko, V. The COOPERATE—Demo power cycle. Energy Convers Manag. 1995, 36, 861–864. [Google Scholar] [CrossRef]
- Mathieu, P.; Nihart, R. Sensitivity analysis of the MATIANT cycle. Energy Convers. Manag. 1999, 40, 1687–1700. [Google Scholar] [CrossRef]
- Feidt, M. Finite Physical Dimensions Optimal Thermodynamics 1 Fundamentals; ISTE Press/Elsevier: London, UK, 2017. [Google Scholar]
- Staicovici, M. Further research zero CO2 emission power production: The ‘COOLENERG’ process. Energy 2002, 27, 831–844. [Google Scholar] [CrossRef]
- Yantovsky, E.; Górski, J.; Smyth, B.; Elshof, J. Zero-emission fuel-fired power plants with ion transport membrane. Energy 2004, 29, 2077–2088. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, T.S.; Sohn, J.L.; Lee, Y.D. An integrated power generation system combining solid oxide fuel cell and oxy-fuel com-bustion for high performance and CO2 capture. Appl. Energy 2011, 88, 1187–1196. [Google Scholar] [CrossRef]
- Budzianowski, W. An oxy-fuel mass-recirculating process for H2 production with CO2 capture by autothermal catalytic ox-yforming of methane. Int. J. Hydrogen Energy 2012, 35, 7454–7469. [Google Scholar] [CrossRef]
- Badur, J.; Lemański, M.; Kowalczyk, T.; Ziółkowski, P.; Kornet, S. Verification of zero-dimensional model of SOFC with internal fuel reforming for complex hybrid energy cycles. Chem. Process Eng. 2018, 39, 113–128. [Google Scholar] [CrossRef]
- Bolland, O.; Kvamsdal, H.M.; Boden, J.C. A Thermodynamic Comparison of Oxy-Fuel Power Cycles Water-Cycle, Graz-Cycle and Matiant-Cycle. 2001. Available online: https://www.semanticscholar.org/paper/A-thermodynamic-comparison-of-the-oxy-fuel-power-Bolland-Kvamsdal/3887db33cefe667646448c253251c5d8683c1b48 (accessed on 30 December 2021).
- Kvamsdal, H.M.; Jordal, K.; Bolland, O. A quantitative comparison of gas turbine cycles with CO2 capture. Energy 2007, 32, 10–24. [Google Scholar] [CrossRef]
- Zhang, N.; Lior, N. Two novel oxy-fuel power cycles integrated with natural gas reforming and CO2 capture. Energy 2008, 33, 340–351. [Google Scholar] [CrossRef]
- Głuch, S.; Ziółkowski, P.; Witanowski, Ł.; Badur, J. Design and computational fluid dynamics analysis of the last stage of inno-vative gas-steam turbine. Arch. Thermodyn. 2021, 42, 1–24. [Google Scholar]
- Gou, C.; Cai, R.; Hong, H. An Advanced Oxy-Fuel Power Cycle with High Efficiency. Proc. Inst. Mech. Eng. Part A J. Power Energy 2006, 220, 315–325. [Google Scholar] [CrossRef]
- Czakiert, T.; Sztekler, K.; Karski, S.; Markiewicz, D.; Nowak, W. Oxy-fuel circulating fluidized bed combustion in a small pi-lot-scale test rig. Fuel Process. Technol. 2010, 91, 1617–1623. [Google Scholar] [CrossRef]
- Liu, C.; Chen, G.; Sipöcz, N.; Assadi, M.; Bai, X. Characteristics of oxy-fuel combustion in gas turbines. Appl. Energy 2011, 89, 387–394. [Google Scholar] [CrossRef]
- Perrin, N.; Dubettier, R.; Lockwood, F.; Court, P.; Tranier, J.-P.; Bourhy-Weber, C.; Devaux, M. Oxycombustion for carbon capture on coal power plants and industrial processes: Advantages, innovative solutions and key projects. Energy Procedia 2013, 37, 1389–1404. [Google Scholar] [CrossRef][Green Version]
- Perrin, N.; Paufique, C.; Leclerc, M. Latest performances and improvement perspective of oxycombustion for carbon capture on coal power plants. Energy Procedia 2014, 63, 524–531. [Google Scholar] [CrossRef]
- Saanum, I.; DiTaranto, M. Experimental study of oxy-fuel combustion under gas turbine conditions. Energy Fuels 2017, 31, 4445–4451. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Paul, J.; Blasiak, W. Studies on low-intensity oxy-fuel burner. Proc. Combust. Inst. 2009, 32, 3139–3146. [Google Scholar] [CrossRef]
- Adamczyk, W.P.; Bialecki, R.A.; Ditaranto, M.; Gladysz, P.; Haugen, N.E.L.; Katelbach-Wozniak, A.; Klimanek, A.; Sladek, S.; Szlek, A.; Wecel, G. CFD modeling and thermodynamic analysis of a concept of a MILD-OXY combustion large scale pulverized coal boiler. Energy 2017, 140, 1305–1315. [Google Scholar] [CrossRef]
- Hjärtstam, S.; Johansson, R.; Andersson, K.; Johnsson, F. Computational fluid dynamics modeling of oxy-fuel flames: The role of soot and gas radiation. Energy Fuels 2012, 26, 2786–2797. [Google Scholar] [CrossRef]
- Ghadamgahi, M.; Ölund, P.; Ekman, T.; Andersson, N.; Jönsson, P. A Comparative CFD study on simulating flameless oxy-fuel combustion in a pilot-scale furnace. J. Combust. 2016, 2016, 6735971. [Google Scholar] [CrossRef]
- Ghadamgahi, M.; Ölund, P.; Lugnet, A.; Pour, M.S.; Yang, W. Design optimization of flameless-oxyfuel soaking pit furnace using CFD technique. Energy Procedia 2014, 61, 611–614. [Google Scholar] [CrossRef]
- Yin, C.; Rosendahl, L.A.; Kær, S.K. Chemistry and radiation in oxy-fuel combustion: A computational fluid dynamics modeling study. Fuel 2011, 90, 2519–2529. [Google Scholar] [CrossRef]
- Lewandowski, M.T.; Ertesvåg, I.S. Analysis of the eddy dissipation concept formulation for MILD combustion modelling. Fuel 2018, 224, 687–700. [Google Scholar] [CrossRef]
- Gładysz, P.; Stanek, W.; Czarnowska, L.; Sładek, S.; Szlęk, A. Thermo-ecological evaluation of an integrated MILD oxy-fuel combustion power plant with CO2 capture, utilisation, and storage—A case study in Poland. Energy 2018, 144, 379–392. [Google Scholar] [CrossRef]
- Gładysz, P.; Stanek, W.; Czarnowska, L.; Węcel, G.; Langørgen, Ø. Thermodynamic assessment of an integrated MILD oxyfuel combustion power plant. Energy 2017, 137, 761–774. [Google Scholar] [CrossRef]
- Ertesvåg, I.S.; Kvamsdal, H.M.; Bolland, O. Exergy analysis of a gas-turbine combined-cycle power plant with precombustion CO2 capture. Energy 2005, 30, 5–39. [Google Scholar] [CrossRef]
- Voldsund, M.; Gardarsdottir, S.O.; De Lena, E.; Pérez-Calvo, J.-F.; Jamali, A.; Berstad, D.; Fu, C.; Romano, M.; Roussanaly, S.; Anantharaman, R.; et al. Comparison of technologies for CO2 capture from cement production—Part 1: Technical evaluation. Energies 2019, 12, 559. [Google Scholar] [CrossRef]
- Skorek-Osikowska, A.; Bartela, Ł.; Kotowicz, J. Economical and ecological evaluation of the advanced electricity production technologies adapter for carbon dioxide capture. J. Energy Sci. 2010, 1, 147–160. [Google Scholar]
- Bartela, Ł.; Skorek-Osikowska, A.; Kotowicz, J. Thermodynamic, ecological and economic aspects of the use of the gas turbine for heat supply to the stripping process in a supercritical CHP plant integrated with a carbon capture installation. Energy Convers. Manag. 2014, 85, 750–763. [Google Scholar] [CrossRef]
- Tlili, N.; Grévillot, G.; Vallières, C. Carbon dioxide capture and recovery by means of TSA and/or VSA. Int. J. Greenh. Gas Control 2009, 3, 519–527. [Google Scholar] [CrossRef]
- Asendrych, D.; Niegodajew, P. Numerical Study of the CO2 Absorber Performance Subjected to the Varying Amine Solvent and Flue Gas Loads. Chem. Eng. Commun. 2016, 204, 580–590. [Google Scholar] [CrossRef]
- Abu Zahra, M.; Schneiders, L.H.; Niederer, J.P.; Feron, P.H.; Versteeg, G.F. CO2 capture from power plants: Part I. A parametric study of the technical performance based on monoethanolamine. Int. J. Greenh. Gas Control 2007, 1, 37–46. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Lemański, M.; Badur, J.; Nastałek, L. Power augmentation of PGE Gorzów gas turbine by steam injection—Ther-modynamic overview. Rynek Energii 2012, 98, 161–167. [Google Scholar]
- Cheng, D.Y. The distinction between Cheng and STIG cycle. In Proceedings of the ASME Turbo Expo 2006: Power for Land, Sea, and Air, Barcelona, Spain, 8–11 May 2006. [Google Scholar]
- Normann, F.; Andersson, K.; Leckner, B.; Johnsson, F. Emission control of nitrogen oxides in the oxy-fuel process. Prog. Energy Combust. Sci. 2009, 35, 385–397. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; McIlveen-Wright, D.; McMullan, J.; Hewitt, N.; Eames, P.; Rezvani, S. A techno-economic analysis of the ap-plication of continuous stage-combustion and flameless oxidation to the combustor design in gas turbines. Fuel Process. Technol. 2006, 87, 727–736. [Google Scholar] [CrossRef]
- Wienchol, P.; Szlęk, A.; Ditaranto, M. Waste-to-energy technology integrated with carbon capture—Challenges and opportuni-ties. Energy 2020, 198, 117352. [Google Scholar] [CrossRef]
- Binnion, M. How the technical differences between shale gas and conventional gas projects lead to a new business model be-ing required to be successful. Mar. Pet. Geol. 2012, 31, 3–7. [Google Scholar] [CrossRef]
- Gabicz, M.; Sokół, H. Polish natural gas market after the arrival of the fiasco with shale deposits in Poland. Rynek Energii 2015, 121, 3–7. (In Polish) [Google Scholar]
- Kinnaman, T.C. The economic impact of shale gas extraction: A review of existing studies. Ecol. Econ. 2011, 70, 1243–1249. [Google Scholar] [CrossRef]
- Rahm, D. Regulating hydraulic fracturing in shale gas plays: The case of Texas. Energy Policy 2011, 39, 2974–2981. [Google Scholar] [CrossRef]
- Jenner, S.; Lamadrid, A.J. Shale gas vs. coal: Policy implications from environmental impact comparisons of shale gas, conventional gas, and coal on air, water, and land in the United States. Energy Policy 2013, 53, 442–453. [Google Scholar] [CrossRef]
- Mendecka, B.; Lombardi, L.; Gladysz, P. Waste to energy efficiency improvements: Integration with solar thermal energy. Waste Manag. Res. J. Sustain. Circ. Econ. 2019, 37, 419–434. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Wnukowski, M.; Niedzwiecki, L.; Czerep, M.; Kowal, M.; Krochmalny, K.; Zgóra, J.; Ostrycharczyk, M.; Baranowski, M.; Tic, W.J.; et al. Torrefaction as a valorization method used prior to the gasification of sewage sludge. Energies 2019, 12, 175. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Wnukowski, M.; Niedzwiecki, L.; Kowal, M.; Krochmalny, K. Gasification of torrefied sewage sludge with the addition of calcium carbonate. J. Energy Resour. Technol. 2020, 142, 070910. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Kowalczyk, T.; Lemański, M.; Badur, J. On energy, exergy, and environmental aspects of a combined gas-steam cycle for heat and power generation undergoing a process of retrofitting by steam injection. Energy Convers. Manag. 2019, 192, 374–384. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Badur, J.; Ziółkowski, P.J. An energetic analysis of a gas turbine with regenerative heating using turbine extrac-tion at intermediate pressure—Brayton cycle advanced according to Szewalski’s idea. Energy 2019, 185, 76–86. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Badur, J. On Navier slip and Reynolds transpiration numbers. Arch. Mech. 2018, 70, 269–300. [Google Scholar]
- Smith, A.; Klosek, J. A review of air separation technologies and their integration with energy conversion processes. Fuel Process. Technol. 2001, 70, 115–134. [Google Scholar] [CrossRef]
- Burdyny, T.; Struchtrup, H. Hybrid membrane/cryogenic separation of oxygen from air for use in the oxy-fuel process. Energy 2010, 35, 1884–1897. [Google Scholar] [CrossRef]
- Darde, A.; Prabhakar, R.; Tranier, J.-P.; Perrin, N. Air separation and flue gas compression and purification units for oxy-coal combustion systems. Energy Procedia 2009, 1, 527–534. [Google Scholar] [CrossRef]
- Banaszkiewicz, T.; Chorowski, M.; Gizicki, W. Comparative Analysis of Oxygen Production for Oxy-combustion Application. Energy Procedia 2014, 51, 127–134. [Google Scholar] [CrossRef]
- Van Der Ham, L.V.; Kjelstrup, S. Exergy analysis of two cryogenic air separation processes. Energy 2010, 35, 4731–4739. [Google Scholar] [CrossRef]
- Zhu, Y.; Legg, S.; Laird, C.D. Optimal design of cryogenic air separation columns under uncertainty. Comput. Chem. Eng. 2010, 34, 1377–1384. [Google Scholar] [CrossRef]
- Fu, Q.; Kansha, Y.; Song, C.; Liu, Y.; Ishizuka, M.; Tsutsumi, A. An elevated-pressure cryogenic air separation unit based on self-heat recuperation technology for integrated gasification combined cycle systems. Energy 2016, 103, 440–446. [Google Scholar] [CrossRef]
- Tesch, S.; Morosuk, T.; Tsatsaronis, G. Advanced exergy analysis applied to the process of regasification of LNG (liquefied natu-ral gas) integrated into an air separation process. Energy 2016, 117, 550–561. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Zonouz, M.J. Analysis of an integrated cryogenic air separation unit, oxy-combustion carbon dioxide power cycle and liquefied natural gas regasification process by exergoeconomic method. Energy Convers. Manag. 2017, 139, 245–259. [Google Scholar] [CrossRef]
- Aneke, M.; Wang, M. Process analysis of pressurized oxy-coal power cycle for carbon capture application integrated with liq-uid air power generation and binary cycle engines. Appl. Energy 2015, 154, 556–566. [Google Scholar] [CrossRef]
- Hanak, D.P.; Powell, D.; Manovic, V. Techno-economic analysis of oxy-combustion coal-fired power plant with cryogenic oxy-gen storage. Appl. Energy 2017, 191, 193–203. [Google Scholar] [CrossRef]
- Fu, C.; Vikse, M.; Gundersen, T. Work and heat integration: An emerging research area. Energy 2018, 158, 796–806. [Google Scholar] [CrossRef]
- Yu, H.; Fu, C.; Gundersen, T. Work Exchange Networks (WENs) and Work and Heat Exchange Networks (WHENs): A Review of the Current State of the Art. Ind. Eng. Chem. Res. 2019, 59, 507–525. [Google Scholar] [CrossRef]
- Blaise, M.; Feidt, M.; Maillet, D. Influence of the working fluid properties on optimized power of an irreversible finite dimensions Carnot engine. Energy Convers. Manag. 2018, 163, 444–456. [Google Scholar] [CrossRef]
- Badur, J.; Kowalczyk, T.; Ziółkowski, P.; Tokarczyk, P.; Woźniak, M. Study of the effectiveness of the turbine condenser air extraction system using hydro ejectors. Trans IFFM 2016, 131, 41–53. [Google Scholar]
- Marto, P.J.; Nunn, R.H. Power Condenser Heat Transfer Technology: Computer modelling/Design/Fouling; Hemisphere Publishing Corporation: Washington, DC, USA, 1981. [Google Scholar]
- Strušnik, D.; Golob, M.; Avsec, J. Effect of non-condensable gas on heat transfer in steam turbine condenser and modelling of ejector pump system by controlling the gas extraction rate through extraction tubes. Energy Convers. Manag. 2016, 126, 228–246. [Google Scholar] [CrossRef]
- Trela, M.; Kwidzinski, R.; Butrymowicz, D.; Karwacki, J. Exergy analysis of two-phase steam–water injector. Appl. Therm. Eng. 2010, 30, 340–346. [Google Scholar] [CrossRef]
- Goliński, J.; Troskaliński, A. Ejectors Theory and Design (Strumienice Teoria i Konstrukcja); WNT: Warsaw, Poland, 1979. (In Polish) [Google Scholar]
- Neve, R. Diffuser performance in two-phase jet pumps. Int. J. Multiph. Flow 1991, 17, 267–272. [Google Scholar] [CrossRef]
- Banasiak, K.; Hafner, A. 1D Computational model of a two-phase R744 ejector for expansion work recovery. Int. J. Therm. Sci. 2011, 50, 2235–2247. [Google Scholar] [CrossRef]
- Śmierciew, K.; Butrymowicz, D.; Kwidziński, R.; Przybyliński, T. Analysis of application of two-phase injector in ejector refriger-ation systems for isobutane. Appl. Therm. Eng. 2015, 78, 630–639. [Google Scholar] [CrossRef]
- He, S.; Li, Y.; Wang, R.Z. Progress of mathematical modelling on ejectors. Renew. Sustain. Energy Rev. 2009, 13, 1760–1780. [Google Scholar] [CrossRef]
- Yuan, G.; Zhang, L.; Zhang, H.; Wang, Z. Numerical and experimental investigation of performance of the liquid–gas and liquid jet pumps in desalination systems. Desalination 2011, 276, 89–95. [Google Scholar] [CrossRef]
- Banasiak, K.; Palacz, M.; Hafner, A.; Buliński, Z.; Smołka, J.; Nowak, A.J.; Fic, A. A CFD-based investigation of the energy perfor-mance of two-phase R744 ejectors to recover the expansion work in refrigeration systems: An irreversibility analysis. Int. J. Refrig. 2014, 40, 328–337. [Google Scholar] [CrossRef]
- Sag, N.B.; Ersoy, H.K.; Hepbasli, A.; Halkaci, H. Energetic and exergetic comparison of basic and ejector expander refrigeration systems operating under the same external conditions and cooling capacities. Energy Convers. Manag. 2015, 90, 184–194. [Google Scholar] [CrossRef]
- Colarossi, M.; Trask, N.; Schmidt, D.P.; Bergander, M.J. Multidimensional modeling of condensing two-phase ejector flow. Int. J. Refrig. 2012, 35, 290–299. [Google Scholar] [CrossRef]
- Ameur, K.; Aidoun, Z.; Ouzzane, M. Modeling and numerical approach for the design and operation of two-phase ejectors. Appl. Therm. Eng. 2016, 109, 809–818. [Google Scholar] [CrossRef]
- Witte, J.H. Mixing Shocks and Their Influence on the Design of Liquid-Gas Ejectors. Ph.D. Thesis, Uitgeverij Waltman, TU Delft, The Netherlands, 1962. [Google Scholar]
- Witte, J.H. Mixing shocks in two-phase flow. J. Fluid Mech. 1969, 36, 639–655. [Google Scholar] [CrossRef]
- Biswas, M.N.; Mitra, A.K. Momentum transfer in horizontal multi-jet liquid-gas ejector. Can. J. Chem. Eng. 1981, 59, 634–637. [Google Scholar] [CrossRef]
- Cunningham, R.G. Gas Compression with the Liquid Jet Pump. J. Fluids Eng. 1974, 96, 203–215. [Google Scholar] [CrossRef]
- Cunningham, R.G.; Hansen, A.G.; Na, T.Y. Jet pump cavitation. J. Fluids Eng. 1970, 3, 483–494. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Badur, J. A theoretical, numerical and experimental verification of the Reynolds thermal transpiration law. Int. J. Numer. Methods Heat Fluid Flow 2018, 28, 64–80. [Google Scholar] [CrossRef]
- Badur, J.; Banaszkiewicz, M. Model of the ideal fluid with scalar microstructure. An application to flashing flow of water. Trans. IFFM 1999, 105, 115–152. [Google Scholar]
- Bilicki, Z.; Badur, J. A thermodynamically consistent relaxation model for a turbulent, binary mixture undergoing phase transi-tion. J. Non-Equilib. Thermodyn. 2003, 28, 145–172. [Google Scholar] [CrossRef]
- Sharma, V.P.; Kumaraswamy, S.; Mani, A. Effect of various nozzle profiles on performance of a two phase flow jet pump. World Acad. Sci. Eng. Technol. 2012, 6, 173–179. [Google Scholar]
- Havelka, P.; Linek, V.; Sinkule, J.; Zahradník, J.; Fialova, M. Effect of the ejector configuration on the gas suction rate and gas hold-up in ejector loop reactors. Chem. Eng. Sci. 1997, 52, 1701–1713. [Google Scholar] [CrossRef]
- Elbel, S. Historical and present developments of ejector refrigeration systems with emphasis on transcritical carbon dioxide air-conditioning applications. Int. J. Refrig. 2011, 34, 1545–1561. [Google Scholar] [CrossRef]
- Biń, A.K. Gas entrainment by plunging liquid jets. Chem. Eng. Sci. 1993, 48, 3585–3630. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Badur, J. Navier number and transition to turbulence. J. Phys. Conf. Ser. 2014, 530, 012035. [Google Scholar] [CrossRef]
- Badur, J.; Ziółkowski, P.; Zakrzewski, W.; Sławiński, D.; Banaszkiewicz, M.; Kaczmarczyk, O.; Kornet, S.; Ziółkowski, P.J. On the surface vis impressa caused by a fluid-solid contact. In Shell Structure Theory and Applications; Pietraszkiewicz, W., Górski, J., Eds.; Taylor & Francis: London, UK, 2014; Volume 3, pp. 53–56. [Google Scholar]
- Badur, J.; Ziółkowski, P.J.; Ziółkowski, P. On the angular velocity slip in nano flows. Microfluid. Nanofluid. 2015, 19, 191–198. [Google Scholar] [CrossRef][Green Version]
- Badur, J.; Ziółkowski, P.; Zakrzewski, W.; Sławiński, D.; Kornet, S.; Kowalczyk, T.; Hernet, J.; Piotrowski, R.; Felincjancik, J. An advanced Thermal-FSI approach to flow heating/cooling. J. Phys. Conf. Ser. 2014, 530, 012039. [Google Scholar] [CrossRef]
- Badur, J.; Ziółkowski, P.; Kornet, S.; Kowalczyk, T.; Banaś, K.; Bryk, M.; Ziółkowski, P.J.; Stajnke, M. Enhanced energy conversion as a result of fluid-solid interaction in micro- and nanoscale. J. Theor. Appl. Mech. 2018, 56, 329–332. [Google Scholar] [CrossRef]
- Butterworth, M.; Sheer, T. High-pressure water as the driving fluid in an ejector refrigeration system. Appl. Therm. Eng. 2007, 27, 2145–2152. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Głuch, J.; Ziółkowski, P. Analysis of Possible Application of High-Temperature Nuclear Reactors to Contemporary Large-Output Steam Power Plants on Ships. Pol. Marit. Res. 2016, 23, 32–41. [Google Scholar] [CrossRef]
- Lemański, M.; Karcz, M. Performance of lignite-syngas operated tubular Solid Oxide Fuell Cell. Chem. Process. Eng. 2008, 29, 233–248. [Google Scholar]
- Szewczuk-Krypa, N.; Drosińska-Komor, M.; Głuch, J.; Breńkacz, Ł. Comparison analysis of selected nuclear power plants supplied with helium from high-temperature gas-cooled reactor. Pol. Marit. Res. 2018, 25, 204–210. [Google Scholar] [CrossRef]
- Martinaitis, V.; Rimdžius, D.; Bielskus, J.; Streckienė, G.; Motuzienė, V. Preliminary comparison of the performance of thermody-namic models of the subsonic ejector and turbofan. J. Mech. Eng. 2020, 66, 325–336. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Badur, J.; Ziółkowski, P. Comparative study of a bottoming SRC and ORC for Joule–Brayton cycle cooling modular HTR exergy losses, fluid-flow machinery main dimensions, and partial loads. Energy 2020, 206, 118072. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Kowalczyk, T.; Kornet, S.; Badur, J. On low-grade waste heat utilization from a supercritical steam power plant using an ORC-bottoming cycle coupled with two sources of heat. Energy Convers. Manag. 2017, 146, 158–173. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Ziółkowski, P.; Badur, J. Exergy Losses in the Szewalski Binary Vapor Cycle. Entropy 2015, 17, 7242–7265. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Zakrzewski, W.; Kaczmarczyk, O.; Badur, J. Thermodynamic analysis of the double Brayton cycle with the use of oxy combustion and capture of CO2. Arch. Thermodyn. 2013, 34, 23–38. [Google Scholar] [CrossRef]
- Topolski, J.; Badur, J. Comparison of the combined cycle efficiencies with different heat recovery steam generators. Trans. IFFM 2002, 111, 5–16. [Google Scholar]
- Chmielniak, T.; Trela, M. Diagnostics of New-Generation Thermal Power Plants; IMP PAN Publisher: Gdańsk, Poland, 2008. [Google Scholar]
- Tindell, R.H.; Alston, T.M.; Sarro, C.A.; Stegmann, G.C.; Gray, L.; Davids, J. Computational Fluid Dynamics Analysis of a Steam Power Plant Low-Pressure Turbine Downward Exhaust Hood. J. Eng. Gas Turbines Power 1996, 118, 214–224. [Google Scholar] [CrossRef]
- Veerabathraswamy, K.; Kumar, A.S. Effective boundary conditions and turbulence modeling for the analysis of steam turbine exhaust hood. Appl. Therm. Eng. 2016, 103, 773–780. [Google Scholar] [CrossRef]
- Śmierciew, K.; Butrymowicz, D.; Przybyliński, T.; Pawluczuk, A. Investigations of heat and momentum transfer in two-phase injector operating with isobutene. Appl. Therm. Eng. 2017, 127, 1495–1505. [Google Scholar] [CrossRef]
- Burton, Z.; Ingram, G.L.; Hogg, S. A literature review of low pressure steam turbine exhaust hood and diffuser studies. J. Eng. Gas Turbines Power 2013, 135, 062001. [Google Scholar] [CrossRef]
- Szulc, O.; Doerffer, P.; Tejero, F. Passive control of rotorcraft high-speed impulsive noise. J. Phys. Conf. Ser. 2016, 760, 012031. [Google Scholar] [CrossRef]
- Ochrymiuk, T. Numerical prediction of film cooling effectiveness over flat plate using variable turbulent Prandtl number clo-sures. J. Therm. Sci. 2016, 25, 280–286. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Witanowski, Ł.; Klonowicz, P.; Głuch, S. Optimizationof the last stage of gas-steam turbine using a hybrid method. In Proceedings of the 14th European Conference on Turbomachinery Fluid Dynamics and Thermodynamics ETC 2021, Gdansk, Poland, 12–16 April 2021. [Google Scholar]
- Ziółkowski, P.; Głuch, S.; Kowalczyk, T.; Badur, J. Revalorisation of the Szewalski’s concept of the law of varying the last-stage blade retraction in a gas-steam turbine. E3S Web Conf. 2021, 323, 34. [Google Scholar] [CrossRef]
- Witanowski, Ł.; Klonowicz, P.; Lampart, P.; Suchocki, T.; Jędrzejewski, Ł.; Zaniewski, D.; Klimaszewski, P. Optimization of an axial turbine for a small scale ORC waste heat recovery system. Energy 2020, 205, 118059. [Google Scholar] [CrossRef]
- Klonowicz, P.; Witanowski, Ł.; Suchocki, T.; Jędrzejewski, Ł.; Lampart, P. Selection of optimum degree of partial admission in a laboratory organic vapour microturbine. Energ. Convers. Manag. 2019, 202, 112189. [Google Scholar] [CrossRef]
- Lampart, P.; Witanowski, Ł.; Klonowicz, P. Efficiency Optimisation of Blade Shape in Steam and ORC Turbines. Mech. Mech. Eng. 2018, 22, 553–564. [Google Scholar] [CrossRef]
- Madejski, P.; Taler, D. Analysis of temperature and stress distribution of superheater tubes after attemperation or sootblower activation. Energy Convers. Manag. 2013, 71, 131–137. [Google Scholar] [CrossRef]
- Badur, J.; Ziółkowski, P.; Sławiński, D.; Kornet, S. An approach for estimation of water wall degradation within pulverized-coal boilers. Energy 2015, 92, 142–152. [Google Scholar] [CrossRef]
- Kantorek, M.; Jesionek, K.; Polesek-Karczewska, S.; Ziółkowski, P.; Badur, J. Thermal utilization of meat and bone meals. Perfor-mance analysis in terms of drying process, pyrolysis and kinetics of volatiles combustion. Fuel 2019, 254, 115548. [Google Scholar] [CrossRef]
- Rybiński, W.; Mikielewicz, J. Analytical 1D models of the wall thermal resistance of rectangular minichannels applied in heat exchangers. Arch. Thermodyn. 2016, 37, 63–78. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Szewczuk-Krypa, N.; Butterweck, A.; Stjanke, M.; Głuch, S.; Drosińska-Komor, M.; Milewska, A.; Głuch, J. Comprehensive thermodynamic analysis of steam storage in a steam cycle in a different regime of work: A zero-dimensional and three-dimensional approach. J. Energy Resour. Technol. Trans. ASME 2021, 143, 050905. [Google Scholar] [CrossRef]
- Perycz, S. Steam and Gas Turbines; Wydawnictwo Politechniki Gdańskiej: Gdańsk, Poland, 1988. (In Polish) [Google Scholar]
- Jaremkiewicz, M.; Dzierwa, P.; Taler, D.; Taler, J. Monitoring of transient thermal stresses in pressure components of steam boilers using an innovative technique for measuring the fluid temperature. Energy 2019, 175, 139–150. [Google Scholar] [CrossRef]
- Jaremkiewicz, M.; Taler, D.; Dzierwa, P.; Taler, J. Determination of transient fluid temperature and thermal stresses in pressure thick-walled elements using a new design thermometer. Energies 2019, 12, 222. [Google Scholar] [CrossRef]
- Hyrzyński, R.; Ziółkowski, P.; Gotzman, S.; Kraszewski, B.; Ochrymiuk, T.; Badur, J. Comprehensive thermodynamic analysis of the CAES system coupled with the underground thermal energy storage taking into account global, central and local level of energy conversion. Renew. Energy 2021, 169, 379–403. [Google Scholar] [CrossRef]
- Rayati, M.; Goodarzi, H.; Ranjbar, A. Optimal bidding strategy of coordinated wind power and gas turbine units in real-time market using conditional value at risk. Int. Trans. Electr. Energy Syst. 2018, 29, e2645. [Google Scholar] [CrossRef]
- Vishwajeet; Pawlak-Kruczek, H.; Baranowski, M.; Czerep, M.; Chorążyczewski, A.; Krochmalny, K.; Ostrycharczyk, M.; Ziółkowski, P.; Madejski, P.; Mączka, T.; et al. Entrained Flow Plasma Gasification of Sewage Sludge–Proof-of-Concept and Fate of Inorganics. Energies 2022, 15, 1948. [Google Scholar] [CrossRef]
- Abam, F.; Diemuodeke, O.; Ekwe, E.; Alghassab, M.; Samuel, O.; Khan, Z.; Imran, M.; Farooq, M. Exergoeconomic and Environmental Modeling of Integrated Polygeneration Power Plant with Biomass-Based Syngas Supplemental Firing. Energies 2020, 13, 6018. [Google Scholar] [CrossRef]
- Kantorek, M.; Jesionek, K.; Polesek-Karczewska, S.; Ziółkowski, P.; Stajnke, M.; Badur, J. Thermal utilization of meat-and-bone meal using the rotary kiln pyrolyzer and the fluidized bed boiler—The performance of pilot-scale installation. Renew. Energy 2020, 164, 1447–1456. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Badur, J.; Kruczek, H.P.; Stasiak, K.; Amiri, M.; Niedzwiecki, L.; Krochmalny, K.; Mularski, J.; Madejski, P.; Mikielewicz, D. Mathematical modelling of gasification process of sewage sludge in reactor of negative CO2 emission power plant. Energy 2021, 244, 122601. [Google Scholar] [CrossRef]
- Ünveren, E.E.; Monkul, B.O.; Sarıoğlan, S.; Karademir, N.; Alper, E. Solid amine sorbents for CO2 capture by chemical adsorption: A review. Petroleum 2017, 3, 37–50. [Google Scholar] [CrossRef]
- Li, H.; Yan, J.; Campana, P.E. Feasibility of integrating solar energy into a power plant with amine-based chemical absorption for CO2 capture. Int. J. Greenh. Gas Control 2012, 9, 272–280. [Google Scholar] [CrossRef]
- Atsonios, K.; Panopoulos, K.D.; Kakaras, E. Investigation of technical and economic aspects for methanol production through CO2 hydrogenation. Int. J. Hydrogen Energy 2016, 41, 2202–2214. [Google Scholar] [CrossRef]
- Bowker, M. Methanol Synthesis from CO2 Hydrogenation. ChemCatChem 2019, 11, 4238–4246. [Google Scholar] [CrossRef]
- Aigba, P.A.; Emovon, I.; Samuel, O.D.; Chintua, E.C.; Abdeljawad, T.; Al-Mdallal, Q.M.; Afzal, A. Exergetic Assessment of Waste Gas to Energy in a Novel Integrated NGL Recovery and Power Generation Plant. Front. Energy Res. 2022, 9, 798896. [Google Scholar] [CrossRef]
- Giwa, S.O.; Nwaokocha, C.N.; Samuel, D.O. Off-grid gasoline-powered generators: Pollutants’ footprints and health risk assessment in Nigeria. Energy Sour. Part A: Recover. Util. Environ. Eff. 2019, 1–18. [Google Scholar] [CrossRef]
- Okwu, M.O.; Samuel, O.D.; Ewim, D.R.E.; Huan, Z. Estimation of biogas yields produced from combination of waste by implementing response surface methodology (RSM) and adaptive neuro-fuzzy inference system (ANFIS). Int. J. Energy Environ. Eng. 2021, 12, 353–363. [Google Scholar] [CrossRef]
- Okwu, M.O.; Tartibu, L.K.; Samuel, O.D.; Omoregbee, H.O.; Ivbanikaro, A.E. Predictive Ability of Response Surface Methodology (RSM) and Artificial Neural Network (ANN) to Approximate Biogas Yield in a Modular Biodigester. Int. Work-Conf. Artif. Neural Netw. 2021, 12861, 202–215. [Google Scholar] [CrossRef]


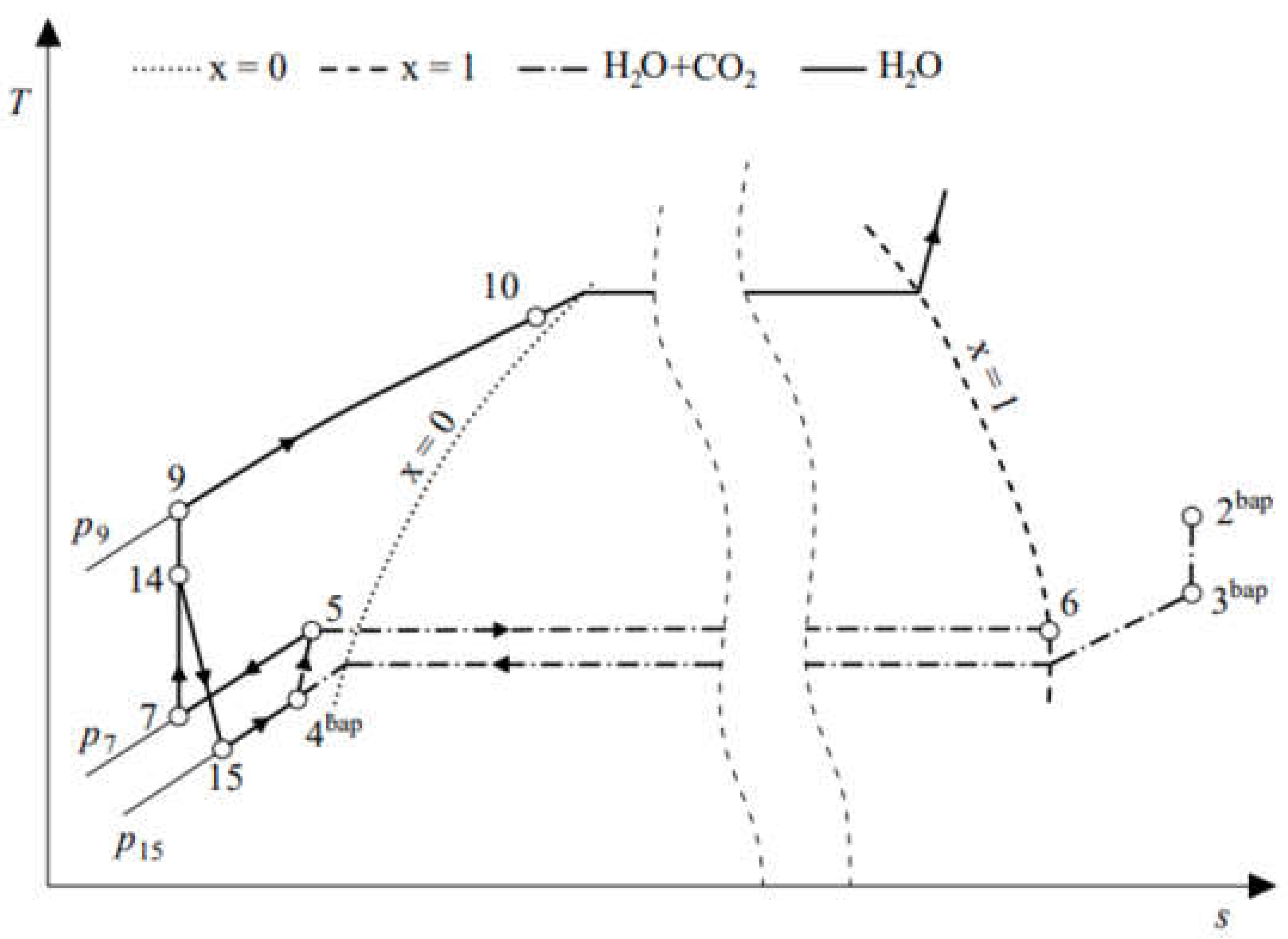

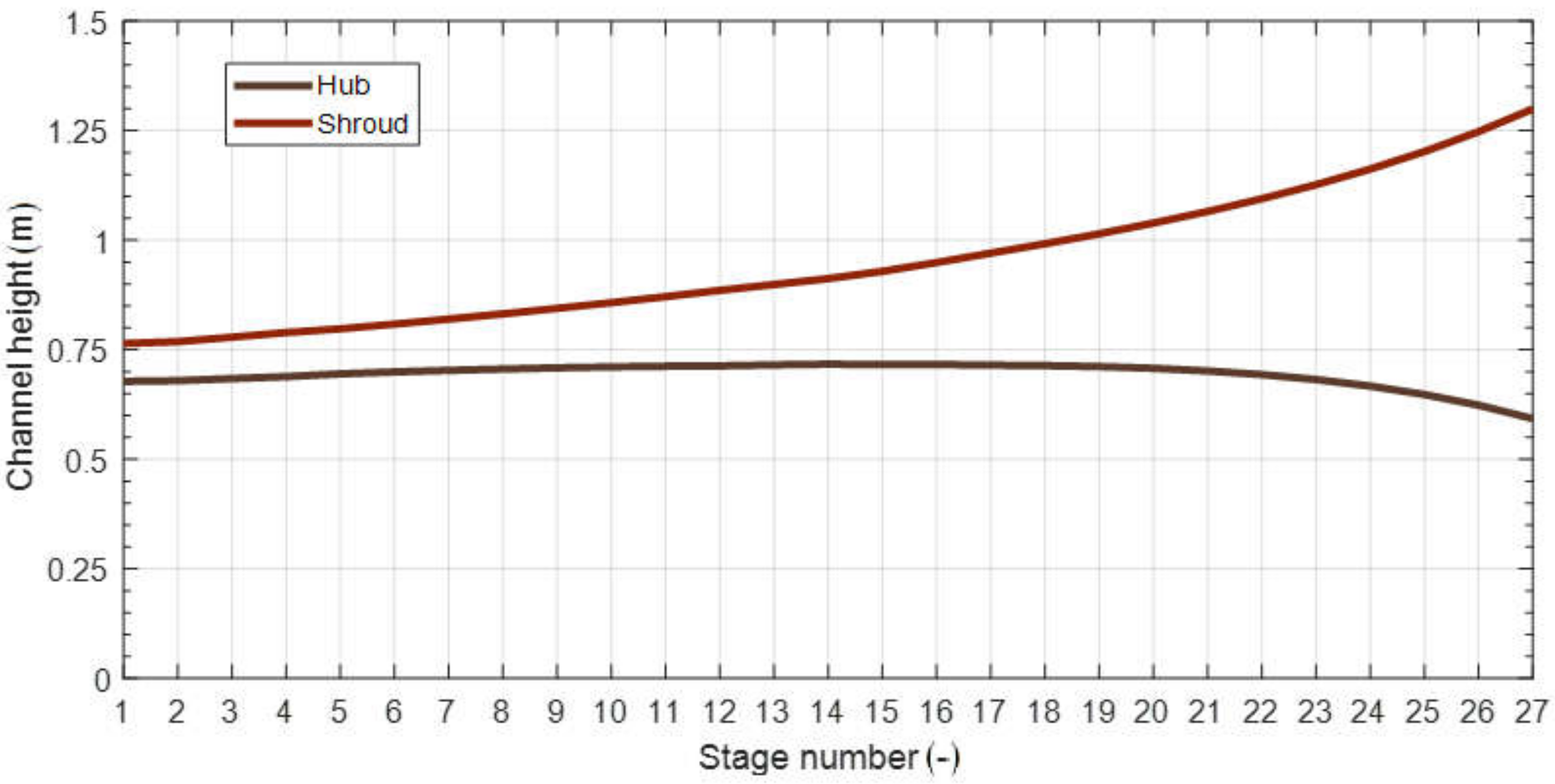
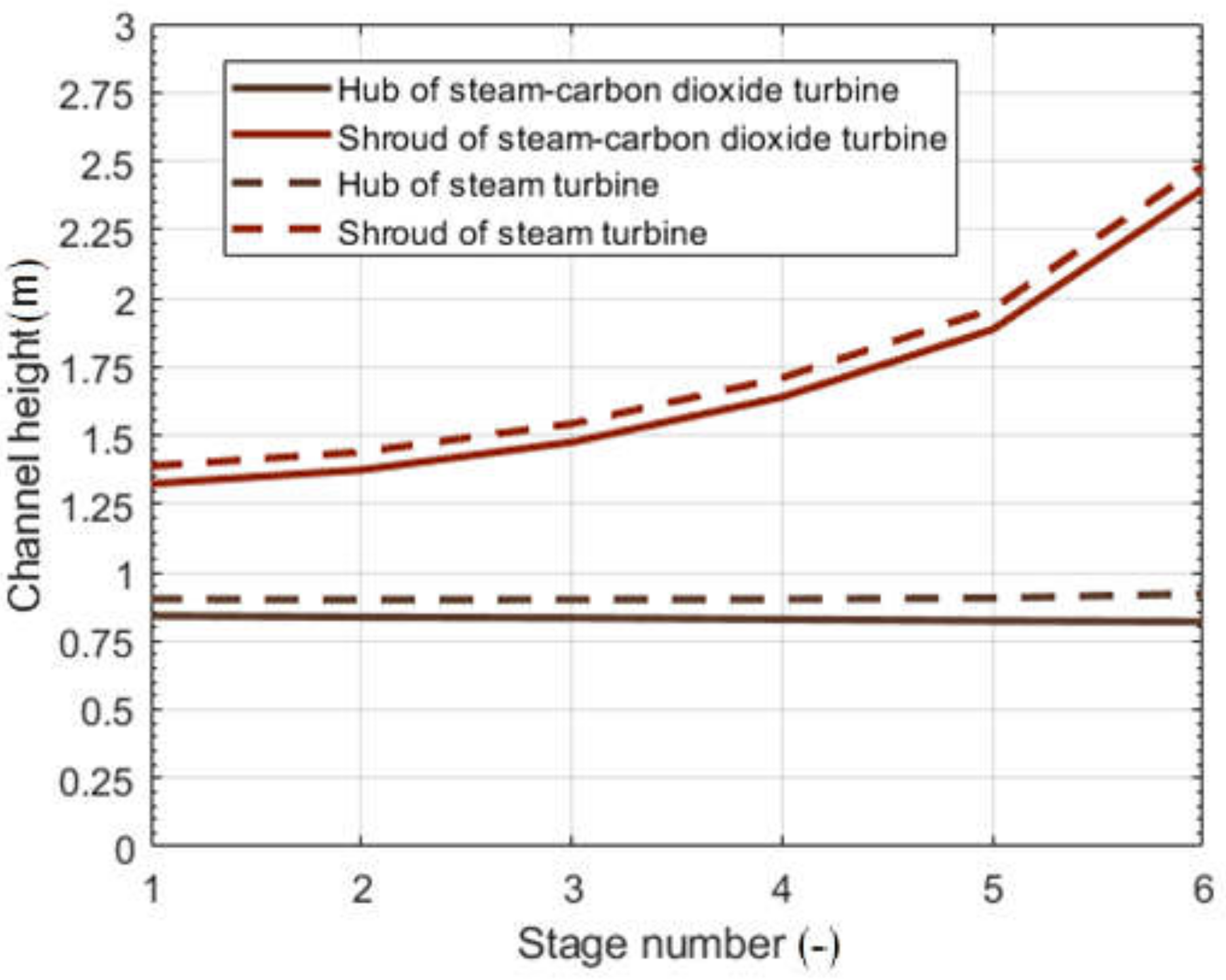
| Parameter | Symbol | Unit | Value | Reference |
|---|---|---|---|---|
| Efficiency and pressure drop in main devices | ||||
| Mechanical efficiency of the turbine and compressor | - | 0.99 | [1,5] | |
| Isentropic efficiency of the turbine | - | 0.89 | [1,5] | |
| Isentropic efficiency of the compressor | - | 0.88 | [1,5] | |
| Electrical motor efficiency | - | 0.95 | [1,5] | |
| Electrical generator efficiency | - | 0.97 | [1,5] | |
| Relative pressure losses in the combustion chamber | - | 0.003 | [1,5] | |
| Efficiency of the combustion chamber with (K) | - | 0.99 | [129] | |
| Isentropic efficiency of pump | - | 0.75 | [1,5] | |
| Mechanical efficiency of pump | 0.98 | [1,5] | ||
| Thermal efficiency of cooling heat exchangers | 0.98 | [1,5] | ||
| Relative pressure losses in regenerative heat exchangers HE | ||||
| HE—the low-temperature (cold) side | - | 0.006 | [130] | |
| HE—the high-temperature (hot) side | - | 0.0075 | [130] | |
| Minimum temperature difference | K | 70 | [130] | |
| Air and fuel thermodynamic parameters | ||||
| Fuel temperature | K | 288.15 | [1,5] | |
| Fuel pressure | MPa | 4.05 | [1,5] | |
| Fuel mass flow rate | kg/s | 12.83 | [129] | |
| Air temperature | K | 288.15 | [1,5] | |
| Air pressure | MPa | 0.101 | [1,5] | |
| Oxygen mass flow rate | kg/s | 51.8 | [129] | |
| Spray-ejector condenser | ||||
| Volumetric entrainment ratio | - | 1 | [94] | |
| Pressure ratio with regard to pressure at the outlet from the spray-ejector condenser | - | 64.1 | [94] | |
| Dimensionless compression ratio | - | 0.19748 | [94] | |
| Efficiency of the nozzle | - | 0.91 | [94] | |
| Efficiency of the suction chamber | - | 0.99 | [94] | |
| Efficiency of the secondary nozzle and mixing chamber | - | 0.99 | [94] | |
| Efficiency of the diffuser | - | 0.7 | [94] | |
| Assumed parameters in thermodynamic points | ||||
| Water injected to combustion chamber mass flow rate | kg/s | 117.7 | [129] | |
| Temperature of condensation | °C | 20 | [129] | |
| Pressure at the outlet from the CCU | bar | 80 | [129] | |
| Pressure at the outlet from the turbine | kPa | 7.7 | [129] | |
| Pressure of condensation | kPa | 7.0 | [94] | |
| Point | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| O2 | H2O | N2 | CO2 | NOX | |||||
| °C | kPa | - | kg/s | - | |||||
| 1 | 32.2 | 515.8 | 1.00 | 51.8 | 0.989 | 0.000 | 0.011 | 0.000 | 0.000 |
| 2 | 822 | 4050.0 | 1.00 | 51.8 | 0.989 | 0.000 | 0.011 | 0.000 | 0.000 |
| 3 | 1338 | 4000.0 | 1.00 | 182.3 | 0.000 | 0.908 | 0.002 | 0.089 | 0.000 |
| 4 | 641 | 101.3 | 1.00 | 182.3 | 0.000 | 0.908 | 0.002 | 0.089 | 0.000 |
| 302 | 7.7 | 1.00 | 182.3 | 0.000 | 0.908 | 0.002 | 0.089 | 0.000 | |
| 101 | 7.7 | 1.00 | 182.3 | 0.000 | 0.908 | 0.002 | 0.089 | 0.000 | |
| 39.0 | 7.0 | 0.93 | 182.3 | 0.000 | 0.9084 | 0.0022 | 0.0894 | 0.000 | |
| 20.83 | 7.0 | 0.001 | 159,192.0 | - | 1 (lq) | - | - | - | |
| 5 | 20.83 | 101.3 | 0.001 | 159,192.0 | - | 1 (lq) | - | - | - |
| 6 | 20 | 101.3 | 1.00 | 36.4 | 0.000 | 0.042 | 0.024 | 0.934 | 0.000 |
| 7 | 20 | 101.3 | 0.00 | 159,155.6 | 0.000 | 1 (lq) | 0.000 | 0.000 | 0.000 |
| 8 | 20 | 101.3 | 0.00 | 159,155.6 | 0.000 | 1 (lq) | 0.000 | 0.000 | 0.000 |
| 9 | 20.05 | 4000.0 | 0.00 | 117.7 | 0.000 | 1 (lq) | 0.000 | 0.000 | 0.000 |
| 10 | 214 | 4000.0 | 0,00 | 117.7 | 0.000 | 1 (lq) | 0.000 | 0.000 | 0.000 |
| 11 | 20 | 101.3 | 0.00 | 159,037.9 | 0.000 | 1 (lq) | 0.000 | 0.000 | 0.000 |
| 12 | 20 | 101.3 | 0.00 | 28.2 | 0.000 | 1 (lq) | 0.000 | 0.000 | 0.000 |
| 13 | 20 | 101.3 | 0.00 | 159,009.6 | 0.000 | 1 (lq) | 0.000 | 0.000 | 0.000 |
| 14 | 20.1 | 500.0 | 0.00 | 159,009.6 | 0.000 | 1 (lq) | 0.000 | 0.000 | 0.000 |
| 15 | 20.1 | 7.0 | 0.00 | 159,009.6 | 0.000 | 1 (lq) | 0.000 | 0.000 | 0.000 |
| 151 | 500.0 | 1.00 | 36.4 | 0.000 | 0.042 | 0.024 | 0.934 | 0.000 | |
| 30 | 500.0 | 0.00 | 0.526 | 0.000 | 1 (lq) | 0.000 | 0.000 | 0.000 | |
| 30 | 500.0 | 1.00 | 35.91 | 0.000 | 0.009 | 0.022 | 0.969 | 0.000 | |
| 172 | 2500.0 | 1.00 | 35.91 | 0.000 | 0.009 | 0.022 | 0.969 | 0.000 | |
| 30 | 2500.0 | 0.00 | 0.099 | 0.000 | 1 (lq) | 0.000 | 0.000 | 0.000 | |
| 30 | 2500.0 | 1.00 | 35.81 | 0.000 | 0.002 | 0.023 | 0.975 | 0.000 | |
| 138 | 8000.0 | 1.00 | 35.81 | 0.000 | 0.002 | 0.023 | 0.975 | 0.000 | |
| Point | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| °C | bar | m/s | kJ/kg | kJ/kg | kg/s | m3/s | m2 | kg/m3 | |
| 101.0 | 0.078 | 50.00 | 1.25 | 3080.79 | 182.3 | 5216.8 | 104.34 | 0.03494 | |
| 39.0 | 0.07 | 559.19 | 156.35 | 2571.76 | 182.3 | 1773.9 | 3.17 | 0.10277 | |
| 14 | 20.0 | 5.00 | 100.00 | 5.00 | 126.20 | 145,332.3 | 145.9 | 1.46 | 995.82 |
| 20.0 | 0.07 | 104.84 | 5.50 | 125.75 | 145,332.3 | 145.9 | 1.39 | 995.82 | |
| 20.83 | 0.07 | 100.13 | 5.01 | 129.48 | 145,514.6 | 146.2 | 1.46 | 995.33 | |
| 5 | 20.83 | 1.05 | 99.14 | 4.91 | 129.66 | 145,514.6 | 146.2 | 1.47 | 995.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziółkowski, P.; Głuch, S.; Ziółkowski, P.J.; Badur, J. Compact High Efficiency and Zero-Emission Gas-Fired Power Plant with Oxy-Combustion and Carbon Capture. Energies 2022, 15, 2590. https://doi.org/10.3390/en15072590
Ziółkowski P, Głuch S, Ziółkowski PJ, Badur J. Compact High Efficiency and Zero-Emission Gas-Fired Power Plant with Oxy-Combustion and Carbon Capture. Energies. 2022; 15(7):2590. https://doi.org/10.3390/en15072590
Chicago/Turabian StyleZiółkowski, Paweł, Stanisław Głuch, Piotr Józef Ziółkowski, and Janusz Badur. 2022. "Compact High Efficiency and Zero-Emission Gas-Fired Power Plant with Oxy-Combustion and Carbon Capture" Energies 15, no. 7: 2590. https://doi.org/10.3390/en15072590
APA StyleZiółkowski, P., Głuch, S., Ziółkowski, P. J., & Badur, J. (2022). Compact High Efficiency and Zero-Emission Gas-Fired Power Plant with Oxy-Combustion and Carbon Capture. Energies, 15(7), 2590. https://doi.org/10.3390/en15072590








