Permeability Modeling and Estimation of Hydrogen Loss through Polymer Sealing Liners in Underground Hydrogen Storage
Abstract
:1. Introduction
2. Methodology and Materials
- Gas permeability coefficient (P) of pure polymer;
- Type of powder additive;
- Amount (volume) of powder additive;
- Size of powder grains;
- Dispersion of powder in the material.
- Additive volume (Φ);
- Permeability coefficient (Pc) of pure polymer (continuous phase);
- Permeability coefficient (Pd) of additive (dispersed phase);
3. Maxwell Model Permeability
4. Hydrogen Leakage Estimation
5. Discussion
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Denholm, P.; Ela, E.; Kirby, B.; Milligan, M. The Role of Energy Storage with Renewable Electricity Generation; Technical Report NREL/TP-6A2-47187; National Renewable Energy Laboratory: Golden, CO, USA, 2010. [Google Scholar]
- European Comission. Renewable Energy Progress Report; European Comission: Brussels, Belgium, 2019. [Google Scholar]
- Matos, C.R.; Carneiro, J.F.; Silva, P.P. Overview of Large-Scale Underground Energy Storage Technologies for Integration of Renewable Energies and Criteria for Reservoir Identification. J. Energy Storage 2018, 21, 241–258. [Google Scholar] [CrossRef]
- Apostolou, D.; Enevoldsen, P. The past, present and potential of hydrogen as a multifunctional storage application for wind power. Renew. Sustain. Energy Rev. 2019, 112, 917–929. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to Gas projects review: Lab, pilot and demo plants for storing renewable energy and CO2. Renew. Sustain. Energy Rev. 2017, 69, 292–312. [Google Scholar] [CrossRef]
- Altfeld, K.; Pinchbeck, D. Admissible Hydrogen Concentrations in Natural Gas Systems. Gas Energy 2013, 2103, 1–2. [Google Scholar]
- Kuczyński, S.; Łaciak, M.; Olijnyk, A.; Szurlej, A.; Włodek, T. Thermodynamic and Technical Issues of Hydrogen and Methane-Hydrogen Mixtures Pipeline Transmission. Energies 2019, 12, 569. [Google Scholar] [CrossRef] [Green Version]
- Hevin, G. Underground Storage of Hydrogen in Salt Caverns; European Workshop on Underground Energy Storage: Paris, France, 2019. [Google Scholar]
- Gillhaus, A. Natural Gas Storage in Salt Caverns–Present Status, Developments and Future Trends in Europe; Springer: Basel, Switzerland, 2007. [Google Scholar]
- Stormont, J.C. In situ gas permeability measurements to delineate damage in rock salt. Int. J. Rock Mech. Min. Sci. 1997, 39, 1055–1064. [Google Scholar] [CrossRef]
- Tengborg, P.; Johansson, J.; Durup, J.G. Storage of Highly Compressed Gases in Underground Lined Rock Caverns–More than 10 years of Experience. In Proceedings of the World Tunnel Congress 2014–Tunnels for a Better Life, Foz do Iguacu, Brazil, 9–15 May 2014. [Google Scholar]
- Gupta, S.B. Innovative Methods of LNG Storage in Underground Lined Rock Caverns. In Natural Gas Extraction to End Use; IntechOpen: London, UK, 2012; pp. 159–180. [Google Scholar] [CrossRef]
- Kim, H.-M.; Rutqvist, J.; Jeong, J.-H.; Choi, B.-H.; Ryu, D.-W.; Song, W.-K. Characterizing Excavation Damaged Zone and Stability of Pressurized Lined Rock Caverns for Underground Compressed Air Energy Storage. Rock Mech. Rock Eng. 2012, 46, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Panfilov, M. 4-Underground and pipeline hydrogen storage. In Compendium of Hydrogen Energy; Gupta, R.B., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Swanston, UK, 2016; pp. 91–115. ISBN 978-1-78242-362-1. [Google Scholar]
- Portarapillo, M.; Di Benedetto, A. Risk Assessment of the Large-Scale Hydrogen Storage in Salt Caverns. Energies 2021, 14, 2856. [Google Scholar] [CrossRef]
- Van Rooyen, L.J.; Karger-Kocsis, J.; Kock, L.D. Improving the helium gas barrier properties of epoxy coatings through the incorporation of graphene nanoplatelets and the influence of preparation techniques. J. Appl. Polym. Sci. 2015, 132, 42584. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Wang, Y.C.; Bailey, C.G.; Istrate, O.M.; Li, Z.; Kinloch, I.A.; Budd, P.M. Quantification of gas permeability of epoxy resin composites with graphene nanoplatelets. Compos. Sci. Technol. 2019, 184, 107875. [Google Scholar] [CrossRef]
- Zeman, S.; Kubík, L. Permeability of Polymeric Packaging Materials. Tech. Sci. 2007, 10, 33–34. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ono, H.; Onoue, K.; Nishimura, S. High-pressure gaseous hydrogen permeation test method -property of polymeric materials for high-pressure hydrogen devices. Int. J. Hydrogen Energy 2020, 45, 29082–29094. [Google Scholar] [CrossRef]
- Maxwell, A.S.; Roberts, S.J. Review of Data on Gas Migration through Polymer Encapsulants. Report to NDA–Radioactive Waste Management Directorate; Serco Ltd.: Oxfordshire, UK, 2008. [Google Scholar]
- Prewitz, M.; Gaber, M.; Müller, R.; Marotztke, C.; Holtappels, K. Polymer coated glass capillaries and structures for high-pressure hydrogen storage: Permeability and hydrogen tightness. Int. J. Hydrogen Energy 2018, 43, 5637–5644. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Nguyen, T.T.; Li, X.; Kim, N.H.; Lee, J.H. Enhanced hydrogen gas barrier performance of diaminoalkane functionalized stitched graphene oxide/polyurethane composites. Compos. Part B Eng. 2017, 117, 101–110. [Google Scholar] [CrossRef]
- Pepin, J.; Lainé, E.; Grandidier, J.-C.; Castagnet, S.; Blanc-Vannet, P.; Papin, P.; Weber, M. Determination of key parameters responsible for polymeric liner collapse in hyperbaric type IV hydrogen storage vessels. Int. J. Hydrogen Energy 2018, 43, 16386–16399. [Google Scholar] [CrossRef]
- Blanc-Vannet, P.; Papin, P.; Weber, M.; Renault, P.; Pepin, J.; Lainé, E.; Tantchou, G.; Castagnet, S.; Grandidier, J.-C. Sample scale testing method to prevent collapse of plastic liners in composite pressure vessels. Int. J. Hydrogen Energy 2018, 44, 8682–8691. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, G.; Xiao, S.; Hua, Z.; Gu, C. Study on fretting behavior of rubber O-ring seal in high-pressure gaseous hydrogen. Int. J. Hydrogen Energy 2019, 44, 22569–22575. [Google Scholar] [CrossRef]
- Gajda, D.; Lutyński, M. Hydrogen Permeability of Epoxy Composites as Liners in Lined Rock Caverns—Experimental Study. Appl. Sci. 2021, 11, 3885. [Google Scholar] [CrossRef]
- Gajda, D. Epoxy Resin for Sealing the Underground Hydrogen Storage Reservoirs. In Proceedings of the 5th International Conference on Energy Harvesting, Storage and Transfer (EHST’21), Niagara Falls, ON, Canada, 21–23 May 2021. [Google Scholar] [CrossRef]
- Choudalakis, G.; Gotsis, A. Permeability of polymer/clay nanocomposites: A review. Eur. Polym. J. 2009, 45, 967–984. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, C.; Zheng, Y.; Ding, H.; Wang, Q.; Shen, Q.; Li, X.; Zhang, L. Characteristics and performance of CaO-based high temperature CO2 sorbents derived from a sol–gel process with different supports. RSC Adv. 2016, 6, 79285–79296. [Google Scholar] [CrossRef]
- Wolf, C.; Angellier-Coussy, H.; Gontard, N.; Doghieri, F.; Guillard, V. How the shape of fillers affects the barrier properties of polymer/non-porous particles nanocomposites: A review. J. Membr. Sci. 2018, 556, 393–418. [Google Scholar] [CrossRef]
- Wu, H.; Zamanian, M.; Kruczek, B.; Thibault, J. Gas Permeation Model of Mixed-Matrix Membranes with Embedded Impermeable Cuboid Nanoparticles. Membranes 2020, 10, 422. [Google Scholar] [CrossRef] [PubMed]
- Gajda, D.; Liu, S.; Lutyński, M. The concept of hydrogen-methane blends storage in underground mine excavations–gas permeability of concrete. E3S Web Conf. 2021, 266, 03007. [Google Scholar] [CrossRef]
- Henager, C.H. Hydrogen Permeation Barrier Coatings. In Materials for the Hydrogen Economy; Jones, R.H., Thomas, G.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 181–190. [Google Scholar]
- Rybak, A.; Rybak, A.; Sysel, P. Modeling of Gas Permeation through Mixed-Matrix Membranes Using Novel Computer Application MOT. Appl. Sci. 2018, 8, 1166. [Google Scholar] [CrossRef] [Green Version]
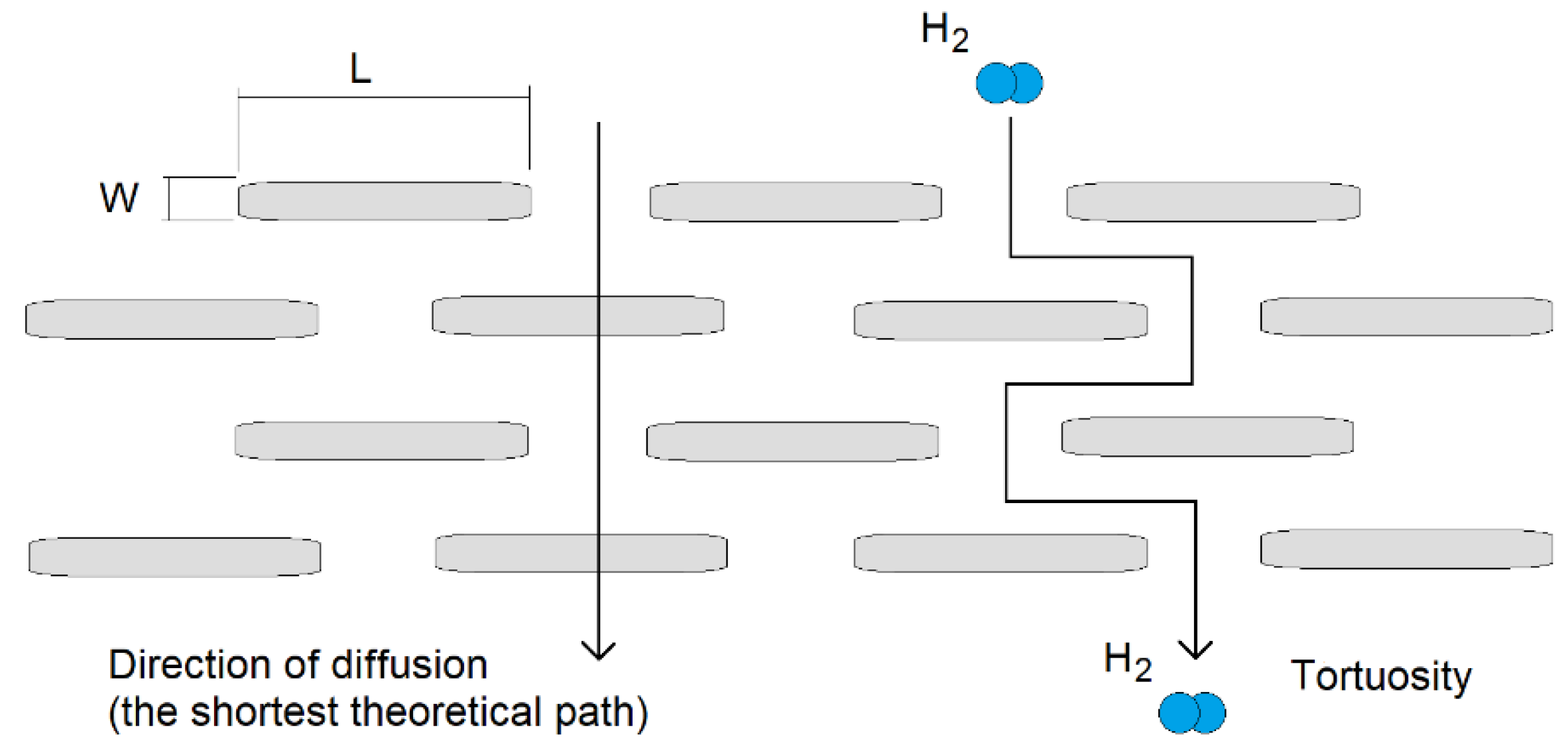
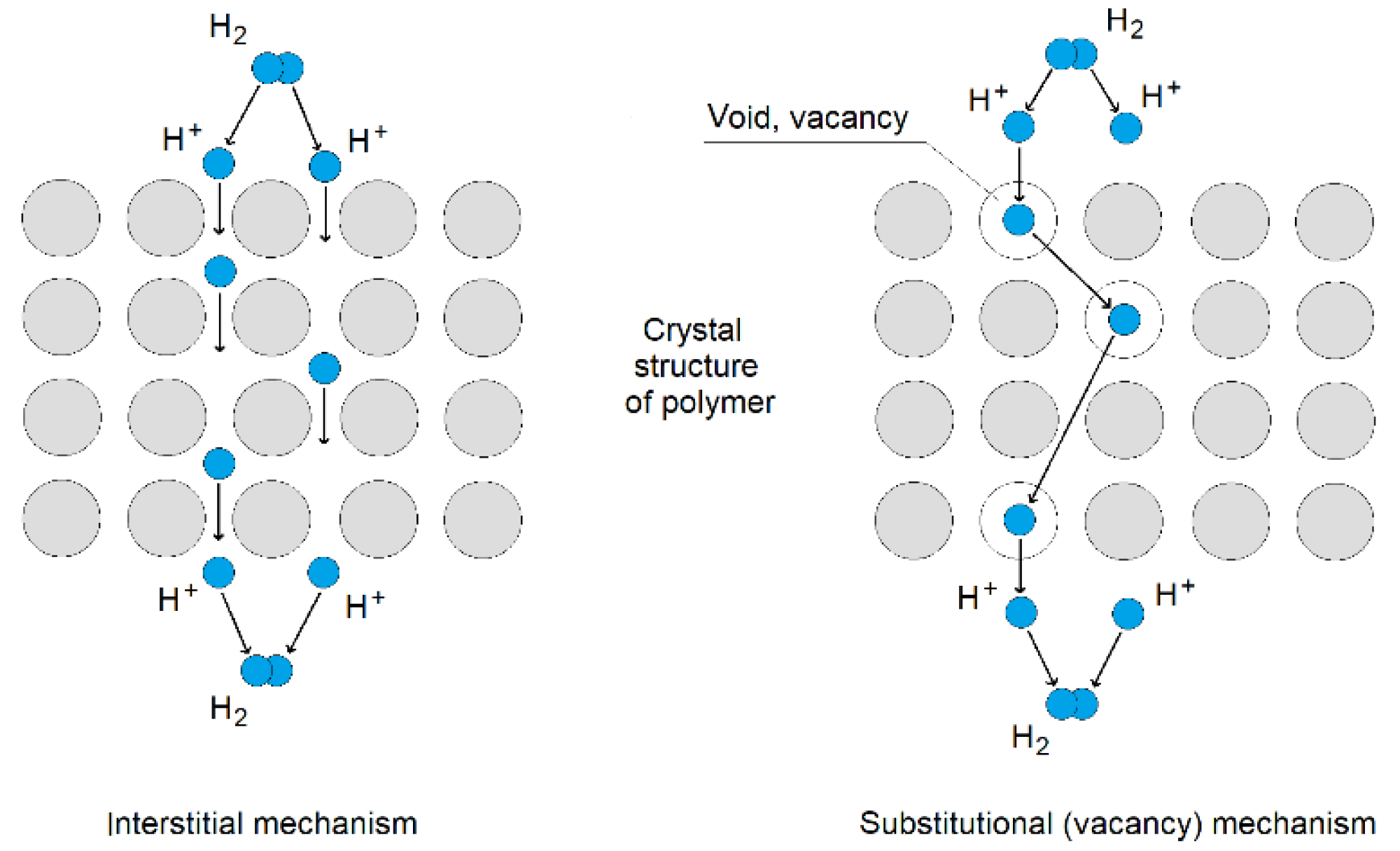





| Sample | Base | Physical Properties | Additives |
|---|---|---|---|
| Epoxy resin | 2,2-Bis (4-hydroxyphenyl) propane with epichlorohydrin resin-hardener ratio: 100:12 | Viscosity: 15,000–30,000 mPa·s Epoxide number: 0.48–0.52 mol/100 g Chlorine content: <0.6% Pot time: 90 min. | Mechanical impurities < 0.03% |
| Epoxy resin + graphite | Amorphous graphite < 50 µm | ||
| Epoxy resin + halloysite | Grinded halloysite < 125 µm | ||
| Epoxy resin + fly ash | Sieved fly ash < 125 µm |
| Parameter | Symbol, Unit | Additive | ||
|---|---|---|---|---|
| Fly Ash | Amorphous Graphite | Grinded Halloysite | ||
| Permeability of pure epoxy resin (continuous phase) | Pc, Barrer | 0.182 | 0.182 | 0.182 |
| Permeability of epoxy resin with additive (effective permeability), experimental | Peff, Barrer | 0.177 | 0.235 | 0.332 |
| Volume of additive | Φ, % | 5 | 5 | 5 |
| Relative permeability | Pr | 0.973 | 1.291 | 1.769 |
| Parameter | Symbol, Unit | Additive | ||
|---|---|---|---|---|
| Fly Ash | Amorphous Graphite | Amorphous Graphite | ||
| Additive volume | % | 30 | 10 | 15 |
| Hydrogen permeability (Maxwell model) | Pmod, Barrer | 0.153 | 0.299 | 0.379 |
| Hydrogen permeability (experimental) | Pexp, Barrer | 0.177 | 0.249 | 0.315 |
| Difference | Barrer | 0.024 | 0.050 | 0.064 |
| Relative difference | 16 | 17 | 17 | |
| Sample | Permeability Coefficient PH2 | |
| (cm3 STP * cm * cm−2 * s−1 * cmHg−1) | Barrer | |
| Concrete | 7.804 × 10−5 | 7.804 × 105 (±2.497 × 104) |
| Polymer–concrete | 3.414 × 10−5 | 3.414 × 105 (±1.092 × 104) |
| Mudstone (Carbon) | 2.330 × 10−7 | 2.330 × 103 (±8.250 × 101) |
| Salt rock (Permian) (before creep) | 4.815 × 10−7 | 4.815 × 103 (±1.823 × 102) |
| Salt rock (Permian) (after creep) | 1.95 × 10−11 | 0.195 (±0.024) |
| Epoxy resin | 1.820 × 10−11 | 0.182 (±0.023) |
| Epoxy resin + graphite (5% vol.) | 2.350 × 10−11 | 0.235 (±0.029) |
| Epoxy resin + halloysite (5% vol.) | 3.220 × 10−11 | 0.322 (±0.040) |
| Epoxy resin + fly ash (5% vol.) | 1.770 × 10−11 | 0,177 (±0.022) |
| Epoxy resin + fly ash (30% vol.) | 1.774 × 10−11 | 0.177 (±0.022) |
| Polyester resin | 4.357 × 10−11 | 0.436 (±0.054) |
| Polyurethane | 2.611 × 10−11 | 0.261 (±0.033) |
| Stainless steel [33] | 4.640 × 10−17 | 4.640 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajda, D.; Lutyński, M. Permeability Modeling and Estimation of Hydrogen Loss through Polymer Sealing Liners in Underground Hydrogen Storage. Energies 2022, 15, 2663. https://doi.org/10.3390/en15072663
Gajda D, Lutyński M. Permeability Modeling and Estimation of Hydrogen Loss through Polymer Sealing Liners in Underground Hydrogen Storage. Energies. 2022; 15(7):2663. https://doi.org/10.3390/en15072663
Chicago/Turabian StyleGajda, Dawid, and Marcin Lutyński. 2022. "Permeability Modeling and Estimation of Hydrogen Loss through Polymer Sealing Liners in Underground Hydrogen Storage" Energies 15, no. 7: 2663. https://doi.org/10.3390/en15072663
APA StyleGajda, D., & Lutyński, M. (2022). Permeability Modeling and Estimation of Hydrogen Loss through Polymer Sealing Liners in Underground Hydrogen Storage. Energies, 15(7), 2663. https://doi.org/10.3390/en15072663







