Indigenous Materials as Catalyst Supports for Renewable Diesel Production in Malaysia
Abstract
:1. Introduction
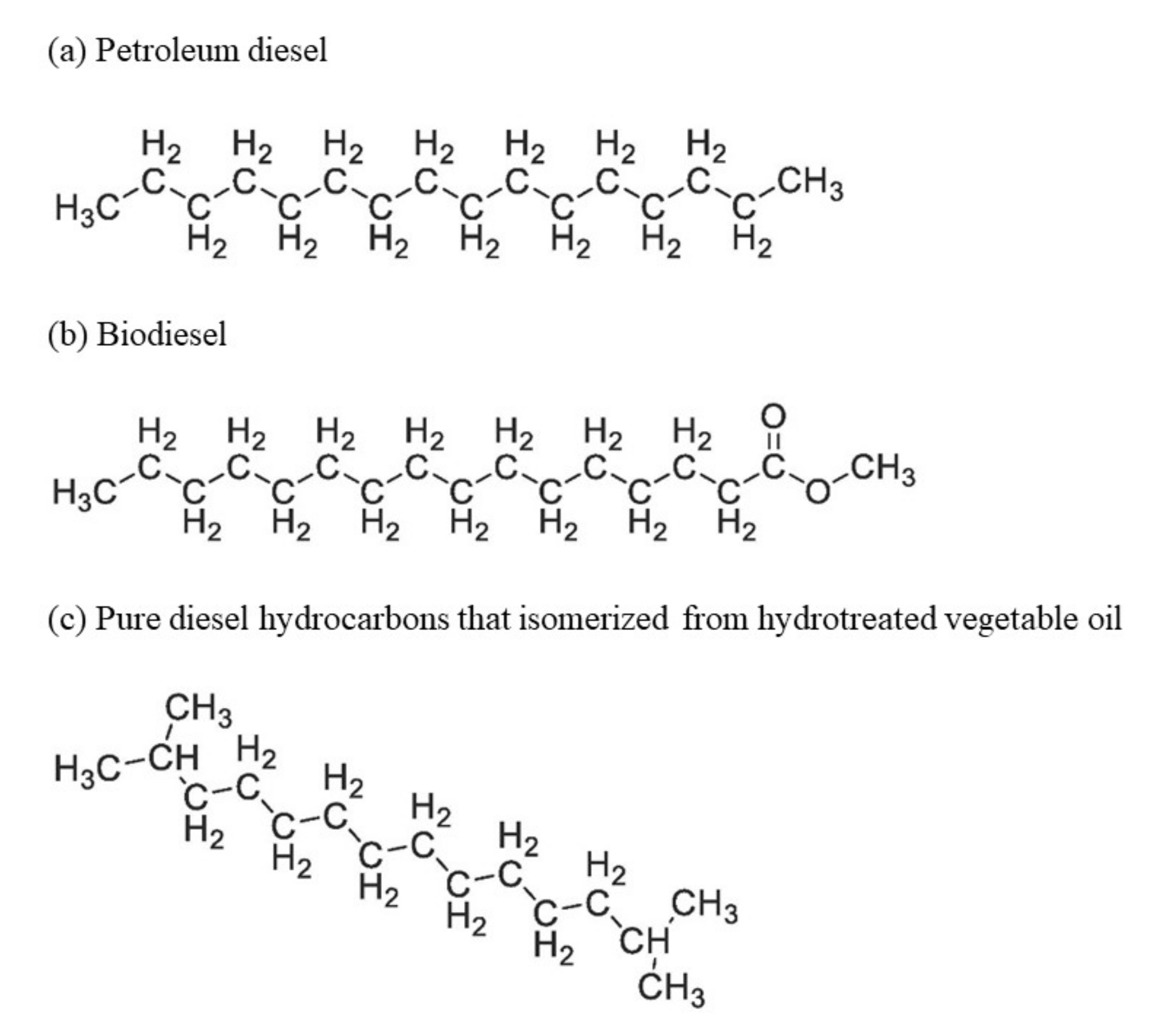
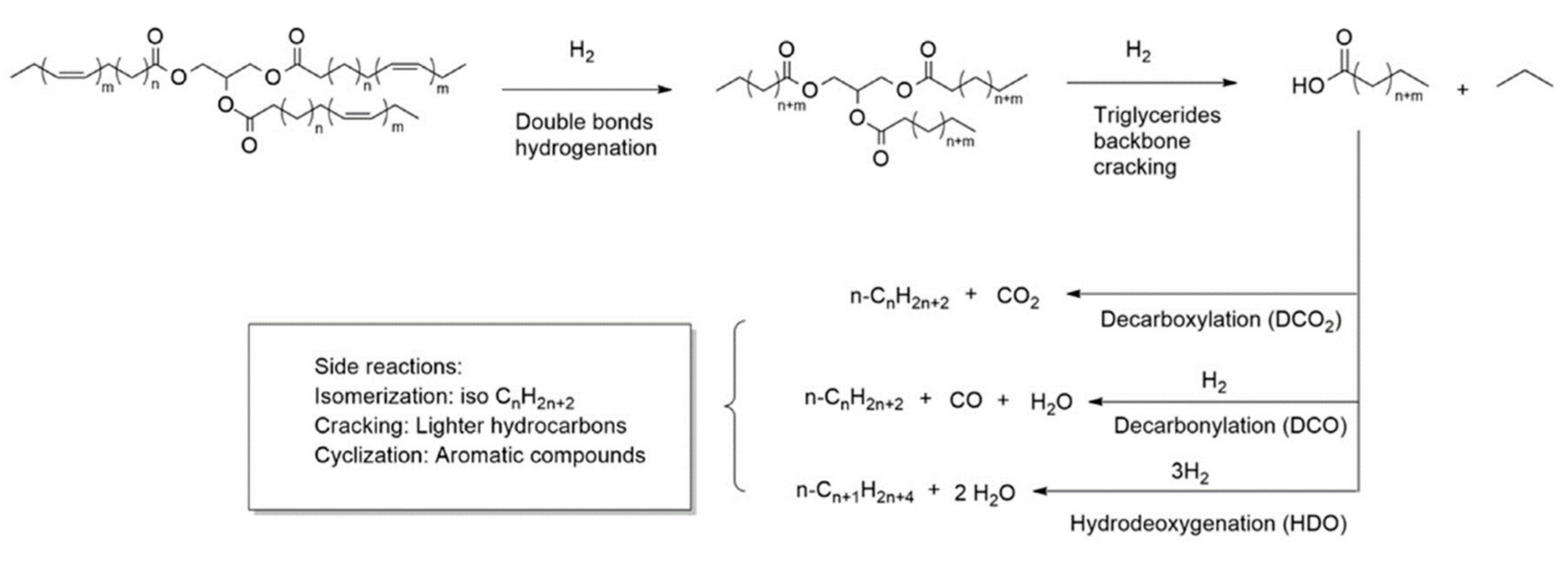
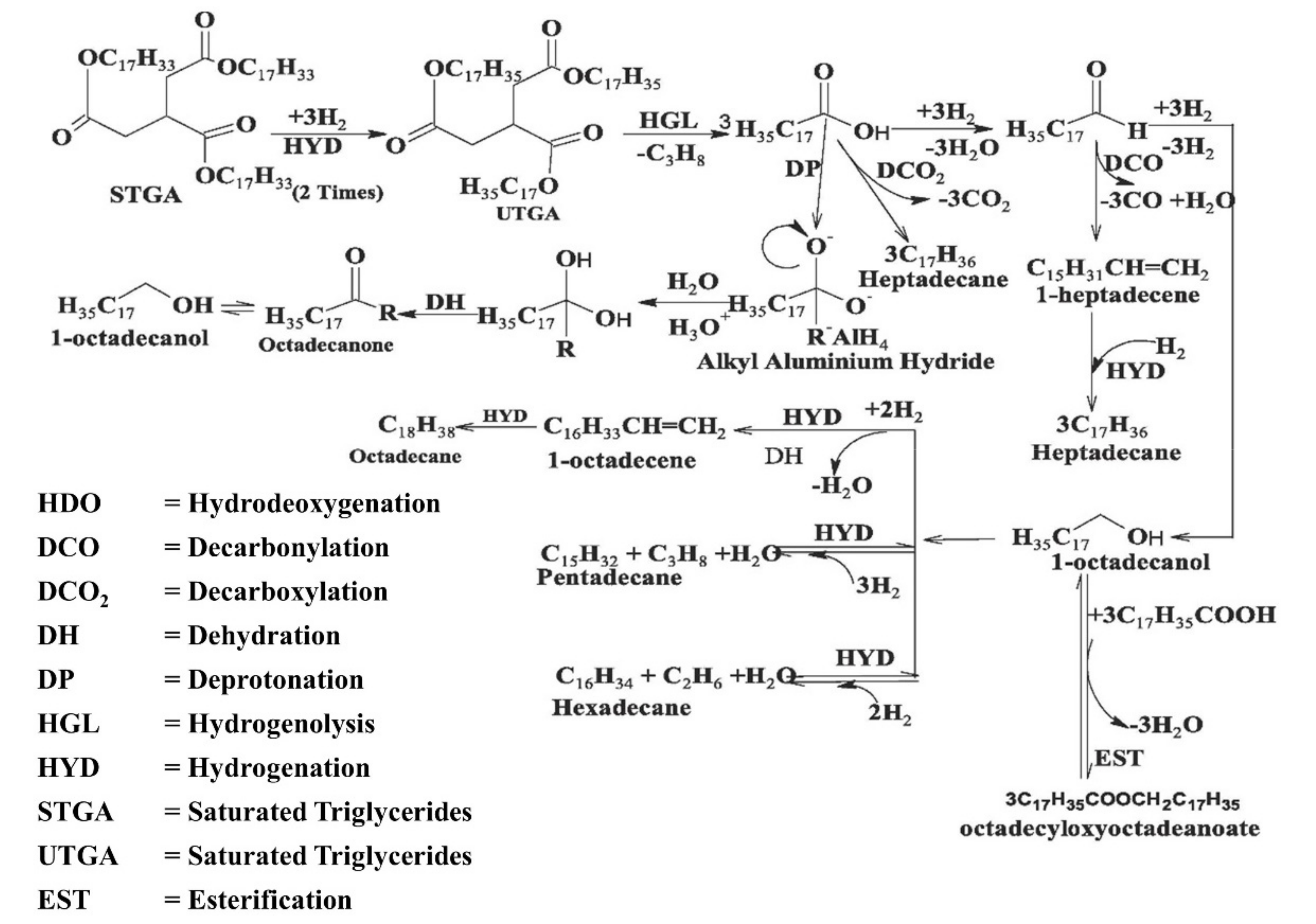
2. Renewable Diesel and Biodiesel Production
3. Type of Supports
3.1. Activated Carbon
| Type of Support | Elemental Composition | Surface Area | Pore Volume | Remarks | Reference | |
|---|---|---|---|---|---|---|
| AC | C: 90.03 % H: 0.557% N: 0.367% S: 0.069% O: 8.98% C/H: 161.6 | Micropore: 775 m2/g External: 15 m2/g | Micro: 0.23 cm3/g Total: 0.26 cm3/g | Charcoals from Iwasaki kiln | [42] | |
| AC | C: 80.71 % H: 1.146% N: 1.094% S: 0.078% O: 16.97% C/H: 70.4 | Micropore: 1202 m2/g External: 20 m2/g | Micro: 0.39 cm3/g Total: 0.42 cm3/g | Charcoals from tube furnace | [42] | |
| AC | - | BET: 1484.33 cm2/g | Total: 1.038 cm3/g | Acid sites: 3.96 mmol NH3/g catalysts | [50] | |
| AC | - | BET: 266.1 m2/g | Total: 0.17 cm3/g | Pre-treated with a nitric acid solution | [51] | |
| AC | C: 88.57 wt% O: 8.01 wt% P: 3.42 wt% | BET: 350 m2/g | Total: 1.88 cm3/g | Total acidity (144 °C): 1055.3 µmol/g Total acidity (852 °C): 2064.7 µmol/g Total basicity (902 °C): 1086.6 µmol/g | [52] | |
| AC | C: 79.1 w/w% H: 0.9 w/w% N: 0.9 w/w% O: 19.2 w/w% | BET: 964 m2/g | Micro: 77.92% Meso: 22.08% Total: 0.57 cm3/g | - | [53] | |
| Type of support | Type of catalyst | Composition of the active phase | Surface area | Pore volume | Remarks | Reference |
| AC | NiP | Ni: 5.14 wt% P: 2.23 wt% | Micropore: 739 m2/g External: 15 m2/g | Micro: 0.22 cm3/g Total: 0.25 cm3/g | Charcoals from Iwasaki kiln | [42] |
| AC | NiP | Ni: 4.66 wt% P: 2.24 wt% | Micropore: 851 m2/g External: 16 m2/g | Micro: 0.26 cm3/g Total: 0.31 cm3/g | Charcoals from tube furnace | [42] |
| AC | Ni2P | - | BET: 612 m2/g | - | Total acidity: 1.3 mmol/g | [54] |
| AC | Ni | O (on the surface): 9.4% | BET: 807.26 cm2/g | Total: 0.185 cm3/g | - | [50] |
| AC | Co-Fe | Co: 8.67 wt% Fe: 3.52 wt% | Micropore: 459.91 m2/g | Micro: 0.22 cm3/g Total: 0.44 cm3/g | - | [48] |
| AC | Mo2C | Mo(II): 52% Mo(IV): 8% Mo(VI): 40% | Total: 417.02 m2/g | Total: 0.22 cm3/g | - | [55] |
| AC | Mo2C | Mo2C (II): 52.17% MoO2 (IV): 8.2% MoO3 (VI): 39.63% | BET: 322.20 m2/g | Total: 0.202 cm3/g | - | [51] |
| AC | Co-Ag | C: 63.41 wt% O: 13.26 wt% P: 1.45 wt% Co: 9.57 wt% Ag: 12.31 wt% | BET: 793 m2/g | Total: 1.67 cm3/g | Acidity: 8502.3 µmol/g Total basicity: 6220.2 µmol/g | [52] |
| AC | CoP | - | BET: 822.9 m2/g | Micro: 68.79% Meso: 31.21% Total 0.43 cm3/g | Acidity: 52.5 µmol/g | [53] |
Oil Palm Wastes in Malaysia
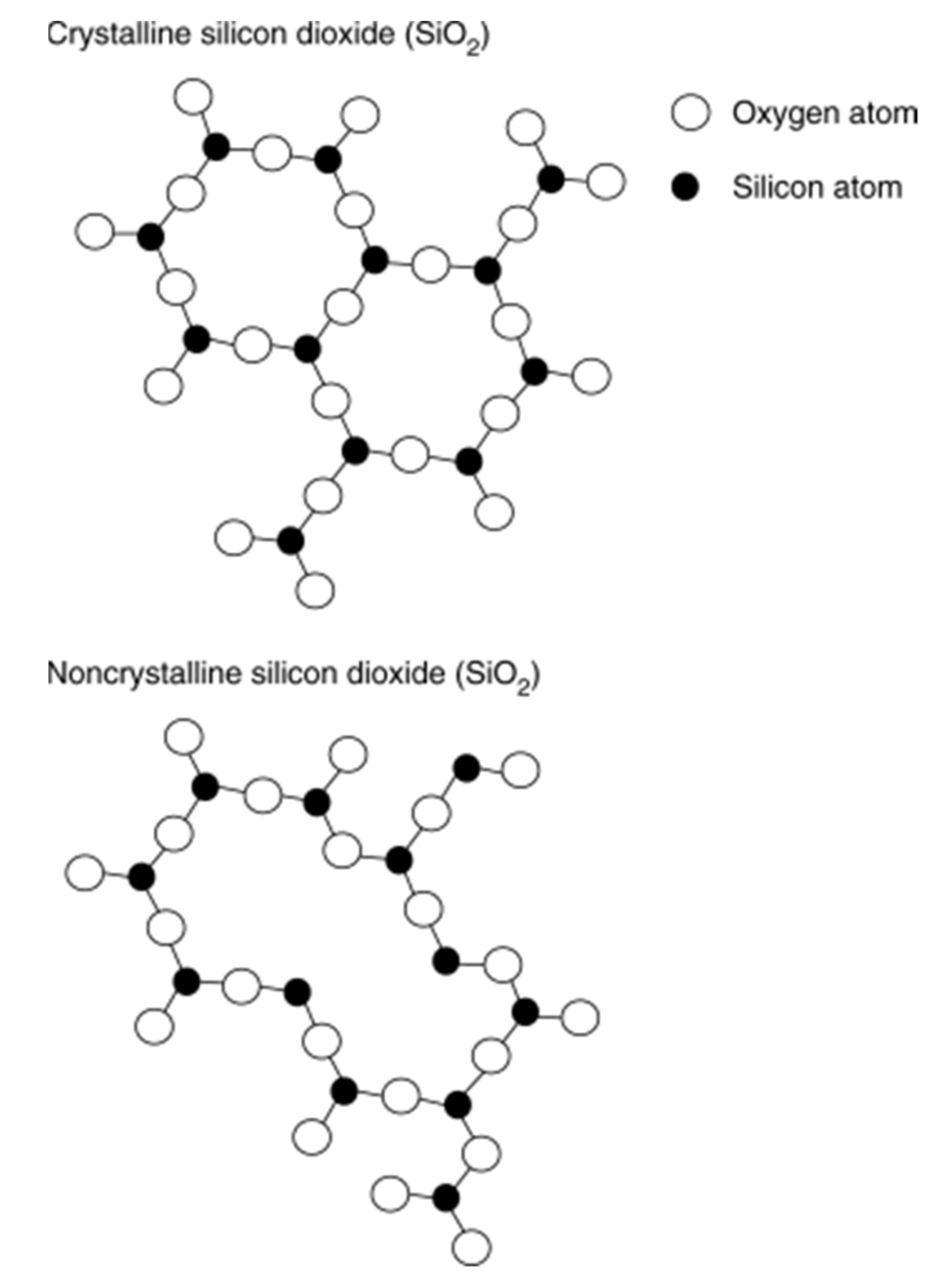
3.2. Metal Oxides
Tin in Malaysia
3.3. Zeolite
3.3.1. Natural Zeolite
3.3.2. Synthetic Zeolite
Kaolin Clay in Malaysia
| Type of Support | Elemental Composition | Surface Area | Pore Volume | Remarks | Reference | |
|---|---|---|---|---|---|---|
| Zeolite | Si: 36.23 wt% O: 47.64 wt% Al: 8.95 wt% Fe: 1.07 wt% | BET: 55.41 m2/g | Micro: 0.09 cc/g | - | [98] | |
| H-ZSM-5 | - | BET: 312.92 cm2/g | Total: 0.047 cm3/g | Acid side amount: 1.39 mmol/g | [50] | |
| Natural mordenite | - | BET: 16 m2/g | Total: 8 m2/g | - | [29] | |
| Activated mordenite | - | BET: 156 m2/g | Total: 131 m2/g | Support activated by acid treatment | [29] | |
| ZrO2 | - | BET: 17.77 cm2/g | Total: 0.053 cm3/g | Acid side amount: 2.13 mmol/g | [50] | |
| Zeolite Y | Si/Al ratio: 2.14 | Micro: 684 m2/g External: 33 m2/g BET: 717 m2/g | Micro: 0.31 cm3/g Total: 0.31 cm3/g | Weak acidity: 0.83 mmol/g Mild acidity: 1.44 mmol/g Medium acidity: 3.01 mmol/g | [115] | |
| Zeolite-Lampung | - | BET: 51.9 m2/g | Total: 0.0045 cc/g | - | [100] | |
| Zeolite beta | O: 51.6 wt% Al: 2.5 wt% Si: 38.1 wt% RM (La/Co/Fe/Mg/Mn/Zn): 4.1 wt% | BET: 648 m2/g | Total: 0.22 cm3/g | Weak + medium acid strength: 1615 µmol/g Strong acid strength: 2883 µmol/g Weak + medium basic strength: 158 µmol/g Strong basic strength: 90 µmol/g | [107] | |
| Type of support | Type of Catalyst | Elemental Composition | Surface Area | Pore Volume | Remarks | Reference |
| Zeolite | Fe | Si: 35.40 wt% O: 49.89 wt% Al: 7.58 wt% Fe: 4.47 wt% | BET: 51.26 m2/g | Micro: 0.07 cc/g | - | [98] |
| Activated mordenite | Ni | - | BET: 96 m2/g | Total: 21 m2/g | 20 wt% of Ni is synthesized with support. | [29] |
| ZrO2 | Ni | O atoms (only on the surface): 35.8% | BET: 19.80 m2/g | Total: 0.585 cm2/g | The binding energy of NiO on ZrO2: 30.90% | [50] |
| H-ZSM-5 | Ni | O atoms (only on the surface): 36.4% | BET: 321.21 m2/g | Total: 0.114 cm2/g | The binding energy of NiO on ZrO2: 32.16% | [50] |
| Zeolite Y | Ni | Si/Al ratio: 2.12 Metal content: 9.44% | Micro: 587 m2/g External: 47 m2/g BET: 634 m2/g | Micro: 0.22 cm3/g Meso: 0.04 cm3/g Total: 0.26 cm3/g | Weak acidity: 1.18 mmol/g Mild acidity: 2.01 mmol/g Medium acidity: 2.72 mmol/g | [115] |
| Zeolite Y | Cu | Si/Al ratio: 2.13 Metal content: 8.90% | Micro: 583 m2/g External: 28 m2/g BET: 611 m2/g | Micro: 0.21 cm3/g Meso: 0.03 cm3/g Total: 0.24 cm3/g | Weak acidity: 0.56 mmol/g Mild acidity: 2.22 mmol/g Medium acidity: 3.55 mmol/g | [115] |
| Zeolite Y | Co | Si/Al ratio: 2.12 Metal content: 9.80% | Micro: 564 m2/g External: 39 m2/g BET: 603 m2/g | Micro: 0.22 cm3/g Meso: 0.04 cm3/g Total: 0.26 cm3/g | Weak acidity: 0.54 mmol/g Mild acidity: 3.80 mmol/g Medium acidity: 1.85 mmol/g | [115] |
| Zeolite Y | Zn | Si/Al ratio: 2.11 Metal content: 8.80% | Micro: 569 m2/g External: 28 m2/g BET: 597 m2/g | Micro: 0.20 cm3/g Meso: 0.03 cm3/g Total: 0.23 cm3/g | Weak acidity: 0.58 mmol/g Mild acidity: 2.92 mmol/g Medium acidity: 1.48 mmol/g | [115] |
| Zeolite Y | Mn | Si/Al ratio: 2.20 Metal content: 9.40% | Micr566 m2/g External: 32 m2/g BET: 598 m2/g | Micro: 0.20 cm3/g Meso: 0.04 cm3/g Total: 0.24 cm3/g | Weak acidity: 1.95 mmol/g Mild acidity: 2.10 mmol/g | [115] |
| Zeolite | Pd | Pd: 4.09 wt% O: 45.67 wt% Al: 8.26 wt% Si: 41.96 wt% | BET: 27.26 m2/g | Total: 0.0139 cc/g | - | [100] |
| Zeolite beta | La2O3 | O: 56 wt% Al: 1.7 wt% Si: 15.1 wt% RM (La/Co/Fe/Mg/Mn/Zn): 7.3 wt% | BET: 556.4 m2/g | Total: 0.29 cm3/g | Weak + medium acid strength: 4700 µmol/g Strong acid strength: 1006 µmol/g Weak + medium basic strength: 97 µmol/g Strong basic strength: 145 µmol/g | [107] |
| Zeolite beta | Co3O4 | O: 63.4 wt% Al: 1.5 wt% Si: 24.3 wt% RM (La/Co/Fe/Mg/Mn/Zn): 8.5 wt% | BET: 556.4 m2/g | Total: 3.6 cm3/g | Weak + medium acid strength: 2594 µmol/g Strong acid strength: 1202 µmol/g Weak + medium basic strength: 130 µmol/g Strong basic strength: 193 µmol/g | [107] |
| Zeolite beta | Fe2O3 | O: 65.7 wt% Al: 1.1 wt% Si: 21.9 wt% RM (La/Co/Fe/Mg/Mn/Zn): 6.2 wt% | BET: 574 m2/g | Total: 3.8 cm3/g | Weak + medium acid strength: 2183 µmol/g Strong acid strength: 692 µmol/g Weak + medium basic strength: 95 µmol/g Strong basic strength: 185 µmol/g | [107] |
| Zeolite beta | MgO | O: 58.4 wt% Al: 1.8 wt% Si: 29.6 wt% RM (La/Co/Fe/Mg/Mn/Zn): 7.7 wt% | BET: 596.8 m2/g | Total: 3.6 cm3/g | Weak + medium acid strength: 7763 µmol/g Strong acid strength: 4105 µmol/g Strong basic strength: 526 µmol/g | [107] |
| Zeolite beta | MnO | O: 48.6 wt% Al: 2.2 wt% Si: 32.7 wt% RM (La/Co/Fe/Mg/Mn/Zn): 9.7 wt% | BET: 440.3 m2/g | Total: 4.7 cm3/g | Weak + medium acid strength: 7186 µmol/g Weak + medium basic strength: 105 µmol/g Strong basic strength: 232 µmol/g | [107] |
| Zeolite beta | ZnO | O: 57.1 wt% Al: 1.4 wt% Si: 29.2 wt% RM (La/Co/Fe/Mg/Mn/Zn): 8.5 wt% | BET: 547.7 m2/g | Total: 3.8 cm3/g | Weak + medium acid strength: 3508 µmol/g Strong acid strength: 521 µmol/g Weak + medium basic strength: 102 µmol/g Strong basic strength: 208 µmol/g | [107] |
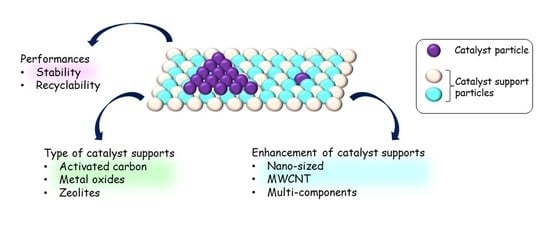
4. Enhancement of Supports
4.1. Pore Size in Nano-Meter Range/Nano-Sized Porous Support
4.2. Synthesis with Other Component(s)
4.3. Multi-Walled Carbon Nanotube
5. Recyclability and Stability of Supported Catalysts
5.1. Recyclability
5.2. Stability
6. Challenges and Future Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalit, W.N.A.W.; Askin-Mijan, N.; Marliza, T.S.; Gamal, M.S.; Shamsuddin, M.R.; Saiman, M.I.; Taufiq-Yap, Y.H. Catalytic deoxygenation of waste cooking oil utilizing nickel oxide catalysts over various supports to produce renewable diesel fuel. Biomass Bioenergy 2021, 154, 106248. [Google Scholar] [CrossRef]
- Garraín, D.; Herrera, I.; Lechόn, Y.; Lago, C. Well-to-Tank environmental analysis of a renewable diesel fuel from vegetable oil through co-processing in a hydrotreatment unit. Biomass Bioenergy 2014, 63, 239–249. [Google Scholar] [CrossRef]
- Patel, M.; Oyedun, A.O.; Kumar, A.; Gupta, R. A Techno-Economic Assessment of Renewable Diesel and Gasoline Production from Aspen Hardwood. Waste Biomass Valorizat. 2019, 10, 2745–2760. [Google Scholar] [CrossRef]
- Aatola, H.; Larmi, M.; Sarjovaara, T.; Mikkonen, S. Hydrotreated Vegetable Oil (HVO) as a Renewable Diesel Fuel: Trade-off between NOₓ, Particulate Emission, and Fuel Consumption of a Heavy Duty Engine. SAE Int. J. Engines 2009, 1, 1251–1262. [Google Scholar] [CrossRef] [Green Version]
- Kalnes, T.; Koers, K.P.; Marker, T.; Shonnard, D.R. Green Diesel and Biodiesel: A technoeconomic and Life Cycle Comparison. In Proceedings of the 1st Alternative Fuels Technology Conference, Prague, Czechoslovakia; 2008. Available online: https://aiche.onlinelibrary.wiley.com/doi/full/10.1002/ep.10319 (accessed on 11 October 2021).
- Hill, S.; Shi, E.; Colletti, P.U.S.; Renewable Diesel Capacity Could Increase due to Announced and Developing Projects. Today in Energy. 2021. Available online: https://www.eia.gov/todayinenergy/detail.php?id=48916 (accessed on 13 October 2021).
- Nickel, R.; Kelly, S.; Plume, K. Plume Renewable Diesel Boom Highlights Challenges in Clean-Energy Transition. 2021. Available online: https://www.reuters.com/article/us-global-oil-biofuels-insight-idUSKBN2AV1BS (accessed on 11 October 2021).
- Niemantsverdriet, J.W. Spectroscopy in Catalysis: An Introduction; John Wiley & Sons: Weinheim, Germany, 2007; Printed in Federal Republic of Germany. [Google Scholar]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the Support in Gold-Containing Nanoparticles as Heterogeneous Catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef] [Green Version]
- Sakata, Y.; Tamaura, Y.; Imamura, H.; Watanabe, M. Preparation of a New Type of CaSiO3 with High Surface Area and Property as a Catalyst Support. In Studies in Surface Science and Catalysis; Gaigneaux, E.M., Devillers, M., De Vos, D.E., Hermans, S., Jacobs, P.A., Martens, J.A., Ruiz, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 331–338. [Google Scholar]
- Chia, S.R.; Nomanbhay, S.; Ong, M.Y.; Chew, K.W.; Show, P.L. Renewable diesel as fossil fuel substitution in Malaysia: A review. Fuel 2022, 314, 123137. [Google Scholar] [CrossRef]
- Gerhard, K.; Krahl, J.; Van Gerpen, J. The Biodiesel Handbook; AOCS Press: Urbana, IL, USA, 2010; Available online: https://www.sciencedirect.com/book/9781893997622/the-biodiesel-handbook?via=ihub=#book-description (accessed on 14 October 2021).
- Thangarasu, V.; Anand, R. Comparative evaluation of corrosion behavior of Aegle Marmelos Correa diesel, biodiesel, and their blends on aluminum and mild steel metals. In Advanced Biofuels; Azad, A.K., Rasul, M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; Chapter 17; pp. 443–471. [Google Scholar]
- Ziolkowska, J.R. Biofuels technologies: An overview of feedstocks, processes, and technologies. In Biofuels for a More Sustainable Future; Ren, J., Scipioni, A., Manzardo, A., Liang, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 1; pp. 1–19. [Google Scholar]
- Venkatesan, M.; Vikram, C.J.; Naveenchandran, P. Performance and emission analysis of pongamia oil methyl ester with diesel blend. Middle East J. Sci. Res. 2012, 12, 1758–1765. [Google Scholar]
- Zahan, K.A.; Kano, M. Biodiesel Production from Palm Oil, Its By-Products and Mill Effluent: A Review. Energies 2018, 11, 2132. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Niu, S.; Han, K.; Li, Y.; Lu, C. Synthesis of the SrO–CaO–Al2O3 trimetallic oxide catalyst for transesterification to produce biodiesel. Renew. Energy 2021, 168, 981–990. [Google Scholar] [CrossRef]
- Molina-Gutiérrez, M.; Alcaraz, L.; Lόpez, F.A.; Rodríguez-Sánchez, L.; Martínez, M.J.; Prieto, A. Immobilized Forms of the Ophiostoma piceae Lipase for Green Synthesis of Biodiesel. Comparison with Eversa Transform 2.0 and Cal A. J. Fungi 2021, 7, 822. [Google Scholar] [CrossRef]
- Ávila, S.N.S.; Collaço, A.C.A.; Greco-Duarte, J.; Aguieiras, E.C.G.; de Castro, A.M.; Gutarra, M.L.E.; Cavalcanti, E.D.C.; Freire, D.M.G. Development of a green integrated process for biodiesel esters production: Use of fermented macaúba cake as biocatalyst for macaúba acid oil transesterification. J. Am. Oil Chem. Soc. 2021, 98, 825–835. [Google Scholar] [CrossRef]
- Kumar, R.; Pal, P. Lipase immobilized graphene oxide biocatalyst assisted enzymatic transesterification of Pongamia pinnata (Karanja) oil and downstream enrichment of biodiesel by solar-driven direct contact membrane distillation followed by ultrafiltration. Fuel Process. Technol. 2021, 211, 106577. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef] [Green Version]
- European Technology and Innovation Platform. Hydrotreatment to HVO. 2021. Available online: https://www.etipbioenergy.eu/value-chains/conversion-technologies/conventional-technologies/hydrotreatment-to-hvo (accessed on 15 October 2021).
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Lee, H.V.; Rashid, U.; Islam, A.; Taufiq-Yap, Y.H. A Review on Thermal Conversion of Plant Oil (Edible and Inedible) into Green Fuel Using Carbon-Based Nanocatalyst. Catalysts 2019, 9, 350. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, C.; Kordouli, E.; Sygellou, L.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. W promoted Ni-Al2O3 co-precipitated catalysts for green diesel production. Fuel Process. Technol. 2021, 217, 106820. [Google Scholar] [CrossRef]
- Burimsitthigul, T.; Yoosuk, B.; Ngamcharussrivichai, C.; Prasassarakich, P. Hydrocarbon biofuel from hydrotreating of palm oil over unsupported Ni–Mo sulfide catalysts. Renew. Energy 2021, 163, 1648–1659. [Google Scholar] [CrossRef]
- Nikolopoulos, I.; Kogkos, G.; Andriopoulou, C.; Kordouli, E.; Dracopoulos, V.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Cobalt–Alumina Coprecipitated Catalysts for Green Diesel Production. Ind. Eng. Chem. Res. 2021, 60, 18672–18683. [Google Scholar] [CrossRef]
- Liu, P.; Chen, C.; Zhou, M.; Xu, J.; Xia, H.; Shang, S.; Jiang, J. Metal–organic framework-derived Ni-based catalyst for the hydrotreatment of triolein into green diesel. Sustain. Energy Fuels 2021, 5, 1809–1820. [Google Scholar] [CrossRef]
- Lycourghiotis, S.; Kordouli, E.; Kordulis, C.; Bourikas, K. Transformation of residual fatty raw materials into third generation green diesel over a nickel catalyst supported on mineral palygorskite. Renew. Energy 2021, 180, 773–786. [Google Scholar] [CrossRef]
- Fani, K.; Lycourghiotis, S.; Bourikas, K.; Kordouli, E. Biodiesel Upgrading to Renewable Diesel over Nickel Supported on Natural Mordenite Catalysts. Ind. Eng. Chem. Res. 2021, 60, 18695–18706. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Ramli, A.; Yusup, S.; Alnarabiji, M.S. Catalytic hydrodeoxygenation of rubber seed oil over sonochemically synthesized Ni-Mo/γ-Al2O3 catalyst for green diesel production. Ultrason. Sonochem. 2019, 51, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Kubička, D.; Tukač, V. Hydrotreating of Triglyceride-Based Feedstocks in Refineries. Adv. Chem. Eng. 2013, 42, 141–194. [Google Scholar]
- Egeberg, R.; Michaelsen, N.; Skyum, L.; Topsoe, P.Z.H. Hydrotreating in the production of green diesel. A novel scheme enables co-processing of light gas oil and tall diesel to produce a renewable diesel meeting EN 590 specifications. Digit. Refin. 2010, 1–11. [Google Scholar]
- Knothe, G. Biodiesel and renewable diesel: A comparison. Prog. Energy Combust. Sci. 2010, 36, 364–373. [Google Scholar] [CrossRef]
- Salaheldeen, M.; Mariod, A.A.; Aroua, M.K.; Raman, S.M.A.; Soudagar, M.E.M.; Fattah, I.M.R. Current State and Perspectives on Transesterification of Triglycerides for Biodiesel Production. Catalysts 2021, 11, 1121. [Google Scholar] [CrossRef]
- Quader, M.A.; Ahmed, S. Bioenergy with Carbon Capture and Storage (BECCS): Future Prospects of Carbon-Negative Technologies, in Clean Energy for Sustainable Development; Rasul, M.G., Azad, A.K., Sharma, S.C., Eds.; Academic Press: Cambridge, MA, USA, 2017; Chapter 4; pp. 91–140. Available online: https://www.sciencedirect.com/science/article/pii/B9780128054239000041 (accessed on 15 October 2021).
- Wisniowski, H.; Zhang, Y. Using Activated Carbon as a Precious Metal Catalyst Carrier. 2021. Available online: https://www.sigmaaldrich.com/MY/en/technical-documents/technical-article/materials-science-and-engineering/solid-state-synthesis/activated-carbon (accessed on 9 November 2021).
- Chandrasekhar, K. Chapter 3.5—Effective and Nonprecious Cathode Catalysts for Oxygen Reduction Reaction in Microbial Fuel Cells. In Microbial Electrochemical Technology; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 485–501. [Google Scholar]
- Nabihah-Fauzi, N.; Asikin-Mijan, N.; Ibrahim, M.L.; Hashim, H.; Yusup, S.; Taufiq-Yap, Y.H.; Mastuli, M.S. Sulfonated SnO 2 nanocatalysts via a self-propagating combustion method for esterification of palm fatty acid distillate. RSC Adv. 2020, 10, 29187–29201. [Google Scholar] [CrossRef]
- Jin, W.; Pastor-Pérez, L.; Villora-Pico, J.J.; Pastor-Blas, M.M.; Sepúlveda-Escribano, A.; Gu, S.; Charisiou, N.D.; Papageridis, K.; Goula, M.A.; Reina, T.R. Catalytic Conversion of Palm Oil to Bio-Hydrogenated Diesel over Novel N-Doped Activated Carbon Supported Pt Nanoparticles. Energies 2020, 13, 132. [Google Scholar] [CrossRef] [Green Version]
- Nie, R.; Yang, H.; Zhang, H.; Yu, X.; Lu, X.; Zhou, D.; Xia, Q. Mild-temperature hydrodeoxygenation of vanillin over porous nitrogen-doped carbon black supported nickel nanoparticles. Green Chem. 2017, 19, 3126–3134. [Google Scholar] [CrossRef]
- Gamal, M.S.; Asikin-Mijan, N.; Khalit, W.N.A.W.; Arumugam, M.; Izham, S.M.; Taufiq-Yap, Y.H. Effective catalytic deoxygenation of palm fatty acid distillate for green diesel production under hydrogen-free atmosphere over bimetallic catalyst CoMo supported on activated carbon. Fuel Process. Technol. 2020, 208, 106519. [Google Scholar] [CrossRef]
- Ruangudomsakul, M.; Osakoo, N.; Keawkumay, C.; Kongmanklang, C.; Butburee, T.; Kiatphuengporn, S.; Faungnawakij, K.; Chanlek, N.; Wittayakun, J.; Khemthong, P. Influential properties of activated carbon on dispersion of nickel phosphides and catalytic performance in hydrodeoxygenation of palm oil. Catal. Today 2021, 367, 153–164. [Google Scholar] [CrossRef]
- Schröder, E.; Thomauske, K.; Weber, C.; Hornung, A.; Tumiatti, V. Experiments on the generation of activated carbon from biomass. J. Anal. Appl. Pyrolysis 2007, 79, 106–111. [Google Scholar] [CrossRef]
- Schrder, E.; Thomauske, K.; Oechsler, B.; Herberger, S. Activated Carbon from Waste Biomass; InTech. Available online: https://www.intechopen.com/chapters/16653 (accessed on 9 November 2021).
- Edeh, I.; Overton, T.; Bowra, S. Catalytic hydrothermal deoxygenation of fatty acids over palladium on activated carbon catalyst (Pd/C) for renewable diesel production. Biofuels 2021, 12, 1075–1082. [Google Scholar] [CrossRef]
- Tapia, J.; Acelas, N.Y.; Lόpez, D.; Moreno, A. NiMo-sulfide supported on activated carbon to produce renewable diesel. Univ. Sci. 2017, 22, 71–85. [Google Scholar] [CrossRef]
- Safa Gamal, M.; Asikin-Mijan, N.; Arumugam, M.; Rashid, U.; Taufiq-Yap, Y.H. Solvent-free catalytic deoxygenation of palm fatty acid distillate over cobalt and manganese supported on activated carbon originating from waste coconut shell. J. Anal. Appl. Pyrolysis 2019, 144, 104690. [Google Scholar] [CrossRef]
- Thangadurai, T.; Tye, C.T. Performance of Activated Carbon Supported Cobalt Oxides and Iron Oxide Catalysts in Catalytic Cracking of Waste Cooking Oil. Period. Polytech. Chem. Eng. 2021, 65, 350–360. [Google Scholar] [CrossRef]
- Mayorga, M.; Cadavid, J.; Suarez, O.; Vargas, J.; Gonzalez, J.; Narvaez, P. Production of Renewable Diesel by Hydrotreating of Palm Oil with Noble Metallic Catalysts. Chem. Eng. Trans. 2019, 74, 7–12. [Google Scholar]
- Hongloi, N.; Prapainainar, P.; Seubsai, A.; Sudsakorn, K.; Prapainainar, C. Nickel catalyst with different supports for green diesel production. Energy 2019, 182, 306–320. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, J.; Wang, K.; Zhai, Q.; Sun, H.; Liu, P.; Feng, J.; Xia, H.; Ye, J.; Li, Z.; et al. Activated carbon supported molybdenum and tungsten carbides for hydrotreatment of fatty acids into green diesel. Fuel 2018, 228, 103–111. [Google Scholar] [CrossRef]
- Safa-Gamal, M.; Asikin-Mijan, N.; Arumugam, M.; Khalit, W.N.A.W.; Nur Azreena, I.; Hafez, F.S.; Taufiq-Yap, Y.H. Catalytic deoxygenation by H2-free single-step conversion of free fatty acid feedstock over a Co-Ag carbon-based catalyst for green diesel production. J. Anal. Appl. Pyrolysis 2021, 160, 105334. [Google Scholar] [CrossRef]
- Kaewtrakulchai, N.; Kaewmeesri, R.; Itthibenchapong, V.; Eiad-Ua, A.; Faungnawakij, K. Palm oil conversion to bio-jet and green diesel fuels over cobalt phosphide on porous carbons derived from palm male flowers. Catalysts 2020, 10, 694. [Google Scholar] [CrossRef]
- Pham, L.K.H.; Tran, T.T.V.; Kongparakul, S.; Reubroycharoen, P.; Karnjanakom, S.; Guan, G.; Samart, C. Formation and activity of activated carbon supported Ni2P catalysts for atmospheric deoxygenation of waste cooking oil. Fuel Process. Technol. 2019, 185, 117–125. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Jiang, J.; Liu, P.; Li, F.; Ye, J.; Zhou, M. Hydrotreatment of vegetable oil for green diesel over activated carbon supported molybdenum carbide catalyst. Fuel 2018, 216, 738–746. [Google Scholar] [CrossRef]
- Jafri, N.; Jimat, D.N.; Azmin, N.F.M.; Sulaiman, S.; Nor, Y.A. The potential of biomass waste in Malaysian palm oil industry: A case study of Boustead Plantation Berhad. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Kuala Lumpur, Malaysia, 2021. [Google Scholar]
- Awalludin, M.F.; Sulaiman, O.; Hashim, R.; Nadhari, W.N.A.W. An overview of the oil palm industry in Malaysia and its waste utilization through thermochemical conversion, specifically via liquefaction. Renew. Sustain. Energy Rev. 2015, 50, 1469–1484. [Google Scholar] [CrossRef]
- Ukanwa, K.S.; Patchigolla, K.; Sakrabani, R.; Anthony, E. Preparation and characterisation of activated carbon from palm mixed waste treated with trona ore. Molecules 2020, 25, 5028. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Chen, J. Applications of lignin-derived catalysts for green synthesis. Green Energy Environ. 2019, 4, 210–244. [Google Scholar] [CrossRef]
- Baroutian, S.; Aroua, M.K.; Raman, A.A.A.; Sulaiman, N.M.N. Potassium hydroxide catalyst supported on palm shell activated carbon for transesterification of palm oil. Fuel Processing Technol. 2010, 91, 1378–1385. [Google Scholar] [CrossRef]
- Liew, R.K.; Chong, M.Y.; Osazuwa, O.U.; Nam, W.L.; Phang, X.Y.; Su, M.H.; Cheng, C.K.; Chong, C.T.; Lam, S.S. Production of activated carbon as catalyst support by microwave pyrolysis of palm kernel shell: A comparative study of chemical versus physical activation. Res. Chem. Intermed. 2018, 44, 3849–3865. [Google Scholar] [CrossRef]
- Krishnan, S.G.; Pua, F.-L.; Zhang, F. Oil palm empty fruit bunch derived microcrystalline cellulose supported magnetic acid catalyst for esterification reaction: An optimization study. Energy Convers. Manag. X 2022, 13, 100159. [Google Scholar] [CrossRef]
- Dechakhumwat, S.; Hongmanorom, P.; Thungyaratchatanon, C.; Smith, S.M.; Boonyuen, S.; Luengnaruemitchai, A. Catalytic activity of heterogeneous acid catalysts derived from corncob in the esterification of oleic acid with methanol. Renew. Energy 2020, 148, 897–906. [Google Scholar] [CrossRef]
- Niu, S.; Ning, Y.; Lu, C.; Han, K.; Yu, H.; Zhou, Y. Esterification of oleic acid to produce biodiesel catalyzed by sulfonated activated carbon from bamboo. Energy Convers. Manag. 2018, 163, 59–65. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B.; Korstad, J. Utilization of lignocellulosic biomass by oleaginous yeast and bacteria for production of biodiesel and renewable diesel. Renew. Sustain. Energy Rev. 2017, 73, 654–671. [Google Scholar] [CrossRef]
- Sagoff, J.; Harmon, J. A Catalytic Support Material Takes a Leading Role. 2018. Available online: https://www.anl.gov/article/a-catalytic-support-material-takes-a-leading-role (accessed on 26 October 2021).
- Aakash, B.Y.J.U. Oxides. 2021. Available online: https://byjus.com/jee/oxide/ (accessed on 26 October 2021).
- Cortés Corberán, V.; Rives, V.; Stathopoulos, V. Chapter 7—Recent Applications of Nanometal Oxide Catalysts in Oxidation Reactions. In Advanced Nanomaterials for Catalysis and Energy; Sadykov, V.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 227–293. [Google Scholar]
- Janampelli, S.; Darbha, S. Metal Oxide-Promoted Hydrodeoxygenation Activity of Platinum in Pt-MOx/Al2O3 Catalysts for Green Diesel Production. Energy Fuels 2018, 32, 12630–12643. [Google Scholar] [CrossRef]
- del Río, J.I.; Cardeño, F.; Pérez, W.; Peña, J.D.; Rios, L.A. Catalytic hydrotreating of jatropha oil into non-isomerized renewable diesel: Effect of catalyst type and process conditions. Chem. Eng. J. 2018, 352, 232–240. [Google Scholar] [CrossRef]
- Sotelo-Boyás, R.; Liu, Y.; Minowa, T. Renewable Diesel Production from the Hydrotreating of Rapeseed Oil with Pt/Zeolite and NiMo/Al2O3 Catalysts. Ind. Eng. Chem. Res. 2011, 50, 2791–2799. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, M.; Wang, K.; Zhang, L.; Xu, X. Catalytic Hydrodeoxygenation of Algae Bio-oil over Bimetallic Ni–Cu/ZrO2 Catalysts. Ind. Eng. Chem. Res. 2015, 54, 890–899. [Google Scholar] [CrossRef]
- Shinde, P.S.; Suryawanshi, P.S.; Patil, K.K.; Belekar, V.M.; Sankpal, S.A.; Delekar, S.D.; Jadhav, S.A. A Brief Overview of Recent Progress in Porous Silica as Catalyst Supports. J. Compos. Sci. 2021, 5, 75. [Google Scholar] [CrossRef]
- Chia, S.R.; Nomanbhay, S.; Ong, M.Y.; Chew, K.W.; Khoo, K.S.; Karimi-Maleh, H.; Show, P.L. Recent Development of Renewable Diesel Production Using Bimetallic Catalysts. Front. Energy Res. 2021, 9, 621. [Google Scholar] [CrossRef]
- Sinton, C.W. Glass and Energy. In Encyclopedia of Energy; Cleveland, C.J., Ed.; Elsevier: New York, NY, USA, 2004; pp. 1–10. [Google Scholar]
- Liu, S.; Simonetti, T.; Zheng, W.; Saha, B. Selective Hydrodeoxygenation of Vegetable Oils and Waste Cooking Oils to Green Diesel Using a Silica-Supported Ir–ReOx Bimetallic Catalyst. ChemSusChem 2018, 11, 1446–1454. [Google Scholar] [CrossRef]
- Zhou, W.; Xin, H.; Yang, H.; Du, X.; Yang, R.; Li, D.; Hu, C. The Deoxygenation Pathways of Palmitic Acid into Hydrocarbons on Silica-Supported Ni12P5 and Ni2P Catalysts. Catalysts 2018, 8, 153. [Google Scholar] [CrossRef] [Green Version]
- Pelemo, J.; Omojola, A.; Inambao, F.; Onuh, E. Development and characterization of coal fly ash reinforced with silica oxide for catalytic green diesel production. Comput. -Aided Des. 2021, 11, 405–420. [Google Scholar]
- Asikin-Mijan, N.; Lee, H.V.; Juan, J.C.; Noorsaadah, A.R.; Abdulkareem-Alsultan, G.; Arumugam, M.; Taufiq-Yap, Y.H. Waste clamshell-derived CaO supported Co and W catalysts for renewable fuels production via cracking-deoxygenation of triolein. J. Anal. Appl. Pyrolysis 2016, 120, 110–120. [Google Scholar] [CrossRef]
- Kubička, D.; Horáček, J.; Setnička, M.; Bulánek, R.; Zukal, A.; Kubičková, I. Effect of support-active phase interactions on the catalyst activity and selectivity in deoxygenation of triglycerides. Appl. Catal. B Environ. 2014, 145, 101–107. [Google Scholar] [CrossRef]
- Leinbach, T.R.; Lockard, C.A.; Ahmad, Z.B.; Ooi, J.B. Malaysia. 2022. Available online: https://www.britannica.com/place/Malaysia/Agriculture-forestry-and-fishing (accessed on 10 February 2022).
- Statista. Tin Mines Production APAC 2020 by Country. 2022. Available online: https://www.statista.com/statistics/1129972/apac-tin-mines-production-by-country/ (accessed on 18 February 2022).
- Bernama. Malaysia to Go into Tin Mining Again. In New Straits Times; Putrajaya, Malaysia. 2019. Available online: https://www.nst.com.my/news/nation/2019/05/484618/malaysia-go-tin-mining-again#:~:text=PUTRAJAYA%3A%20The%20government%20is%20planning,tin%20producer%20in%20the%20world (accessed on 21 February 2022).
- Min, E.Y.K. Tin Mining in Malaysia-is There Any Revival. Feature in Jurutera. Univ. Sains Malays. Pulau Pinang. 2007, pp. 12–18. Available online: http://dspace.unimap.edu.my/bitstream/handle/123456789/15965/feature%20tin%20mining%205pp.pdf?sequence=1#:~:text=Tin%20mining%20is%20indeed%20a,and%20reworking%20of%20the%20tailings (accessed on 21 February 2022).
- Srinivas, M.; Raveendra, G.; Parameswaram, G.; Prasad, P.S.S.; Lingaiah, N. Cesium exchanged tungstophosphoric acid supported on tin oxide: An efficient solid acid catalyst for etherification of glycerol with tert-butanol to synthesize biofuel additives. J. Mol. Catal. A Chem. 2016, 413, 7–14. [Google Scholar] [CrossRef]
- de Almeida, R.M.; Souza, F.T.C.; Júnior, M.A.C.; Albuquerque, N.J.A.; Meneghetti, S.M.P.; Meneghetti, M.R. Improvements in acidity for TiO2 and SnO2 via impregnation with MoO3 for the esterification of fatty acids. Catal. Commun. 2014, 46, 179–182. [Google Scholar] [CrossRef]
- da Silva, M.A.; dos Santos, A.S.S.; dos Santos, T.V.; Meneghetti, M.R.; Meneghetti, S.M.P. Organotin(iv) compounds with high catalytic activities and selectivities in the glycerolysis of triacylglycerides. Catal. Sci. Technol. 2017, 7, 5750–5757. [Google Scholar] [CrossRef]
- Harino, H.; Arai, T.; Ohji, M.; Ismail, A.; Miyazaki, N. Organotin Contaminations in Malaysia. Coast. Mar. Sci 2008, 32, 96–101. [Google Scholar]
- Kristiani, A.; Sudiyarmanto, S.; Aulia, F.; Hidayati, L.N.; Abimanyu, H. Metal supported on natural zeolite as catalysts for conversion of ethanol to gasoline. In MATEC Web of Conferences; EDP Sciences: Lez Ili, France, 2017; Available online: https://www.matec-conferences.org/articles/matecconf/abs/2017/15/matecconf_sicest2017_01001/matecconf_sicest2017_01001.html#:~:text=Metal%20supported%20on%20natural%20zeolite%20as%20catalysts%20for%20conversion%20of%20ethanol%20to%20gasoline,-Anis%20Kris (accessed on 25 October 2021).
- Wise, W.S. MINERALS|Zeolites☆. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Buekenhoudt, A.; Kovalevsky, A.; Luyten, J.; Snijkers, F. 1.11—Basic Aspects in Inorganic Membrane Preparation. In Comprehensive Membrane Science and Engineering; Drioli, E., Giorno, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 217–252. [Google Scholar]
- Kalvachev, Y.; Todorova, T.; Popov, C. Recent Progress in Synthesis and Application of Nanosized and Hierarchical Mordenite—A Short Review. Catalysts 2021, 11, 308. [Google Scholar] [CrossRef]
- Verdoliva, V.; Saviano, M.; De Luca, S. Zeolites as Acid/Basic Solid Catalysts: Recent Synthetic Developments. Catalysts 2019, 9, 248. [Google Scholar] [CrossRef] [Green Version]
- Abdullahi, T.; Harun, Z.; Othman, M.H.D. A review on sustainable synthesis of zeolite from kaolinite resources via hydrothermal process. Adv. Powder Technol. 2017, 28, 1827–1840. [Google Scholar] [CrossRef]
- Yoshida, K.; Toyoura, K.; Matsunaga, K.; Nakahira, A.; Kurata, H.; Ikuhara, Y.H.; Sasaki, Y. Atomic sites and stability of Cs+ captured within zeolitic nanocavities. Sci. Rep. 2013, 3, 2457. [Google Scholar] [CrossRef] [Green Version]
- Król, M. Natural vs. Synthetic Zeolites; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2020; Available online: https://www.mdpi.com/2073-4352/10/7/622/htm (accessed on 25 October 2021).
- Conservation OnLine, Zeolites. 1996. Available online: https://cool.culturalheritage.org/byorg/abbey/an/an20/an20-7/an20-702.html (accessed on 25 October 2021).
- Putra, R.; Lestari, W.W.; Wibowo, F.R.; Susanto, B.H. Fe/Indonesian natural zeolite as hydrodeoxygenation catalyst in green diesel production from palm oil. Bull. Chem. React. Eng. Catal. 2018, 13, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Rostamizadeh, M.; Yaripour, F. Bifunctional and bimetallic Fe/ZSM-5 nanocatalysts for methanol to olefin reaction. Fuel 2016, 181, 537–546. [Google Scholar] [CrossRef]
- Susanto, B.H.; Nasikin, M.; Sukirno; Wiyo, A. A. Synthesis of renewable diesel through hydrodeoxygenation using Pd/zeolite catalysts. Procedia Chem. 2014, 9, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X. 17—Porous materials for direct and indirect evaporative cooling in buildings. In Materials for Energy Efficiency and Thermal Comfort in Buildings; Hall, M.R., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 399–426. [Google Scholar]
- Monzón, J.D.; Gonzalez, M.R.; Mardones, L.E.; Conconi, M.S.; Pereyra, A.M.; Basaldella, E.I. The role of alkaline activation in the structural transformations of aluminosiliceous industrial wastes towards zeolite production. Mater. Today Commun. 2019, 21, 100624. [Google Scholar] [CrossRef]
- Ojumu, T.V.; Du Plessis, P.W.; Petrik, L.F. Synthesis of zeolite A from coal fly ash using ultrasonic treatment—A replacement for fusion step. Ultrason. Sonochem. 2016, 31, 342–349. [Google Scholar] [CrossRef]
- Krisnandi, Y.; Parmanti, I.Y.; Yunarti, R.T.; Sihombing, R.; Saragi, I.R. Synthesis and Characterization of Zeolite NaY from kaolin Bangka Belitung with variation of synthesis composition and crystallization time. J. Phys. Conf. Ser. 2018, 1095, 012043. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Shi, C.; Wu, D.; He, S.; Ren, B. A Simple Method of Preparation of High Silica Zeolite Y and Its Performance in the Catalytic Cracking of Cumene. J. Nanotechnol. 2016, 2016, 1486107. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, N.; Li, G.; Li, L.; Wang, A.; Cong, Y.; Wang, X.; Xu, G.; Zhang, T. Protonated titanate nanotubes as a highly active catalyst for the synthesis of renewable diesel and jet fuel range alkanes. Appl. Catal. B Environ. 2015, 170-171, 124–134. [Google Scholar] [CrossRef]
- Azreena, I.N.; Lau, H.L.N.; Asikin-Mijan, N.; Hassan, M.A.; Saiman Mohd, I.; Kennedy, E.; Stockenhuber, M.; Mastuli, M.S.; Alharthi, F.A.; Alghamdi, A.A.; et al. A promoter effect on hydrodeoxygenation reactions of oleic acid by zeolite beta catalysts. J. Anal. Appl. Pyrolysis 2021, 155, 105044. [Google Scholar] [CrossRef]
- Sousa, G. Top 12 Kaolin Exporting Countries. Economics. 2017. Available online: https://www.worldatlas.com/articles/top-12-kaolin-exporting-countries.html (accessed on 19 March 2022).
- Abdullahi, T.; Harun, Z.; Othman, M.H.D.; Yusof, K.N.; Rahmawati, A.; Yunos, M.Z.; Lajis, M.A.; Yusof, Y. Preliminary studies on hydrothermal synthesis of zeolite from Malaysian kaolinite clays. Malays J. Fundam. Appl. Sci. 2019, 15, 421–425. [Google Scholar] [CrossRef]
- Somderama, S.; Aziza, A.S.A.; Abdullaha, A.H.; Matb, R. Characterisation of NaA zeolite made from Malaysian Kaolin. Chem. Eng. 2019, 72, 325–330. [Google Scholar]
- Sazali, N.; Harun, Z.; Abdullahi, T.; Kamarudin, N.H.; Sazali, N.; Jamalludin, M.R.; Hubadillah, S.K.; Alias, S.S. The Route of Hydrothermal Synthesis Zeolite—A from the Low-Grade Perak kaolin, Malaysia. Silicon. 2022, pp. 1–17. Available online: https://link.springer.com/article/10.1007/s12633-021-01620-4 (accessed on 14 January 2022).
- Abdullahi, T.; Harun, Z.; Othman, M.H.D.; Nuhu, A.H.; Usman, J. Conversion and characterization of synthetic zeolite using low grade kaolin from Mersing area of Johor. AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019. Available online: https://aip.scitation.org/doi/abs/10.1063/1.5089401 (accessed on 19 March 2022).
- Abdullahi, T.; Harun, Z.; Sazali, N.; Hairom, N.H.H.; Jamalludin, M.R.; Hubadillah, S.K.; Sazali, N.; Alias, S.S.; Azhar, F.H. Synthesizing of Zeolite Particle Using Alkaline Plant Extract. Emerg. Adv. Integr. Technol. 2021, 2, 1–6. [Google Scholar] [CrossRef]
- Abdullah, A.H.; Zulkefli, N.; Abd Aziz, A.S.; Mat, R. Synthesis of zeolite Na-A from local kaolin for bioethanol purification. Indian J. Sci. Technol. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Choo, M.-Y.; Oi, L.E.; Ling, T.C.; Ng, E.; Lin, Y.; Centi, G.; Juan, J.C. Deoxygenation of triolein to green diesel in the H2-free condition: Effect of transition metal oxide supported on zeolite Y. J. Anal. Appl. Pyrolysis 2020, 147, 104797. [Google Scholar] [CrossRef]
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Mustafa-Alsultan, G.; Lee, H.V.; Wilson, K.; Taufiq-Yap, Y.H. Efficient deoxygenation of waste cooking oil over Co3O4–La2O3-doped activated carbon for the production of diesel-like fuel. RSC Adv. 2020, 10, 4996–5009. [Google Scholar] [CrossRef] [Green Version]
- Intarasiri, S.; Ratana, T.; Sornchamni, M.; Phongaksorn, M.; Tungkamani, S. Effect of pore size diameter of cobalt supported catalyst on gasoline-diesel selectivity. Energy Procedia 2017, 138, 1035–1040. [Google Scholar] [CrossRef]
- Papageridis, K.N.; Charisiou, N.D.; Douvartzides, S.L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKhoori, S.; Polychronopoulou, K.; Goula, M.A. Effect of operating parameters on the selective catalytic deoxygenation of palm oil to produce renewable diesel over Ni supported on Al2O3, ZrO2 and SiO2 catalysts. Fuel Process. Technol. 2020, 209, 106547. [Google Scholar] [CrossRef]
- Wang, H.; Yan, S.; Salley, S.O.; Simon Ng, K.Y. Support effects on hydrotreating of soybean oil over NiMo carbide catalyst. Fuel 2013, 111, 81–87. [Google Scholar] [CrossRef]
- Veriansyah, B.; Han, J.Y.; Kim, S.K.; Hong, S.; Kim, Y.J.; Lim, J.S.; Shu, Y.; Oh, S.; Kim, J. Production of renewable diesel by hydroprocessing of soybean oil: Effect of catalysts. Fuel 2012, 94, 578–585. [Google Scholar] [CrossRef]
- Liu, J.; Fan, K.; Tian, W.; Liu, C.; Rong, L. Hydroprocessing of Jatropha oil over NiMoCe/Al2O3 catalyst. Int. J. Hydrogen Energy 2012, 37, 17731–17737. [Google Scholar] [CrossRef]
- Janampelli, S.; Darbha, S. Highly efficient Pt-MoOx/ZrO2 catalyst for green diesel production. Catal. Commun. 2019, 125, 70–76. [Google Scholar] [CrossRef]
- Charisiou, N.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Roles of Ni, Co and Cu Monometallic Catalysts Supported on ZrO2 for Green Diesel Production via the Palm Oil Hydrodeoxygenation. In 2019-Sustainable Industrial Processing Summit; Flogen Star Outreach. 2019. Available online: https://www.flogen.org/sips2019/paper-12-249.html (accessed on 28 October 2021).
- Moretti, E.; Molina, A.I.; Sponchia, G.; Talon, A.; Frattini, R.; Rodriguez-Castellon, E.; Storaro, L. Low-temperature carbon monoxide oxidation over zirconia-supported CuO–CeO2 catalysts: Effect of zirconia support properties. Appl. Surf. Sci. 2017, 403, 612–622. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Polychronopoulou, K.; Goula, M.A. Hydrogen production via the glycerol steam reforming reaction over nickel supported on alumina and lanthana-alumina catalysts. Int. J. Hydrogen Energy 2017, 42, 13039–13060. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Tzounis, L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Investigating the correlation between deactivation and the carbon deposited on the surface of Ni/Al2O3 and Ni/La2O3-Al2O3 catalysts during the biogas reforming reaction. Appl. Surf. Sci. 2019, 474, 42–56. [Google Scholar] [CrossRef] [Green Version]
- Papageridis, K.N.; Charisiou, N.D.; Douvartzides, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKhoori, A.A.; AlKhoori, S.I.; Polychronopoulou, K.; Goula, M.A. Continuous selective deoxygenation of palm oil for renewable diesel production over Ni catalysts supported on Al2O3 and La2O3–Al2O3. RSC Adv. 2021, 11, 8569–8584. [Google Scholar] [CrossRef]
- Gomes, L.C.; de Oliveira Rosas, D.; Chistone, R.C.; Zotin, F.M.Z.; de Araujo, L.R.R.; Zotin, J.L. Hydroisomerization of n-hexadecane using Pt/alumina-Beta zeolite catalysts for producing renewable diesel with low pour point. Fuel 2017, 209, 521–528. [Google Scholar] [CrossRef]
- Palanisamy, S.; Kandasamy, K. Direct Hydrogenation and Hydrotreating of Neat Vegetal Oil into Renewable Diesel Using Alumina Binder with Zeolite. Rev. Chim 2020, 71, 98–112. [Google Scholar] [CrossRef]
- Vázquez-Garrido, I.; Lόpez-Benítez, A.; Guevara-Lara, A.; Berhault, G. Synthesis of NiMo catalysts supported on Mn-Al2O3 for obtaining green diesel from waste soybean oil. Catal. Today 2021, 365, 327–340. [Google Scholar] [CrossRef]
- Malins, K. Production of renewable hydrocarbons from vegetable oil refining by-product/waste soapstock over selective sulfur-free high metal loading SiO2–Al2O3 supported Ni catalyst via hydrotreatment. J. Clean. Prod. 2021, 283, 125306. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; Lee, H.V.; Taufiq-Yap, Y.H.; Abdulkrem-Alsultan, G.; Mastuli, M.S.; Ong, H.C. Optimization study of SiO2-Al2O3 supported bifunctional acid–base NiO-CaO for renewable fuel production using response surface methodology. Energy Convers. Manag. 2017, 141, 325–338. [Google Scholar] [CrossRef]
- Hanafi, S.A.; Elmelawy, M.S.; Ahmed, H.A. Solvent-free deoxygenation of low-cost fat to produce diesel-like hydrocarbons over Ni–MoS2/Al2O3–TiO2 heterogenized catalyst. Int. J. Energy Water Resour. 2021, 6, 1–3. [Google Scholar] [CrossRef]
- Papageridis, K.N.; Charisiou, N.D.; Douvartzides, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKhoori, S.; Polychronopoulou, K.; Goula, M.A. Promoting effect of CaO-MgO mixed oxide on Ni/γ-Al2O3 catalyst for selective catalytic deoxygenation of palm oil. Renew. Energy 2020, 162, 1793–1810. [Google Scholar] [CrossRef]
- Vikár, A.; Solt, H.E.; Novodárszki, G.; Mihályi, M.R.; Barthos, R.; Domján, A.; Hancsόk, J.; Valyon, J.; Lόnyi, F. A study of the mechanism of triglyceride hydrodeoxygenation over alumina-supported and phosphatized-alumina-supported Pd catalysts. J. Catal. 2021, 404, 67–79. [Google Scholar] [CrossRef]
- Markets and Markets. Carbon Nanotubes (CNT) Market by Type (Single Walled & Multi Walled), End-Use Industry (Electronics & Semiconductors, Chemical Materials & Polymers, Structural Composites, Energy & Storage, Medical), Method, and Region—Global Forecast to 2026. Available online: https://www.marketsandmarkets.com/Market-Reports/carbon-nanotubes-139.html (accessed on 9 December 2021).
- Aliana-Nasharuddin, N.; Asikin-Mijan, N.; Abdulkareem-Alsultan, G.; Saiman, M.I.; Alharthi, F.A.; Alghamdi, A.A.; Taufiq-Yap, Y.H. Production of green diesel from catalytic deoxygenation of chicken fat oil over a series binary metal oxide-supported MWCNTs. RSC Adv. 2020, 10, 626–642. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Li, X.; Liu, J.; Rong, L. A clean hydroprocessing of jatropha oil into biofuels over a high performance Ni-HPW/CNT catalyst. Nano 2017, 12, 1750142. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; Rosman, N.A.; Abdulkareem-Alsultan, G.; Mastuli, M.S.; Lee, H.V.; Nabihah-Fauzi, N.; Lokman, I.M.; Alharthi, F.A.; Alghamdi, A.A.; Aisyahi, A.A.; et al. Production of renewable diesel from Jatropha curcas oil via pyrolytic-deoxygenation over various multi-wall carbon nanotube-based catalysts. Process Saf. Environ. Prot. 2020, 142, 336–349. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; Lee, H.V.; Abdulkareem-Alsultan, G.; Afandi, A.; Taufiq-Yap, Y.H. Production of green diesel via cleaner catalytic deoxygenation of Jatropha curcas oil. J. Clean. Prod. 2017, 167, 1048–1059. [Google Scholar] [CrossRef]
- Malins, K. Synthesis of renewable hydrocarbons from vegetable oil feedstock by hydrotreatment over selective sulfur-free SiO2-Al2O3 supported monometallic Pd, Pt, Ru, Ni, Mo and bimetallic NiMo catalysts. Fuel 2021, 285, 119129. [Google Scholar] [CrossRef]
- Alsultan, G.A.; Asikin-Mijan, N.; Lee, H.V.; Albazzaz, A.S.; Taufiq-Yap, Y.H. Deoxygenation of waste cooking to renewable diesel over walnut shell-derived nanorode activated carbon supported CaO-La2O3 catalyst. Energy Convers. Manag. 2017, 151, 311–323. [Google Scholar] [CrossRef]
- Nur Azreena, I.; Lau, H.L.N.; Asikin-Mijan, N.; Hassan, M.A.; Izham, S.M.; Safa Gamal, M.; Nor Adira Wan Khalit, W.; Arumugam, M.; Kennedy, E.; Stockenhuber, M.; et al. Hydrodeoxygenation of fatty acid over La-modified HZSM5 for premium quality renewable diesel production. J. Anal. Appl. Pyrolysis 2022, 161, 105406. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Yang, J.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Synthesis of renewable diesel with the 2-methylfuran, butanal and acetone derived from lignocellulose. Bioresour. Technol. 2013, 134, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Pérez, W.; Marín, J.; del Río, J.; Peña, J.; Rios, L. Upgrading of palm oil renewable diesel through hydroisomerization and formulation of an optimal blend. Fuel 2017, 209, 442–448. [Google Scholar] [CrossRef]


| Process | Advantages | Disadvantages |
|---|---|---|
| Transesterification |
|
|
| Hydroprocessing |
|
|
| Type of Support | Elemental Composition | Surface Area | Pore Volume | Remarks | Reference | |
|---|---|---|---|---|---|---|
| γ-Al2O3 | - | BET: 352 m2/g | Total: 0.68 cc/g | Total acidity: 0.99 mmol/g Acid sites distribution Weak (100–200 °C): 0.40 mmol/g Moderate (200–350 °C): 0.21 mmol/g Strong (>350 °C): 0.37 mmol/g | [69] | |
| SiO2 | - | BET: 342 m2/g | Total: 0.97 cm3/g | - | [77] | |
| CaO | Ca: 98.91 atomic% Other (include S, Sr, Cu, Br, K, Fe): 1.09 atomic% | BET: 9.8 m2/g | - | Basic sites: 548.52 µmol/g | [79] | |
| Type of Support | Type of Catalyst | Elemental Composition | Surface Area | Pore Volume | Remarks | Reference |
| Al2O3 | Pt | - | BET: 292 m2/g | Total: 0.66 cc/g | Total acidity: 1.27 mmol/g Acid sites distribution Weak (100–200 °C): 0.56 mmol/g Moderate (200–350 °C): 0.63 mmol/g Strong (>350 °C): 0.07 mmol/g | [69] |
| Al2O3 | Pt-WOx | Pt: 2.4 wt% Mo/W: 10.3 wt% Al: 42.7 wt% O: 44.4 wt% | BET: 240 m2/g | Total: 0.23 cc/g | Total acidity: 1.39 mmol/g Acid sites distribution Weak (100–200 °C): 0.55 mmol/g Moderate (200–350 °C): 0.47 mmol/g Strong (>350 °C): 0.37 mmol/g | [69] |
| Al2O3 | Pt-MoOx | Pt: 3.4 wt% Mo/W:7.6 wt% Al: 43.2 wt% O: 45.8 wt% | BET: 269 m2/g | Total: 0.19 cc/g | Total acidity: 1.70 mmol/g Acid sites distribution Weak (100–200 °C): 0.64 mmol/g Moderate (200–350 °C): 0.80 mmol/g Strong (>350 °C): 0.25 mmol/g | [69] |
| Al2O3 | Pt-ReOx | - | BET: 242 m2/g | Total: 0.21 cc/g | Total acidity: 1.40 mmol/g Acid sites distribution Weak (100–200 °C): 0.68 mmol/g Moderate (200–350 °C): 0.64 mmol/g Strong (>350 °C): 0.07 mmol/g | [69] |
| Al2O3 | Pt-SnOx | - | BET: 187 m2/g | Total: 0.16 cc/g | Total acidity: 1.26 mmol/g Acid sites distribution Weak (100–200 °C): 0.35 mmol/g Moderate (200–350 °C): 0.63 mmol/g Strong (>350 °C): 0.27 mmol/g | [69] |
| Al2O3 | Ni-Mo | NiO: 3.5 wt% Ni: 2.75 wt% MoO3: 10.5 wt% Mo: 6.7 wt% | Micro: 262 m2/g External: 30.7 m2/g | Total: 0.7 cm3/g | External surface area is the metallic surface area Dispersion of CO: 7% | [70] |
| Al2O3 | Ni-Mo | S: 9.8 wt% C: 5.9 wt% | BET: 177 m2/g | - | Desorbed NH3: 98.3 mmol at maximum temperature, 167 °C | [80] |
| Al2O3 | Co-Mo | CoO: 4.4 wt% Co: 3.46 wt% MoO3: 11.5 wt% Mo: 7.67 wt% | Micro: 242 m2/g External: 20.8 m2/g | Total: 0.6 cm3/g | External surface area is the metallic surface area Dispersion of CO: 4% | [70] |
| SiO2 | Ni | Ni: 2.82 wt% O: 54.93 wt% Si: 42.25 wt% | BET: 237 m2/g | Total: 0.66 cm3/g | Active site: 0.03 mmol/g Metal exposed: 0.34% | [77] |
| SiO2 | Ni12P5 | Ni: 5.22 wt% P: 2.06 wt% O: 56.90 wt% Si: 35.82 wt% | BET: 218 m2/g | Total: 0.55 cm3/g | Active site: 0.03 mmol/g Metal exposed: 0.45% | [77] |
| SiO2 | Ni2P | Ni: 4.02 wt% P: 5.27 wt% O: 57.12 wt% Si: 33.59 wt% | BET: 88 m2/g | Total: 0.38 cm3/g | Active site: 0.02 mmol/g Metal exposed: 0.26% | [77] |
| SiO2 | Coal fly ash | - | External: 32.58 m2/g BET: 35.11 m2/g | Micro: 0.000852 cm3/g Total: 0.13 cm3/g | Micropore area: 2.53 m2/g | [78] |
| SiO2 | Ni-Mo | S: 15.6 wt% C: 5.6 wt% | BET: 343 m2/g | - | Desorbed NH3: 108.1 mmol at maximum temperature, 160 °C | [80] |
| TiO2 | Ni-Mo | S: 7.1 wt% C: 5.5 wt% | BET: 117 m2/g | - | Desorbed NH3: 54.1 mmol at maximum temperature, 195 °C | [80] |
| CaO | Co | Ca: 80.29 atomic% Co: 19.37 atomic% Other (include S, Sr, Cu, Br, K, Fe): 0.34 atomic% | BET: 7.46 m2/g | - | Basic sites: 260.79, 307.77 µmol/g (603, 826 °C) Acid sites: 257, 486.91 µmol/g (488, 840 °C) | [79] |
| CaO | W | Ca: 83.04 atomic% W: 16.24 atomic% Other (include S, Sr, Cu, Br, K, Fe): 0.72 atomic% | BET: 7.48 m2/g | - | Basic sites: 450.55 µmol/g (638 °C) Acid sites: 77.22, 10340.49 µmol/g (505, 734 °C) | [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chia, S.R.; Nomanbhay, S.; Chew, K.W.; Show, P.L.; Milano, J.; Shamsuddin, A.H. Indigenous Materials as Catalyst Supports for Renewable Diesel Production in Malaysia. Energies 2022, 15, 2835. https://doi.org/10.3390/en15082835
Chia SR, Nomanbhay S, Chew KW, Show PL, Milano J, Shamsuddin AH. Indigenous Materials as Catalyst Supports for Renewable Diesel Production in Malaysia. Energies. 2022; 15(8):2835. https://doi.org/10.3390/en15082835
Chicago/Turabian StyleChia, Shir Reen, Saifuddin Nomanbhay, Kit Wayne Chew, Pau Loke Show, Jassinnee Milano, and Abd Halim Shamsuddin. 2022. "Indigenous Materials as Catalyst Supports for Renewable Diesel Production in Malaysia" Energies 15, no. 8: 2835. https://doi.org/10.3390/en15082835
APA StyleChia, S. R., Nomanbhay, S., Chew, K. W., Show, P. L., Milano, J., & Shamsuddin, A. H. (2022). Indigenous Materials as Catalyst Supports for Renewable Diesel Production in Malaysia. Energies, 15(8), 2835. https://doi.org/10.3390/en15082835