Helianthus salicifolius as a New Biomass Source for Biogas Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Works Design
2.2. Feedstock Origin and Silage Preparation
2.3. Analytical Methods
2.4. Fermentation Measurements
2.5. Statistical Analysis
3. Results and Discussion
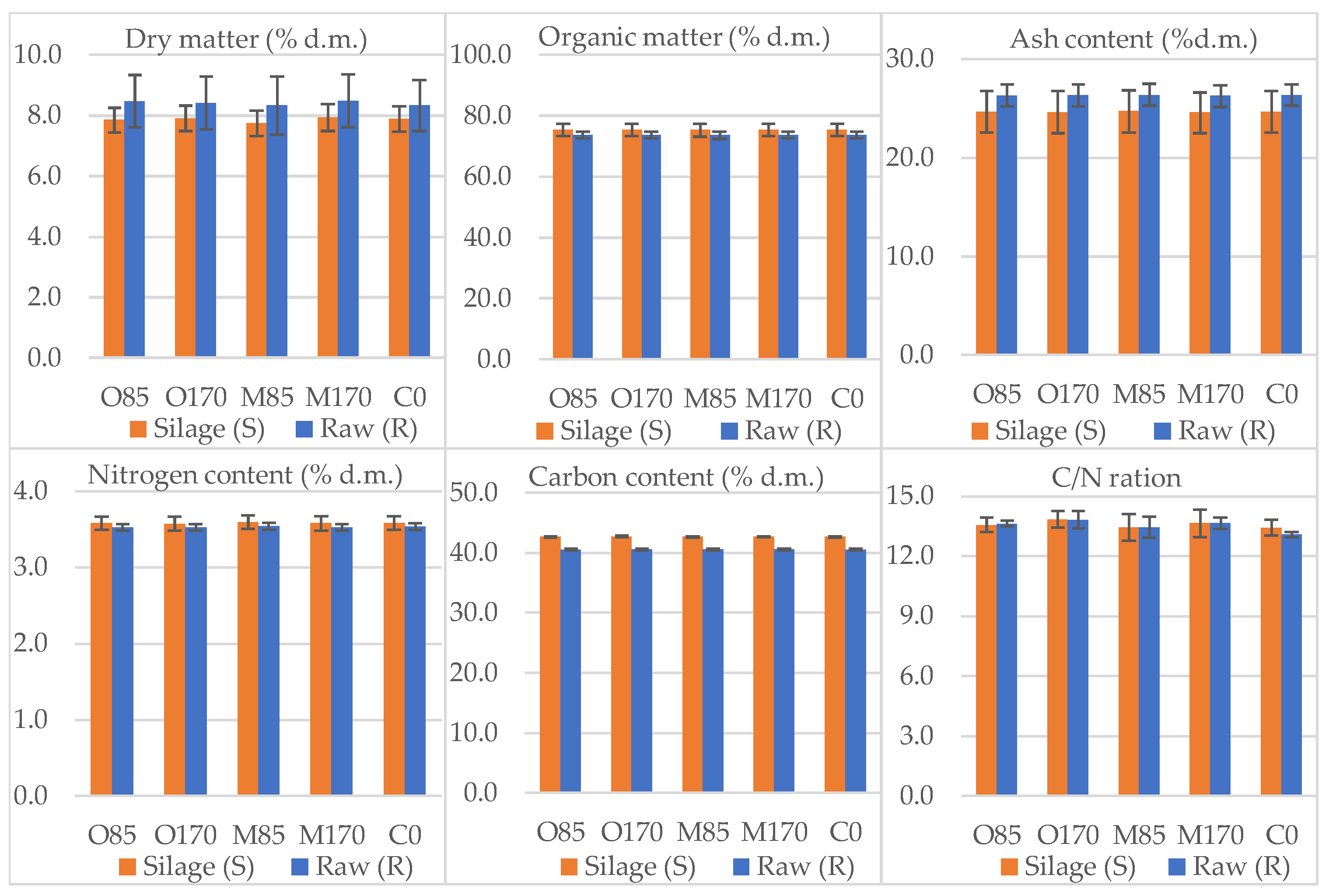
3.1. Feedstock Characteristics and Correlation
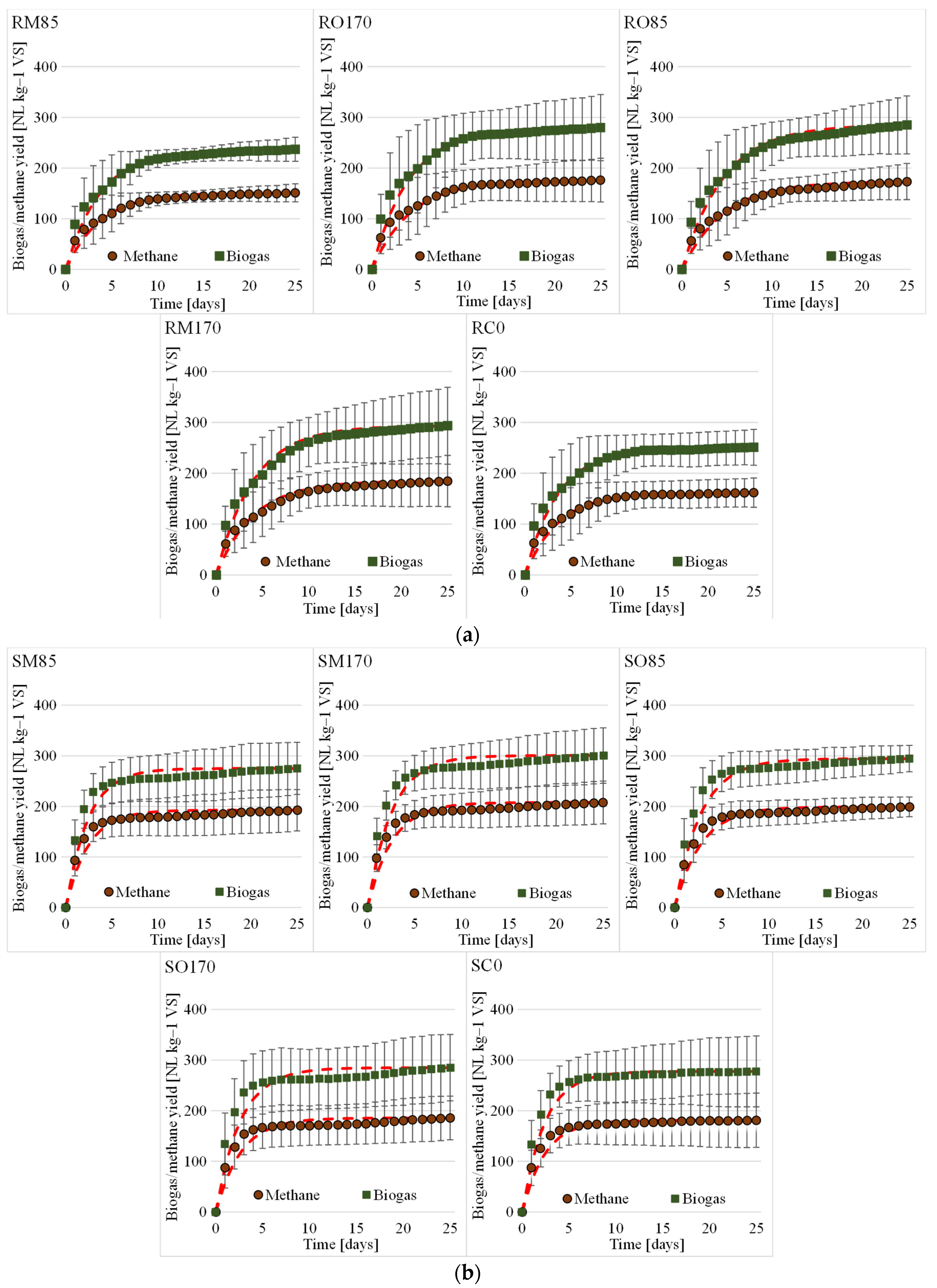
3.2. Biogas and Biomethane Production
3.3. Digestate Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- European Commission Bioeconomy Strategy. A Sustainable Bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment; European Commission: Brussels, Belgium, 2018. Available online: https://ec.europa.eu/research/bioeconomy/pdf/ec_bioeconomy_strategy_2018.pdf (accessed on 25 February 2020).
- Eurostat. Strategy Bioeconomy, Share of Energy from Renewable Sources. 2021. Available online: https://ec.europa.eu/eurostat/databrowser/view/nrg_ind_ren/default/bar?lang=en (accessed on 7 November 2021).
- European Parliament. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC; European Parliament: Strasbourg, France, 2009.
- Scarlat, N.; Dallemand, J.F.; Taylor, N.; Banja, M. Brief on Biomass for Energy in the European Union; Lopez, J.S., Avraamides, M., Eds.; Publications Office of the European Union: Luxembourg, 2019; ISBN 978-92-79-77235-1. [CrossRef]
- Wellinger, A.; Murphy, J.D.; Baxter, D. The Biogas Handbook: Science, Production and Applications; Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar]
- Plochl, M.; Heiermann, M. Biogas farming in central and northern Europe: A strategy for developing countries? Agric. Eng. Int. 2006, 8, 1–15. [Google Scholar]
- EBA. Statistical Report. European Biogas Association Report. 2020. Available online: https://www.europeanbiogas.eu/eba-statistical-report-2020 (accessed on 9 November 2021).
- Ruf, T.; Audu, V.; Holzhauser, K.; Emmerling, C. Bioenergy from periodically waterlogged cropland in Europe: A first assessment of the potential of five perennial energy crops to provide biomass and their interactions with soil. Agronomy 2019, 9, 374. [Google Scholar] [CrossRef]
- Altieri, M.A. The ecological impacts of large-scale agrofuel monoculture production systems in the Americas. Bull. Sci. Technol. Sci. 2009, 29, 236–244. [Google Scholar] [CrossRef]
- Mudryk, K.; Wróbel, M. Willow-leaved sunflower Helianthus salicifolius A. Dietr. for energy purposes. Agric. Eng. 2012, 136, 249–256. (In Polish) [Google Scholar]
- Thompson, T.E.; Zimmerman, D.C.; Rogers, C.E. Wild Helianthus as a genetic resource. Field Crops Res. 1981, 4, 333–343. [Google Scholar] [CrossRef]
- Faure, N.; Serieys, H.; Bervillé, A. Potential gene flow from cultivated sunflower to volunteer, wild Helianthus species in Europe. Agric. Ecosyst. Environ. 2002, 89, 183–190. [Google Scholar] [CrossRef]
- Hind, N.; Smith, A. 901. Helianthus salicifolius: Compositae. Curtis’s Bot. Mag. 2018, 35, 444–460. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Warmiński, K.; Tworkowski, J.; Szczukowski, S.; Olba–Zięty, E.; Gołaszewski, J. Energy efficiency of perennial herbaceous crops production depending on the type of digestate and mineral fertilizers. Energy 2017, 134, 50–60. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Śnieg, M.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S. Short rotation coppices, grasses and other herbaceous crops: Productivity and yield energy value versus 26 genotypes. Biomass Bioenergy 2018, 119, 109–120. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Warmiński, K.; Tworkowski, J.; Szczukowski, S. Perennial herbaceous crops as a feedstock for energy and industrial purposes: Organic and mineral fertilizers versus biomass yield and efficient nitrogen utilization. Ind. Crops Prod. 2017, 107, 244–259. [Google Scholar] [CrossRef]
- Lava, S.S.; Zipper, R.; Spring, O. Spring, Sunflower white blister rust—Host specificity and fungicide effects on infectivity and early infection stages. Crop Prot. 2015, 67, 214–222. [Google Scholar] [CrossRef]
- Malm, A.; Grzegorczyk, A.; Biernasiuk, A.; Baj, T.; Rój, E.; Tyśkiewicz, K.; Dębczak, A.; Stolarski, M.J.; Krzyżaniak, M.; Olba-Zięty, E. Could supercritical extracts from the aerial parts of Helianthus salicifolius A. Dietr. and Helianthus tuberosus L. be regarded as potential raw materials for biocidal purposes? Agriculture 2021, 11, 10. [Google Scholar] [CrossRef]
- Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J.; Peni, D. Growth Potential of Yellow Mealworm Reared on Industrial Residues. Agriculture 2020, 10, 599. [Google Scholar] [CrossRef]
- Dutta, N.; Usman, M.; Luo, G.; Zhang, S. An Insight into Valorization of Lignocellulosic Biomass by Optimization with the Combination of Hydrothermal (HT) and Biological Techniques: A Review. Sustain. Chem. 2022, 3, 3. [Google Scholar] [CrossRef]
- Piccitto, A.; Scordia, D.; Corinzia, S.A.; Cosentino, S.L.; Testa, G. Advanced Biomethane Production from Biologically Pretreated Giant Reed under Different Harvest Times. Agronomy 2022, 12, 712. [Google Scholar] [CrossRef]
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Zborowska, M.; Waliszewska, H.; Waliszewska, B.; Borysiak, S.; Brozdowski, J.; Stachowiak-Wencek, A. Conversion of Carbohydrates in Lignocellulosic Biomass after Chemical Pretreatment. Energies 2022, 15, 254. [Google Scholar] [CrossRef]
- Singh, H.; Tomar, S.; Qureshi, K.A.; Jaremko, M.; Rai, P.K. Recent Advances in Biomass Pretreatment Technologies for Biohydrogen Production. Energies 2022, 15, 999. [Google Scholar] [CrossRef]
- Wu, Z.; Ferreira, D.F.; Crudo, D.; Bosco, V.; Stevanato, L.; Costale, A.; Cravotto, G. Plant and Biomass Extraction and Valorisation under Hydrodynamic Cavitation. Processes 2019, 7, 965. [Google Scholar] [CrossRef]
- Zieliński, M.; Kisielewska, M.; Dudek, M.; Rusanowska, P.; Nowicka, A.; Krzemieniewski, M.; Kazimierowicz, J.; Dębowski, M. Comparison of microwave thermohydrolysis and liquid hot water pretreatment of energy crop Sida hermaphrodita for enhanced methane production. Biomass Bioenergy 2019, 128, 105324. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Bartkowska, I.; Walery, M. Effect of Low-Temperature Conditioning of Excess Dairy Sewage Sludge with the Use of Solidified Carbon Dioxide on the Efficiency of Methane Fermentation. Energies 2021, 14, 150. [Google Scholar] [CrossRef]
- Dębowski, M.; Kisielewska, M.; Kazimierowicz, J.; Rudnicka, A.; Dudek, M.; Romanowska-Duda, Z.; Zieliński, M. The effects of Microalgae Biomass Co-Substrate on Biogas Production from the Common Agricultural Biogas Plants Feedstock. Energies 2020, 13, 2186. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dzienis, L.; Dębowski, M.; Zieliński, M. Optimisation of methane fermentation as a valorisation method for food waste products. Biomass Bioenergy 2021, 144, 105913. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Zieliński, M.; Dębowski, M. Influence of the Heating Method on the Efficiency of Biomethane Production from Expired Food Products. Fermentation 2021, 7, 12. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. The Effect of Static Magnetic Field on Methanogenesis in the Anaerobic Digestion of Municipal Sewage Sludge. Energies 2021, 14, 590. [Google Scholar] [CrossRef]
- Kiesel, A.; Lewandowski, I. Miscanthus as biogas substrate—Cutting tolerance and potential for anaerobic digestion. GCB-Bioenergy 2017, 9, 153–167. [Google Scholar] [CrossRef]
- Paul, S.; Dutta, A. Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour. Conserv. Recycl. 2018, 130, 164–174. [Google Scholar] [CrossRef]
- Drosg, B.; Braun, R.; Bochmann, G.; Al Saedi, T. Analysis and characterisation of biogas feedstocks. In The Biogas Handbook: Science, Production and Applications; Murphy, J.D., Baxter, D., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 52–84. ISBN 9780857097415. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, K.; Liu, P.; Khan, A.; Xiong, J.; Tian, F.; Li, X. A critical review on the interaction of substrate nutrient balance and microbial community structure and function in anaerobic co-digestion. Bioresour. Technol. 2018, 247, 1119–1127. [Google Scholar] [CrossRef]
- Schnurer, A.; Jarvis, A. Microbiological Handbook for Biogas Plants; Swedish Gas Centre Report 207; Swedish Gas Centre: Malmö, Sweden, 2010; pp. 13–138. [Google Scholar]
- Kazimierowicz, J.; Dzienis, L. Giant miscanthus as a substrate for biogas production. J. Ecol. Eng. 2015, 16, 139–142. [Google Scholar] [CrossRef]
- Ma, S.; Wang, H.; Li, J.; Fu, Y.; Zhu, W. Methane production performances of different compositions in lignocellulosic biomass through anaerobic digestion. Energy 2019, 189, 116190. [Google Scholar] [CrossRef]
- Prapinagsorn, W.; Sittijunda, S.; Reungsang, A. Co-Digestion of Napier Grass and Its Silage with Cow Dung for Bio-Hydrogen and Methane Production by Two-Stage Anaerobic Digestion Process. Energies 2018, 11, 47. [Google Scholar] [CrossRef]
- Galván, M.J.; Degano, S.; Cagnolo, M.; Becker, A.; Hilbert, J.; Fuentes, M.; Acevedo, D. Batch optimization of biogas yield from pasteurized slaughterhouse by-products incorporating residues from corn sieving. Biomass Bioenergy 2021, 151, 106136. [Google Scholar] [CrossRef]
- Pakarinen, O.; Lehtomäki, A.; Rissanen, S.; Rintala, J. Storing energy crops for methane production: Effects of solids content and biological additive. Bioresour. Technol. 2008, 99, 7074–7082. [Google Scholar] [CrossRef] [PubMed]
- Surendra, K.C.; Khanal, S.K. Effects of crop maturity and size reduction on digestibility and methane yield of dedicated energy crop. Bioresour. Technol. 2015, 178, 187–193. [Google Scholar] [CrossRef]
- Menardo, S.; Bauer, A.; Theuretzbacher, F.; Piringer, G.; Nilsen, P.J.; Balsari, P.; Pavliska, O.; Amon, T. Biogas production from steam-exploded Miscanthus and utilization of biogas energy and CO2 in greenhouses. Bioenergy Res. 2013, 6, 620–630. [Google Scholar] [CrossRef]
- Klimiuk, E.; Pokoj, T.; Budzyński, W.; Dubis, B. Theoretical and observed biogas production from plant biomass of different fibre contents. Bioresour. Technol. 2010, 101, 9527–9535. [Google Scholar] [CrossRef]
- Kupryś-Caruk, M.; Podlaski, S. The comparison of single and double cut harvests on biomass yield, quality and biogas production of Miscanthus × giganteus. Plant Soil Environ. 2019, 65, 369–376. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Dubis, B.; Budzyński, W.S.; Bórawski, P.; Bułkowska, K. Energy efficiency of crops grown for biogas production in a large-scale farm in Poland. Energy 2016, 109, 277–286. [Google Scholar] [CrossRef]
- Schievano, A.; D’Imporzano, G.; Orzi, V.; Colombo, G.; Maggiore, T.; Adani, F. Biogas from dedicated energy crops in Northern Italy: Electric energy generation costs. GCB Bioenergy 2015, 7, 899–908. [Google Scholar] [CrossRef]
- Ferrero, F.; Dinuccio, E.; Rollé, L.; Tabacco, E.; Borreani, G. Suitability of cardoon (Cynara cardunculus L.) harvested at two stages of maturity to ensiling and methane production. Biomass Bioenergy 2020, 142, 105776. [Google Scholar] [CrossRef]
- McEniry, J.; O’Kiely, P. Anaerobic methane production from five common grassland species at sequential stages of maturity. Bioresour. Technol. 2013, 127, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kupryś-Caruk, M.; Podlaski, S.; Kotyrba, D. Influence of double-cut harvest system on biomass yield, quality and biogas production from C4 perennial grasses. Biomass Bioenergy 2019, 130, 105376. [Google Scholar] [CrossRef]
- Jurgutis, L.; Šlepetienė, A.; Amalevičiūtė-Volungė, K.; Volungevičius, J.; Šlepetys, J. The effect of digestate fertilisation on grass biogas yield and soil properties in field-biomass-biogas-field renewable energy production approach in Lithuania. Biomass Bioenergy 2021, 153, 106211. [Google Scholar] [CrossRef]
- Tilvikiene, V.; Venslauskas, K.; Povilaitis, V.; Navickas, K.; Zuperka, V.; Kadziuliene, Z. The effect of digestate and mineral fertilisation of cocksfoot grass on greenhouse gas emissions in a cocksfoot-based biogas production system. Energy Sustain. Soc. 2020, 10, 13. [Google Scholar] [CrossRef]
- Herrmann, C.; Heiermann, M.; Idler, C. Effects of ensiling, silage additives and storage period on methane formation of biogas crops. Bioresour. Technol. 2011, 102, 5153–5161. [Google Scholar] [CrossRef] [PubMed]
- Kreuger, E.; Nges, I.A.; Björnsson, L. Ensiling of crops for biogas production: Effects on methane yield and total solids determination. Biotechnol. Biofuels 2011, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Kisielewska, M.; Rusanowska, P.; Dudek, M.; Nowicka, A.; Krzywik, A.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M. Evaluation of ultrasound pretreatment for enhanced anaerobic digestion of Sida hermaphrodita. Bioenergy Res. 2020, 13, 824–832. [Google Scholar] [CrossRef]
- Zieliński, M.; Rusanowska, P.; Krzywik, A.; Dudek, M.; Nowicka, A.; Dębowski, M. Application of hydrodynamic cavitation for improving methane fermentation of Sida hermaphrodita silage. Energies 2019, 12, 526. [Google Scholar] [CrossRef]
- Głowacka, A.; Szostak, B.; Klebaniuk, R. Effect of biogas digestate and mineral fertilisation on the soil properties and yield and nutritional value of switchgrass forage. Agronomy 2020, 10, 490. [Google Scholar] [CrossRef]
- Pokój, T.; Bułkowska, K.; Gusiatin, Z.M.; Klimiuk, E.; Jankowski, K.J. Semi-continuous anaerobic digestion of different silage crops: VFAs formation, methane yield from fiber and non-fiber components and digestate composition. Bioresour. Technol. 2015, 190, 201–210. [Google Scholar] [CrossRef]
- Różyło, K.; Oleszczuk, P.; Jośko, I.; Kraska, P.; Kwiecińska-Poppe, E.; Andruszczak, S. An ecotoxicological evaluation of soil fertilized with biogas residues or mining waste. Environ. Sci. Pollut. Res. 2015, 22, 7833–7842. [Google Scholar] [CrossRef] [PubMed]
- Kazimierowicz, J. Biogas acquisition from selected mixtures of expired food products. J. Ecol. Eng. 2017, 18, 118–122. [Google Scholar] [CrossRef][Green Version]
- Visvanathan, C. Evaluation of anaerobic digestate for greenhouse gas emissions at various stages of its management. Int. Biodeterior. Biodegrad. 2014, 95, 167–175. [Google Scholar] [CrossRef]
- Sheets, J.P.; Yang, L.; Ge, X.; Wang, Z.; Li, Y. Beyond land application: Emerging technologies for the treatment and reuse of anaerobically digested agricultural and food waste. Waste Manag. 2015, 44, 94–115. [Google Scholar] [CrossRef]

| Feedstock Type from Helianthus salicifolius | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Raw Biomass (R) | Silage (S) | ||||||||
| Fertilization Type | |||||||||
| Mineral Fertilizer (M) | Organic Fertilizer (O) | Without Fertilization—Control (C) | Mineral Fertilizer (M) | Organic Fertilizer (O) | Without Fertilization—Control (C) | ||||
| Nitrogen dose | |||||||||
| 85 | 170 | 85 | 170 | 0 | 85 | 170 | 85 | 170 | 0 |
| Variant | |||||||||
| RM85 | RM170 | RO85 | RO170 | RC0 | SM85 | SM170 | SO85 | SO170 | SC0 |
| Composition Trait | Anaerobic Sludge |
|---|---|
| DM (%) | 6.8 ± 0.2 |
| OM (% d.m.) | 73.5 ± 0.9 |
| Ash (% d.m.) | 26.5 ± 0.9 |
| C (% d.m.) | 41.1 ± 0.8 |
| N (% d.m.) | 3.7 ± 0.0 |
| C/N ratio | 11.2 ± 0.2 |
| Source of Variation | Dry Matter | Organic Matter | Ash Content | C Content | N Content | C/N Ratio | CO2 | CH4 | Biogas Production | Biomethane Production |
|---|---|---|---|---|---|---|---|---|---|---|
| Feedstock type | 0.000 * | 0.657 | 0.657 | 0.000 * | 0.000 * | 0.001 * | 0.000 * | 0.000 * | 0.317 | 0.097 |
| Fertilization type | 0.005 * | 0.000 * | 0.000 * | 0.154 | 0.000 * | 0.000 * | 0.005 * | 0.016 * | 0.632 | 0.685 |
| Nitrogen (N) dose | 0.690 | 0.115 | 0.115 | 0.755 | 0.511 | 0.454 | 0.800 | 0.561 | 0.552 | 0.639 |
| Feedstock type × Fertilization type | 0.014 * | 0.846 | 0.846 | 0.684 | 0.266 | 0.101 | 0.001 * | 0.002 * | 0.902 | 0.884 |
| Feedstock type × N dose | 0.183 | 0.140 | 0.140 | 0.464 | 0.875 | 0.754 | 0.382 | 0.235 | 0.756 | 0.667 |
| Fertilization type × N dose | 0.768 | 0.308 | 0.308 | 0.719 | 0.610 | 0.344 | 0.981 | 0.818 | 0.523 | 0.630 |
| Feedstock type × Fertilization type × N dose | 0.532 | 0.414 | 0.414 | 0.871 | 0.896 | 0.970 | 0.123 | 0.208 | 0.934 | 0.953 |
| Item | CO2 | CH4 | Biomethane Production | Biogas Production | Organic Matter | Ash Content | Dry Matter Content | C Content | N Content | C/N Ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| CO2 % | 1.00 | |||||||||
| CH4 % | −0.99 * | 1.00 | ||||||||
| Biomethane production | −0.63 * | 0.64 * | 1.00 | |||||||
| Biogas production | −0.45 * | 0.47 * | 0.98 * | 1.00 | ||||||
| Organic matter | −0.11 | 0.06 | −0.22 | −0.26 | 1.00 | |||||
| Ash content | 0.11 | −0.06 | 0.22 | 0.26 | −1.00 * | 1.00 | ||||
| Dry matter content | 0.55 * | −0.58 * | −0.38 * | −0.28 | 0.53 * | −0.53 * | 1.00 | |||
| C content | −0.60 * | 0.59 * | 0.47 * | 0.39 * | 0.09 | −0.09 | −0.58 * | 1.00 | ||
| N content | −0.60 * | 0.62 * | 0.52 * | 0.41 * | −0.49 * | 0.49 * | −0.87 * | 0.48 * | 1.00 | |
| C/N ratio | 0.47 * | −0.49 * | −0.43 * | −0.35 * | 0.59 * | −0.59 * | 0.83 * | −0.28 | −0.97 * | 1.00 |
| Variant | CO2 (%) | CH4 (%) | rm (cm3 day−1) | rb (cm3 day−1) | km,b (L day−1) |
|---|---|---|---|---|---|
| RO85 | 39.30 ± 0.31 | 60.70 ± 0.31 | 38.1 | 62.8 | 0.22 |
| RO170 | 37.51 ± 0.69 | 62.83 ± 0.76 | 42.3 | 69.9 | 0.25 |
| RM85 | 36.36 ± 1.12 | 63.64 ± 1.12 | 40.8 | 64.0 | 0.27 |
| RM170 | 37.35 ± 1.00 | 62.65 ± 1.00 | 46.1 | 73.4 | 0.25 |
| RC0 | 35.83 ± 2.39 | 64.17 ± 2.39 | 45.3 | 70.3 | 0.28 |
| SO85 | 32.43 ± 0.69 | 67.57 ± 0.69 | 71.7 | 106.0 | 0.36 |
| SO170 | 34.93 ± 0.16 | 65.08 ± 0.16 | 70.5 | 108.3 | 0.38 |
| SM85 | 30.37 ± 1.90 | 69.64 ± 1.90 | 80.9 | 115.6 | 0.42 |
| SM170 | 29.95 ± 1.21 | 68.83 ± 1.48 | 81.0 | 117.3 | 0.39 |
| SC0 | 35.59 ± 3.11 | 64.41 ± 3.11 | 76.0 | 116.6 | 0.42 |
| Composition Trait | RO85 | RO170 | RM85 | RM170 | RC0 | SO85 | SO170 | SM85 | SM170 | SC0 | I.A.A.D. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OM (% d.m.) | 72.9 ± 0.3 | 73.0 ± 0.3 | 72.6 ± 0.0 | 73.2 ± 0.3 | 72.7 ± 0.0 | 69.5 ± 3.1 | 69.5 ± 2.3 | 68.7 ± 2.1 | 69.4 ± 2.8 | 70.7 ± 0.2 | 69.8 ± 1.3 |
| DM (%) | 6.7 ± 0.4 | 6.6 ± 0.5 | 6.6 ± 0.4 | 6.6 ± 0.5 | 6.6 ± 0.6 | 6.3 ± 0.0 | 6.2 ± 0.0 | 6.1 ± 0.1 | 6.2 ± 0.2 | 6.2 ± 0.2 | 5.9 ± 0.1 |
| Ash (% d.m.) | 27.1 ± 0.3 | 27.0 ± 0.3 | 27.4 ± 0.0 | 26.8 ± 0.3 | 27.3 ± 0.0 | 30.5 ± 3.1 | 30.5 ± 2.3 | 31.3 ± 2.1 | 30.6 ± 2.8 | 29.3 ± 0.2 | 30.2 ± 1.3 |
| C (% d.m.) | 40.8 ± 0.5 | 40.6 ± 0.6 | 40.9 ± 0.4 | 40.1 ± 0.7 | 39.8 ± 0.4 | 39.8 ± 1.6 | 39.6 ± 0.8 | 39.2 ± 1.3 | 39.1 ± 0.6 | 39.9 ± 0.2 | 40.3 ± 0.1 |
| N (% d.m.) | 3.3 ± 0.2 | 3.3 ± 0.2 | 3.4 ± 0.1 | 3.3 ± 0.2 | 3.3 ± 0.2 | 3.3 ± 0.2 | 3.4 ± 0.2 | 3.3 ± 0.2 | 3.4 ± 0.2 | 3.4 ± 0.3 | 3.5 ± 0.1 |
| C/N ratio | 12.5 ± 0.6 | 12.2 ± 0.5 | 12.0 ± 0.4 | 12.1 ± 0.4 | 12.2 ± 0.6 | 12.0 ± 1.1 | 11.9 ± 0.8 | 11.8 ± 1.1 | 11.5 ± 0.7 | 11.7 ± 0.9 | 11.4 ±0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peni, D.; Dębowski, M.; Stolarski, M.J. Helianthus salicifolius as a New Biomass Source for Biogas Production. Energies 2022, 15, 2921. https://doi.org/10.3390/en15082921
Peni D, Dębowski M, Stolarski MJ. Helianthus salicifolius as a New Biomass Source for Biogas Production. Energies. 2022; 15(8):2921. https://doi.org/10.3390/en15082921
Chicago/Turabian StylePeni, Dumitru, Marcin Dębowski, and Mariusz J. Stolarski. 2022. "Helianthus salicifolius as a New Biomass Source for Biogas Production" Energies 15, no. 8: 2921. https://doi.org/10.3390/en15082921
APA StylePeni, D., Dębowski, M., & Stolarski, M. J. (2022). Helianthus salicifolius as a New Biomass Source for Biogas Production. Energies, 15(8), 2921. https://doi.org/10.3390/en15082921








