Fouling Behavior and Dispersion Stability of Nanoparticle-Based Refrigeration Fluid
Abstract
:1. Introduction
- Elevated specific surface area and hence higher heat transfer surface between fluid and particles.
- Better stability of the dispersion, in which the Brownian motion of the particles prevails, compared to a normal solid/liquid suspension.
- Decreased pumping power compared to obtaining a quite similar heat transfer.
- Decreased particle obstruction, for heat transfer intensification, in comparison to conventional solid–liquid suspensions (such as fluids containing micro-sized metallic particles or as well as conventional solid/fluid mixtures).
- Facilitated system miniaturization.
- Variety of properties by changing NPs concentration.
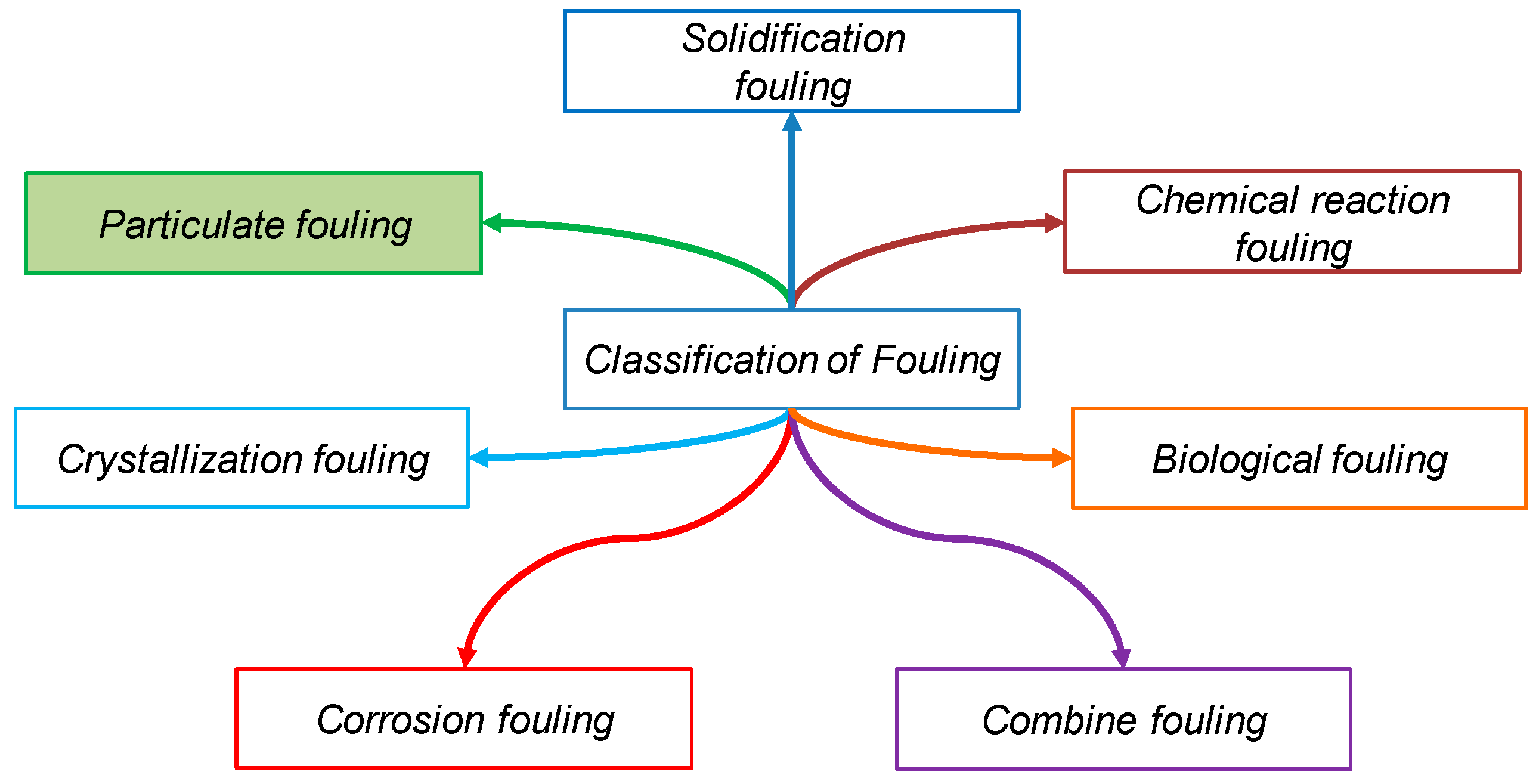
2. Particulate Fouling: Towards a Description
- Deposition: operated by the contacts between the fluid and particles and then also by the contacts between the wall surfaces and particles.
- Re-entrainment: Happens after the deposition. Indeed, the settled NPs can be re-suspended. This phenomenon is determined by contact forces and hydrodynamic forces: whether the particles stay on the surfaces or are re-suspended depending on the equilibrium between these forces.
- Agglomeration: It intervenes only when the NPs’ concentration is high enough to permit surface–surface interactions to occur repeatedly. Similar to the deposition phenomenon, the frequency of particle–particle collisions is governed by the hydrodynamic transport of the particles, while the resulting adhesion between two particles can be limited by the interactions between the short-range particles. Furthermore, when adhesion forces are lower than hydrodynamic forces, the break-up of aggregates can happen.
- Clogging: Occurs when several NP layers collect on the surface leading to a multilayer formation. After the growth of several layers of particles, the deposit disturbs the hydrodynamic flow, and, when the deposit reaches a size similar to the section of the pipe, the blockage occurs.
- Flow velocity. Indeed, the fouling resistance decreases continuously with the increasing velocity, except for a very low velocity.
- Heat flux. With an increasing heat flux, the resistance to fouling phenomena reaches a maximum, which drops in amplitude and moves towards higher heat fluxes depending on whether the wall shear stress is increased.
- Suspension pH. The fouling resistance is pH-dependent, showing a maximum at a specific pH value. Attachment and agglomeration are due to electrical double layer forces and Van der Waals forces. Repulsion due to the first-mentioned force is due to the charges building up on the surface of hydroxides and metal oxides in solutions. These charges are closely related to the solution pH and its ionic content.
- Surface temperature. This effect is lower than in the case of the other fouling mechanisms. In general, the fouling resistance increases with the temperature more rapidly at higher temperatures and slowly at low temperatures.
- Surface roughness. Non-wetting surfaces delay fouling and a better surface finish leads to the delay of fouling and easiness of cleaning. On the other hand, rough surfaces boost particulate deposition. During fouling, the roughness will be influenced by the deposit itself.
- Particle concentration. Fouling resistance increases with particle increases reaching a constant value.
- Particle size. The fouling resistance is also dependent on the particles’ size.
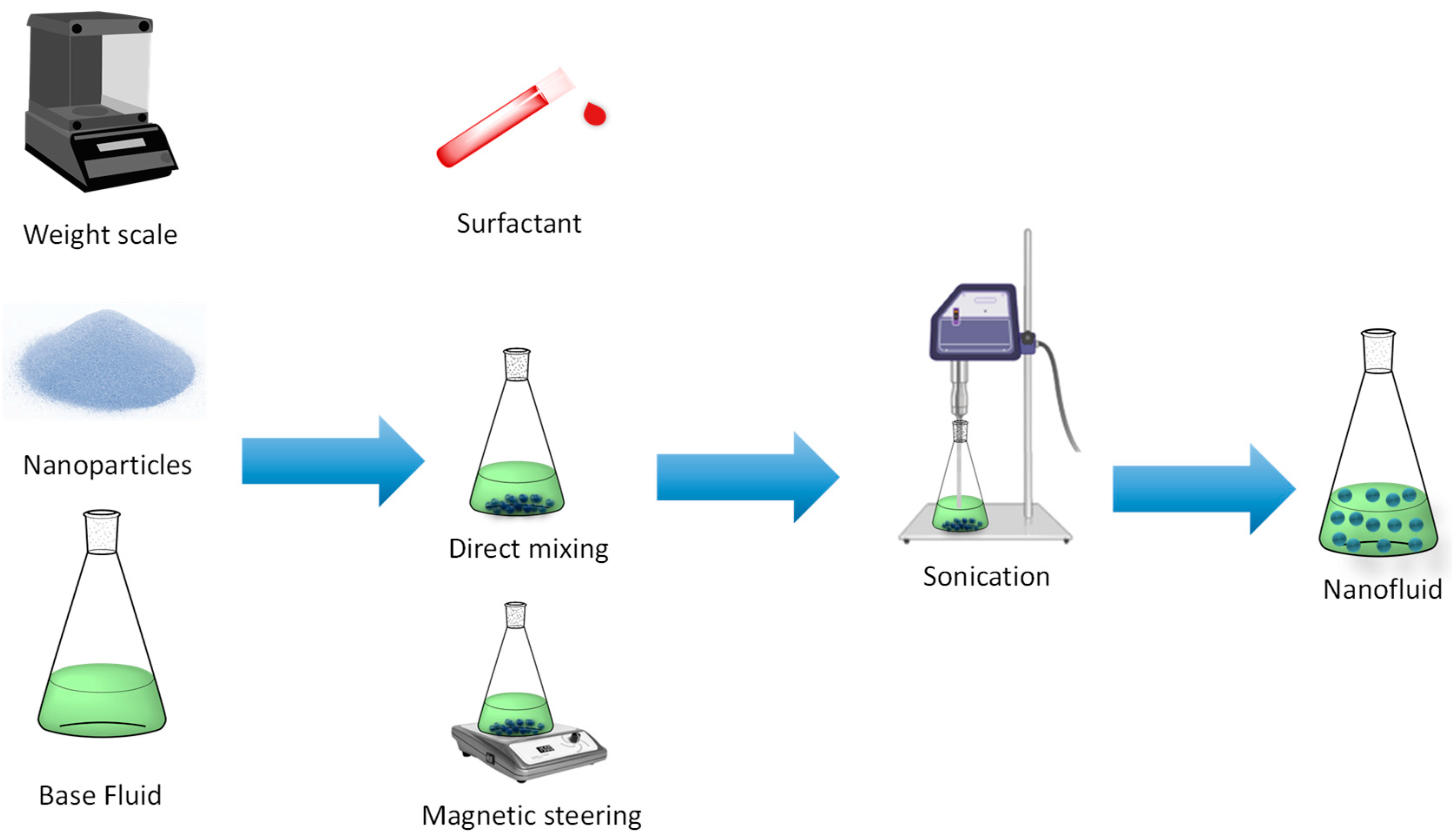
3. Preparation Procedures
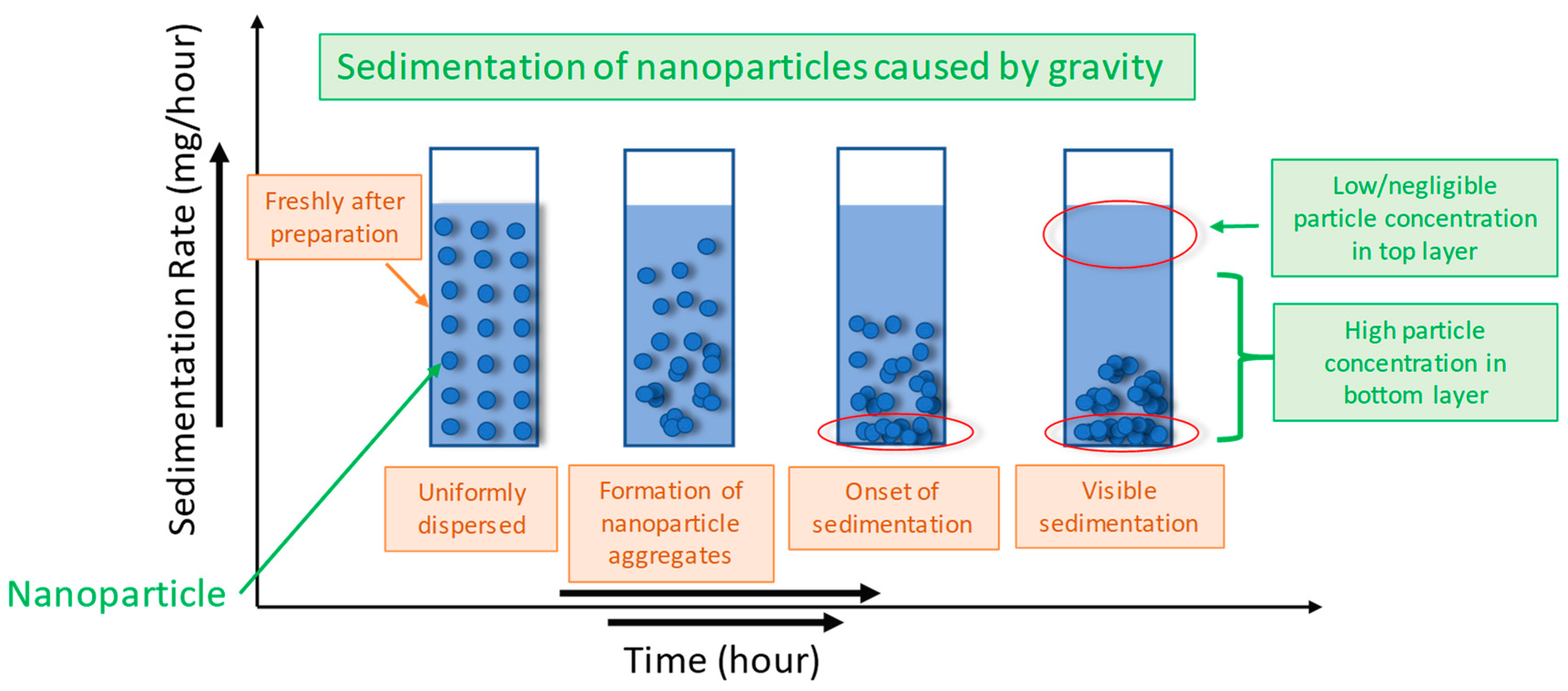
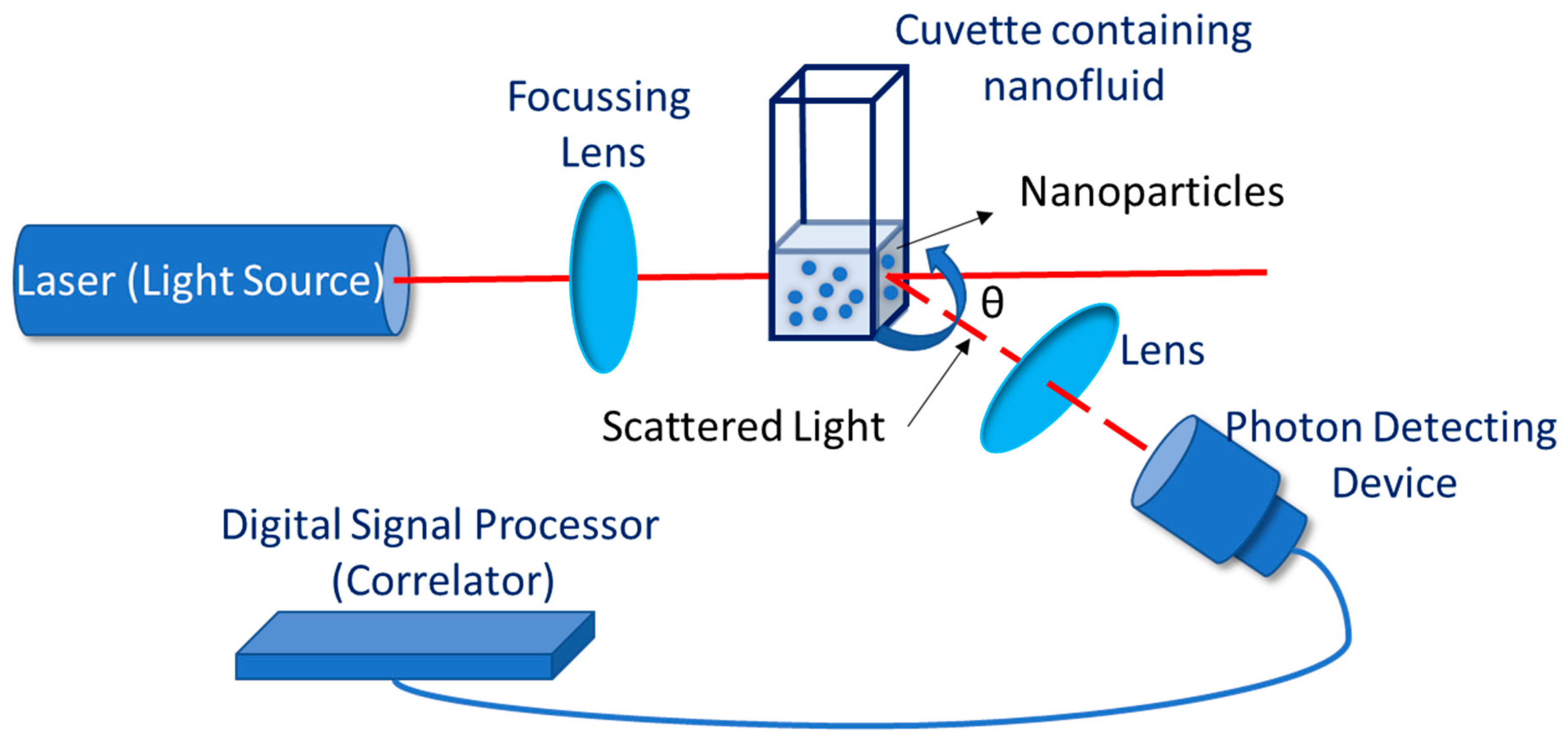
4. Stability Evaluation
5. Nanofluid Stabilization Methods
6. Nanoparticles in Nanofluids
| NPs | Size | Loading | Base Fluid | Lubricant/ Surfactant | [Ref.] | |
|---|---|---|---|---|---|---|
| Carbon-based | Diamond | 10 nm | 0 ÷ 5 wt.% | R113 | VG68 | [89] |
| Diamond | 10 nm | 2.6 vol.% | R134a | RL68H | [91] | |
| CNT | 100 ar, 667.7 ar, 18.8 ar, 125 ar | 0.2 ÷ 1 vol.% | R113 | - | [38] | |
| CNT | 20 ÷ 50 nm | 0.1% Au + 0.005% CNT; 0.2% Au + 0.005% CNT (vol.%) | R134a | - | [102] | |
| CNT | 15 ÷ 80 nm | 0 ÷ 0.1 vol.% | R113 | - | [122] | |
| MWCNTs | 8 ÷ 15 nm (length: 10–50 µm); 10 ÷ 30 nm (length: 0.5–40 µm) | 0.25 ÷ 1 vol.% | HFE-7500 | Fluorocarbon stabilizer: Krytox 157 FSL | [97] | |
| MWCNTs | 20 nm | 1 vol.% | R124, R134a | - | [101] | |
| GNS | 10 (length: 2–15 µm) | 0.25 ÷ 1 vol.% | HFE-7500 | Fluorocarbon stabilizer: Krytox 157 FSL | [97] | |
| Metal-based | Au | 10 nm | 0.1 ÷ 0.2 vol.% | R134a | polyalkylene glycol | [102] |
| Cu | 40 nm | 0.1 ÷ 0.3 wt.% | R141b | Span 80 | [107] | |
| Al | 40 nm | 0.1 ÷ 0.3 wt.% | R141b | Span 80 | [107] | |
| Metal-oxide Based | CuO | 40 nm | 0.1 ÷ 0.3 wt.% | R141b | Span 80 | [107] |
| CuO | 40 nm | 0 ÷ 1 wt.% | R600a | POE | [123] | |
| CuO | 30 nm | 0.02 ÷ 0.08% vol.% | R134a | POE | [111] | |
| CuO | 60 nm | 0.05 ÷ 0.8 vol.% | H2O/ethylene glycol (40/60) | - | [110] | |
| CuO | 60 nm | 0.05 ÷ 0.8 vol.% | H2O/ethylene glycol (40/60) | - | [109] | |
| SiO2@HMD | 0.05% ÷ 0.5% vol.% | R134a | - | [123] | ||
| NiFe2O4 | 35 nm | R134a R407c R410a R425a | Naphthene-based mineral oil B32 lubricant/Span-80 | [114] | ||
| ZnO | 29.1 nm | 10 vol.% | R134a | - | [115] | |
| TiO2 | 40 nm | 1.7 ÷ 8.3 wt./vol.% | R141b | Lubricating oil | [124] | |
| TiO2 | <25 nm | R600a | Mineral oil/oleic acid | [117] | ||
| TiO2 | 21 nm | 0.5 ÷ 2 vol.% | R123 | - | [116] | |
| TiO2 | <25 nm | 0.25 ÷ 1 vol.% | HFE-7500 | Fluorocarbon stabilizer: Krytox 157 FSL | [97] | |
| TiO2 | 50 nm | 0.06 ÷ 0.1 wt.% | R134a | SUNISO 3GS | [11] | |
| TiO2 | 21 nm | 0.01 ÷ 0.05 vol.% | R141b | - | [125] | |
| Al2O3 | 40 nm | 0.1 ÷ 0.3 wt.% | R141b | Span 80 | [107] | |
| Al2O3 | 13 nm | 0.05 ÷ 0.15 vol.% | R141b | - | [126] | |
| Al2O3 | 30 nm | 5 vol.% | R134a | - | [120] | |
| Al2O3 | <50 nm | R600a | Mineral oil/oleic acid | [117] | ||
| Al2O3 | 2 ÷ 6 vol.% | Water/ethylene glycol (50/50) | - | [127] | ||
| Al2O3 | ~10 nm | 1 ÷ 2 wt.% | R134a | - | [119] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Choi, S.U.S.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles; ASME IMECE: San Francisco, CA, USA, 1995. [Google Scholar]
- Saidur, R.; Leong, K.; Mohammad, H. A review on applications and challenges of nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Wong, K.V.; De León, O. Applications of nanofluids: Current and future. Adv. Mech. Eng. 2010, 2010, 519659. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xu, X.; Choi, S.U.S. Thermal conductivity of nanoparticle-fluid mixture. J. Thermophys. Heat Transf. 1999, 13, 474–480. [Google Scholar] [CrossRef]
- Yoo, D.H.; Hong, K.S.; Yang, H.S. Study of thermal conductivity of nanofluids for the application of heat transfer fluids. Thermochim. Acta 2007, 455, 66–69. [Google Scholar] [CrossRef]
- Kamyar, A.; Saidur, R.; Hasanuzzaman, M. Application of Computational Fluid Dynamics (CFD) for nanofluids. Int. J. Heat Mass Transf. 2012, 55, 4104–4115. [Google Scholar] [CrossRef]
- Bharathwaj, P.R.; Javeed, S.; Raju, J.J.; Padmanathan, P.; Satheesh, A. A critical review on nanorefrigerants: Boiling, condensation and tribological properties. Int. J. Refrig. 2021, 128, 139–152. [Google Scholar]
- Muhammad, A.; Arafat, A.B.; Sayedus, S.; Mohammad, M.E.; Basit, K.; Md Hamidur, R. Synthesis, heat transport mechanisms and thermophysical properties of nanofluids: A critical overview. Int. J. Therm. 2021, 10, 100086. [Google Scholar]
- Surendran, V.S.; Hansoo, K.; Joonho, L. A Review on Thermophysical Property Assessment of Metal Oxide-Based Nanofluids: Industrial Perspectives. Metals 2022, 12, 165. [Google Scholar]
- Choi, S.U.S. Development and Application of Non-Newtonian Flows, 1st ed.; Siginer, D.A., Wang, H.-P., Eds.; ASME Press: New York, NY, USA, 1995; Volume 231, pp. 99–105. [Google Scholar]
- Bi, S.; Shi, L.; Zhang, L. Application of nanoparticles in domestic refrigerators. Appl. Therm. Eng. 2008, 28, 1834–1843. [Google Scholar] [CrossRef]
- Awais, M.; Bhuiyan, A.A. Recent advancements in impedance of fouling resistance and particulate depositions in heat exchangers. Int. J. Heat Mass Transf. 2019, 141, 580–603. [Google Scholar] [CrossRef]
- Tawfik, A.S.; Vinod, K.G. Membrane Fouling and Strategies for Cleaning and Fouling Control. In Nanomaterial and Polymer Membranes Synthesis, Characterization, and Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 25–53. [Google Scholar]
- Kazi, S.N. Fouling and Fouling Mitigation on Heat Exchanger Surfaces. In Heat Exchangers—Basics Design Applications; Mitrovic, J., Ed.; InTech: Bondi Junction, NSW, Australia, 2012. [Google Scholar]
- Salim, N.K. Fouling and fouling mitigation of calcium compounds on heat exchangers by novel colloids and surface modifications. Rev. Chem. Eng. 2020, 36, 653–685. [Google Scholar]
- Kakac, S.; Liu, H. Heat Exchanger Selection Rating and Thermal Design, 2nd ed.; CRC Press: Boca, FL, USA, 2002. [Google Scholar]
- Sarma, P.K.; Krishna, K.R.; Subrahmanyam, T.; Prasad, L.S.V.; Sharma, K.V.; Srinivas, V.; Vedula, D.R.; Prasad, V.S.R.K. Fouling and its effect on the thermal performance of heat exchanger tubes. Int. J. Heat Technol. 2017, 35, 509–519. [Google Scholar] [CrossRef]
- Awais, M.; Ullah, N.; Ahmad, J.; Sikandar, F.; Ehsan, M.M.; Salehin, S.; Bhuiyan, A.A. Heat transfer and pressure drop performance of Nanofluid: A state-of-the-art review. Int. J. Thermofluids 2021, 9, 100065. [Google Scholar] [CrossRef]
- Arya, H.; Sarafraz, M.M.; Pourmehran, O.; Arjomandi, M. Performance index improvement of a doublepipe cooler with MgO/water-ethylene glycol (50:50) nano-suspension. Propuls. Power Res. 2020, 9, 75–86. [Google Scholar] [CrossRef]
- Sajid, M.U.; Ali, H.M. Thermal conductivity of hybrid nanofluids: A critical review. Int. J. Heat Mass Transf. 2018, 126, 211–234. [Google Scholar] [CrossRef]
- Babar, H.; Ali, H.M. Towards hybrid nanofluids: Preparation, thermophysical properties, applications, and challenges. J. Mol. Liq. 2019, 281, 598–633. [Google Scholar] [CrossRef]
- Murshed, S.S.; Estelle, P. A state of the art review on viscosity of nano fluids. Renew. Sust. Energ. Rev. 2017, 76, 1134–1152. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.H.; Nabil, M.F.; Mamat, R.; Sharma, K.V. Experimental investigation of thermal conductivity and dynamic viscosity on nanoparticle mixture ratios of TiO2-SiO2 nanofluids. Int. J. Heat Mass Transf. 2018, 116, 1143–1152. [Google Scholar] [CrossRef]
- Qi, G.; Jiang, F. Numerical investigation on prevention of fouling in the horizontal tube heat exchanger: Particle distribution and pressure drop. Desalination 2015, 367, 112–125. [Google Scholar] [CrossRef]
- Master, B.I.; Chunangad, K.S.; Pushpanathan, V. Fouling mitigation using helixchanger heat exchangers. In Proceedings of the Heat Exchanger Fouling and Cleaning: Fundamentals and Applications, Santa Fe, NM, USA, 18–22 May 2003. [Google Scholar]
- Mueller, S.H.; Malayeri, M.R.; Watkinson, A.P. Fouling of heat exchanger-New approaches to solve old problem. Heat Transf. Eng. 2005, 26, 1–4. [Google Scholar] [CrossRef]
- Epstein, N. Thinking about heat transfer fouling: A 5–5 matrix. Heat Transf. Eng. 1983, 4, 43–56. [Google Scholar] [CrossRef]
- Bryan Research and Engineering Inc. 1997 Cause of Edmeston Heat Exchanger Failure: Unit 2 Altamira; Bryan Research and Engineering Inc.: Bryan, TX, USA, 1998. [Google Scholar]
- Awad, M.M. Fouling of Heat Transfer Surfaces, Heat Transfer. In Theoretical Analysis, Experimental Investigations and Industrial Systems; Belmiloudi, A., Ed.; InTech: Bondi Junction, NSW, Australia, 2011. [Google Scholar]
- Roy, U. Fouling and Its Effect on Heat Exchangers. In Advanced Analytic and Control Techniques for Thermal Systems with Heat Exchangers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 233–246. [Google Scholar]
- Müller-Steinhagen, H.; Malayeri, M.R.; Watkinson, A.P. Heat Exchanger Fouling: Environmental Impacts. Heat Transf. Eng. 2010, 30, 773–776. [Google Scholar] [CrossRef]
- Sarafraz, M.; Hormozi, F. Heat transfer, pressure drop and fouling studies of multi-walled carbon nanotube nano-fluids inside a plate heat exchanger. Exp. Therm. Fluid Sci. 2016, 72, 1–11. [Google Scholar] [CrossRef]
- Sarafraz, M.; Hormozi, F.; Peyghambarzadeh, S. Role of nanofluid fouling on thermal performance of a thermosyphon: Are nanofluids reliable working fluid? Appl. Therm. Eng. 2015, 82, 212–224. [Google Scholar] [CrossRef]
- Sarafraz, M.; Nikkhah, V.; Madani, S.; Jafarian, M.; Hormozi, F. Low-frequency vibration for fouling mitigation and intensification of thermal performance of a plate heat exchanger working with CuO/water nanofluid. Appl. Therm. Eng. 2017, 121, 388–399. [Google Scholar] [CrossRef]
- Nikkhah, V.; Sarafraz, M.M.; Hormozi, F.; Peyghambarzadeh, S.M. Particulate fouling of CuO–water nanofluid at isothermal diffusive condition inside the conventional heat exchanger-experimental and modeling. Exp. Therm. Fluid Sci. 2015, 60, 83–95. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Pendyala, R.; Marneni, N. Preparation, Sedimentation, and Agglomeration of Nanofluids. Chem. Eng. Technol. 2014, 37, 2011–2021. [Google Scholar] [CrossRef]
- Wang, K.; Ding, G.; Jiang, W. Development of nanorefrigerant and its rudiment property. In Proceedings of the 8th International Symposium on Fluid Control, Measurement and Visualization, Chengdu, China, 22−25 August 2005; China Aerodynamics Research Society: Chengdu, China, 2005; pp. 1–6. [Google Scholar]
- Jiang, W.; Ding, G.; Peng, H. Measurement and model on thermal conductivities of carbon nanotube nanorefrigerants. Int. J. Therm. Sci. 2009, 48, 1108–1115. [Google Scholar] [CrossRef]
- Peng, H.; Ding, G.; Jiang, W.; Hu, H.; Gao, Y. Heat transfer characteristics of refrigerant-based nanofluid flow boiling inside a horizontal smooth tube. Int. J. Refrig. 2009, 32, 1259–1270. [Google Scholar] [CrossRef]
- Wang, R.X.; Hao, B.; Xie, G.Z.; Li, H.Q. A refrigerating-system using HFC134a and mineral lubricant appended with N-TIO2 (R) as working fluids. In Proceedings of the 4th International Symposium on HAVC; Tsinghua University Press: Beijing, China, 2003; pp. 882–892. [Google Scholar]
- Babarinde, T.O.; Akinlabi, S.A.; Madyira, D.M. Enhancing the performance of vapour compression refrigeration system using nano refrigerants: A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 413, 012068. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Wang, R.; Cong, W.; Li, Q.; Wu, Y. Experiment study on performance of refrigerator using nano-particle additive. Hsi-Chiao Tung Ta Hsueh/J. Xi’an Jiaotong Univ. 2008, 42, 852–854. [Google Scholar]
- Sabareesh, R.K.; Gobinath, N.; Sajith, V.; Das, S.; Sobhan, C.B. Application of TiO2 nanoparticles as a lubricant-additive for vapor compression refrigeration systems—An experimental investigation. Int. J. Refrig. 2012, 35, 1989–1996. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Tung, S.; Schneider, E.; Xi, S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009, 196, 89–101. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H. A Review on Nanofluids: Preparation, Stability Mechanisms, and Applications. J. Nanometer. 2012, 2012, 435873. [Google Scholar] [CrossRef] [Green Version]
- Devendiran, D.K.; Amirtham, V.A. A review on preparation, characterization, properties and applications of nanofluids. Renew. Sustain. Energy Rev. 2016, 60, 21–40. [Google Scholar] [CrossRef]
- Angayarkanni, S.A.; Philip, J. Review on thermal properties of nanofluids: Recent developments. Adv. Colloid Interface Sci. 2015, 225, 146–176. [Google Scholar] [CrossRef] [PubMed]
- Trisaksri, V.; Wongwises, S. Critical review of heat transfer characteristics of nanofluids. Renew. Sustain Energy Rev. 2007, 11, 512–523. [Google Scholar] [CrossRef]
- Wang, X.Q.; Mujumdar, X.Q. Heat transfer characteristics of nanofluids: A review. Int. J. Therm. Sci. 2007, 46, 1–19. [Google Scholar] [CrossRef]
- Sarkar, J. A critical review on convective heat transfer correlations of nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 3271–3277. [Google Scholar] [CrossRef]
- Yu, W.; France, D.M.; Routbort, J.L.; Choi, S.U.S. Review and Comparison of Nanofluid Thermal Conductivity and Heat Transfer Enhancements. Heat Transf. Eng. 2008, 29, 432–460. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; De Castro, C.A.N. Superior thermal features of carbon nanotubes-based nanofluids—A review.Renew. Sustain. Energy Rev. 2014, 37, 155–167. [Google Scholar] [CrossRef]
- Kasaeian, A.; Eshghi, A.T.; Sameti, M. A review on the applications of nanofluids in solar energy systems Renew. Sustain. Energy Rev. 2015, 43, 584–598. [Google Scholar] [CrossRef]
- Mahian, O.; Kianifar, A.; Kalogirou, S.A.; Pop, I.; Wongwises, I. A review of the applications of nanofluids in solar energy. Int. J. Heat Mass Transf. 2013, 57, 582–594. [Google Scholar] [CrossRef]
- Wen, D.; Lin, G.; Vafaei, S.; Zhang, K. Review of nanofluids for heat transfer applications. Particuology 2009, 7, 141–150. [Google Scholar] [CrossRef]
- Verma, S.K.; Tiwari, A.K. Progress of nanofluid application in solar collectors: A review. Energy Convers. Manag. 2015, 100, 324–346. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Progress of Nanofluid Application in Machining: A Review. Mater. Manuf. Process. 2015, 30, 813–828. [Google Scholar] [CrossRef]
- Bacchin, P.; Aimar, P.; Sanchez, V. Model for colloidal fouling of membranes. AIChE J. 1995, 41, 368–376. [Google Scholar] [CrossRef]
- Elimelech, M.; Gregory, J.; Jia, X.; Williams, R. Particle Deposition and Aggregation: Measurement, Modelling and Simulation, 1st ed.; Butterworth-Heinemann: Woburn, MA, USA, 1995. [Google Scholar]
- Adomeit, P.; Renz, U. Deposition of fine particles from a turbulent liquid flow: Experiments and numerical predictions. Chem. Eng. Sci. 1996, 51, 3491–3503. [Google Scholar] [CrossRef]
- Hunter, R.J. Foundations of Colloid Science, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2001. [Google Scholar]
- Gudmundsson, J.S. Particulate Fouling. In Fouling of Heat Trunsfer Equipment; Somerscales, E.F.C., Knudsen, J., Eds.; Hemisphere: Washington, DC, USA, 1981; Volume 1, pp. 251–291. [Google Scholar]
- Müller-Steinhagen, H. Cooling-Water Fouling in Heat Exchangers. Adv. Heat Transf. 1999, 33, 415–496. [Google Scholar]
- Ali, H.M.; Babar, H.; Shah, T.R.; Sajid, M.U.; Qasim, M.A.; Javed, S. Preparation Techniques of TiO2 Nanofluids and Challenges: A Review. Appl. Sci. 2018, 8, 587. [Google Scholar] [CrossRef] [Green Version]
- Ba, T.L.; Mahian, O.; Wongwises, S.; Szilágyi, I.M. Review on the recent progress in the preparation and stability of graphene-based nanofluids. J. Therm. Anal. Calorim. 2020, 142, 1145–1172. [Google Scholar]
- Mohammed, H.; Al-Aswadi, A.; Shuaib, N.; Saidur, R. Convective heat transfer and fluid flow study over a step using nanofluids: A review. Renew. Sustain. Energy Rev. 2011, 15, 2921–2939. [Google Scholar] [CrossRef]
- Shenoy, U.S.; Shetty, A.N. A simple single-step approach towards synthesis of nanofluids containing cuboctahedral cuprous oxide particles using glucose reduction. Front. Mater. Sci. 2018, 12, 74–82. [Google Scholar] [CrossRef]
- Kong, L.; Sun, J.; Bao, Y. Preparation, characterization and tribological mechanism of nanofluids. RSC Adv. 2017, 7, 12599–12609. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.; Lee, J.K.; Lee, J.K.; Jeong, Y.M.; Cheong, S.I.; Ahn, Y.C.; Kim, S.H. Production and dispersion stability of nanoparticles in nanofluids. Powder Technol. 2008, 186, 145–153. [Google Scholar] [CrossRef]
- Worthen, A.J.; Tran, V.; Cornell, K.A.; Truskett, T.M.; Johnston, K.P. Steric stabilization of nanoparticles with grafted low molecular weight ligands in highly concentrated brines including divalent ions. Soft Matter. 2016, 12, 2025–2039. [Google Scholar] [CrossRef]
- Askar, A.H.; Kadham, S.A.; Mshehid, S.H. The surfactants effect on the heat transfer enhancement and stability of nanofluid at constant wall temperature. Heliyon 2020, 6, e04419. [Google Scholar] [CrossRef]
- Ghadimi, A.; Metselaar, H.S.C.; Lotfizadehdehkordi, B. Nanofluid stability optimization based on UV-Vis spectrophotometer measurement. J. Eng. Sci. Technol. 2015, 10, 32–40. [Google Scholar]
- Kim, G.; Baek, S.; Choi, W.; Lee, A.; Lee, S.; Jeong, H.; Sung, Y. Stability, surface tension, and thermal conductivity of Al2O3/water nanofluids according to different types of alcohol and their proportion. Case Stud. Therm. Eng. 2021, 28, 101385. [Google Scholar] [CrossRef]
- Chamsa-Ard, W.; Brundavanam, S.; Fung, C.C.; Fawcett, D.; Poinern, G. Nanofluid types, their synthesis, properties and incorporation in direct solar thermal collectors: A review. Nanomaterials 2017, 7, 131. [Google Scholar] [CrossRef]
- Oh, D.W.; Kwon, O.; Lee, J.S. Transient Thermal Conductivity and Colloidal Stability Measurements of Nanofluids by Usingthe 3ω Method. J. Nanosci. Nanotechnol. 2008, 8, 4923–4929. [Google Scholar] [CrossRef] [PubMed]
- Naser, A.; Joao, A.T.; Abdulmajid, A. A Review on Nanofluids: Fabrication, Stability, and Thermophysical Properties. J. Nanomater. 2018, 2018, 6978130. [Google Scholar]
- Kim, H.S.; Yilmaz, F.; Dharmaiah, P.; Lee, D.J.; Lee, T.H.; Hong, S.J. Characterization of Cu and Ni Nano-Fluids Synthesized by Pulsed Wire Evaporation Method. Arch. Metall. Mater. 2017, 62, 999–1004. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Paria, S. Preparation and stability of nanofluids—A review. IOSR-JMCE 2013, 9, 63–69. [Google Scholar] [CrossRef]
- Fedele, L.; Colla, L.; Bobbo, S.; Barison, S.; Agresti, F. Experimental stability analysis of different water-based nanofluids. Nanoscale Res. Lett. 2011, 6, 300. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, A.; Mohammadi, A. Environmentally responsive surface-modified silica nanoparticles for enhanced oil recovery. J. Nanoparticle Res. 2016, 18, 266. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, V.; Kumar, R.; Said, Z. A review on thermophysical properties of nanofluids and heat transfer applications. Renew. Sust. Energ. Rev. 2017, 74, 638–670. [Google Scholar] [CrossRef]
- Krishnan, B.P.; Vijayan, R.; Gokulnath, K. Performance analysis of a nano refrigerant mixtures in a domestic refrigeration system. Adv. Nat. Appl. Sci. 2017, 11, 508–516. [Google Scholar]
- Haque, M.E.; Bakar, R.A.; Kadirgama, K.; Noor, M.M.; Shakaib, M. Performance of a domestic refrigerator using nanoparticles-based polyolester oil lubricant. J. Mech. Eng. Sci. 2016, 10, 1778–1791. [Google Scholar] [CrossRef]
- Kannan, P.; Manivanana, A. Theoretical analysis of a vapour compression refrigeration system with R134a, R290, R600a & various ratio of R290/R600a. Int. J. Res. Appl. Sci. Eng. Technol. 2016, 547, 143–146. [Google Scholar]
- Soliman, A.M.A.; Ali, K.; Rahman, A.; Ookawara, S. Theoretical investigation of vapor compression cycle performance using different nanomaterials additives. In Proceedings of the International Conference on Advances in Mechanical Engineering Istanbul 2016—ICAME2016, Istanbul, Turkey, 11–13 May 2016; pp. 721–725. [Google Scholar]
- Subramani, N.; Mohan, A. Performance studies on a vapour compression refrigeration system using nano-lubricant. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 522–530. [Google Scholar]
- Mahbubul, I.M.; Fadhilah, S.A.; Saidur, R.; Leong, K.Y.; Amalina, M.A. Thermophysical properties and heat transfer performance of Al2O3/R-134a nanorefrigerants. Int. J. Heat Mass Transf. 2013, 57, 100–108. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Imai, T.; Tanabe, K.; Tsuno, T.; Kumazawa, Y.; Fujimori, N. The measurement of thermal properties of diamond. Diam. Relat. Mater. 1997, 6, 1057–1061. [Google Scholar] [CrossRef]
- Peng, H.; Ding, G.; Hu, H.; Jiang, W.; Zhuang, D.; Wang, K. Nucleate pool boiling heat transfer characteristics of refrigerant/oil mixture with diamond nanoparticles. Int. J. Refrig. 2010, 33, 347–358. [Google Scholar] [CrossRef]
- Rohsenow, W.M. A method of correlating heat transfer data for surface boiling of liquids. Trans. ASME 1952, 74, 969–976. [Google Scholar]
- Kedzierski, M.A. Effect of Diamond Nanolubricant on R134a Pool Boiling Heat Transfer. J. Heat Transf. 2012, 134, 051001. [Google Scholar] [CrossRef]
- Bianco, V.; Vafai, K.; Manca, O.; Nardini, S. Heat Transfer Enhancement with Nanofluids, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Bahiraei, M.; Heshmatian, S. Electronics cooling with nanofluids: A critical review. Energy Convers. Manag. 2018, 172, 438–456. [Google Scholar] [CrossRef]
- Nasiri, A.; Shariaty-Niasar, M.; Rashidi, A.M.; Khodafarin, R. Effect of CNT structures on thermal conductivity and stability of nanofluid. Int. J. Heat Mass Transf. 2012, 55, 1529–1535. [Google Scholar] [CrossRef]
- Talaei, Z.; Mahjoub, A.R.; Morad Rashidi, A.; Amrollahi, A.; Meibodi, M.E. The effect of functionalized group concentration on the stability and thermal conductivity of carbon nanotube fluid as heat transfer media. Int. Commun. Heat Mass Trans. 2011, 38, 513–517. [Google Scholar] [CrossRef]
- Sardarabadi, H.; Heris, S.Z.; Ahmadpour, A.; Passandideh-Fard, M. Experimental investigation of a novel type of two-phase closed thermosyphon filled with functionalized carbon nanotubes/water nanofluids for electronic cooling application. Energy Convers. Manag. 2019, 188, 321–332. [Google Scholar] [CrossRef]
- Ozturk, S.; Hassan, Y.A.; Ugaz, V.M. Graphene-enhanced nanorefrigerants. Nanoscale 2013, 5, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Vadukumpully, S.; Gupta, J.; Zhang, Y.P.; Xu, G.Q.; Valiyaveettil, S. Functionalization of surfactant wrapped graphene nanosheets with alkylazides for enhanced dispersibility. Nanoscale 2011, 3, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Rosell, A.; Storti, G.; Morbidelli, M.; Bratton, D.; Howdle, S.M. Dispersion Polymerization of Methyl Methacrylate in Supercritical Carbon Dioxide Using a Pseudo-Graft Stabilizer: Role of Reactor Mixing. Macromolecules 2004, 37, 2996–3004. [Google Scholar] [CrossRef]
- Moore, V.C.; Strano, M.S.; Haroz, E.H.; Hauge, R.H.; Smalley, R.E. Individually suspended single-walled carbon nanotubes in various surfactants. Nano Lett. 2003, 3, 1379–1382. [Google Scholar] [CrossRef]
- Park, K.J.; Jung, D. Boiling heat transfer enhancement with carbon nanotubes for refrigerants used in building air-conditioning. Energy Build. 2007, 39, 1061–1064. [Google Scholar] [CrossRef]
- Mohan, K.; Sundararaj, S.; Gopi Kannan, K.; Kannan, A. Experimental analysis on refrigeration system using CNT, gold & HAUCL4 nano fluids. Mater Today Proc. 2020, 33, 360–366. [Google Scholar]
- Chen, H.; Ding, Y.; He, Y.; Tan, C. Rheological behaviour of ethylene glycol based titania nanofluids. Chem. Phys. Lett. 2007, 444, 333–337. [Google Scholar] [CrossRef]
- Senthilraja, S.; Vijayakumar, K.; Gangadevi, R. A comparative study on thermal conductivity of Al2O3/water, CuO/water and Al2O3—CuO/water nanofuids. Dig. J. Nanomater. Biostruct. 2015, 10, 1449–1458. [Google Scholar]
- Senthilkumar, A.; Praveen, R. Performance analysis of a domestic refrigerator using CuO–R600a nano refrigerant as working fluid. J. Chem. Pharm. Sci. ISSN 2015, 974, 2115. [Google Scholar]
- Ding, G.; Peng, H.; Jiang, W.; Gao, Y. The migration characteristics of nanoparticles in the pool boiling process of nano refrigerant and nano refrigerant–oil mixture. Int. J. Refrig. 2009, 32, 114–123. [Google Scholar] [CrossRef]
- Sun, B.; Yang, D. Flow boiling heat transfer characteristics of nano-refrigerants in a horizontal tube. Int. J. Refrig. 2014, 38, 206–214. [Google Scholar] [CrossRef]
- Peng, H.; Fan, S.; Sun, S. Study on enhancement of thermal conductivity of hydrate containing carbon nanotubes. J. Refrig. 2006, 27, 39–43. [Google Scholar]
- Samira, P.; Saeed, Z.H.; Motahare, S.; Mostafa, K. Pressure drop and thermal performance of CuO/ethylene glycol (60%)-water (40%) nanofluid in car radiator. Korean J. Chem. Eng. 2014, 324, 609–616. [Google Scholar] [CrossRef]
- Zeinali Heris, S.; Shokrgozar, M.; Poorpharhang, S.; Shanbedi, M.; Noie, S.H. Experimental Study of Heat Transfer of a Car Radiator with CuO/Ethylene Glycol-Water as a Coolant. J. Dispers. Sci. Technol. 2014, 35, 677–684. [Google Scholar] [CrossRef]
- Henderson, K.; Park, Y.G.; Liu, L.; Jacobi, A.M. Flow-boiling heat transfer of R-134a-based nanofluids in a horizontal tube. Int. J. Heat Mass Transf. 2010, 53, 944–951. [Google Scholar] [CrossRef]
- Zhu, M.S. Application Technology of HFC134a as Refrigerant; Chinese Vision; Tsinghua University Publishers: Beijing, China, 1994. [Google Scholar]
- Park, K.J.; Jung, D. Thermodynamic performance of HCFC22 alternative refrigerants for residential air-conditioning applications. Energy Build. 2007, 39, 675–680. [Google Scholar] [CrossRef]
- Wang, R.; Qingping, W.; Wu, Y. Use of nanoparticles to make mineral oil lubricants feasible for use in a residential air conditioner employing hydro-fluorocarbons refrigerants. Energy Build. 2010, 42, 2111–2117. [Google Scholar] [CrossRef]
- Maheshwary, P.B.; Handa, C.C.; Nemade, K.R. Effect of Shape on Thermophysical and Heat Transfer Properties of ZnO/R-134a Nanorefrigerant. Mater. Today Proc. 2018, 5, 1635–1639. [Google Scholar] [CrossRef]
- Mahbubul, I.M.; Saidur, R.; Amalina, M.A. Investigation of viscosity of R123-TIO2 nanorefrigerant. Int. J. Mech. Mater. Eng. 2012, 7, 146–151. [Google Scholar]
- Zhelezny, V.P.; Lukianov, N.N.; Khliyeva, O.Y.; Nikulina, A.S.; Melnyk, A.V. A complex investigation of the nanofluids R600a-mineral oil-Al2O3 and R600a mineral oil-TiO2. Thermophysical Properties. Int. J. Refrig. 2017, 74, 486–502. [Google Scholar] [CrossRef]
- Fedele, L.; Colla, L.; Bobbo, S. Viscosity and thermal conductivity measurements of water-based nanofluids containing titanium oxide nanoparticles. Int. J. Refrig. 2012, 35, 1359–1366. [Google Scholar] [CrossRef]
- Jwo, C.S.; Jeng, L.Y.; Chang, H.; Teng, T.P. Experimental study on thermal conductivity of lubricant containing nanoparticles. Rev. Adv. Mater. Sci. 2008, 18, 660–666. [Google Scholar]
- Mahbubul, I.M.; Saadah, A.; Saidur, R.; Khairul, M.A.; Kamyar, A. Thermal performance analysis of Al2O3/R-134a nanorefrigerant. Int. J. Heat Mass Transf. 2015, 85, 1034–1040. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Meng, F.; Yanga, B.; Xiao, P. Research Progress of Nano-refrigerant and Its Application in Refrigeration. In Proceedings of the 3rd International Conference on Architectural Engineering and New Materials, Guangzhou, China, 21–22 December 2018; Volume 978, pp. 308–312. [Google Scholar]
- Rashidi, H.; Nikou, M.R.K. Modeling of Thermal conductivity of carbon nanotubes-refrigerants fluids. Am. J. Sci. 2012, 8, 654–658. [Google Scholar]
- Sheikholeslami, M.; Rezaeianjouybari, B.; Darzi, M.; Shafee, A.; Li, Z.; Nguyen, T.K. Application of nano-refrigerant for boiling heat transfer enhancement employing an experimental study. Int. J. Heat Mass Transf. 2019, 141, 974–980. [Google Scholar] [CrossRef]
- Mahbubul, I.M.; Kamyar, A.; Saidur, R.; Amalina, M.A. Migration Properties of TiO2 Nanoparticles during the Pool Boiling of Nanorefrigerants. Ind. Eng. Chem. Res. 2013, 52, 6032–6038. [Google Scholar] [CrossRef]
- Trisaksri, V.; Wongwises, S. Nucleate pool boiling heat transfer of TiO2–R141b nanofluids. Int. J. Heat Mass Transf. 2009, 52, 1582–1588. [Google Scholar] [CrossRef]
- Mahbubul, I.M.; Khaleduzzaman, S.S.; Saidur, R.; Amalina, M.A. Rheological behavior of Al2O3/R141b nanorefrigerant. Int. J. Heat Mass Transf. 2014, 73, 118–123. [Google Scholar] [CrossRef]
- Kulkarni, D.P.; Vajjha, R.S.; Das, D.K.; Oliva, D. Application of aluminum oxide nanofluids in diesel electric generator as jacket water coolant. Appl. Therm. Eng. 2008, 28, 1774–1781. [Google Scholar] [CrossRef]
- Mishra, R.S. Method for improving thermodynamic performance of vapour compression refrigeration system using nanofluids—A Review. Int. J. Res. Eng. Innov. 2018, 2, 374–383. [Google Scholar]




| Type | Refrigerant |
|---|---|
| HFC | R23 |
| R32 | |
| R125 | |
| R134a | |
| R143a | |
| R152a | |
| HFC blend | R410a |
| R407c | |
| R425a | |
| CFC | R11 |
| R12 | |
| R13 | |
| R113 | |
| R114 | |
| R115 | |
| R500 | |
| HCFC | R22 |
| R123 | |
| R124 | |
| R141b | |
| R142b | |
| HC | R600 |
| R600a | |
| R290 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponticorvo, E.; Iuliano, M.; Cirillo, C.; Maiorino, A.; Aprea, C.; Sarno, M. Fouling Behavior and Dispersion Stability of Nanoparticle-Based Refrigeration Fluid. Energies 2022, 15, 3059. https://doi.org/10.3390/en15093059
Ponticorvo E, Iuliano M, Cirillo C, Maiorino A, Aprea C, Sarno M. Fouling Behavior and Dispersion Stability of Nanoparticle-Based Refrigeration Fluid. Energies. 2022; 15(9):3059. https://doi.org/10.3390/en15093059
Chicago/Turabian StylePonticorvo, Eleonora, Mariagrazia Iuliano, Claudia Cirillo, Angelo Maiorino, Ciro Aprea, and Maria Sarno. 2022. "Fouling Behavior and Dispersion Stability of Nanoparticle-Based Refrigeration Fluid" Energies 15, no. 9: 3059. https://doi.org/10.3390/en15093059
APA StylePonticorvo, E., Iuliano, M., Cirillo, C., Maiorino, A., Aprea, C., & Sarno, M. (2022). Fouling Behavior and Dispersion Stability of Nanoparticle-Based Refrigeration Fluid. Energies, 15(9), 3059. https://doi.org/10.3390/en15093059










