Comparative Study of Stability against Moisture for Solid Garnet Electrolytes with Different Dopants
Abstract
:1. Introduction
2. Materials and Methods
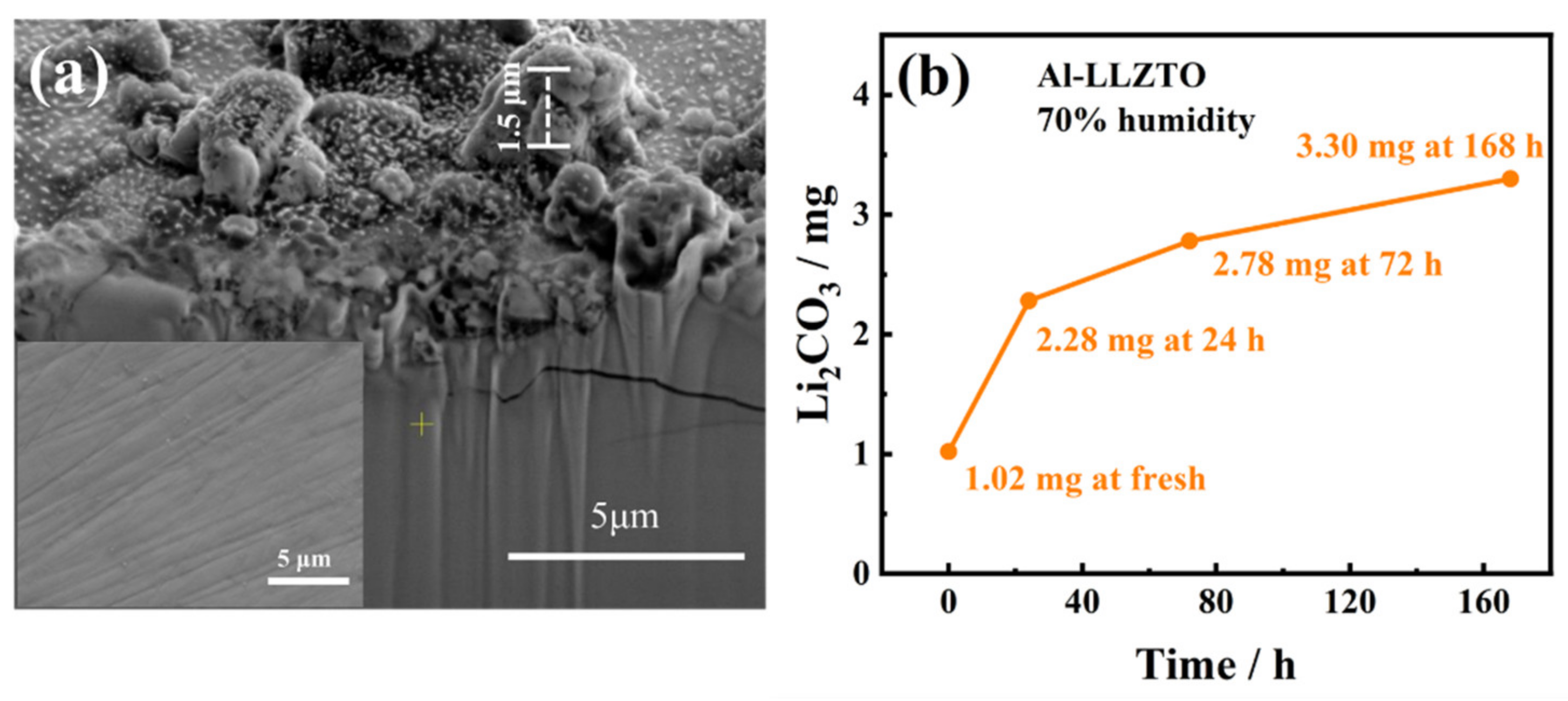
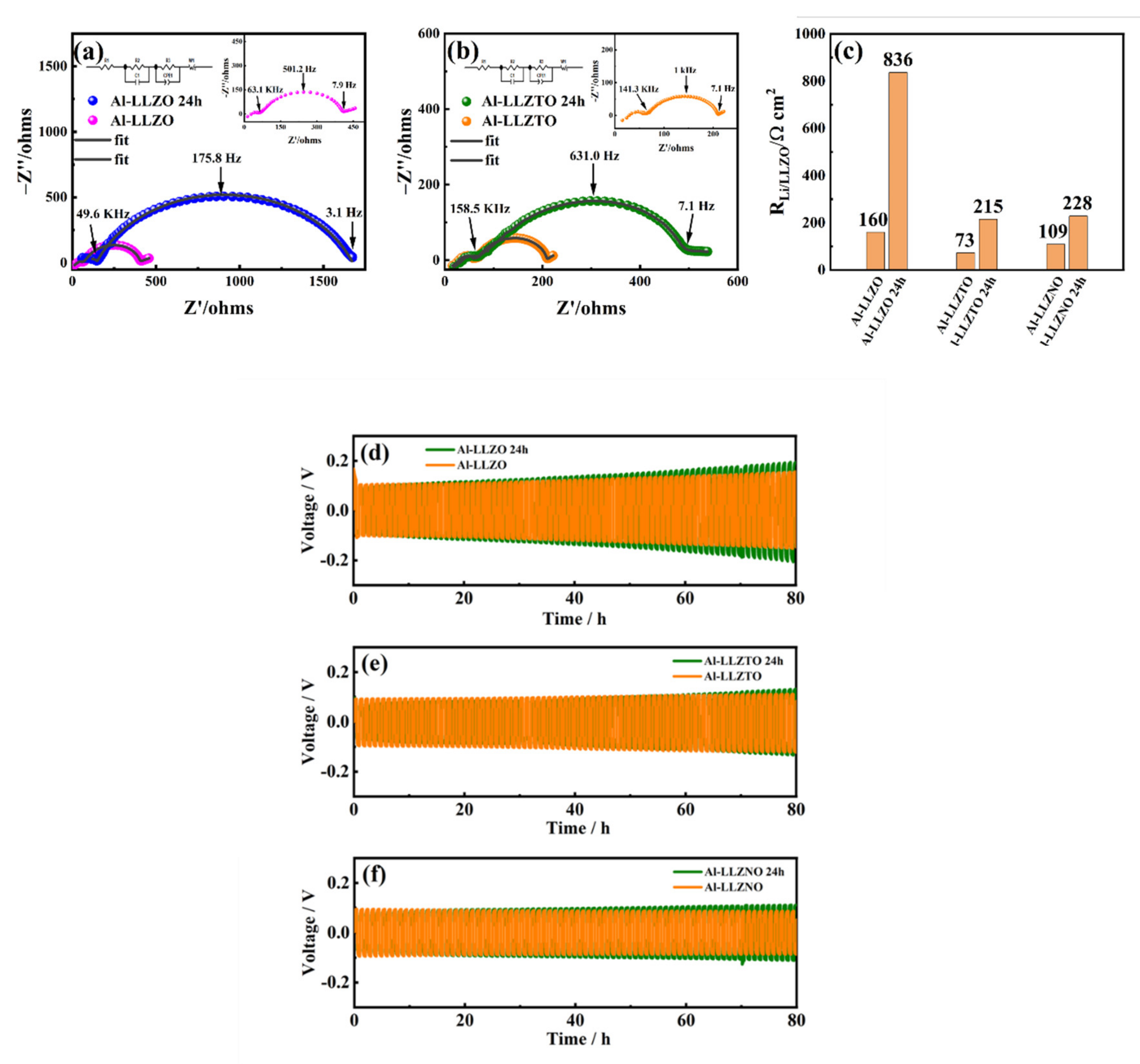
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fu, K.; Gong, Y.; Dai, J.; Gong, A.; Han, X.; Yao, Y.; Wang, C.; Wang, Y.; Chen, Y.; Yan, C.; et al. Flexible, solid-state, ion-conducting membrane with 3D garnet nanofiber networks for lithium batteries. Proc. Natl. Acad. Sci. USA 2016, 113, 7094–7099. [Google Scholar] [CrossRef] [Green Version]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Han, F.; Westover, A.S.; Yue, J.; Fan, X.; Wang, F.; Chi, M.; Leonard, D.N.; Dudney, N.; Wang, H.; Wang, C. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 2019, 4, 187–196. [Google Scholar] [CrossRef]
- Bi, Z.; Zhao, N.; Ma, L.; Fu, Z.; Xu, F.; Wang, C.; Guo, X. Interface engineering on cathode side for solid garnet batteries. Chem. Eng. J. 2020, 387, 124089. [Google Scholar] [CrossRef]
- Bi, Z.; Zhao, N.; Ma, L.; Shi, C.; Fu, Z.; Xu, F.; Guo, X. Surface coating of LiMn2O4 cathodes with garnet electrolytes for improving cycling stability of solid lithium batteries. J. Mater. Chem. A 2020, 8, 4252–4256. [Google Scholar] [CrossRef]
- Bi, Z.; Huang, W.; Mu, S.; Sun, W.; Zhao, N.; Guo, X. Dual-interface reinforced flexible solid garnet batteries enabled by in-situ solidified gel polymer electrolytes. Nano Energy 2021, 90, 106498. [Google Scholar] [CrossRef]
- Bi, Z.; Mu, S.; Zhao, N.; Sun, W.; Huang, W.; Guo, X. Cathode supported solid lithium batteries enabling high energy density and stable cyclability. Energy Storage Mater. 2021, 35, 512–519. [Google Scholar] [CrossRef]
- Liu, Q.; Geng, Z.; Han, C.; Fu, Y.; Li, S.; He, Y.-B.; Kang, F.; Li, B. Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries. J. Power Sources 2018, 389, 120–134. [Google Scholar] [CrossRef]
- Zhao, N.; Khokhar, W.; Bi, Z.; Shi, C.; Guo, X.; Fan, L.-Z.; Nan, C.-W. Solid Garnet Batteries. Joule 2019, 3, 1190–1199. [Google Scholar] [CrossRef]
- Jia, M.; Bi, Z.; Shi, C.; Zhao, N.; Guo, X. Air-stable dopamine-treated garnet ceramic particles for high-performance composite electrolytes. J. Power Sources 2021, 486, 229363. [Google Scholar] [CrossRef]
- Gai, J.; Zhao, E.; Ma, F.; Sun, D.; Ma, X.; Jin, Y.; Wu, Q.; Cui, Y. Improving the Li-ion conductivity and air stability of cubic Li7La3Zr2O12 by the co-doping of Nb, Y on the Zr site. J. Eur. Ceram. Soc. 2018, 38, 1673–1678. [Google Scholar] [CrossRef]
- Huo, H.; Luo, J.; Thangadurai, V.; Guo, X.; Nan, C.-W.; Sun, X. Li2CO3: A Critical Issue for Developing Solid Garnet Batteries. ACS Energy Lett. 2020, 5, 252–262. [Google Scholar] [CrossRef]
- Wu, J.F.; Pu, B.W.; Wang, D.; Shi, S.Q.; Zhao, N.; Guo, X.; Guo, X. In Situ Formed Shields Enabling Li2CO3-Free Solid Electrolytes: A New Route to Uncover the Intrinsic Lithiophilicity of Garnet Electrolytes for Dendrite-Free Li-Metal Batteries. ACS Appl. Mater. Interfaces 2019, 11, 898–905. [Google Scholar] [CrossRef]
- Duan, H.; Zheng, H.; Zhou, Y.; Xu, B.; Liu, H. Stability of garnet-type Li ion conductors: An overview. Solid State Ion. 2018, 318, 45–53. [Google Scholar] [CrossRef]
- Galven, C.; Fourquet, J.-L.; Crosnier-Lopez, M.-P.; Le Berre, F. Instability of the Lithium Garnet Li7La3Sn2O12: Li+/H+ Exchange and Structural Study. Chem. Mater. 2011, 23, 1892–1900. [Google Scholar] [CrossRef]
- Larraz, G.; Orera, A.; Sanjuán, M.L. Cubic phases of garnet-type Li7La3Zr2O12: The role of hydration. J. Mater. Chem. A 2013, 1, 11419–11428. [Google Scholar] [CrossRef] [Green Version]
- Jia, M.; Zhao, N.; Huo, H.; Guo, X. Comprehensive Investigation into Garnet Electrolytes Toward Application-Oriented Solid Lithium Batteries. Electrochem. Energy Rev. 2020, 3, 656–689. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-Type Solid-State Electrolytes: Materials, Interfaces, and Batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef]
- Cheng, L.; Crumlin, E.J.; Chen, W.; Qiao, R.; Hou, H.; Lux, S.F.; Zorba, V.; Russo, R.; Kostecki, R.; Liu, Z.; et al. The origin of high electrolyte-electrode interfacial resistances in lithium cells containing garnet type solid electrolytes. Phys. Chem. Chem. Phys. 2014, 16, 18294–18300. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, C.H.; Jarry, A.; Chen, W.; Ye, Y.F.; Zhu, J.F.; Kostecki, R.; Persson, K.; Guo, J.H.; Salmeron, M.; et al. Interrelationships among Grain Size, Surface Composition, Air Stability, and Interfacial Resistance of Al-Substituted Li7La3Zr2O12 Solid Electrolytes. ACS Appl. Mater. Interfaces 2015, 7, 17649–17655. [Google Scholar] [CrossRef]
- Ishiguro, K.; Nemori, H.; Sunahiro, S.; Nakata, Y.; Sudo, R.; Matsui, M.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Ta-Doped Li7La3Zr2O12 for Water-Stable Lithium Electrode of Lithium-Air Batteries. J. Electrochem. Soc. 2014, 161, A668–A674. [Google Scholar] [CrossRef]
- Huo, H.; Chen, Y.; Zhao, N.; Lin, X.; Luo, J.; Yang, X.; Liu, Y.; Guo, X.; Sun, X. In-situ formed Li2CO3-free garnet/Li interface by rapid acid treatment for dendrite-free solid-state batteries. Nano Energy 2019, 61, 119–125. [Google Scholar] [CrossRef]
- Fu, K.; Gong, Y.; Liu, B.; Zhu, Y.; Xu, S.; Yao, Y.; Luo, W.; Wang, C.; Lacey, S.D.; Dai, J.; et al. Toward garnet electrolyte-based Li metal batteries: An ultrathin, highly effective, artificial solid-state electrolyte/metallic Li interface. Sci. Adv. 2017, 3, e1601659. [Google Scholar] [CrossRef] [Green Version]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Schmidt, R.D.; Garcia-Mendez, R.; Herbert, E.; Dudney, N.J.; Wolfenstine, J.B.; Sakamoto, J.; Siegel, D.J. Elastic Properties of the Solid Electrolyte Li7La3Zr2O12 (LLZO). Chem. Mater. 2016, 28, 197–206. [Google Scholar] [CrossRef]
- Zhao, N.; Fang, R.; He, M.-H.; Chen, C.; Li, Y.-Q.; Bi, Z.-J.; Guo, X.-X. Cycle stability of lithium/garnet/lithium cells with different intermediate layers. Rare Met. 2018, 37, 473–479. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, J.; Li, X.; Li, J.; Guo, X.; Li, H.; Zhou, W. Rational Design of Mixed Electronic-Ionic Conducting Ti-Doping Li7La3Zr2O12 for Lithium Dendrites Suppression. Adv. Funct. Mater. 2020, 31, 2001918. [Google Scholar] [CrossRef]
- Samson, A.J.; Hofstetter, K.; Bag, S.; Thangadurai, V. A bird’s-eye view of Li-stuffed garnet-type Li7La3Zr2O12 ceramic electrolytes for advanced all-solid-state Li batteries. Energy Environ. Sci. 2019, 12, 2957–2975. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Li, C.; Cao, Y.; Guo, X. Densification and ionic-conduction improvement of lithium garnet solid electrolytes by flowing oxygen sintering. J. Power Sources 2014, 248, 642–646. [Google Scholar] [CrossRef]
- Zhang, X.; Weng, S.; Yang, G.; Li, Y.; Li, H.; Su, D.; Gu, L.; Wang, Z.; Wang, X.; Chen, L. Interplay between solid-electrolyte interphase and (in)active LixSi in silicon anode. Cell Rep. Phys. Sci. 2021, 2, 100668. [Google Scholar] [CrossRef]
- Fang, C.; Li, J.; Zhang, M.; Zhang, Y.; Yang, F.; Lee, J.Z.; Lee, M.H.; Alvarado, J.; Schroeder, M.A.; Yang, Y.; et al. Quantifying inactive lithium in lithium metal batteries. Nature 2019, 572, 511–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, W.; Cao, Z.; Wang, L.; Fu, J.; Chi, X.; Gao, W.; Wang, L.-W. The analysis of a plane wave pseudopotential density functional theory code on a GPU machine. Comput. Phys. Commun. 2013, 184, 9–18. [Google Scholar] [CrossRef]
- Jia, W.; Fu, J.; Cao, Z.; Wang, L.; Chi, X.; Gao, W.; Wang, L.-W. Fast plane wave density functional theory molecular dynamics calculations on multi-GPU machines. J. Comput. Phys. 2013, 251, 102–115. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlipf, M.; Gygi, F. Optimization algorithm for the generation of ONCV pseudopotentials. Comput. Phys. Commun. 2015, 196, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Hamann, D.R. Erratum: Optimized norm-conserving Vanderbilt pseudopotentials. Phys. Rev. B Condens. Matter Mater. Phys. 2013, 88, 085117. [Google Scholar] [CrossRef] [Green Version]
- Sharafi, A.; Yu, S.; Naguib, M.; Lee, M.; Ma, C.; Meyer, H.M.; Nanda, J.; Chi, M.; Siegel, D.J.; Sakamoto, J. Impact of air exposure and surface chemistry on Li-Li7La3Zr2O12 interfacial resistance. J. Mater. Chem. A 2017, 5, 13475–13487. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Yu, C.; Yan, X.; Xu, B.; Wang, L.-m. Lithium halide coating as an effective intergrain engineering for garnet-type solid electrolytes avoiding high temperature sintering. Electrochim. Acta 2018, 289, 254–263. [Google Scholar] [CrossRef]
- Shalev, T.; Gopin, A.; Bauer, M.; Stark, R.W.; Rahimipour, S. Non-leaching antimicrobial surfaces through polydopamine bio-inspired coating of quaternary ammonium salts or an ultrashort antimicrobial lipopeptide. J. Mater. Chem. 2012, 22, 2026–2032. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, Z.; Sui, J.; Jiang, X.; Waterhouse, G.I.N.; Zhang, Z.; Yu, L. Microwave-based synthesis of (NiCo)x/(MnO)y/C composites and their tunable wave absorption properties in the K band. Ceram. Int. 2020, 46, 9353–9362. [Google Scholar] [CrossRef]
- Nam, C.; Zimudzi, T.J.; Geise, G.M.; Hickner, M.A. Increased Hydrogel Swelling Induced by Absorption of Small Molecules. ACS Appl. Mater. Interfaces 2016, 8, 14263–14270. [Google Scholar] [CrossRef] [PubMed]
- De Geyter, N.; Morent, R.; Leys, C. Surface characterization of plasma-modified polyethylene by contact angle experiments and ATR-FTIR spectroscopy. Surf. Interface Anal. 2008, 40, 608–611. [Google Scholar] [CrossRef]
- Yow, Z.F.; Oh, Y.L.; Gu, W.; Rao, R.P.; Adams, S. Effect of Li+/H+ exchange in water treated Ta-doped Li7La3Zr2O12. Solid State Ion. 2016, 292, 122–129. [Google Scholar] [CrossRef]
- Qiao, Y.; Yi, J.; Wu, S.; Liu, Y.; Yang, S.; He, P.; Zhou, H. Li-CO2 Electrochemistry: A New Strategy for CO2 Fixation and Energy Storage. Joule 2017, 1, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Guo, X.; Li, Y.; Ma, Z.; Guo, X.; Li, H.; Zhu, Y.; Zhou, W. The Ab Initio Calculations on the Areal Specific Resistance of Li-Metal/Li7La3Zr2O12 Interphase. Adv. Theory Simul. 2019, 2, 1900028. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Wu, C.; Gao, J.; Xu, H.; Li, Y.; Guo, X.; Li, H.; Zhou, W. A Multilayer Ceramic Electrolyte for All-Solid-State Li Batteries. Angew. Chem. Int. Ed. Engl 2021, 60, 3781–3790. [Google Scholar] [CrossRef]



| Samples | Energy keV/f.u. |
|---|---|
| Li2O | −0.825603 |
| Li6Zr2O7 | −6.811825 |
| La2O3 | −3.053699 |
| LiTaO3 | −3.101137 |
| LiNbO3 | −3.057770 |
| Al2O3 | −5.079534 |
| Li6.25Al0.25La3Zr2O12 | −12.13005 |
| Li5.625Al0.25La3Zr1.375Ta0.625O12 | −12.19829 |
| Li5.625Al0.25La3Zr1.375Nb0.625O12 | −12.17118 |
| Samples | Decomposition Equations | Δ Energy meV/atom |
|---|---|---|
| Li6.25Al0.25La3Zr2O12 | 1/8Li2O + Li6Zr2O7 + 3/2La2O3 + 1/8Al2O3 | −19.89 |
| Li5.625Al0.25La3Zr1.375Ta0.625O12 | 7/16Li2O + 11/16Li6Zr2O7 + 5/8LiTaO3 + 3/2La2O3 + 1/8Al2O3 | +11.08 |
| Li5.625Al0.25La3Zr1.375Nb0.625O12 | 7/16Li2O + 11/16Li6Zr2O7 + 5/8LiNbO3 + 3/2La2O3 + 1/8Al2O3 | +11.07 |
| Samples | Calculated Lattice Parameter | Refinement Lattice Parameter | ||||||
|---|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | a = b = c (Å) | α = β = γ (°) | |
| Al-LLZO | 12.9441 | 12.9707 | 13.0724 | 89.9 | 90.1 | 89.9 | 12.9387 | 90 |
| Al-LLZTO | 13.0032 | 12.9055 | 12.9431 | 90.0 | 90.0 | 89.9 | 12.9243 | 90 |
| Al-LLZNO | 13.0167 | 12.9176 | 12.9291 | 90.1 | 90.1 | 89.9 | 12.9288 | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Gao, J.; Bi, Z.; Zhao, N.; Wu, J.; Fang, Q.; Wang, X.; Wan, Y.; Guo, X. Comparative Study of Stability against Moisture for Solid Garnet Electrolytes with Different Dopants. Energies 2022, 15, 3206. https://doi.org/10.3390/en15093206
Huang L, Gao J, Bi Z, Zhao N, Wu J, Fang Q, Wang X, Wan Y, Guo X. Comparative Study of Stability against Moisture for Solid Garnet Electrolytes with Different Dopants. Energies. 2022; 15(9):3206. https://doi.org/10.3390/en15093206
Chicago/Turabian StyleHuang, Li, Jian Gao, Zhijie Bi, Ning Zhao, Jipeng Wu, Qiu Fang, Xuefeng Wang, Yong Wan, and Xiangxin Guo. 2022. "Comparative Study of Stability against Moisture for Solid Garnet Electrolytes with Different Dopants" Energies 15, no. 9: 3206. https://doi.org/10.3390/en15093206





