Photocatalytic Fuel Cells for Simultaneous Wastewater Treatment and Power Generation: Mechanisms, Challenges, and Future Prospects
Abstract
:1. Introduction
2. Development of Single and Dual Photoelectrode PEC
3. Electrodes Used in PFC
4. Mechanism of Photocatalytic Fuel Cell
4.1. Photoreduction Reaction
4.2. Photooxidation Reaction
4.3. Decomposition of Organic Compound
5. Recent Development in the Design of PFC
6. Applications of PFC
- a.
- Wastewater treatment
- b.
- Recovery of chemical energy from organic matter
- c.
- Hydrogen production
- d.
- Carbon dioxide reduction
7. Future Prospect and Challenges
8. Conclusions and Recommendations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPA | Bisphenol A |
| CB | Conduction band |
| COD | Chemical oxygen demand |
| DSPFC | Dye self-photosensitization photo fuel cell |
| Eg | Band gap |
| FTO | Fluorine doped tin oxide |
| FF | Fill factor |
| HER | Hydrogen evolution reaction |
| HOMO | Highest occupied molecular orbital |
| Isc | Short circuit current |
| Jsc | Short circuit current density |
| (JV) max | Maximum power density |
| LUMO | Lowest unoccupied molecular orbital |
| LDH | Layered double hydroxide |
| MB | Methylene blue |
| MFC | Microbial fuel cell |
| MPD | Maximum power density |
| OCV | Open circuit voltage |
| ORR | Oxygen reduction reaction |
| PCD | Photocurrent density |
| PEC | Photoelectrocatalytic cell |
| PEC | Photoelectrochemical cells |
| PFC | Photocatalytic fuel cell |
| PLD | Pulsed laser deposition |
| RGO | Reduced graphene oxide |
| SCC | Short circuit current |
| VB | Valence band |
| Voc | Open circuit voltage |
| WRR | Water reduction reaction |
References
- Xu, P.; Xu, H.; Zheng, D. Simultaneous electricity generation and wastewater treatment in a photocatalytic fuel cell integrating electro-Fenton process. J. Power Sources 2019, 421, 156–161. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Recent Advances in TiO2 Films Prepared by Sol-Gel Methods for Photocatalytic Degradation of Organic Pollutants and Antibacterial Activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef] [Green Version]
- Pant, B.; Barakat, N.A.M.; Pant, H.R.; Park, M.; Saud, P.S.; Kim, J.-W.; Kim, H.-Y. Synthesis and photocatalytic activities of CdS/TiO2 nanoparticles supported on carbon nanofibers for high efficient adsorption and simultaneous decomposition of organic dyes. J. Colloid Interface Sci. 2014, 434, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, D.P.; Pant, B.; Acharya, J.; Park, M.; Ojha, G.P. Recent Progress in Metal–Organic Framework-Derived Nanostructures in the Removal of Volatile Organic Compounds. Molecules 2021, 26, 4948. [Google Scholar] [CrossRef]
- Hayworth, J.S.; Clement, T.P. BP’s Operation Deep Clean—Could Dilution be the Solution to Beach Pollution? Environ. Sci. Technol. 2011, 45, 4201–4202. [Google Scholar] [CrossRef]
- Marí-Guaita, J.; Bouich, A.; Marí, B. Shedding Light on Phase Stability and Surface Engineering of Formamidinium Lead Iodide (FaPbI3) Thin Films for Solar Cells. Eng. Proc. 2021, 12, 1. [Google Scholar]
- Bouich, A.; Marí-Guaita, J.; Bouich, A.; Pradas, I.G.; Marí, B. Towards Manufacture Stable Lead Perovskite APbI3 (A = Cs, MA, FA) Based Solar Cells with Low-Cost Techniques. Eng. Proc. 2021, 12, 81. [Google Scholar]
- Chang, X.; Fang, J.; Fan, Y.; Luo, T.; Su, H.; Zhang, Y.; Lu, J.; Tsetseris, L.; Anthopoulos, T.D.; Liu, S.; et al. Printable CsPbI3 Perovskite Solar Cells with PCE of 19% via an Additive Strategy. Adv. Mater. 2020, 32, 2001243. [Google Scholar] [CrossRef]
- Mani, S.; Bharagava, R.N. Textile industry wastewater: Environmental and health hazards and treatment approaches. In Recent Advances in Environmental Management; CRC Press: Boca Raton, FL, USA, 2018; pp. 47–69. [Google Scholar]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef] [Green Version]
- Hunge, Y.M.; Yadav, A.A.; Dhodamani, A.G.; Suzuki, N.; Terashima, C.; Fujishima, A.; Mathe, V.L. Enhanced photocatalytic performance of ultrasound treated GO/TiO2 composite for photocatalytic degradation of salicylic acid under sunlight illumination. Ultrason. Sonochem 2020, 61, 104849. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Verma, P. Microbial fuel cell: A green approach for the utilization of waste for the generation of bioelectricity. Bioresour. Bioprocess. 2016, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Omine, K.; Sivasankar, V.; Sano, H.; Chicas, S.D. Development of low-cost solid phase microbial fuel cell using organic waste and recycling of materials after power generation: Characterization of carbon anode. Biomass Bioenergy 2021, 154, 106266. [Google Scholar] [CrossRef]
- Li, B.; Xu, D.; Feng, L.; Liu, Y.; Zhang, L. Advances and prospects on the aquatic plant coupled with sediment microbial fuel cell system. Environ. Pollut. 2022, 297, 118771. [Google Scholar] [CrossRef]
- Dhawle, R.; Mantzavinos, D.; Lianos, P. UV/H2O2 degradation of diclofenac in a photocatalytic fuel cell. Appl. Catal. B Environ. 2021, 299, 120706. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.B.; Jothinathan, D.; Rajkumar, M. Microbial Fuel Cells: Fundamentals, Types, Significance and Limitations. In Microbial Fuel Cell Technology for Bioelectricity; Sivasankar, V., Mylsamy, P., Omine, K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 23–48. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Gu, Z.; Gu, Y.; Cen, Z.; Wei, L.; Zhou, Y.; Zhang, C.; Yang, C. Trash to treasure: A novel chemical route to synthesis of NiO/C for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 16144–16153. [Google Scholar] [CrossRef]
- Selihin, N.M.; Tay, M.G. A review on future wastewater treatment technologies: Micro-nanobubbles, hybrid electro-Fenton processes, photocatalytic fuel cells, and microbial fuel cells. Water Sci. Technol. 2021, 85, 319–341. [Google Scholar] [CrossRef]
- Ameta, R.; Solanki, M.S.; Benjamin, S.; Ameta, S.C. Chapter 6—Photocatalysis. In Advanced Oxidation Processes for Waste Water Treatment; Ameta, S.C., Ameta, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 135–175. [Google Scholar] [CrossRef]
- Wong, R.J.; Scott, J.; Low, G.K.C.; Feng, H.; Du, Y.; Hart, J.N.; Amal, R. Investigating the effect of UV light pre-treatment on the oxygen activation capacity of Au/TiO2. Catal. Sci. Technol. 2016, 6, 8188–8199. [Google Scholar] [CrossRef]
- Wong, R.J.; Scott, J.; Kappen, P.; Low, G.K.C.; Hart, J.N.; Amal, R. Enhancing bimetallic synergy with light: The effect of UV light pre-treatment on catalytic oxygen activation by bimetallic Au–Pt nanoparticles on a TiO2 support. Catal. Sci. Technol. 2017, 7, 4792–4805. [Google Scholar] [CrossRef]
- Wong, R.J.; Tsounis, C.; Scott, J.; Low, G.K.-C.; Amal, R. Promoting Catalytic Oxygen Activation by Localized Surface Plasmon Resonance: Effect of Visible Light Pre-treatment and Bimetallic Interactions. Chem. Cat Chem. 2018, 10, 287–295. [Google Scholar] [CrossRef]
- Pant, B.; Pant, H.R.; Barakat, N.A.M.; Park, M.; Jeon, K.; Choi, Y.; Kim, H.-Y. Carbon nanofibers decorated with binary semiconductor (TiO2/ZnO) nanocomposites for the effective removal of organic pollutants and the enhancement of antibacterial activities. Ceram. Int. 2013, 39, 7029–7035. [Google Scholar] [CrossRef]
- Pant, B.; Saud, P.S.; Park, M.; Park, S.-J.; Kim, H.-Y. General one-pot strategy to prepare Ag–TiO2 decorated reduced graphene oxide nanocomposites for chemical and biological disinfectant. J. Alloy. Compd. 2016, 671, 51–59. [Google Scholar] [CrossRef]
- He, F.; Jeon, W.; Choi, W. Photocatalytic air purification mimicking the self-cleaning process of the atmosphere. Nat. Commun. 2021, 12, 2528. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Kim, H.-Y.; Park, S.-J. Ag-ZnO photocatalyst anchored on carbon nanofibers: Synthesis, characterization, and photocatalytic activities. Synth. Met. 2016, 220, 533–537. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lai, Y.-H.; Mersch, D.; Reisner, E. Cu2O|NiOx nanocomposite as an inexpensive photocathode in photoelectrochemical water splitting. Chem. Sci. 2012, 3, 3482–3487. [Google Scholar] [CrossRef]
- Larminie, J.; Dicks, A.; McDonald, M.S. Fuel Cell Systems Explained; J. Wiley: Chichester, UK, 2003; Volume 2. [Google Scholar]
- Lianos, P. Production of electricity and hydrogen by photocatalytic degradation of organic wastes in a photoelectrochemical cell: The concept of the Photofuelcell: A review of a re-emerging research field. J. Hazard. Mater. 2011, 185, 575–590. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, G.; Zhang, Y.; Liu, J.; Zhang, Y.-n.; Shi, H. A solar-driven photocatalytic fuel cell with dual photoelectrode for simultaneous wastewater treatment and hydrogen production. J. Mater. Chem. A 2015, 3, 3416–3424. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Zhao, C.; Nie, E.; Wang, J.; Zhou, J.; Zhao, Q. Efficient Dye Contaminant Elimination and Simultaneously Electricity Production via a Bi-Doped TiO2 Photocatalytic Fuel Cell. Nanomaterials 2022, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; He, Y.; Xiao, M.; Zhang, Y.; Wang, Z.; Qin, Z.; Chai, B.; Yan, J.; Li, J.; Li, J. A solar-light driven photocatalytic fuel cell for efficient electricity generation and organic wastewater degradation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128205. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Lee, J.H.; Kim, H.-Y.; Park, S.-J. Novel magnetically separable silver-iron oxide nanoparticles decorated graphitic carbon nitride nano-sheets: A multifunctional photocatalyst via one-step hydrothermal process. J. Colloid Interface Sci. 2017, 496, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ng, Y.H.; Wen, X.; Chung, H.Y.; Wong, R.J.; Du, Y.; Dou, S.X.; Amal, R.; Scott, J. Construction of a Bi2MoO6:Bi2Mo3O12 heterojunction for efficient photocatalytic oxygen evolution. Chem. Eng. J. 2018, 353, 636–644. [Google Scholar] [CrossRef]
- Queiroz, B.D.; Fernandes, J.A.; Martins, C.A.; Wender, H. Photocatalytic fuel cells: From batch to microfluidics. J. Environ. Chem. Eng. 2022, 10, 107611. [Google Scholar] [CrossRef]
- Hu, C.; Kelm, D.; Schreiner, M.; Wollborn, T.; Mädler, L.; Teoh, W.Y. Designing Photoelectrodes for Photocatalytic Fuel Cells and Elucidating the Effects of Organic Substrates. Chem. Sus. Chem. 2015, 8, 4005–4015. [Google Scholar] [CrossRef]
- Imran, M.; Yousaf, A.B.; Farooq, M.; Kasak, P. Enhancement of visible light-driven hydrogen production over zinc cadmium sulfide nanoparticles anchored on BiVO4 nanorods. Int. J. Hydrogen Energy 2022, 47, 8327–8337. [Google Scholar] [CrossRef]
- Asiri, A.M.; Nawaz, T.; Tahir, M.B.; Fatima, N.; Khan, S.B.; Alamry, K.A.; Alfifi, S.Y.; Marwani, H.M.; Al-Otaibi, M.M.; Chakraborty, S. Fabrication of WO3 based nanocomposites for the excellent photocatalytic energy production under visible light irradiation. Int. J. Hydrogen Energy 2021, 46, 39058–39066. [Google Scholar] [CrossRef]
- Mishra, P.; Saravanan, P.; Packirisamy, G.; Jang, M.; Wang, C. A subtle review on the challenges of photocatalytic fuel cell for sustainable power production. Int. J. Hydrogen Energy 2021, 46, 22877–22906. [Google Scholar] [CrossRef]
- Chung, H.Y.; Toe, C.Y.; Chen, W.; Wen, X.; Wong, R.J.; Amal, R.; Abdi, F.F.; Ng, Y.H. Manipulating the Fate of Charge Carriers with Tungsten Concentration: Enhancing Photoelectrochemical Water Oxidation of Bi2WO6. Small 2021, 17, 2102023. [Google Scholar] [CrossRef]
- Tan, H.L.; Tahini, H.A.; Wen, X.; Wong, R.J.; Tan, X.; Iwase, A.; Kudo, A.; Amal, R.; Smith, S.C.; Ng, Y.H. Interfacing BiVO4 with Reduced Graphene Oxide for Enhanced Photoactivity: A Tale of Facet Dependence of Electron Shuttling. Small 2016, 12, 5295–5302. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Wen, X.; Amal, R.; Ng, Y.H. BiVO4 {010} and {110} Relative Exposure Extent: Governing Factor of Surface Charge Population and Photocatalytic Activity. J. Phys. Chem. Lett. 2016, 7, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; El-Kalliny, A.S.; Squadrito, G. Stacked titanium dioxide nanotubes photoanode facilitates unbiased hydrogen production in a solar-driven photoelectrochemical cell powered with a microbial fuel cell treating animal manure wastewater. Energy Convers. Manag. 2022, 254, 115225. [Google Scholar] [CrossRef]
- Kaneko, M.; Gokan, N.; Katakura, N.; Takei, Y.; Hoshino, M. Artificial photochemical nitrogen cycle to produce nitrogen and hydrogen from ammonia by platinized TiO2 and its application to a photofuel cell. Chem. Commun. 2005, 12, 1625–1627. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Zhou, B.; Chen, H.; Wang, Z.; Cai, W. A TiO2-nanotube-array-based photocatalytic fuel cell using refractory organic compounds as substrates for electricity generation. Chem. Commun. 2011, 47, 10314–10316. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, D.P.; Shrestha, S.; Shrestha, B.K.; Park, C.H.; Kim, C.S. A controlled surface geometry of polyaniline doped titania nanotubes biointerface for accelerating MC3T3-E1 cells growth in bone tissue engineering. Chem. Eng. J. 2018, 350, 57–68. [Google Scholar] [CrossRef]
- Rezk, A.I.; Bhattarai, D.P.; Park, J.; Park, C.H.; Kim, C.S. Polyaniline-coated titanium oxide nanoparticles and simvastatin-loaded poly (ε-caprolactone) composite nanofibers scaffold for bone tissue regeneration application. Colloids Surf. B Biointerfaces 2020, 192, 111007. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Dong, L.; Shen, C.; Li, F.; Huang, M.; Ma, C.; Yang, B.; An, X.; Sand, W. Recent advances on photocatalytic fuel cell for environmental applications—The marriage of photocatalysis and fuel cells. Sci. Total Environ. 2019, 668, 966–978. [Google Scholar] [CrossRef]
- Dubey, P.K.; Kumar, R.; Tiwari, R.S.; Srivastava, O.N.; Pandey, A.C.; Singh, P. Surface modification of aligned TiO2 nanotubes by Cu2O nanoparticles and their enhanced photo electrochemical properties and hydrogen generation application. Int. J. Hydrogen Energy 2018, 43, 6867–6878. [Google Scholar] [CrossRef]
- Tao, S.; Wang, F.; Zhang, J.; Shi, J.; Guo, W.; Lu, J. Visible-Light-Responsive TiO2/NiFe Mixed Metal Oxide-Carbon Photocatalytic Fuel Cell with Synchronous Hydrogen Peroxide Production. Eur. J. Inorg. Chem. 2021, 2021, 1230–1239. [Google Scholar] [CrossRef]
- Marami, M.B.; Farahmandjou, M.; Khoshnevisan, B. Sol–Gel Synthesis of Fe-Doped TiO2 Nanocrystals. J. Electron. Mater. 2018, 47, 3741–3748. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, M.; Wang, Y.; Lv, J.; Liu, Y.; He, G.; Sun, Z. Low-temperature strategy for vapor phase hydrothermal synthesis of C\N\S-doped TiO2 nanorod arrays with enhanced photoelectrochemical and photocatalytic activity. J. Ind. Eng. Chem. 2021, 98, 130–139. [Google Scholar] [CrossRef]
- Thor, S.-H.; Ho, L.-N.; Ong, S.-A.; Abidin, C.Z.A.; Heah, C.-Y.; Nordin, N.; Ong, Y.-P.; Yap, K.-L. Advanced oxidation treatment of amaranth dye synchronized with electricity generation using carbon-based cathodes in a sustainable photocatalytic fuel cell integrated electro-fenton system. J. Environ. Chem. Eng. 2021, 9, 106439. [Google Scholar] [CrossRef]
- Pant, B.; Prasad Ojha, G.; Acharya, J.; Park, M. Ag3PO4-TiO2-Carbon nanofiber Composite: An efficient Visible-light photocatalyst obtained from eelectrospinning and hydrothermal methods. Sep. Purif. Technol. 2021, 276, 119400. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Hydrothermal synthesis of Ag2CO3-TiO2 loaded reduced graphene oxide nanocomposites with highly efficient photocatalytic activity. Chem. Eng. Commun. 2020, 207, 688–695. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: The effect of doping elements on photocatalytic activity. Mater. Adv. 2020, 1, 1193–1201. [Google Scholar] [CrossRef]
- Monfort, O.; Roch, T.; Gregor, M.; Satrapinskyy, L.; Raptis, D.; Lianos, P.; Plesch, G. Photooxidative properties of various BiVO4/TiO2 layered composite films and study of their photocatalytic mechanism in pollutant degradation. J. Environ. Chem. Eng. 2017, 5, 5143–5149. [Google Scholar] [CrossRef]
- Sui, M.; Dong, Y.; Wang, Z.; Wang, F.; You, H. A biocathode-driven photocatalytic fuel cell using an Ag-doped TiO2/Ti mesh photoanode for electricity generation and pollutant degradation. J. Photochem. Photobiol. A Chem. 2017, 348, 238–245. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Lu, X.-Y.; Xuan, J.; Leung, M.K.H. Solar photocatalytic fuel cell using CdS–TiO2 photoanode and air-breathing cathode for wastewater treatment and simultaneous electricity production. Chem. Eng. J. 2014, 253, 174–182. [Google Scholar] [CrossRef]
- He, X.; Chen, M.; Chen, R.; Zhu, X.; Liao, Q.; Ye, D.; Zhang, B.; Zhang, W.; Yu, Y. A solar responsive photocatalytic fuel cell with the membrane electrode assembly design for simultaneous wastewater treatment and electricity generation. J. Hazard. Mater. 2018, 358, 346–354. [Google Scholar] [CrossRef]
- Górska, P.; Zaleska, A.; Kowalska, E.; Klimczuk, T.; Sobczak, J.W.; Skwarek, E.; Janusz, W.; Hupka, J. TiO2 photoactivity in vis and UV light: The influence of calcination temperature and surface properties. Appl. Catal. B Environ. 2008, 84, 440–447. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Li, X.; Huang, K.; Zhou, B.; Cai, W.; Shangguan, W. Visible-Light Responsive Photocatalytic Fuel Cell Based on WO3/W Photoanode and Cu2O/Cu Photocathode for Simultaneous Wastewater Treatment and Electricity Generation. Environ. Sci. Technol. 2012, 46, 11451–11458. [Google Scholar] [CrossRef]
- Zeng, Q.; Chang, S.; Wang, M.; Li, M.; Deng, Q.; Xiong, Z.; Zhou, B.; Liu, Y. Highly-active, metal-free, carbon-based ORR cathode for efficient organics removal and electricity generation in a PFC system. Chin. Chem. Lett. 2021, 32, 2212–2216. [Google Scholar] [CrossRef]
- Sfaelou, S.; Lianos, P. Photoactivated Fuel Cells (PhotoFuelCells). An alternative source of renewable energy with environmental benefits. AIMS Mater. Sci. 2016, 3, 270–288. [Google Scholar] [CrossRef]
- Khalik, W.F.; Ho, L.-N.; Ong, S.-A.; Voon, C.-H.; Wong, Y.-S.; Yusuf, S.Y.; Yusoff, N.; Lee, S.-L. Reactive Black 5 as electron donor and/or electron acceptor in dual chamber of solar photocatalytic fuel cell. Chemosphere 2018, 202, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, Y.; He, Y.; Yang, C.; Wang, Y.; Jia, J. Photocatalytic fuel cell (PFC) and dye self-photosensitization photocatalytic fuel cell (DSPFC) with BiOCl/Ti photoanode under UV and visible light irradiation. Environ. Sci. Technol. 2013, 47, 3490–3497. [Google Scholar] [CrossRef]
- Masudy-Panah, S.; Siavash Moakhar, R.; Chua, C.S.; Kushwaha, A.; Dalapati, G.K. Stable and Efficient CuO Based Photocathode through Oxygen-Rich Composition and Au–Pd Nanostructure Incorporation for Solar-Hydrogen Production. ACS Appl. Mater. Interfaces 2017, 9, 27596–27606. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Khan, S.; Takagi, K.; Suzuki, N.; Teshima, K.; Terashima, C.; Fujishima, A. Photocatalytic degradation of bisphenol A using titanium dioxide@nanodiamond composites under UV light illumination. J. Colloid Interface Sci. 2021, 582, 1058–1066. [Google Scholar] [CrossRef]
- Pant, H.R.; Pant, B.; Pokharel, P.; Kim, H.J.; Tijing, L.D.; Park, C.H.; Lee, D.S.; Kim, H.Y.; Kim, C.S. Photocatalytic TiO2–RGO/nylon-6 spider-wave-like nano-nets via electrospinning and hydrothermal treatment. J. Membr. Sci. 2013, 429, 225–234. [Google Scholar] [CrossRef]
- Trapalis, A.; Todorova, N.; Giannakopoulou, T.; Boukos, N.; Speliotis, T.; Dimotikali, D.; Yu, J. TiO2/graphene composite photocatalysts for NOx removal: A comparison of surfactant-stabilized graphene and reduced graphene oxide. Appl. Catal. B Environ. 2016, 180, 637–647. [Google Scholar] [CrossRef]
- Tang, B.; Chen, H.; Peng, H.; Wang, Z.; Huang, W. Graphene Modified TiO2 Composite Photocatalysts: Mechanism, Progress and Perspective. Nanomaterials 2018, 8, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pant, H.R.; Adhikari, S.P.; Pant, B.; Joshi, M.K.; Kim, H.J.; Park, C.H.; Kim, C.S. Immobilization of TiO2 nanofibers on reduced graphene sheets: Novel strategy in electrospinning. J. Colloid Interface Sci. 2015, 457, 174–179. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J.; Kim, H.-Y. One-pot synthesis of CdS sensitized TiO2 decorated reduced graphene oxide nanosheets for the hydrolysis of ammonia-borane and the effective removal of organic pollutant from water. Ceram. Int. 2016, 42, 15247–15252. [Google Scholar] [CrossRef]
- Tan, H.L.; Denny, F.; Hermawan, M.; Wong, R.J.; Amal, R.; Ng, Y.H. Reduced graphene oxide is not a universal promoter for photocatalytic activities of TiO2. J. Mater. 2017, 3, 51–57. [Google Scholar] [CrossRef]
- Bai, J.; Wang, R.; Li, Y.; Tang, Y.; Zeng, Q.; Xia, L.; Li, X.; Li, J.; Li, C.; Zhou, B. A solar light driven dual photoelectrode photocatalytic fuel cell (PFC) for simultaneous wastewater treatment and electricity generation. J. Hazard. Mater. 2016, 311, 51–62. [Google Scholar] [CrossRef]
- Kee, M.W.; Soo, J.W.; Lam, S.M.; Sin, J.C.; Mohamed, A.R. Evaluation of photocatalytic fuel cell (PFC) for electricity production and simultaneous degradation of methyl green in synthetic and real greywater effluents. J. Environ. Manag. 2018, 228, 383–392. [Google Scholar] [CrossRef]
- Divyapriya, G.; Singh, S.; Martínez-Huitle, C.A.; Scaria, J.; Karim, A.V.; Nidheesh, P.V. Treatment of real wastewater by photoelectrochemical methods: An overview. Chemosphere 2021, 276, 130188. [Google Scholar] [CrossRef]
- Matarrese, R.; Mascia, M.; Vacca, A.; Mais, L.; Usai, E.M.; Ghidelli, M.; Mascaretti, L.; Bricchi, B.R.; Russo, V.; Casari, C.S.; et al. Integrated Au/TiO2 Nanostructured Photoanodes for Photoelectrochemical Organics Degradation. Catalysts 2019, 9, 340. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Bruning, H.; Li, X.; Yntema, D.; Rijnaarts, H.H.M. Significant enhancement of micropollutant photocatalytic degradation using a TiO2 nanotube array photoanode based photocatalytic fuel cell. Chem. Eng. J. 2018, 354, 553–562. [Google Scholar] [CrossRef]
- Lee, S.-L.; Ho, L.-N.; Ong, S.-A.; Wong, Y.-S.; Voon, C.-H.; Khalik, W.F.; Yusoff, N.A.; Nordin, N. A highly efficient immobilized ZnO/Zn photoanode for degradation of azo dye Reactive Green 19 in a photocatalytic fuel cell. Chemosphere 2017, 166, 118–125. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Y.; Li, L.; Shen, W.; Li, J. An efficient optofluidic photocatalytic fuel cell with dual-photoelectrode for electricity generation from wastewater treatment. J. Solid State Chem. 2021, 293, 121780. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Mahadik, M.A.; Bulakhe, R.N.; Yadav, S.P.; Shim, J.J.; Moholkar, A.V.; Bhosale, C.H. Oxidative degradation of benzoic acid using spray deposited WO3/TiO2 thin films. J. Mater. Sci. Mater. Electron. 2017, 28, 17976–17984. [Google Scholar] [CrossRef]
- Lui, G.; Jiang, G.; Fowler, M.; Yu, A.; Chen, Z. A high performance wastewater-fed flow-photocatalytic fuel cell. J. Power Sources 2019, 425, 69–75. [Google Scholar] [CrossRef]
- Nahyoon, N.A.; Liu, L.; Rabe, K.; Thebo, K.H.; Yuan, L.; Sun, J.; Yang, F. Significant photocatalytic degradation and electricity generation in the photocatalytic fuel cell (PFC) using novel anodic nanocomposite of Fe, graphene oxide, and titanium phosphate. Electrochim. Acta 2018, 271, 41–48. [Google Scholar] [CrossRef]
- Scharber, M.C.; Sariciftci, N.S. Low Band Gap Conjugated Semiconducting Polymers. Adv. Mater. Technol. 2021, 6, 2000857. [Google Scholar] [CrossRef]
- Sitt, A.; Hadar, I.; Banin, U. Band-gap engineering, optoelectronic properties and applications of colloidal heterostructured semiconductor nanorods. Nano Today 2013, 8, 494–513. [Google Scholar] [CrossRef]
- Sinha, B.; Goswami, T.; Paul, S.; Misra, A. The impact of surface structure and band gap on the optoelectronic properties of Cu2O nanoclusters of varying size and symmetry. RSC Adv. 2014, 4, 5092–5104. [Google Scholar] [CrossRef]
- Vojkovic, S.; Fernandez, J.; Elgueta, S.; Vega, F.E.; Rojas, S.D.; Wheatley, R.A.; Seifert, B.; Wallentowitz, S.; Cabrera, A.L. Band gap determination in multi-band-gap CuFeO2 delafossite epitaxial thin film by photoconductivity. SN Appl. Sci. 2019, 1, 1322. [Google Scholar] [CrossRef] [Green Version]
- Persson, C.; Lindefelt, U.; Sernelius, B.E. Band gap narrowing in n-type and p-type 3C-, 2H-, 4H-, 6H-SiC, and Si. J. Appl. Phys. 1999, 86, 4419–4427. [Google Scholar] [CrossRef]
- Haram, S.K. 9—Semiconductor Electrodes. In Handbook of Electrochemistry; Zoski, C.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 329–389. [Google Scholar] [CrossRef]
- Kohl, P.A.; Bard, A.J. Semiconductor Electrodes: XVII. Electrochemical Behavior of n-and p-Type Electrodes in Acetonitrile Solutions. J. Electrochem. Soc. 1979, 126, 598. [Google Scholar] [CrossRef]
- Pant, B.; Ojha, G.P.; Kim, H.-Y.; Park, M.; Park, S.-J. Fly-ash-incorporated electrospun zinc oxide nanofibers: Potential material for environmental remediation. Environ. Pollut. 2019, 245, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E.J.; Wain, A.J. The Butler-Volmer equation in electrochemical theory: Origins, value, and practical application. J. Electroanal. Chem. 2020, 872, 114145. [Google Scholar] [CrossRef]
- He, Y.; Chen, K.; Leung, M.K.; Zhang, Y.; Li, L.; Li, G.; Xuan, J.; Li, J. Photocatalytic fuel cell–A review. Chem. Eng. J. 2022, 428, 131074. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Khataee, A.; Dragoi, E.-N.; Moradi, M.; Nabavifard, S.; Conti, G.O.; Khaneghah, A.M. Pollutants degradation and power generation by photocatalytic fuel cells: A comprehensive review. Arabian J. Chem. 2020, 13, 8458–8480. [Google Scholar] [CrossRef]
- Ammar, S.H.; Shafi, R.F.; Ali, A.D. A novel airlift photocatalytic fuel cell (APFC) with immobilized CdS coated zerovalent iron (Fe@ CdS) and g-C3N4 photocatalysts film as photoanode for power generation and organics degradation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125164. [Google Scholar] [CrossRef]
- Li, L.; Chen, R.; Zhu, X.; Liao, Q.; Ye, D.; Zhang, B.; He, X.; Jiao, L.; Feng, H.; Zhang, W. A ternary hybrid CdS/SiO2/TiO2 photoanode with enhanced photoelectrochemical activity. Renew. Energy 2018, 127, 524–530. [Google Scholar] [CrossRef]
- Ješić, D.; Lašič Jurković, D.; Pohar, A.; Suhadolnik, L.; Likozar, B. Engineering photocatalytic and photoelectrocatalytic CO2 reduction reactions: Mechanisms, intrinsic kinetics, mass transfer resistances, reactors and multi-scale modelling simulations. Chem. Eng. J. 2021, 407, 126799. [Google Scholar] [CrossRef]
- Liu, X.-H.; Xing, Z.-H.; Chen, Q.-Y.; Wang, Y.-H. Multi-functional photocatalytic fuel cell for simultaneous removal of organic pollutant and chromium (VI) accompanied with electricity production. Chemosphere 2019, 237, 124457. [Google Scholar] [CrossRef]
- Yao, H.; Xu, Y.; Zhong, D.; Zeng, Y.; Zhong, N. Efficient rhodamine B degradation and stable electricity generation performance of visible-light photocatalytic fuel cell with g-C3N4/WO3/TiO2/Ti photoanode. Ionics 2021, 27, 4875–4884. [Google Scholar] [CrossRef]
- He, Y.; Yuan, R.; Yan, J.; Li, J. A highly efficient NiFe-layer double hydroxide/TiO2 heterojunction photoanode-based high-performance bifunctional photocatalytic fuel cell. Mater. Today Commun. 2021, 26, 102177. [Google Scholar] [CrossRef]
- Yong, Z.-J.; Lam, S.-M.; Sin, J.-C.; RahmanMohamed, A. Feasibility study of municipal wastewater removal synchronized with electricity generation via solar-driven photocatalytic fuel cell with Bi2WO6/ZnO nanorods array photoanode. IOP Conf. Ser. Earth Environ. Sci. 2021, 945, 012004. [Google Scholar]
- Xie, F.; Chen, R.; Zhu, X.; Liao, Q.; Ye, D.; Zhang, B.; Yu, Y.; Li, J. CO2 utilization: Direct power generation by a coupled system that integrates photocatalytic reduction of CO2 with photocatalytic fuel cell. J. CO2 Util. 2019, 32, 31–36. [Google Scholar] [CrossRef]
- Li, K.; Zhang, H.; Tang, T.; Xu, Y.; Ying, D.; Wang, Y.; Jia, J. Optimization and application of TiO2/Ti–Pt photo fuel cell (PFC) to effectively generate electricity and degrade organic pollutants simultaneously. Water Res. 2014, 62, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xie, F.; Chen, R.; Zhu, X.; Liao, Q.; Ye, D.; Li, J.; Song, S. New insights into the role of CO2 in a photocatalytic fuel cell. J. Power Sources 2021, 487, 229438. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Hwang, T.I.; Kim, J.I.; Lee, J.H.; Chun, S.; Kim, B.-S.; Park, C.H.; Kim, C.S. Synthesis of polypyrrole nanorods via sacrificial removal of aluminum oxide nanopore template: A study on cell viability, electrical stimulation and neuronal differentiation of PC12 cells. Mater. Sci. Eng. C 2020, 107, 110325. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Kim, B.S. NIR-triggered hyperthermal effect of polythiophene nanoparticles synthesized by surfactant-free oxidative polymerization method on colorectal carcinoma cells. Cells 2020, 9, 2122. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Pandeya, S.; Bhattarai, D.P.; Joshi, M.K. Biomimetic Mineralization of Electrospun PCL-Based Composite Nanofibrous Scaffold for Hard Tissue Engineering. In Nanoscale Engineering of Biomaterials: Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 683–704. [Google Scholar]
- Bhattarai, D.P.; Pokharel, P.; Xiao, D. Surface functionalization of polymers. In Reactive and Functional Polymers Volume Four; Springer: Berlin/Heidelberg, Germany, 2020; pp. 5–34. [Google Scholar]
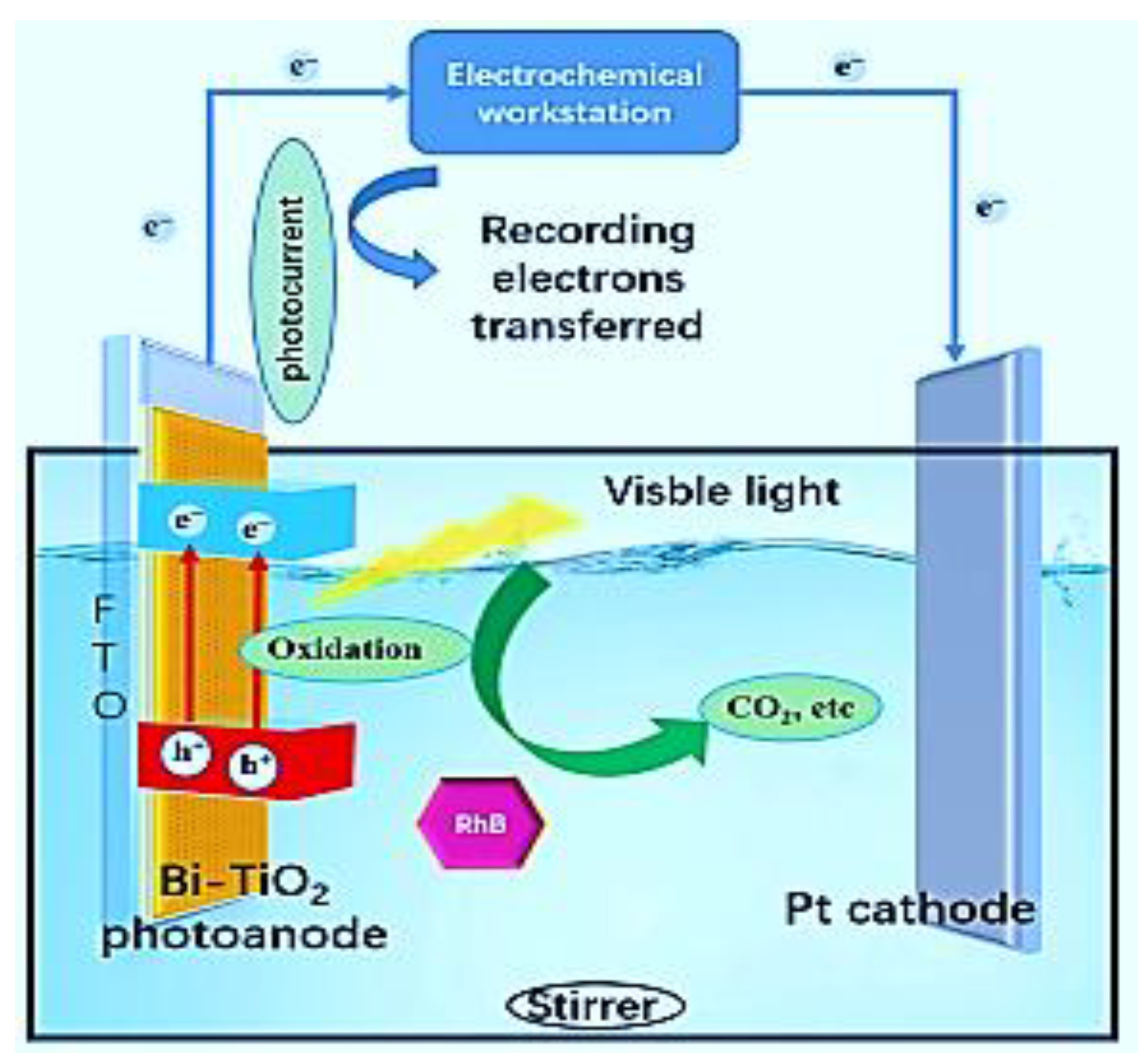

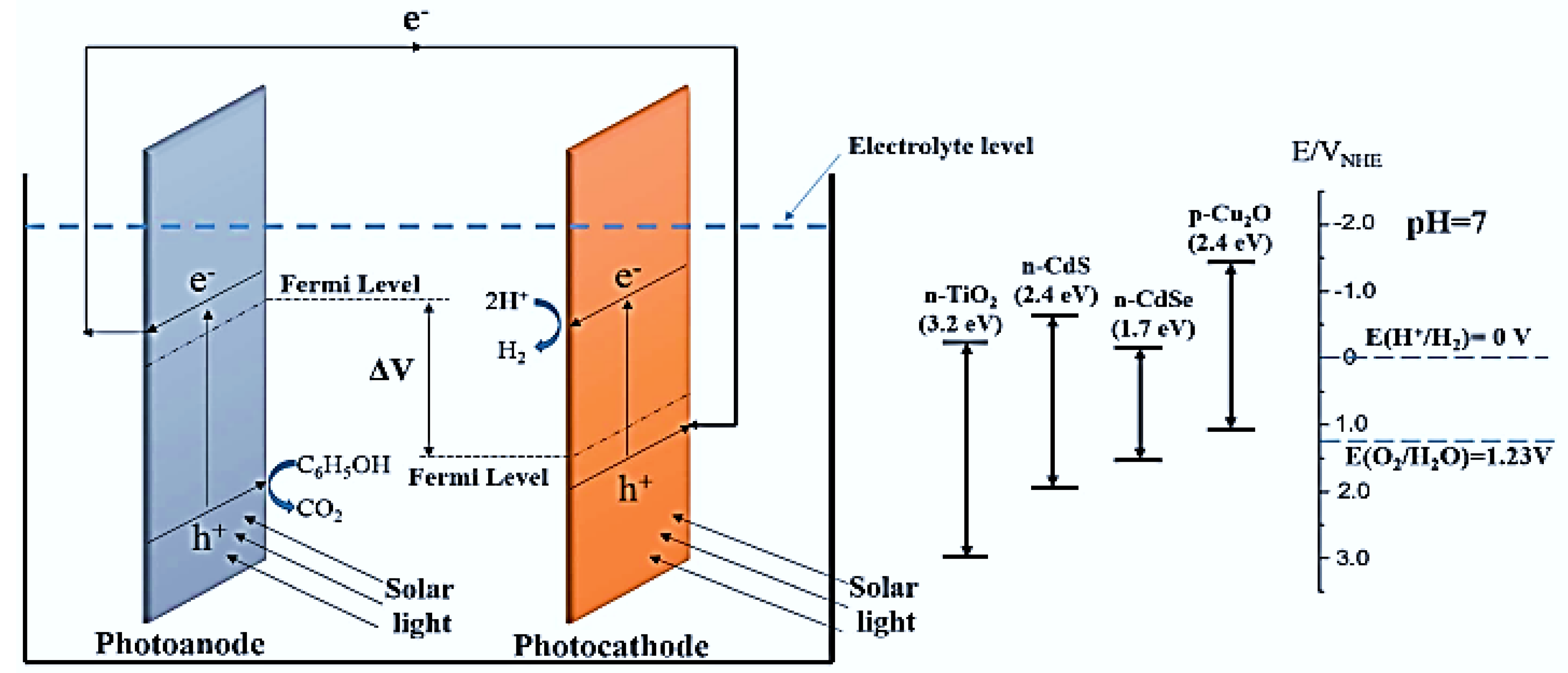
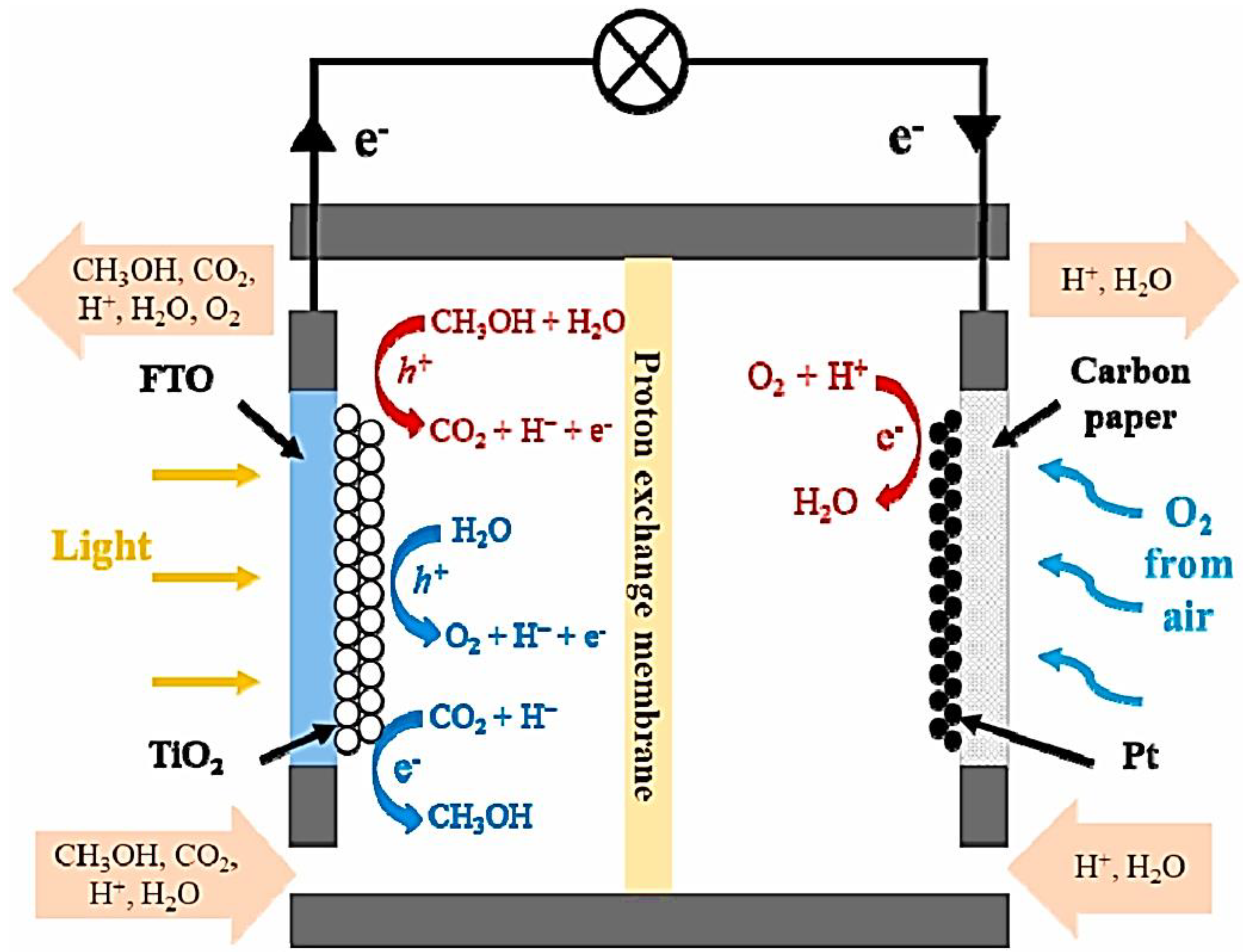
| Photoanode Materials | Cathode/Photocathode | Methods of Preparation | Special Features | Performance | Ref. |
|---|---|---|---|---|---|
| TiO2 NRs/FTO | C/Cu2O NW As/Cu | Hydrothermal/anodization | Phenol degradation and simultaneous hydrogen production | TOC removal rate 84.2% and H2 production rate 86.8 μmol cm−2 | [34] |
| Au/TiO2 | Pt | Pulsed laser deposition (PLD) | Bisphenol A (BPA) oxidation, water splitting, hydrogen production | Photocurrent density = 58 μA/cm2 | [83] |
| TiO2 nanotube array | Cu | Electrochemical anodization | Micropollutant removal from water | 62% MCPA removal | [84] |
| ZnO/Zn | Pt/C | Heat attachment method | Degradation of reactive green 19 | 100% removal of RG19, Jsc = 0.0427 mAcm−2, and (JV)max = 0.0102 mW cm−2 | [85] |
| BiVO4 bismuth vandate | Cu2O | - | Good stability, Electricity generation and waste water treatment. | Voc = 0.463 V and Jsc = 0.113 mA cm−2 | [86] |
| CdS-ZnS-TiO2 | Pt black on carbon black | Successive ionic layer adsorption and reaction | Organic compound degradation and electricity generation. | JVmax = 1.01 mW cm−2 at a current density of 1.4 mA cm−2. FF = 0.45 | [65] |
| WO3/W | Fe@Fe2O3/carbon felt electro-fenton | Hydrothermal process | Waste water treatment and electricity generation. | Jsc = 0.59 mA cm−2, JVmax = 0.34 mW cm−2 | [1] |
| WO3/TiO2 | - | Spray pyrolysis method | Photoelectrolytic degradation of benzoic acid | Benzoic acid degradation rate of 46.56%, Voc = 0.485 V Isc = 0.575 mA | [87] |
| Ag-TiO2 | Pt/C on carbon cloth | Solvothermal process | Coulombic efficiency of 9.4% | JVmax = 1.85 Wm−2 COD removal = 14.8% | [88] |
| MoS2/TiO2 | MoS2/Ni foam | Hydrothermal process | Higher light harvesting, electron transport efficiency, Rhodamine B (20 ppm, pH =7) degradation efficiency of 69.16%. | Voc = 0.7 V Isc = 1.04 mA cm−2 JVmax = 0.114 mW cm−2. | [36] |
| WO3/W | Cu2O/Cu | Hydrothermal process | Waste water treatment and electricity generation | Degradation rate of phenol, Rhodamine B and Congo red were 58%, 63%, 74%, respectively. | [67] |
| BiOCl/Ti | Pt | Wet chemical synthesis and dip coating method | Rhodamine B and salicylic acid degradation and electricity generation | Jsc = 0.0116, Voc = 0.655 V, ff = 0.39 for Salicylic acid under UV irradiation. | [71] |
| Bi doped TiO2 | Pt | Sol-gel method | Rhodamine B degradation and photocurrent generation | 91.2% degradation of Rhodamine B | [35] |
| NiFe layered double oxide/TiO2 | Carbon black | co-precipitation/calcination | Hydrogen peroxide production | Voc = 0.78 V Jsc = 1093 μA cm−2. Pmax = 169 μW cm−2. | [55] |
| BiVO4/TiO2 NTs | ZnO/CuO NWs | Hydrothermal/liquid phase deposition | Degradations of methyl orange, Congo red and methylene blue are 76%, 83%, and 90%, respectively. | JVmax = 0.116 mW cm−2. | [80] |
| Fe-graphene oxide-titanium phosphate | ZnIn2S4 | Hummer’s modified method, wet chemical method | Degradation of Rhodamine B | 90% removal capacity for Rhodamine B at pH 1. | [89] |
| SN | Photoanode | Cathode/ Photocathode | OCV (Voc) | SCC (Jsc) mA cm−2 | MPD (Pmax) μW cm−2 | PCD mA cm−2 | Degradation Profile | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | g-C3N4/WO3/TiO2/Ti | Pt | - | 0.20 | 19.35 | 2.27 at 1.1 V vs. SCE | RhB degradation by 87.7% | [105] |
| 2 | NiFe-layered double hydroxide/TiO2 | Cu2O | 0.690 | 0.622 | 206 | 1.85 at 1.6 V vs RHE | MB degradation (10 ppm) 94.6% | [106] |
| 3 | Bi2WO6/ZnO nanorods | Pt | 563 mv | 37.10 | 3.30 | [107] | ||
| 4 | ZnO/C | Pt/C | 1000 mV | 0.0069 | 2.34 | - | Degradation of Azo dye reactive black 5 (13.6% at anode and 8.7% at cathode) | [70] |
| 5 | BiVO4/TiO2 NTs | ZnO/CuO NWs | 0.53 V | 0.43 | 0.116 mW cm−2 | - | Degradation of Methyl orange (76%), Congo red (90%), and Methylene blue (83%) | [80] |
| 6 | TiO2NRs/FTO | C/Cu2O/Cu | 0.41 V | 0.50 | - | - | Phenol removal (84.2%) | [34] |
| 7 | ZnO/Zn | Pt/C | 1050 mV | 0.0327 | 0.0076 mW cm−2 | - | Degradation of reactive green 19 | [85] |
| 8 | TiO2/FTO | TiO2 | 0.98 V | 0.175 | 0.110 mW cm−2 | - | CO2 utilization | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oli, H.B.; Kim, A.A.; Park, M.; Bhattarai, D.P.; Pant, B. Photocatalytic Fuel Cells for Simultaneous Wastewater Treatment and Power Generation: Mechanisms, Challenges, and Future Prospects. Energies 2022, 15, 3216. https://doi.org/10.3390/en15093216
Oli HB, Kim AA, Park M, Bhattarai DP, Pant B. Photocatalytic Fuel Cells for Simultaneous Wastewater Treatment and Power Generation: Mechanisms, Challenges, and Future Prospects. Energies. 2022; 15(9):3216. https://doi.org/10.3390/en15093216
Chicago/Turabian StyleOli, Hari Bhakta, Allison A. Kim, Mira Park, Deval Prasad Bhattarai, and Bishweshwar Pant. 2022. "Photocatalytic Fuel Cells for Simultaneous Wastewater Treatment and Power Generation: Mechanisms, Challenges, and Future Prospects" Energies 15, no. 9: 3216. https://doi.org/10.3390/en15093216









