Experimental Investigation on the Performance of an Aviation Piston Engine Fueled with Bio-Jet Fuel Prepared via Thermochemical Conversion of Triglyceride
Abstract
:1. Introduction
2. Experimental Methods
2.1. Bio-Jet Fuel Preparation
- Hyperthermia catalytical pyrolysis:
- 2.
- Distillation:
- 3.
- Molecule structure adjustment:

2.2. Experimental Details
- Preheat the cylinder to 70 °C.
- Start the engine and run the engine at idle speed until the cylinder temperature reaches 135 °C.
- Adjust control parameters to make the engine work on the specified working conditions.
- Measure the data when the cylinder temperature stabilizes at 170 °C and the rotating speed is steady.
3. Results
3.1. Performances of Power and Economy
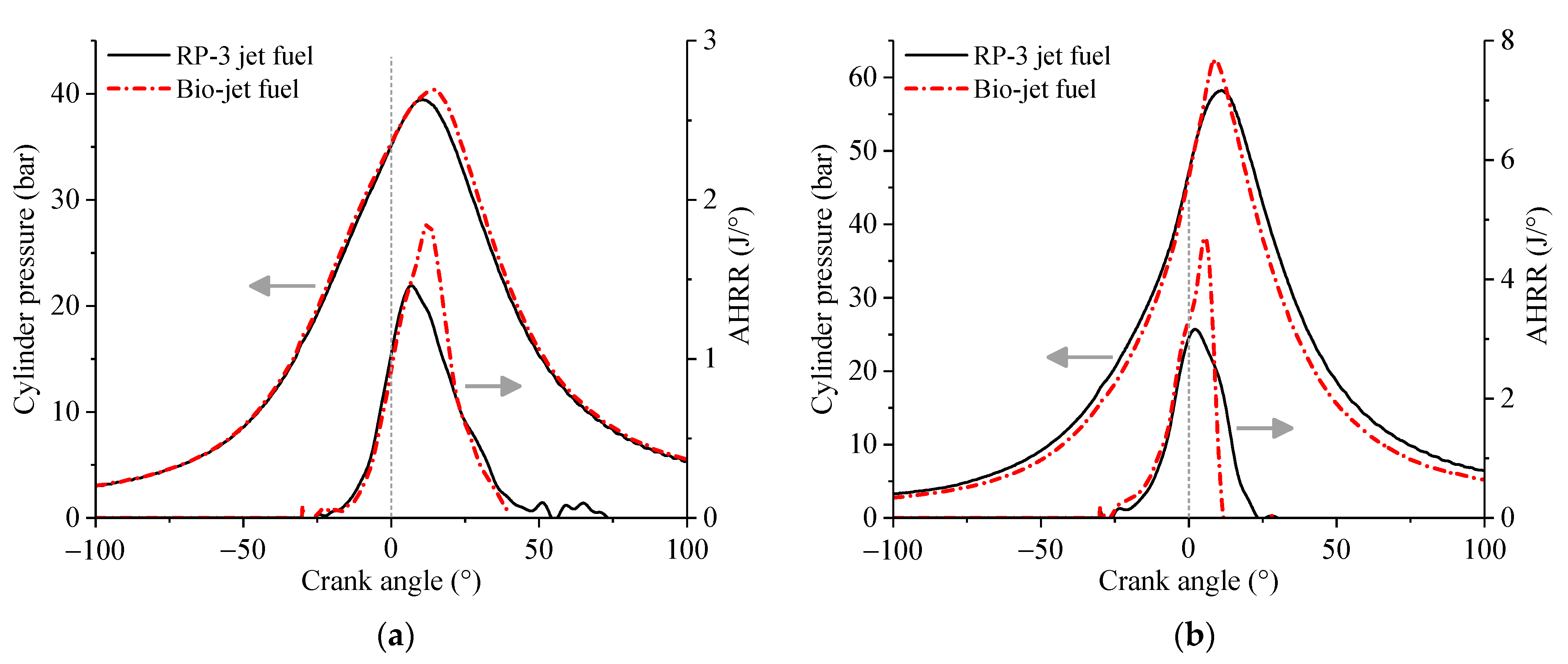
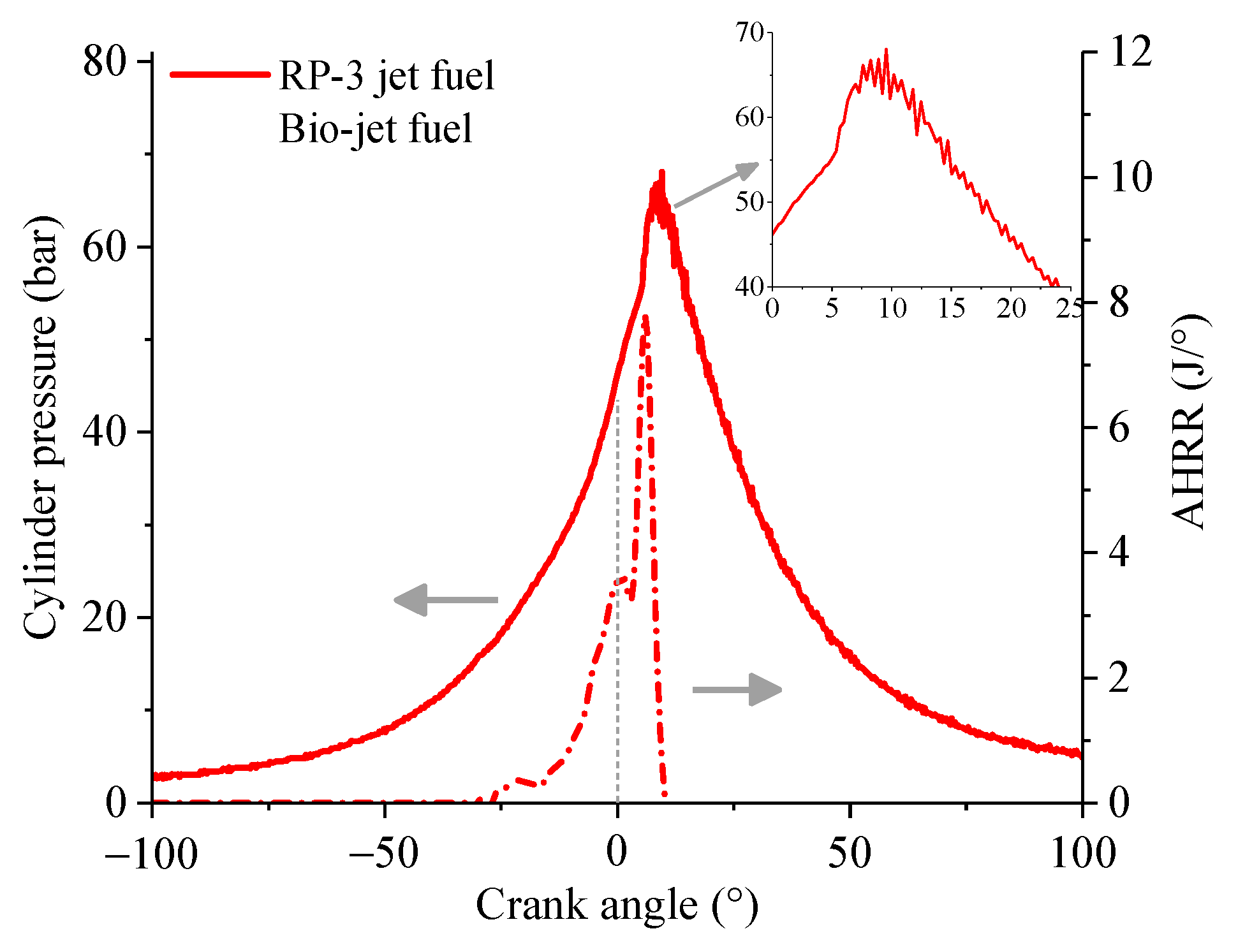
3.2. Heat Release Law
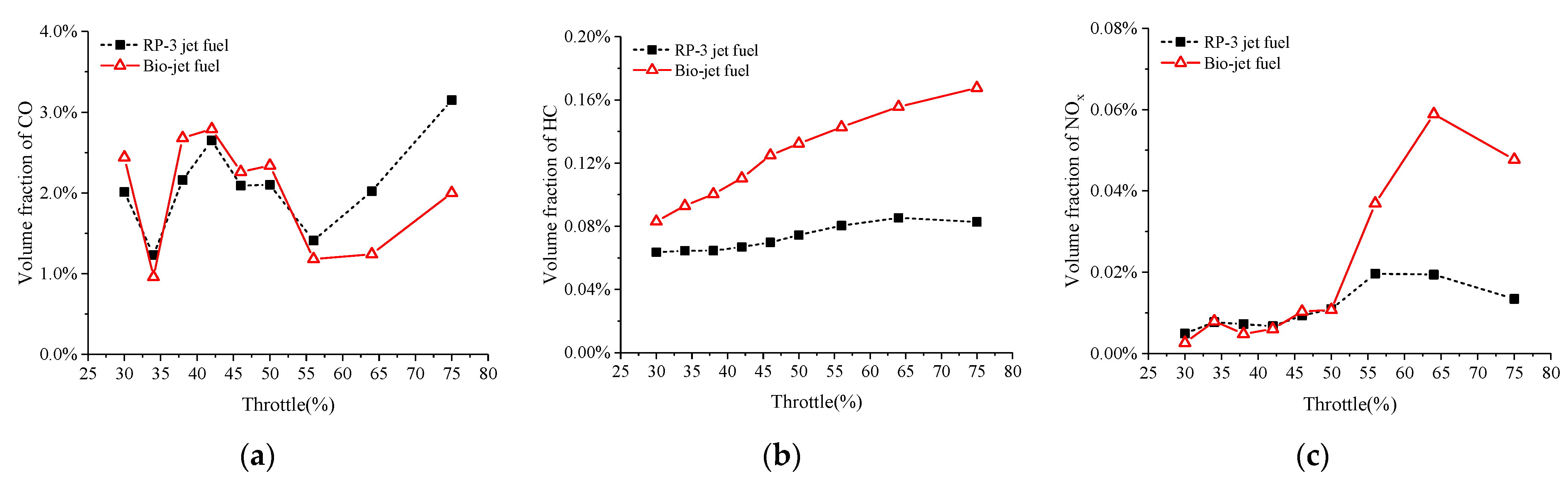
3.3. Emission Performance
4. Discussion and Conclusions
- When the cooling requirement of the cylinder was satisfied, the performances of the power and the economy of the engine were not degraded by burning the bio-jet fuel at small and medium throttle openings, and the performance even increased slightly at medium throttle openings. For throttle openings larger than 60%, the performances of power and economy degraded. At 75% throttle opening, the brake power decreased by about 5%, and the BSFC increased by about 10% when the engine was fueled with the bio-jet fuel.
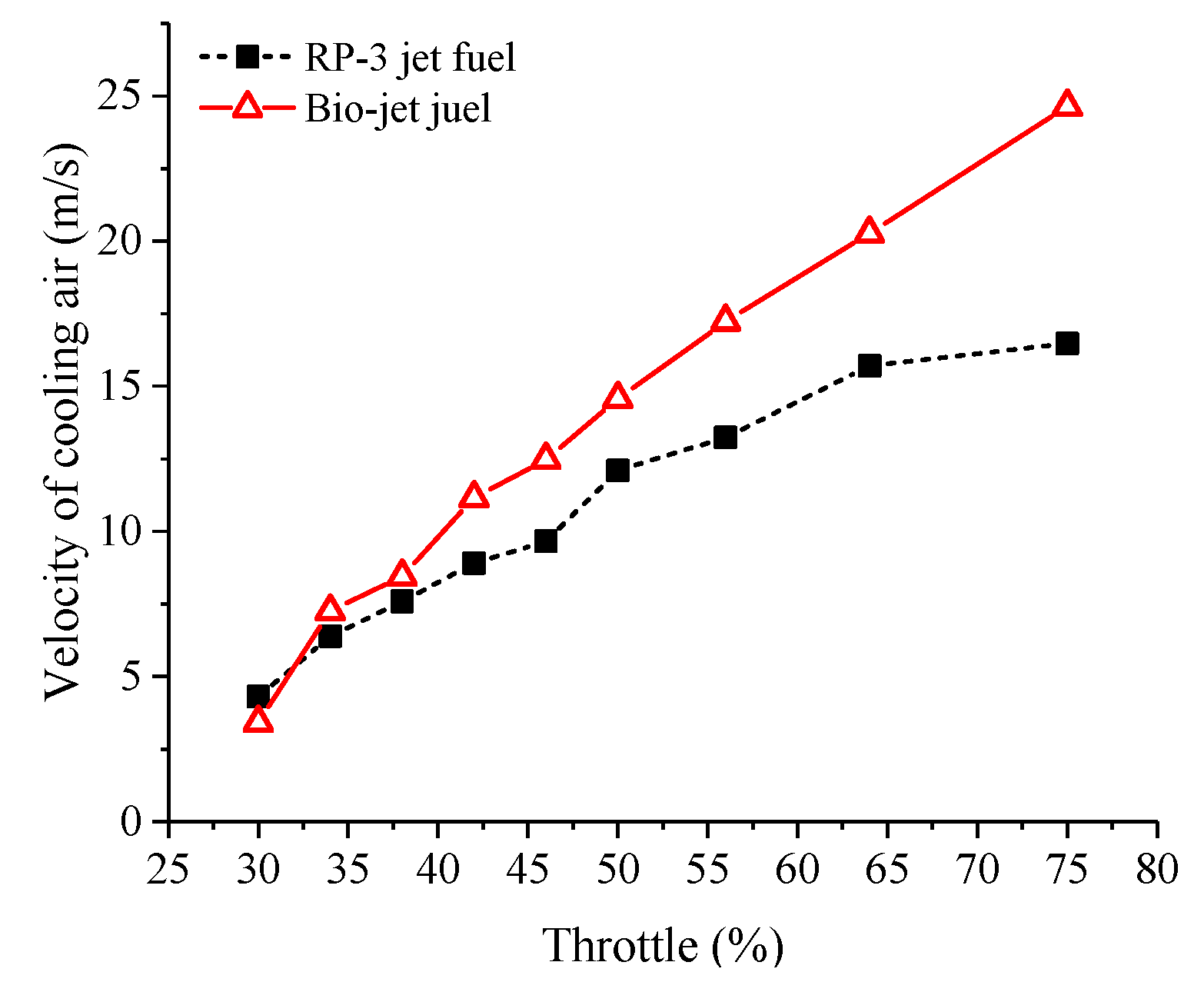
- The bio-jet fuel is more prone to spontaneous combustion than RP-3 jet fuel. Spontaneous combustion helped to increase the maximum cylinder pressure and the power at medium throttle openings but led to knock combustion and thus decreased the performances of power and economy at large throttle openings. Spontaneous combustion also increased heat release and then increased the cylinder temperature, so larger cooling air flux was required when the engine was fueled with the bio-jet fuel.
- Burning the bio-jet fuel leads to higher HC emissions than burning the RP-3 jet fuel. The emissions of CO and NOx from burning the bio-jet fuel were close to those from burning RP-3 jet fuel at small and medium throttle openings, but for throttle openings larger than 50%, burning the bio-jet fuel significantly increased the NOx emissions and decreased the CO emissions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiong, M.C.; Chong, C.T.; Ng, J.; Lam, S.S.; Tran, M.; Chong, W.W.F.; Jaafar, M.N.M.; Valera-Medina, A. Liquid biofuels production and emissions performance in gas turbines: A review. Energy Convers. Manag. 2018, 173, 640–658. [Google Scholar] [CrossRef] [Green Version]
- Nam, V.D.; Lim, M.T.; Lim, O. Study on auto-ignition characteristics of gasoline-biodiesel blend fuel in a rapid compression expansion machine. Energy Procedia 2017, 105, 1789–1795. [Google Scholar] [CrossRef]
- Putrasari, Y.; Lim, O. A study on combustion and emission of GCI engines fueled with gasoline-biodiesel blends. Fuel 2017, 189, 141–154. [Google Scholar] [CrossRef]
- Adams, C.A.; Loeper, P.; Krieger, R.; Andrie, M.J.; Foster, D.E. Effects of biodiesel–gasoline blends on gasoline direct-injection compression ignition (GCI) combustion. Fuel 2013, 111, 784–790. [Google Scholar] [CrossRef]
- Subramaniam, M.; Solomon, J.M.; Nadanakumar, V.; Anaimuthu, S.; Sathyamurthy, R. Experimental investigation on performance, combustion and emission characteristics of DI diesel engine using algae as a biodiesel. Energy Rep. 2020, 6, 1382–1392. [Google Scholar] [CrossRef]
- Datta, A.; Mandal, B.K. Numerical prediction of the performance, combustion and emission characteristics of a CI engine using different biodiesels. Clean. Technol. Environ. 2018, 8, 1773–1790. [Google Scholar] [CrossRef]
- Muralidharan, K.; Vasudevan, D. Performance, emission and combustion characteristics of a variable compression ratio engine using methyl esters of waste cooking oil and diesel blends. Appl. Energy 2011, 88, 3959–3968. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernández, J.; Agudelo, J.R. Diesel particulate emissions from used cooking oil biodiesel. Bioresour. Technol. 2008, 99, 731–740. [Google Scholar] [CrossRef]
- Xuan, T.; Cao, J.; He, Z.; Wang, Q.; Zhong, W.; Leng, X.; Li, D.; Shang, W. A study of soot quantification in diesel flame with hydrogenated catalytic biodiesel in a constant volume combustion chamber. Energy 2018, 145, 691–699. [Google Scholar] [CrossRef]
- Derick, A.; Mei, D.; Zuo, L.; Zhang, Q.; Wang, J. A review on partial hydrogenation of biodiesel and its influence on fuel property. Fuel 2019, 251, 660–668. [Google Scholar]
- Ilya, G.; Ritvik, S.; Xuesong, Z.; César, I.R.; L, G.K.; Philip, R.G. Sustainable bioenergy production from marginal lands in the US Midwest. Nature 2013, 493, 514–517. [Google Scholar]
- Zhan, L. Optimization Study of Combustion and Emission Characteristics for a Compression Ignition Engine Fueled by Gasoline/Hydrogenated Catalytic Biodiesel Blends. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2020. [Google Scholar]
- Cremonez, P.A.; Feroldi, M.; de Araújo, A.V.; Borges, M.N.; Meier, T.W.; Feiden, A.; Teleken, J.G. Biofuels in Brazilian aviation: Current scenario and prospects. Renew. Sustain. Energy Rev. 2015, 43, 1063–1072. [Google Scholar] [CrossRef]
- Liu, G.; Shen, H.; Qu, H.; Zhang, X.; Mi, Z. Chemical composition-property relation of jet fuels. J. Fuel Chem. Technol. 2007, 6, 737–742. [Google Scholar]
- Chu, P.L.; Vanderghem, C.; MacLean, H.L.; Saville, B.A. Process modeling of hydrodeoxygenation to produce renewable jet fuel and other hydrocarbon fuels. Fuel 2017, 196, 298–305. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Q.; Guan, Q.; He, L.; Li, W. Bio-aviation fuel production from hydroprocessing castor oil promoted by the nickel-based bifunctional catalysts. Bioresour. Technol. 2015, 183, 93–100. [Google Scholar] [CrossRef]
- Wang, T.; Li, K.; Liu, Q.; Zhang, Q.; Qiu, S.; Long, J.; Chen, L.; Ma, L.; Zhang, Q. Aviation fuel synthesis by catalytic conversion of biomass hydrolysate in aqueous phase. Appl. Energy 2014, 136, 775–780. [Google Scholar]
- Zhang, C.; Hui, X.; Lin, Y.; Sung, C. Recent development in studies of alternative jet fuel combustion: Progress, challenges, and opportunities. Renew. Sustain. Energy Rev. 2016, 54, 120–138. [Google Scholar] [CrossRef] [Green Version]
- Bester, N.; Yates, A. Assessment of the operational performance of Fischer-Tropsch synthetic-paraffinic kerosene in a T63 gas turbine compared to conventional jet A-1 fuel. In Proceedings of the ASME Turbo Expo 2009: Power for Land, Sea and Air, Orlando, FL, USA, 8–12 June 2009. [Google Scholar]
- Bulzan, D.; Anderson, B.; Wey, C.; Howard, R.; Winstead, E.; Beyersdorf, A.; Et, A. Gaseous and particulate emissions results of the NASA Alternative Aviation Fuel Experiment (AAFEX). In Proceedings of the ASME Turbo Expo 2010: Power for Land, Sea and Air, Glasgow, Scotland, 14–18 June 2010. [Google Scholar]
- Klingshirn, C.D.; Dewitt, M.J.; Striebich, R.; Anneken, D.; Brigalli, D. Hydroprocessed renewable jet fuel evaluation, performance, and emissions in a T63 turbine engine. In Proceedings of the ASME Turbo Expo 2011: Turbine Technical Conference & Exposition, Vancouver, BC, Canada, 6–10 June 2011. [Google Scholar]
- Khandelwal, B.; Roy, S.; Lord, C.; Blakey, S. Comparison of vibrations and emissions of conventional jet fuel with stressed 100% SPK and fully formulated synthetic jet fuel. Aerospace 2014, 1, 52–66. [Google Scholar] [CrossRef]
- Badami, M.; Nuccio, P.; Pastrone, D.; Signoretto, A. Performance of a small-scale turbojet engine fed with traditional and alternative fuels. Energy Convers. Manag. 2014, 82, 219–228. [Google Scholar] [CrossRef]
- Xu, J.; Li, F.; Jiang, J.; Liu, P.; Zhai, Q.; Wang, F. A Method of Preparing Bio-Jet Fuel via Thermochemical Conversion of Triglyceride. China Patent CN201711052081.X, 27 March 2018. [Google Scholar]
- Li, F.; Jiang, J.; Liu, P.; Zhai, Q.; Wang, F.; Hse, C.; Xu, J. Catalytic cracking of triglycerides with a base catalyst and modification of pyrolytic oils for production of aviation fuels. Sustain. Energy Fuels 2018, 2, 1206–1215. [Google Scholar] [CrossRef]
- Li, F. Study on the Preparation of Aviation Hydrocarbon Fuel via Thermochemical Conversion of Triglycerides. Master’s Thesis, Chinese Academy of Forestry, Nanjing, China, 2018. [Google Scholar]
- Xu, J.; Long, F.; Jiang, J.; Li, F.; Zhai, Q.; Wang, F.; Liu, P.; Li, J. Integrated catalytic conversion of waste triglycerides to liquid hydrocarbons for aviation biofuels. J. Clean. Prod. 2019, 222, 784–792. [Google Scholar] [CrossRef]
- Yang, F. Study on the Basic Characteristics of Bio-Aviation Kerosene. Master’s Thesis, Nanjing University of Aeronautics and Astronautics, Nanjing, China, 2019. [Google Scholar]
- Guo, L.; Hao, K.; Wen, Y.; Wang, D.; Fu, Z.; Xu, Z. Chemical composition analysis of commercial jet fuel. Chin. J. Anal. Lab. 2021, 3, 330–335. [Google Scholar]
- Zhang, L. Study on the Quantitative Mapping Relationship from Fuel Structure Molecular to Combustion Characteristic Parameters. Master’s Thesis, Jilin University, Changchun, China, 2019. [Google Scholar]
- Rui, L.; Sheng, J.; Ma, J.; Yang, G.; Dong, X.; Liang, Y. Knock combustion investigation on a two-stroke spark ignition UAV engine burning RP-3 kerosene fuel. Aircr. Eng. Aerosp. Technol. 2019, 10, 1278–1284. [Google Scholar]
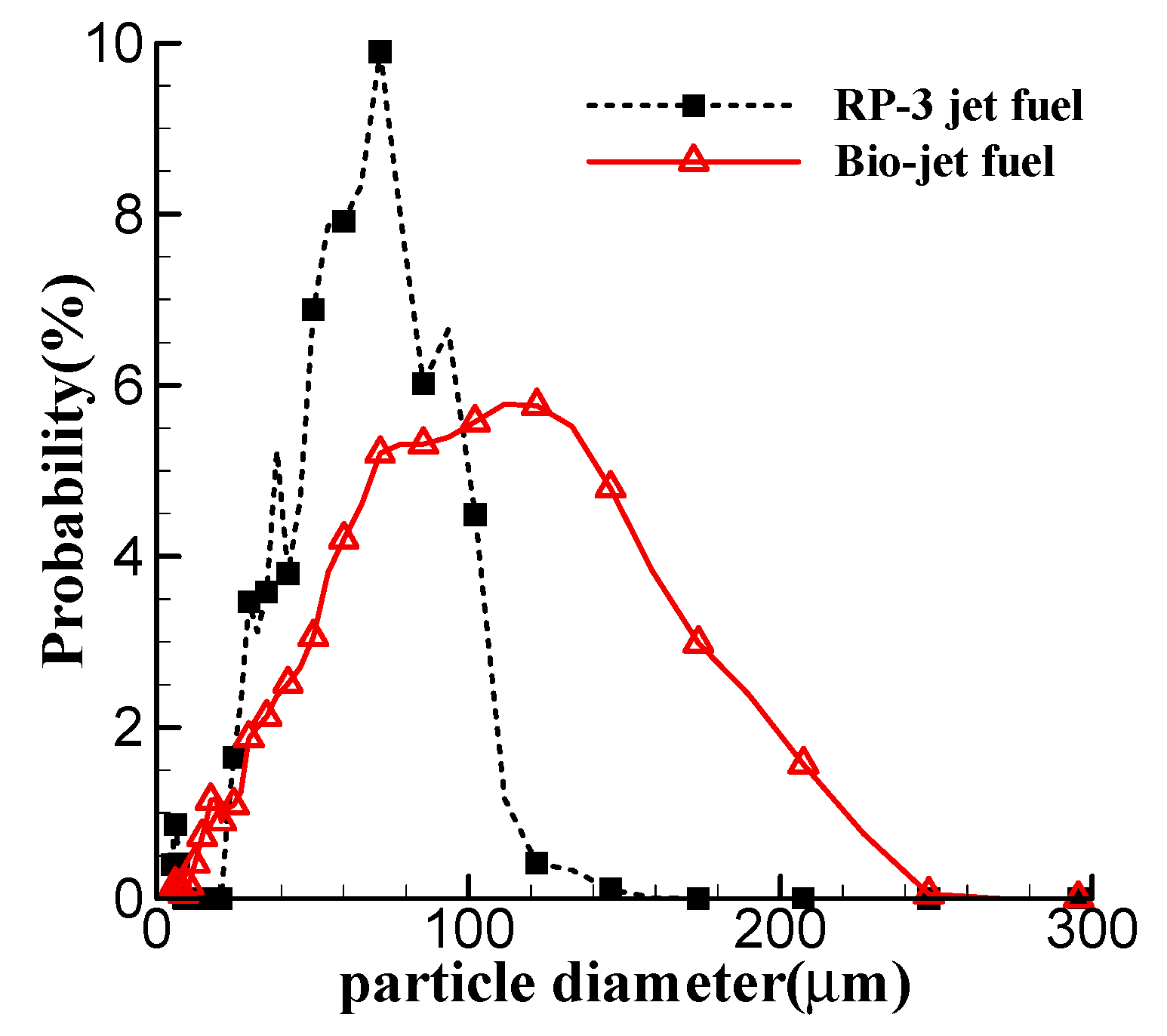
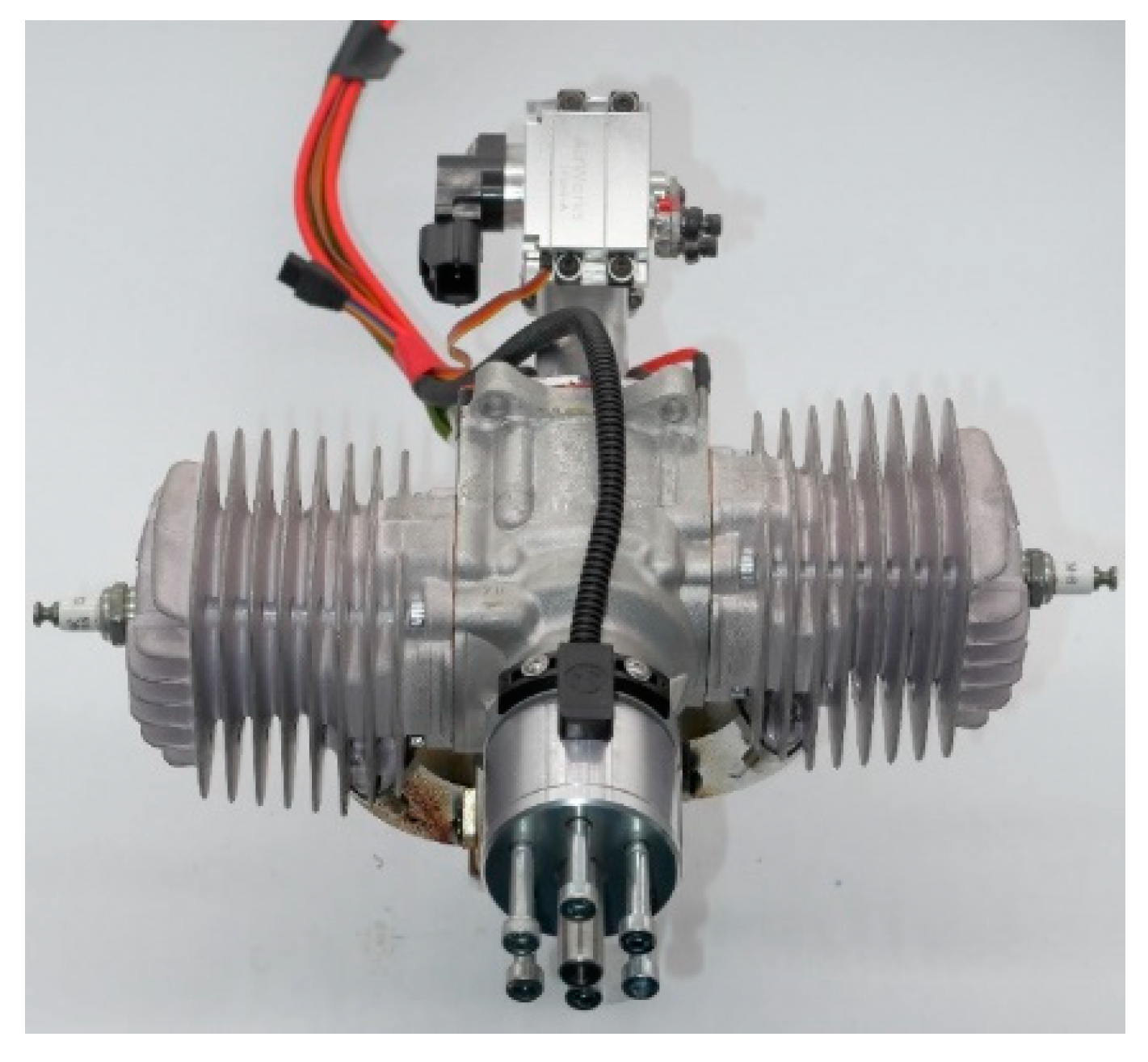


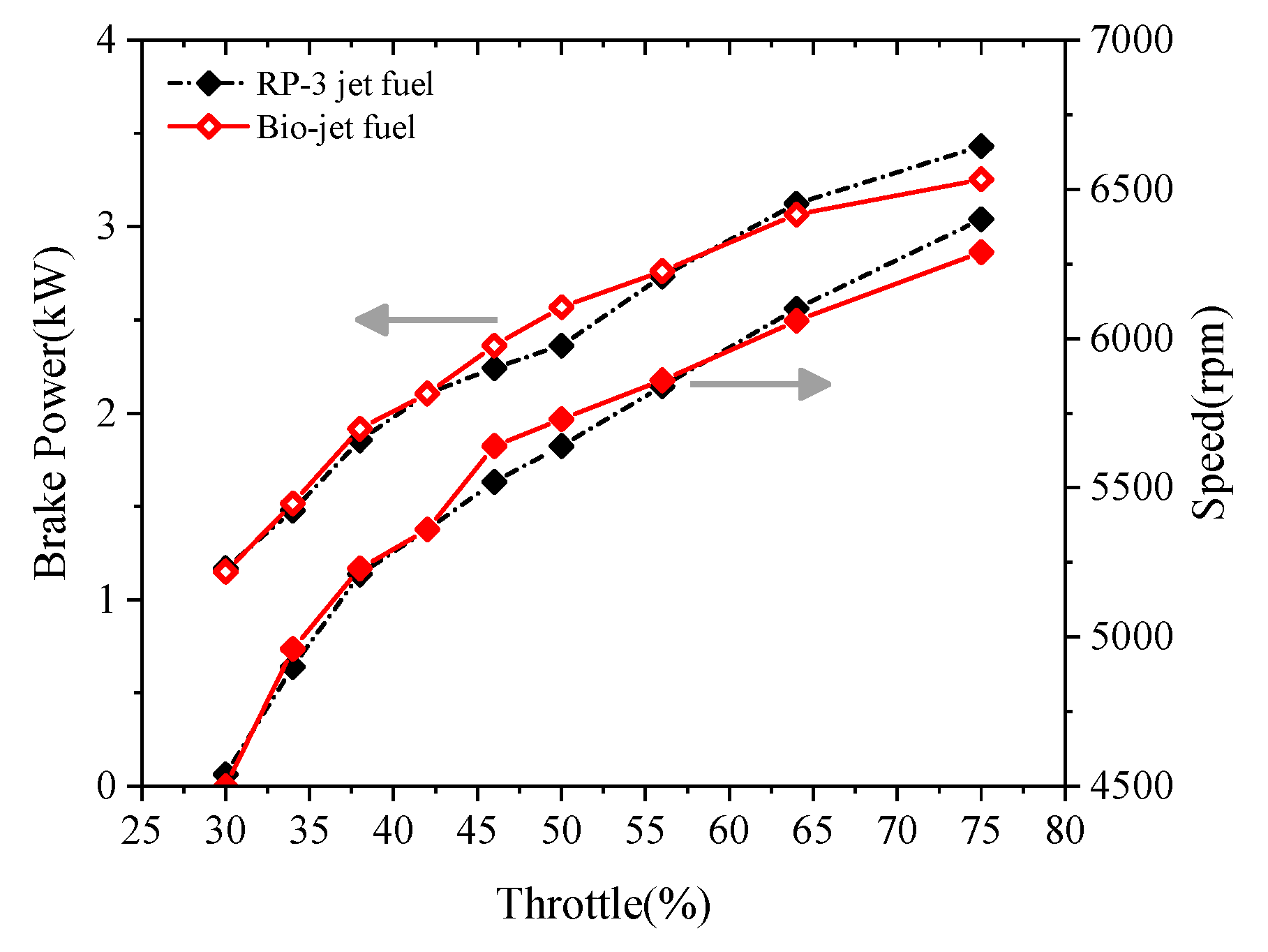
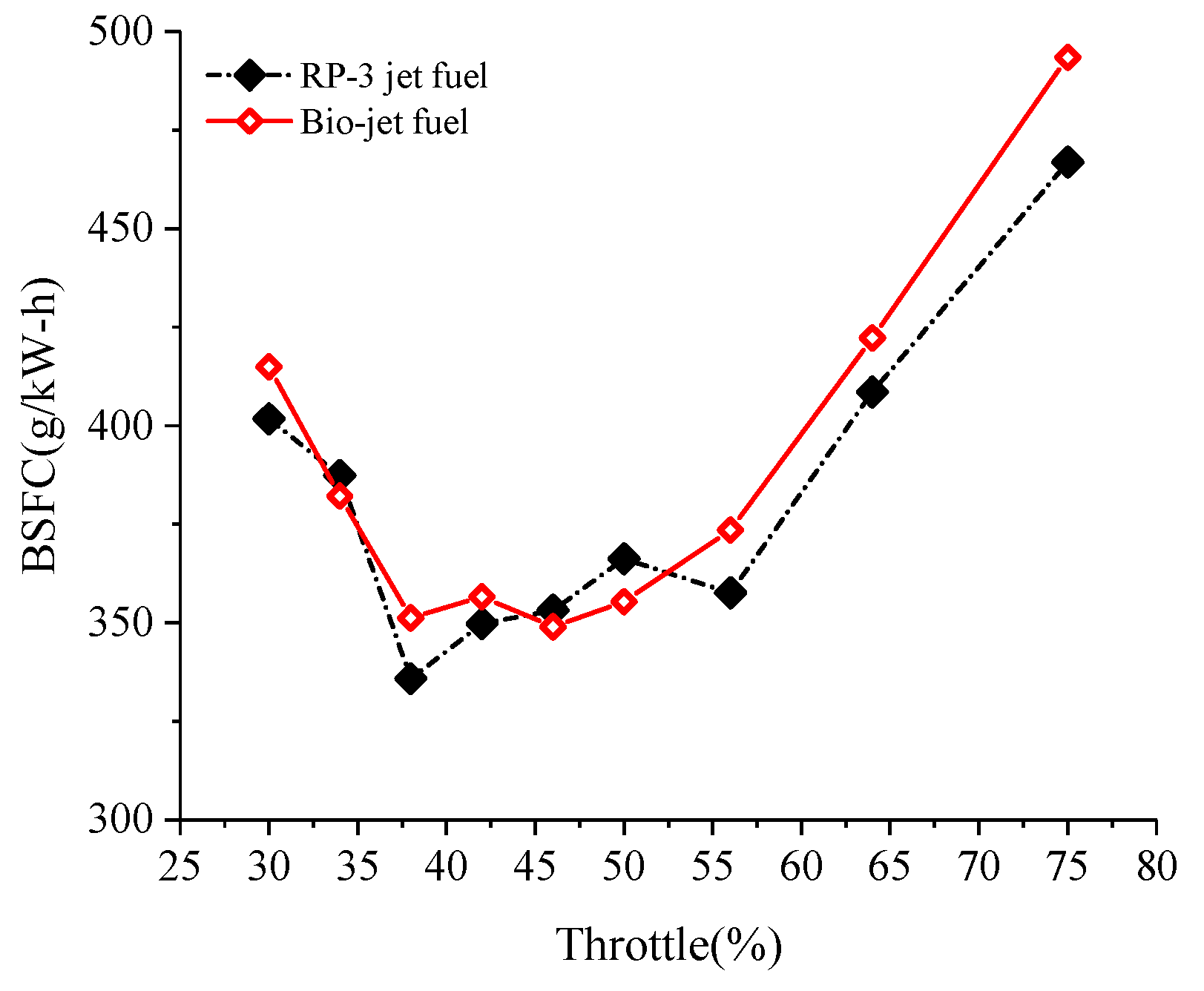
| Properties | Bio-Jet Fuel | Standard Limit of RP-3 Jet Fuel |
|---|---|---|
| Density/kg·m−3 | 808 | 775~830 |
| Heat value/MJ·kg−1 | 44.4 | >42.8 |
| Viscosity/mm2·s−1 | 2.11 | ≥1.25 |
| Acid value/mg KOH·g−1 | 0 | <0.015 |
| Freezing point/°C | −48 | ≤−47 |
| Sulphur content/% | <0.0001 | ≤0.2 |
| Closed-cup Flash Point/°C | 38 | ≥38 |
| Parameters | Values |
|---|---|
| Rotating speed | 3000 rpm~7000 rpm |
| Rated power | 3.6 kW |
| Compression ratio | 10.2:1 |
| Displacement | 75 cc |
| Assembled propeller | JXF 22 × 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Luo, L.; Chen, W.; Yang, F.; Luo, G.; Xu, J. Experimental Investigation on the Performance of an Aviation Piston Engine Fueled with Bio-Jet Fuel Prepared via Thermochemical Conversion of Triglyceride. Energies 2022, 15, 3246. https://doi.org/10.3390/en15093246
Zhang C, Luo L, Chen W, Yang F, Luo G, Xu J. Experimental Investigation on the Performance of an Aviation Piston Engine Fueled with Bio-Jet Fuel Prepared via Thermochemical Conversion of Triglyceride. Energies. 2022; 15(9):3246. https://doi.org/10.3390/en15093246
Chicago/Turabian StyleZhang, Chen, Lei Luo, Wei Chen, Fei Yang, Gang Luo, and Junming Xu. 2022. "Experimental Investigation on the Performance of an Aviation Piston Engine Fueled with Bio-Jet Fuel Prepared via Thermochemical Conversion of Triglyceride" Energies 15, no. 9: 3246. https://doi.org/10.3390/en15093246
APA StyleZhang, C., Luo, L., Chen, W., Yang, F., Luo, G., & Xu, J. (2022). Experimental Investigation on the Performance of an Aviation Piston Engine Fueled with Bio-Jet Fuel Prepared via Thermochemical Conversion of Triglyceride. Energies, 15(9), 3246. https://doi.org/10.3390/en15093246






