Safety Considerations of Hydrogen Application in Shipping in Comparison to LNG
Abstract
:1. Introduction
Definitions of Safety, Hazard and Risk
2. Materials and Methods
3. Results
3.1. Properties of Hydrogen Related to Safety
3.2. Hazards Arising from Hydrogen
3.2.1. Hydrogen in General
3.2.2. Pressurized Hydrogen Storage in Vessels
3.2.3. Storage as Liquefied Hydrogen at Cryogenic Temperature
3.2.4. Cryo-Compressed Storage
3.2.5. Chemical Hydrogen Storage as a Metal Hydride
3.2.6. Chemical Hydrogen Storage as a Liquid Organic Hydrogen Carrier
3.3. Rules and Regulations
Rules and Regulations by the International Maritime Organization
3.4. Ongoing Hydrogen Projects in Shipping
3.4.1. Suiso Frontier
3.4.2. Elektra
3.4.3. Hydra
3.4.4. Topeka
3.4.5. LH2 FGSS (MAN Cryo)
3.5. Proposal of a Method for the Alternative Design Approach
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BinSchUO | Binnen-Schiffsuntersuchungsordnung |
| BLEVE | boiling liquid expanding vapor explosion |
| CESNI | European Committee for Drawing up Standards in the Field of Inland Navigation |
| CO | carbon monoxide |
| CO2 | carbon dioxide |
| DBT | dibenzyltoluene |
| DSB | Direktoratet for samfunnssikkerhet og beredskap (Norwegian Maritime Directorate) |
| EU | European Union |
| FPSO | Floating Production, Storage and Offloading |
| GHG | greenhouse gas |
| H2 | hydrogen |
| HAZID | Hazard Identification Study |
| HySTRA | Hydrogen Energy Supply Chain Technology Research Association |
| IEC | International Electrotechnical Commission |
| IGC Code | International Code for the Construction and Equipment of Ships Carrying Liquefied Gases in Bulk |
| IGF Code | International Code for Safety for Ships Using Gases or Other Low-Flashpoint Fuels |
| IMO | International Maritime Organization |
| IR | infrared |
| ISO | International Organization for Standardization |
| KHI | Kawasaki Heavy Industries |
| LEL | lower explosive limit |
| LFL | lower flammability limit |
| LH2 | liquified hydrogen |
| LH2 FGSS | liquid-hydrogen fuel-gas-supply systems |
| LNG | liquified natural gas |
| LOHC | liquid organic hydrogen carrier |
| MIR | multi-spectrum-infrared |
| MSC | Maritime Safety Committee |
| MSC104 | 104th Session of the Maritime Safety Committee |
| NOX | nitrogen oxide |
| PEM | proton exchange membrane |
| PRD | pressure relief device |
| RoRo | Roll on Roll off |
| SOLAS | International Convention of Safety of Life at Sea |
| TNT | trinitrotoluene |
| TPRD | thermally activated pressure relief device |
| UEL | upper explosive limit |
| UFL | upper flammability limit |
| UV | ultraviolet |
References
- Faber, J.; Hanayama, S.; Zhang, S.; Pereda, P.; Comer, B.; Hauerhof, E.; van der Loeff, W.S.; Smith, T.; Zhang, Y.; Kosaka, H.; et al. Fourth IMO GHG Study 2020: Full Report, London. 2021. Available online: https://wwwcdn.imo.org/localresources/en/OurWork/Environment/Documents/Fourth%20IMO%20GHG%20Study%202020%20-%20Full%20report%20and%20annexes.pdf (accessed on 7 December 2021).
- UNCTAD United Nations Conference on Trade and Development. Review of Maritime Transport 2019, New York, United States of America. 2019. Available online: https://unctad.org/system/files/official-document/rmt2019_en.pdf (accessed on 7 December 2021).
- International Maritime Organization (IMO) Resolution MEPC.304(72) Initial IMO Strategy on Reduction of GHG Emissions from Ships: MEPC.304(72), 2018. Available online: https://wwwcdn.imo.org/localresources/en/KnowledgeCentre/IndexofIMOResolutions/MEPCDocuments/MEPC.304(72).pdf (accessed on 10 March 2022).
- Wallner, T.; Lohse-Busch, H.; Shidore, N. Operating strategy for a hydrogen engine for improved drive-cycle efficiency and emissions behavior. Int. J. Hydrogen Energy 2009, 34, 4617–4625. [Google Scholar] [CrossRef]
- International Maritime Organization (IMO) Guidelines for the Approval of Alternatives and Equivalents as Provided for in Various IMO Instruments: MSC.1/Circ.1455, 2013. Available online: https://puc.overheid.nl/nsi/doc/PUC_2017_14/1/ (accessed on 10 March 2022).
- International Maritime Organization (IMO) Revised Guidelines on Alternative Design and Arrangements for SOLAS Chapters II-1 and III: MSC.1/Circ.1212/Rev.1, 2019. Available online: https://puc.overheid.nl/nsi/doc/PUC_1648_14/1/ (accessed on 10 March 2022).
- McGuinness, E.; Utne, I.B. A systems engineering approach to implementation of safety management systems in the Norwegian fishing fleet. Reliab. Eng. Syst. Saf. 2014, 121, 221–239. [Google Scholar] [CrossRef]
- ISO International Organization for Standardization. Safety Aspects—Guidelines for Their Inclusion in Standards, 2014 (ISO/IEC Guide 51:2014). Available online: https://www.iso.org/obp/ui/#iso:std:iso-iec:guide:51:ed-3:v1:en (accessed on 15 March 2022).
- Engebø, A.; Nilsen, S.; Kirchsteiger, C.; Perrette, L.; Merad, M.; Andersen, H.B. Biennal Report On Hydrogen Safety: Chapter IV: Risk Assessment, 2007. Available online: http://www.hysafe.org/download/1199/BRHS_Chap4_V1p2.pdf (accessed on 15 March 2022).
- Klebanoff, L.E.; Pratt, J.W.; LaFleur, C.B. Comparison of the safety-related physical and combustion properties of liquid hy-drogen and liquid natural gas in the context of the SF-BREEZE high-speed fuel-cell ferry. Int. J. Hydrogen Energy 2017, 42, 757–774. [Google Scholar] [CrossRef] [Green Version]
- Saaty, T.L. Decision making with the analytic hierarchy process. Int. J. Serv. Sci. 2008, 1, 83–98. [Google Scholar] [CrossRef] [Green Version]
- Feldhusen, J.; Grote, K.-H. (Eds.) Konstruktionslehre: Methoden und Anwendung Erfolgreicher Produktentwicklung, 8. Vollständig überarbeitete Auflage; Springer Vieweg: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-29569-0. [Google Scholar]
- Zadeh, L.A. Fuzzy sets. Inf. Control 1965, 1965, 338–353. [Google Scholar] [CrossRef] [Green Version]
- Zadeh, L.A. Quantitative fuzzy semantics. Inf. Sci. 1971, 3, 159–176. [Google Scholar] [CrossRef]
- Zadeh, L.A. The concept of a linguistic variable and its application to approximate reasoning—I. Inf. Sci. 1975, 8, 199–249. [Google Scholar] [CrossRef]
- Ehlers, U.C.; Ryeng, E.O.; McCormack, E.; Khan, F.; Ehlers, S. Assessing the safety effects of cooperative intelligent transport systems: A bowtie analysis approach. Accid. Anal. Prev. 2017, 99, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Khan, F.; Shaikh, A.; Ahmed, S.; Imtiaz, S. Development of risk model for marine logistics support to offshore oil and gas operations in remote and harsh environments. Ocean Eng. 2019, 174, 125–134. [Google Scholar] [CrossRef]
- van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815–843. [Google Scholar] [CrossRef]
- EIGA European Industrial Gases Association AISBL. Gaseous Hydrogen Installations: EIGA Doc 15/21 15/21, Brussels, 2021. Available online: https://www.eiga.eu/uploads/documents/DOC015.pdf (accessed on 23 November 2021).
- EIGA European Industrial Gases Association AISBL. Safety in Storage, Handling and Distribution of Liquid Hydrogen: Doc 06/19 06/19, Brussels, 2019. Available online: https://www.eiga.eu/uploads/documents/DOC006.pdf (accessed on 23 November 2021).
- Verfondern, K. Biennal Report on Hydrogen Safety: Chapter I: Hydrogen Fundamentals, 2007. Available online: http://www.hysafe.org/download/1196/BRHS_Chap1_V1p2.pdf (accessed on 7 December 2021).
- Verfondern, K. Safety Considerations on Liquid Hydrogen; Forschungszentrum Zentralbibliothek: Jülich, Germany, 2008; ISBN 978-3-89336-530-2. [Google Scholar]
- Raucci, C.; Calleya, J.; Suárez de la Fuente, S.; Pawling, R. Hydrogen on board ship: A first analysis of key parameters and implications. In Proceedings of the International Conference on Shipping in Changing Climates 2015, Glasgow, UK, 24–26 November 2015. [Google Scholar]
- Aceves, S.M.; Petitpas, G.; Espinosa-Loza, F.; Matthews, M.J.; Ledesma-Orozco, E. Safe, long range, inexpensive and rapidly refuelable hydrogen vehicles with cryogenic pressure vessels. Int. J. Hydrogen Energy 2013, 38, 2480–2489. [Google Scholar] [CrossRef]
- Engineering ToolBox. Fuels-Higher and Lower Calorific Values: Higher and Lower Calorific Values (Heating Values) for Fuels Like Coke, Oil, Wood, Hydrogen and Others. Available online: https://www.engineeringtoolbox.com/fuels-higher-calorific-values-d_169.html (accessed on 16 December 2021).
- Balcombe, P.; Brierley, J.; Lewis, C.; Skatvedt, L.; Speirs, J.; Hawkes, A.; Staffell, I. How to decarbonise international shipping: Options for fuels, technologies and policies. Energy Convers. Manag. 2019, 182, 72–88. [Google Scholar] [CrossRef]
- Cheliotis, M.; Boulougouris, E.; Trivyza, N.L.; Theotokatos, G.; Livanos, G.; Mantalos, G.; Stubos, A.; Stamatakis, E.; Ve-netsanos, A. Review on the safe use of ammonia fuel cells in the maritime industry. Energies 2021, 14, 3023. [Google Scholar] [CrossRef]
- Müller, K.; Stark, K.; Emel’yanenko, V.N.; Varfolomeev, M.A.; Zaitsau, D.H.; Shoifet, E.; Schick, C.; Verevkin, S.P.; Arlt, W. Liquid organic hydrogen carriers: Thermophysical and thermochemical studies of benzyl- and dibenzyl-toluene derivatives. Ind. Eng. Chem. Res. 2015, 54, 7967–7976. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen storage for mobility: A review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [Green Version]
- Rigas, F. Hydrogen Safety, Online-Ausg; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2012; ISBN 978-1-4398-6232-2. [Google Scholar]
- SEA-LNG Ltd. LNG-Fuelled Vessels Approaching 30% of Orders. Available online: https://sea-lng.org/2021/09/lng-fuelled-vessels-approaching-30-of-orders/ (accessed on 30 November 2021).
- IFA Institute for Occupational Safety and Health of the German Social Accident Insurance. Methane: GESTIS-Substance Database. Available online: https://gestis.dguv.de/data?name=010000&lang=en (accessed on 23 December 2021).
- Drägerwerk AG & Co. KGaA. Hydrogen: How to Meet the Safety Challenges, 2021. Available online: https://www.draeger.com/Library/Content/hydrogen-safety-challenges-ebk-11064-en-master.pdf (accessed on 23 December 2021).
- HyResponder. Properties of Hydrogen Relevant to Safety: Lecture 2. Level IV Specialist Officer, 2021. Available online: https://hyresponder.eu/e-platform/training-materials/educational-training/lecture-2-properties-of-hydrogen-relevant-to-safety/ (accessed on 9 December 2021).
- IFA Institute for Occupational Safety and Health of the German Social Accident Insurance. Hydrogen: GESTIS-Substance Database. Available online: https://gestis.dguv.de/data?name=007010&lang=en (accessed on 3 December 2021).
- Boon-Brett, L.; Bousek, J.; Black, G.; Moretto, P.; Castello, P.; Hübert, T.; Banach, U. Identifying performance gaps in hydrogen safety sensor technology for automotive and stationary applications. Int. J. Hydrogen Energy 2010, 35, 373–384. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Buttner, W.J.; Post, M.B.; Burgess, R.; Rivkin, C. An overview of hydrogen safety sensors and requirements. Int. J. Hydrogen Energy 2011, 36, 2462–2470. [Google Scholar] [CrossRef]
- H2 MOBILITY Deutschland GmbH & Co. KG. FAQs. Available online: https://h2-mobility.de/en/faq/ (accessed on 27 December 2021).
- BEHALA Berliner Hafen- und Lagerhausgesellschaft mbH. Versorgung der “ELEKTRA” mit grünem Wasserstoff bis Ende 2024 vertraglich gesichert, 2021. Available online: https://www.behala.de/wp-content/uploads/2021/07/PM_ELEKTRA_Wasserstoffversorgung_14_07_2021.pdf (accessed on 12 January 2022).
- Weyandt, N.; Janssens, M.L. Analysis of Induced Catastrophic Failure of a 5000 PSIG Type IV Hydrogen Cylinder: Final Report, San Antonio, Texas, USA, 2005. Available online: https://www.mvfri.org/contracts/Final%20Reports/SwRI%20Final%20Type%203%20H2%20Tank%20Test.pdf (accessed on 13 December 2021).
- HyResponder. Hydrogen Storage: Lecture 3. Level IV Specialist Officer, 2021. Available online: https://hyresponder.eu/e-platform/training-materials/educational-training/lecture-3-hydrogen-storage/ (accessed on 14 December 2021).
- Zalosh, R. Blast Waves and Fireballs Generated by Hydrogen Fuel Tank Rupture During Fire Exposure, Wellesley, MA, USA, 2007. Available online: https://www.mvfri.org/contracts/Final%20Reports/Zalosh-HydrogenTankFirePaper.pdf (accessed on 14 December 2021).
- Dryer, F.L.; Chaos, M.; Zhao, Z.; Stein, J.N.; Alpert, J.Y.; Homer, C.J. Spontaneous ignition of pressur-ized releases of hydrogen and natural gas into air. Combust. Sci. Technol. 2007, 179, 663–694. [Google Scholar] [CrossRef]
- Mogi, T.; Wada, Y.; Ogata, Y.; Koichi Hayashi, A. Self-ignition and flame propagation of high-pressure hydrogen jet during sudden discharge from a pipe. Int. J. Hydrogen Energy 2009, 34, 5810–5816. [Google Scholar] [CrossRef]
- Pagliaro, M.; Iulianelli, A. Hydrogen Refueling Stations: Safety and Sustainability. Gen. Chem. 2019, 6, 190029. [Google Scholar] [CrossRef]
- Venetsanos, A.; Benard, P.; Papanikolaou, E.; Verfondern, K.; Gallego, E.; Martin-Valdepeñas, J.; Jimenez, M. Biennal Report on Hydrogen Safety: Chapter III: Accidental Phenomena and Consequences, 2007. Available online: http://www.hysafe.org/download/1198/BRHS_Chap3_V1p2.pdf (accessed on 16 December 2021).
- Ustolin, F.; Paltrinieri, N.; Landucci, G. An innovative and comprehensive approach for the consequence analysis of liquid hydrogen vessel explosions. J. Loss Prev. Process Ind. 2020, 68, 104323. [Google Scholar] [CrossRef]
- Prichard, D.K.; Rattigan, W.M. Hazards of Liquid Hydrogen: Position Paper, 2010. Available online: https://www.hse.gov.uk/research/rrpdf/rr769.pdf (accessed on 22 December 2021).
- Aziz, M. Liquid hydrogen: A review on liquefaction, storage, transportation, and safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- IFA Institute for Occupational Safety and Health of the German Social Accident Insurance. Oxygen: GESTIS-Substance Da-tabase. Available online: https://gestis.dguv.de/data?name=007080&lang=en (accessed on 12 January 2022).
- Petitpas, G.; Aceves, S.M. Modeling of sudden hydrogen expansion from cryogenic pressure vessel failure. Int. J. Hydrogen Energy 2013, 38, 8190–8198. [Google Scholar] [CrossRef] [Green Version]
- Modi, P.; Aguey-Zinsou, K.-F. Room temperature metal hydrides for stationary and heat storage applications: A review. Front. Energy Res. 2021, 9, 616115. [Google Scholar] [CrossRef]
- Bellosta von Colbe, J.; Ares, J.-R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Krummrich, S. Fuel cell methanol reformer system for submarines. In Proceedings of the 18th World Hydrogen Energy Conference 2010—WHEC 2010, Essen, Germany, 16–21 May 2010; Forschungszentrum IEF-3: Jülich, Germany, 2010. ISBN 978-3-89336-653-8. [Google Scholar]
- Teichmann, D.; Arlt, W.; Wasserscheid, P.; Freymann, R. A future energy supply based on liquid organic hydrogen carriers (LOHC). Energy Environ. Sci. 2011, 4, 2767. [Google Scholar] [CrossRef]
- Brückner, N.; Obesser, K.; Bösmann, A.; Teichmann, D.; Arlt, W.; Dungs, J.; Wasserscheid, P. Evaluation of industrially applied heat-transfer fluids as liquid organic hydrogen carrier systems. ChemSusChem 2014, 7, 229–235. [Google Scholar] [CrossRef]
- IFA Institute for Occupational Safety and Health of the German Social Accident Insurance. Dibenzyltoluene, Isomers: GES-TIS-Substance Database. Available online: https://gestis.dguv.de/data?name=492182&lang=en (accessed on 12 January 2022).
- IFA Institute for Occupational Safety and Health of the German Social Accident Insurance. Diesel Fuel: GESTIS-Substance Database. Available online: https://gestis.dguv.de/data?name=536303&lang=en (accessed on 12 January 2022).
- IMO International Maritime Organization. Introduction to IMO. Available online: https://www.imo.org/en/About/Pages/Default.aspx (accessed on 15 December 2021).
- IMO International Maritime Organization. IMO What It Is, London, UK, 2013. Available online: https://wwwcdn.imo.org/localresources/en/About/Documents/What%20it%20is%20Oct%202013_Web.pdf (accessed on 15 December 2021).
- Deutsche Flagge. BG Verkehr. Available online: https://www.deutsche-flagge.de/en/german-flag/flag-state/bg-verkehr/bg-verkehr?set_language=en (accessed on 23 December 2021).
- Deutsche Flagge. Classification Societies. Available online: https://www.deutsche-flagge.de/en/german-flag/flag-state/classification-societies-2/classification-societies?set_language=en (accessed on 23 December 2021).
- IMO International Maritime Organization. International Convention for the Safety of Life at Sea (SOLAS), 1974. Available online: https://www.imo.org/en/About/Conventions/Pages/International-Convention-for-the-Safety-of-Life-at-Sea-(SOLAS),-1974.aspx (accessed on 27 December 2021).
- IMO International Maritime Organization. Maritime Safety Committee (MSC), 104th Session, 4–8 October 2021. Available online: https://www.imo.org/en/MediaCentre/MeetingSummaries/Pages/MSC-104th-session.aspx (accessed on 27 December 2021).
- International Maritime Organization (IMO) International Code of Safety for Ships Using Gases or Other Low-Flashpoint Fuels: IGF-Code, 2015. Available online: https://www.imo.org/en/OurWork/Safety/Pages/IGF-Code.aspx (accessed on 10 March 2022).
- International Maritime Organization (IMO) International Code for the Construction and Equipment of Ships Carrying Liquefied Gases in Bulk: IGC-Code, 2014. Available online: https://www.imo.org/en/OurWork/Environment/Pages/IGCCode.aspx (accessed on 10 March 2022).
- International Maritime Organization (IMO) Resolution MSC.420(97) Interim Recommendations for Carriage of Liquefied Hydrogen in Bulk: MSC.420(97), 2016. Available online: https://wwwcdn.imo.org/localresources/en/KnowledgeCentre/IndexofIMOResolutions/MSCResolutions/MSC.420(97).pdf (accessed on 10 March 2022).
- FleetMon. Suiso Frontier. Available online: https://www.fleetmon.com/vessels/suiso-frontier_9860154_2165466/ (accessed on 24 January 2022).
- Kudou, J.; Ota, S.; Senga, Y. Safety requirements for liquedied hydrogen tankers. In Proceedings of the International Conference on Hydrogen Safety, Yokohama, Japan, 19–21 October 2015. [Google Scholar]
- ClassNK. ClassNK Adds Liquefied Hydrogen Carrier to Its Register: World’s First Liquefied Hydrogen Carrier Built by Kawasaki Heavy Industries; ClassNK: Tokyo, Japan, 2021; Available online: https://www.classnk.or.jp/hp/en/hp_news.aspx?id=6982&type=press_release (accessed on 25 January 2022).
- Kawasaki Heavy Industries, Ltd. Kawasaki Develops Cargo Containment System for Large Liquefied Hydrogen Carrier with World’s Highest Carrying Capacity—AiP Obtained from ClassNK; Kawasaki Heavy Industries, Ltd.: Tokyo, Japan, 2021; Available online: https://global.kawasaki.com/en/corp/newsroom/news/detail/?f=20210506_9983 (accessed on 24 January 2022).
- ShipInsight. ClassNK Gives AiP for Liquid Hydrogen Containment System. ShipInsight [Online], 10 May 2021. Available online: https://shipinsight.com/articles/classnk-gives-aip-for-liquid-hydrogen-cargo-containment-system (accessed on 24 January 2022).
- BEHALA Berliner Hafen- und Lagerhausgesellschaft mbH. The Push Boat with a Whole New Energy System: Lighthouse Project: ELEKTRA. Available online: https://www.behala.de/en/the-push-boat-with-a-whole-new-energy-system/ (accessed on 24 January 2022).
- Lichtfuß, K.-G. Erfolgreicher Stapellauf der “ELEKTRA” in Derben; BEHALA—Berliner Hafen- und Lagerhausgesellschaft mbH: Berlin, Germany, 2021; Available online: https://www.behala.de/wp-content/uploads/2021/05/BEHALA_PM_ELEKTRA_Stapellauf_27052021.pdf (accessed on 26 April 2022).
- Kräft, M.; Andreas, J.; Segieth, P. Vorzeige-Projekt für Wasserstoff-Technologie. Binnenschifffahrt [Online], 1 September 2020. Available online: https://binnenschifffahrt-online.de/2020/09/schiffstechnik/16744/ (accessed on 24 January 2022).
- Larsen, S.; Markussen, H.M. MF ‘Hydra’ Named Ship of The Year: Minister of Transport and Communications Knut Arild Hareide Hands Out This Year’s Ship of the Year on Board MF ‘Hydra’. Skipsrevyen [Online], 9 September 2021. Available online: https://www.skipsrevyen.no/article/mf-hydra-named-ship-of-the-year/ (accessed on 25 January 2022).
- Norway: MF “Hydra”, the World’s First Hydrogen Operated Ferry Wins Ship of the Year 2021. FuelCellWorks [Online], 28 De-cember 2021. Available online: https://fuelcellsworks.com/news/norway-mf-hydra-the-worlds-first-hydrogen-operated-ferry-wins-ship-of-the-year-2021/ (accessed on 25 January 2022).
- LMG Marin AS. Hydra. Available online: https://www.lmgmarin.no/references/485/hydra (accessed on 25 January 2022).
- Radowitz, B. World’s First Hydrogen-Powered Ferry in Norway to Run on Green Gas from Germany: Linde to Provide H2 from an Electrolyser at Facility in Leuna Chemicals Complex Despite Cheaper Power Prices in Norway. Recharge [Online], 9 March 2021. Available online: https://www.rechargenews.com/technology/worlds-first-hydrogen-powered-ferry-in-norway-to-run-on-green-gas-from-germany/2-1-976939 (accessed on 25 January 2022).
- Turner, J. HySHIP: Inside Europe’s Flagship Hydrogen Ship Demonstrator Project. Ship Technology [Online], 22 December 2020. Available online: https://www.ship-technology.com/features/hydrogen-vessel/ (accessed on 25 January 2022).
- Wilh. Wilhelmsen Holding ASA. Topeka’s Hydrogen Vessels One Step Closer to Reality, 2021. Available online: https://www.wilhelmsen.com/media-news-and-events/press-releases/2021/topekas-hydrogen-vessels-one-step-closer-to-reality/ (accessed on 25 January 2022).
- Søholt, N. MAN Cryo Announces Series of Hydrogen Projects: Company Takes Lead in Fuel-Gas-Supply Systems for Alternative Fuel with Great Potential; MAN Energy Solutions: Augsburg, Germany, 2020. [Google Scholar]
- MAN Energy Solutions. Hydrogen in Shipping; MAN Energy Solutions: Augsburg, Germany, 2021. [Google Scholar]
- Ferdous, R.; Khan, F.; Sadiq, R.; Amyotte, P.; Veitch, B. Handling and updating uncertain information in bow-tie analysis. J. Loss Prev. Process Ind. 2012, 25, 8–19. [Google Scholar] [CrossRef]
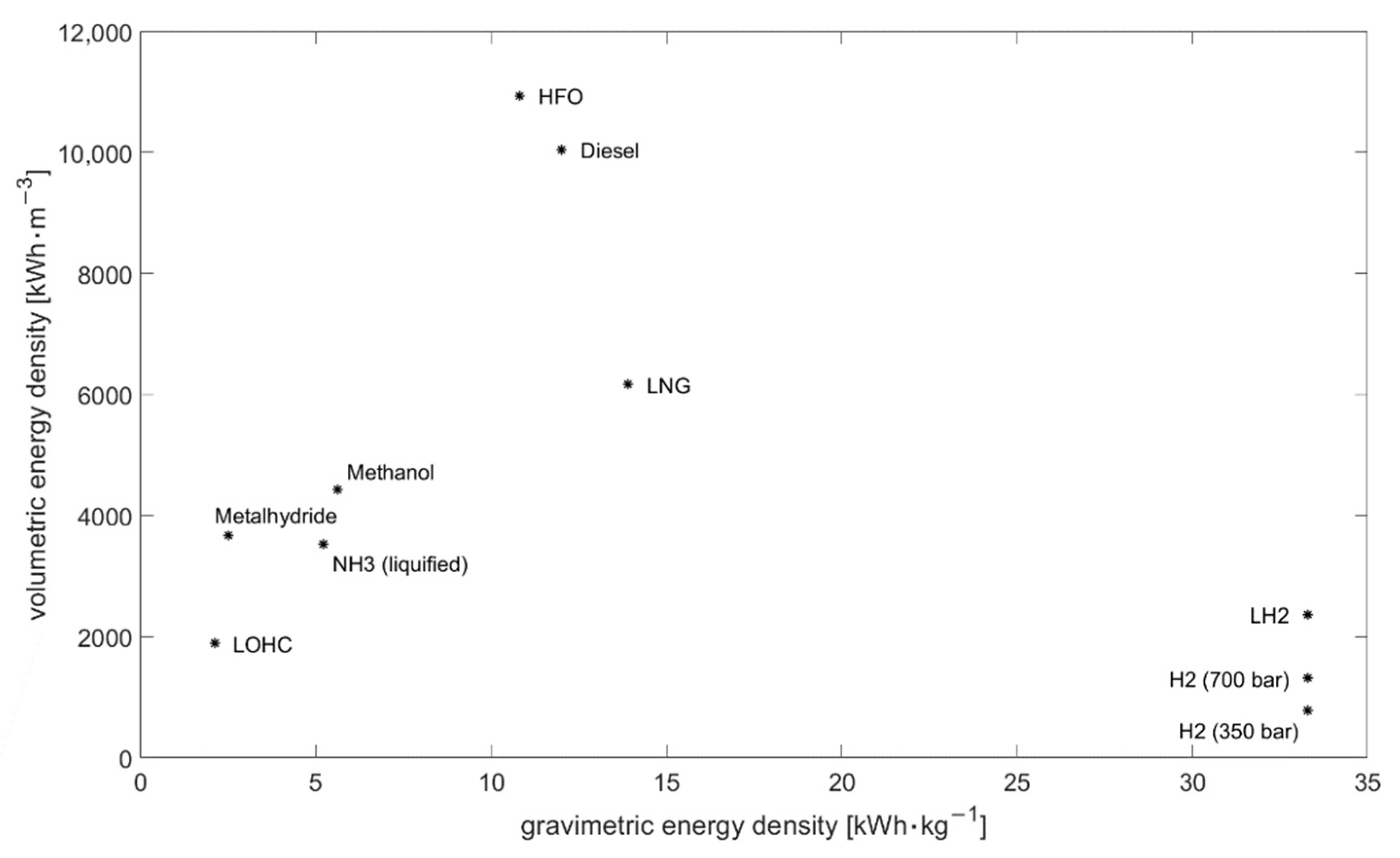





| Fuel | Volumetric Energy Density (kWh/m3) | Gravimetric Energy Density (kWh/kg) | Density (kg/m3) |
|---|---|---|---|
| Diesel | 10,044 | 12.0 [18] | 837 [18] |
| Heavy Fuel Oil | 10,938 | 10.8 [25] | 1010 [26] |
| Liquefied Natural Gas (111 K) | 6165 | 13.9 [18] | 443.5 [18] |
| Liquefied Ammonia (10 bar or 239 K) | 3528 [27] | 5.2 [18] | 678.5 |
| Hydrogen (350 bar) | 766 | 33.3 [22] | 23.3 [23] |
| Hydrogen (700 bar) | 1309 | 33.3 [22] | 39.3 [23] |
| Liquefied Hydrogen (20 K) | 2363 | 33.3 [22] | 70.96 [22] |
| Methanol | 4424 | 5.6 [18] | 790 [18] |
| Liquid Organic Hydrogen Carrier (here: Dibenzyltoluene) | 1886 | 2.1 | 913.4 [28] |
| Metalhydride (here: MgH2) | 3672 [29] | 2.5 | 1450 [29] |
| Chapter | Name | Location | Keywords |
|---|---|---|---|
| Section 3.4.1 | Suiso Frontier | Japan | operating tanker for 1250 m3 liq. hydrogen |
| Section 3.4.2 | Elektra | Germany | operating push boat for inland shipping |
| Section 3.4.3 | Hydra | Norway | operating liquid hydrogen ferry |
| Section 3.4.4 | Topeka | Norway | planned liq. hydrogen RoRo freighter |
| Section 3.4.5 | LH2 FGSS | Sweden | liq. hydrogen fuel-gas-supply systems |
| Aspect 1 | Aspect 2 | Aspect 3 | … | Aspect n | Σi | ||
|---|---|---|---|---|---|---|---|
| aspect 1 | 1 | W1,2 | W1,3 | … | W1,n | ||
| aspect 2 | W2,1 | 1 | W2,3 | … | W2,n | ||
| aspect 3 | W3,1 | W3,2 | 1 | … | W3,n | ||
| … | … | … | 1 | … | … | … | |
| aspect n | Wn,1 | Wn,2 | Wn,3 | … | 1 | ||
| Σ | n2 | 1 | |||||
| Novel System | Existing System | |||||||
|---|---|---|---|---|---|---|---|---|
| Criterion | Value | Evaluation | Value | Criterion | ||||
| “Significantly Safer” | “A Little Safer” | “Neutral” | “A Little Safer” | “Significantly Safer” | ||||
| criterion 1 | X | criterion 1 | ||||||
| … | … | |||||||
| criterion n | X | criterion n | ||||||
| Linguistic Statement | Lower Boundary | Most Likely Value | Upper Boundary |
|---|---|---|---|
| Significantly unsafer | 0 | 0.1 | 0.2 |
| A little unsafer | 0.175 | 0.275 | 0.375 |
| neutral | 0.35 | 0.5 | 0.65 |
| A little safer | 0.625 | 0.725 | 0.825 |
| Significantly safer | 0.8 | 0.9 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Depken, J.; Dyck, A.; Roß, L.; Ehlers, S. Safety Considerations of Hydrogen Application in Shipping in Comparison to LNG. Energies 2022, 15, 3250. https://doi.org/10.3390/en15093250
Depken J, Dyck A, Roß L, Ehlers S. Safety Considerations of Hydrogen Application in Shipping in Comparison to LNG. Energies. 2022; 15(9):3250. https://doi.org/10.3390/en15093250
Chicago/Turabian StyleDepken, Jorgen, Alexander Dyck, Lukas Roß, and Sören Ehlers. 2022. "Safety Considerations of Hydrogen Application in Shipping in Comparison to LNG" Energies 15, no. 9: 3250. https://doi.org/10.3390/en15093250
APA StyleDepken, J., Dyck, A., Roß, L., & Ehlers, S. (2022). Safety Considerations of Hydrogen Application in Shipping in Comparison to LNG. Energies, 15(9), 3250. https://doi.org/10.3390/en15093250







