Evaluation of Bi-Functional Electrochemical Catalytic Activity of Co3O4-CoFe2O4 Composite Spinel Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
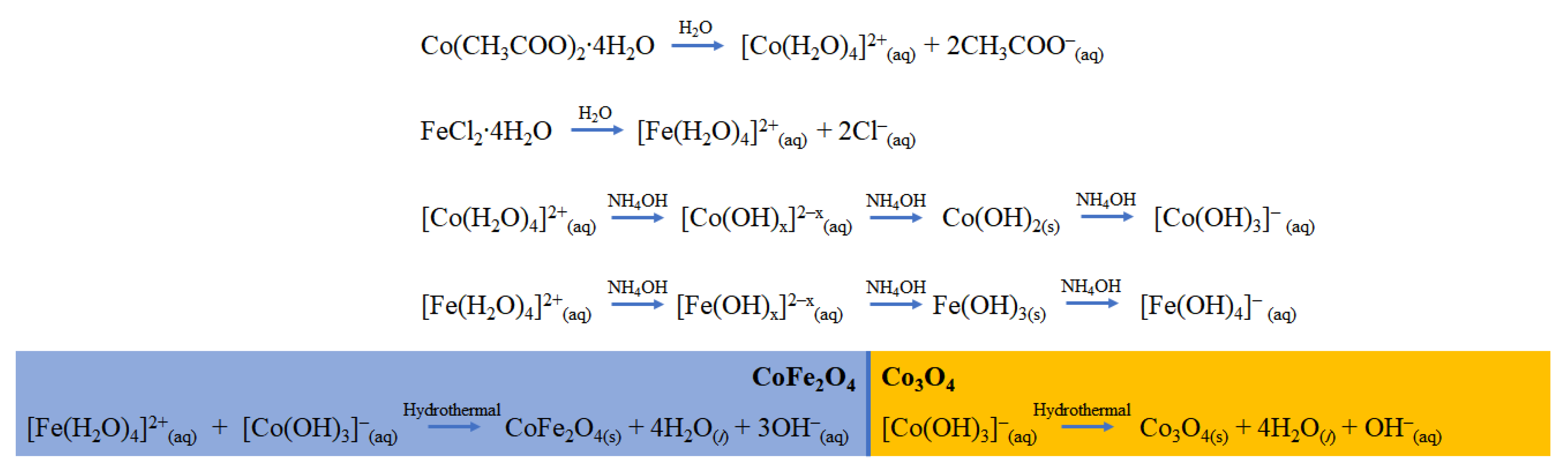
2.2. Synthesis of Composite Co3O4-CoFe2O4-X via Hydrothermal Method
2.3. Characterization
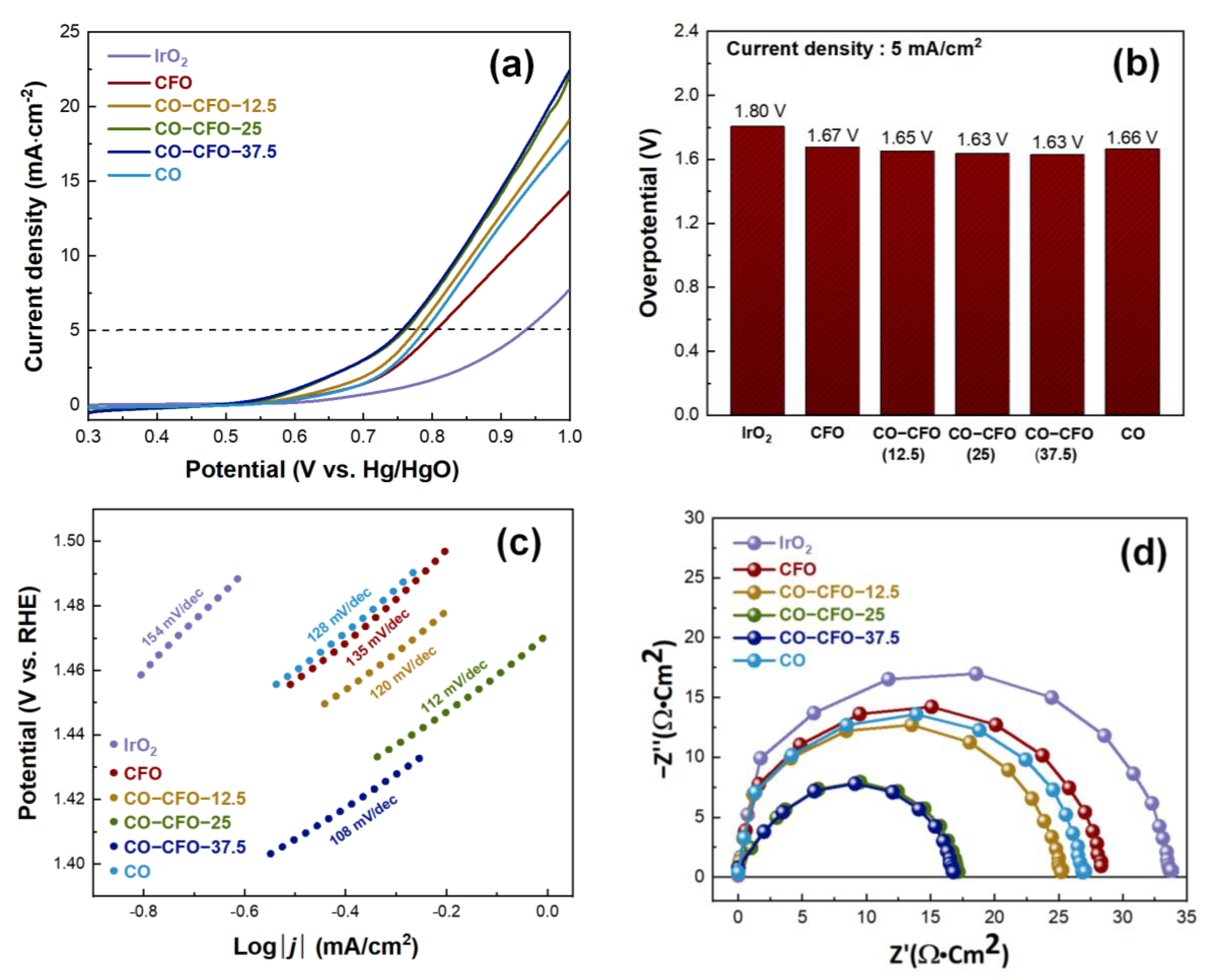
3. Results and Discussion
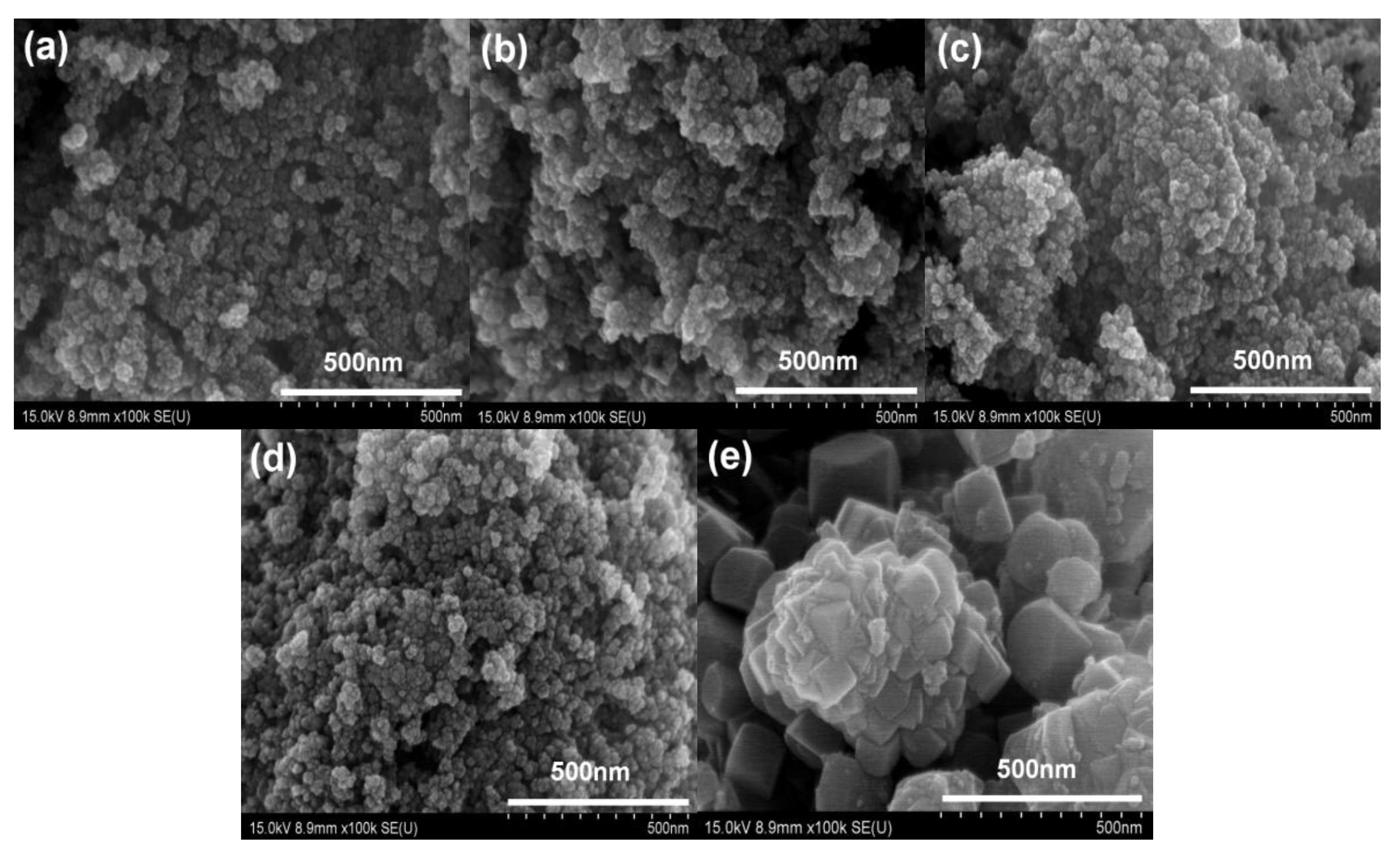
3.1. Characterization
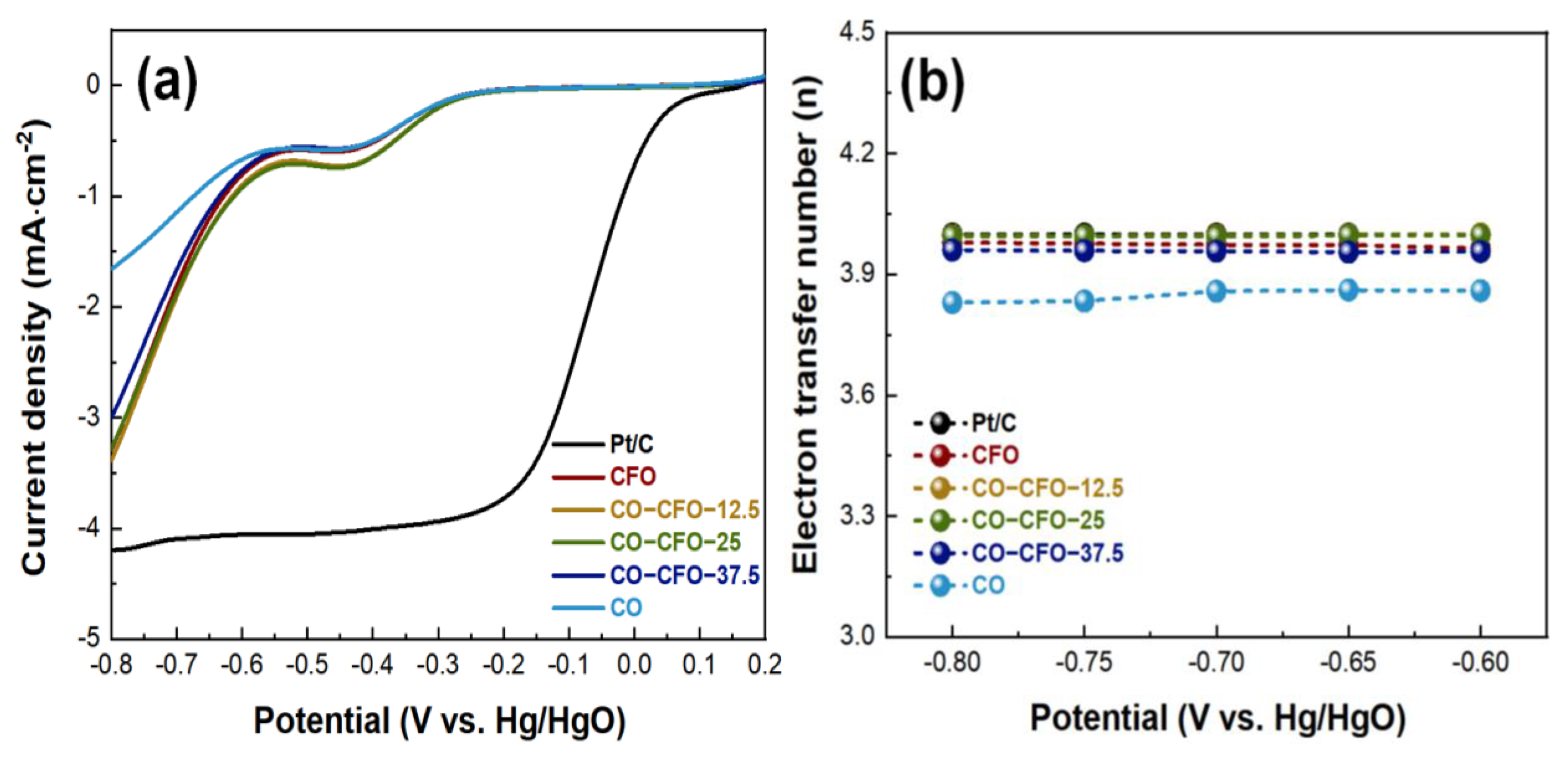
3.2. Electrochemical Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Xie, X.; Pan, L.; Bao, Z.; Cui, Y. Hybrid nanostructured materials for high-performance electrochemical capacitors. Nano Energy 2013, 2, 213–234. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Rausch, B.; Symes, M.D.; Chisholm, G.; Cronin, L. Decoupled catalytic hydrogen evolution from a molecular metal oxide redox mediator in water splitting. Science 2014, 345, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, Y.; Zhang, M.; Chen, Y. Nitrogen-doped graphene materials for supercapacitor applications. J. Nanosci. Nanotechnol. 2014, 14, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J.E.; Wang, H.; Dai, H. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 2013, 4, 1805. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Kim, S.T.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal-Air Batteries with High Energy Density: Li-Air versus Zn-Air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, J.; Yu, J.; Yu, H.; Wang, K.; Yang, X.; Li, J.; Li, W. Hierarchically porous N-doped carbon derived from biomass as oxygen reduction electrocatalyst for high-performance Al–air battery. J. Energy Chem. 2020, 45, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Sengodan, S.; Chol, S.; Jun, A.; Shin, T.H.; Ju, Y.W.; Jeong, H.Y.; Shin, J.; Irvine, J.T.S.; Kim, G.T. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 2015, 14, 205–209. [Google Scholar] [CrossRef]
- Ju, Y.W.; Eto, H.; Inagaki, T.; Ida, S.; Ishihara, T. Preparation of Ni-Fe bimetallic porous anode support for solid oxide fuel cells using LaGaO3 based electrolyte film with high power density. J. Power Sources 2010, 195, 6294–6300. [Google Scholar] [CrossRef]
- Wang, D.; Xia, Y.; Lv, H.; Miao, L.; Bi, L.; Liu, W. PrBaCo2−xTaxO5+δ based composite materials as cathodes for proton-conducting solid oxide fuel cells with high CO2 resistance. Int. J. Hydrogen Energy 2020, 45, 31017–31026. [Google Scholar] [CrossRef]
- Jahan, M.; Liu, Z.; Loh, K.P. A Graphene oxide and copper-centered metal organic framework composite as a tri-functional catalyst for HER, OER, and ORR. Adv. Funct. Mater. 2013, 23, 5363–5372. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef]
- Ge, X.; Liu, Y.; Goh, F.T.; Hor, T.A.; Zong, Y.; Xiao, P.; Zhang, Z.; Lim, S.H.; Li, B.; Wang, X.; et al. Dual-phase spinel MnCo2O4 and spinel MnCo2O4/nanocarbon hybrids for electrocatalytic oxygen reduction and evolution. ACS Appl. Mater. Interfaces 2014, 6, 12684–12691. [Google Scholar] [CrossRef]
- Vukmirovic, M.B.; Zhang, J.; Sasaki, K.; Nilekar, A.U.; Uribe, F.; Mavrikakis, M.; Adzic, R.R. Platinum monolayer electrocatalysts for oxygen reduction. Electrochim. Acta 2007, 52, 2257–2263. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, T.; Zhang, Y.; Du, J.; Han, X.; Chen, J. Enhancing electrocatalytic oxygen reduction on MnO2 with vacancies. Angew. Chem. Int. Ed. 2013, 52, 2474–2477. [Google Scholar] [CrossRef]
- Oh, T.; Park, D.; Kim, J. CoFe2O4 nanoparticles anchored on N/S co-doped mesoporous carbon spheres as efficient bifunctional electrocatalysts for oxygen catalytic reactions. Int. J. Hydrogen Energy 2019, 44, 2645–2655. [Google Scholar] [CrossRef]
- Bian, W.; Yang, Z.; Strasser, P.; Yang, R. A CoFe2O4/graphene nanohybrid as an efficient bi-functional electrocatalyst for oxygen reduction and oxygen evolution. J. Power Sources 2014, 250, 196–203. [Google Scholar] [CrossRef]
- Malko, D.; Kucernak, A.; Lopes, T. In situ electrochemical quantification of active sites in Fe-N/C non-precious metal catalyst. Nat. Commun. 2016, 7, 13285. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, C.; Pérez-Alonso, F.J.; Salam, M.A.; De La Fuente, J.L.G.; Al-Thabaiti, S.A.; Basahel, S.N.; Peña, M.A.; Fierro, J.L.G.; Rojas, S. Effect of transition metal (M: Fe, Co or Mn) for the oxygen reduction reaction with non-precious metal catalysts in acid medium. Int. J. Hydrogen Energy 2014, 39, 5309–5318. [Google Scholar] [CrossRef]
- Jiang, S.; Zhu, C.; Dong, S. Cobalt and nitrogen-cofunctionalized graphene as a durable non-precious metal catalyst with enhanced ORR activity. J. Mater. Chem. A 2013, 1, 3593–3599. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, X.; Li, J.; Qian, J.; Liu, Y.; Yang, S.; Wang, Y.; Gao, D.; Xue, D. Interfacial engineering of NiO/NiCo2O4 porous nanofibers as efficient bifunctional catalysts for rechargeable zinc–air batteries. ACS Appl. Mater. Interfaces 2020, 12, 21661–21669. [Google Scholar] [CrossRef] [PubMed]
- Agyeman, D.A.; Zheng, Y.; Lee, T.H.; Park, M.H.; Tamakloe, W.; Lee, G.H.; Jang, H.W.; Cho, K.J.; Kang, Y.M. Synergistic Catalysis of the Lattice Oxygen and Transition Metal Facilitating ORR and OER in Perovskite Catalysts for Li-O2 Batteries. ACS Catal. 2020, 11, 424–434. [Google Scholar] [CrossRef]
- Hong, Q.; Lu, H.; Wang, J. CuO Nanoplatelets with Highly Dispersed Ce-Doping Derived from Intercalated Layered Double Hydroxides for Synergistically Enhanced Oxygen Reduction Reaction in Al-Air Batteries. ACS Sustain. Chem. Eng. 2017, 5, 9169–9175. [Google Scholar] [CrossRef]
- Majidi, M.R.; Farahani, F.S.; Hosseini, M.; Ahadzadeh, I. Low-cost nanowired α-MnO2/C as an ORR catalyst in air-cathode microbial fuel cell. Bioelectrochemistry 2019, 125, 38–45. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Ponraj, J.; Mansour, S.A. Preparation of mesoporous/microporous MnCo2O4 and nanocubic MnCr2O4 using a single step solution combustion synthesis for bifunction oxygen electrocatalysis. J. Electrochem. Soc. 2020, 167, 054507. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhou, H.; Jian, Z.; Li, H.; Guan, X.; Xing, Y.; Zhang, S.; Xu, H. Improved Electrocatalytic Activity of Three-Dimensional Open-Structured Co3O4@ MnO2 Bifunctional Catalysts of Li-O2 Batteries by Inducing the Oriented Growth of Li2O2. ACS Sustain. Chem. Eng. 2021, 9, 5334–5344. [Google Scholar] [CrossRef]
- Li, Y.C.; Jin, F.L.; Park, S.J. Oxygen-vacancy-rich spinel CoFe2O4 nanocrystals anchored on cage-like carbon for high-performance oxygen electrocatalysis. Korean J. Chem. Eng. 2021, 38, 2134–2140. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Wang, L.; Zhang, Z.; Wang, S. Spinel CoFe2O4 supported by three dimensional graphene as high-performance bi-functional electrocatalysts for oxygen reduction and evolution reaction. Int. J. Hydrogen Energy 2019, 44, 1610–1619. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled preparation, oxygen reduction/evolution reaction application, and beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef]
- Niu, Y.; Huang, X.; Zhao, L.; Hu, W.; Li, C.M. One-pot synthesis of Co/CoFe2O4 nanoparticles supported on N-doped graphene for efficient bifunctional oxygen electrocatalysis. ACS Sustain. Chem. Eng. 2018, 6, 3556–3564. [Google Scholar] [CrossRef]
- Liu, W.; Rao, D.; Bao, J.; Xu, L.; Lei, Y.; Li, H. Strong coupled spinel oxide with N-rGO for high-efficiency ORR/OER bifunctional electrocatalyst of Zn-air batteries. J. Energy Chem. 2021, 57, 428–435. [Google Scholar] [CrossRef]
- Yin, J.; Shen, L.; Li, Y.; Lu, M.; Sun, K.; Xi, P. CoFe2O4 nanoparticles as efficient bifunctional catalysts applied in Zn–air battery. J. Mater. Res. 2018, 33, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.F.; Gu, L.F.; Wang, J.W.; Wu, J.X.; Liao, P.Q.; Li, G.R. Bimetal-organic framework derived CoFe2O4/C porous hybrid nanorod arrays as high-performance electrocatalysts for oxygen evolution reaction. Adv. Mater. 2017, 29, 1604437. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, L.; Yan, J.; Yan, W.; Wu, C.; Lian, J.; Huang, Y.; Bao, J.; Qiu, J.; Xu, L.; et al. Facile preparation of NiFe2O4/MoS2 composite material with synergistic effect for high performance supercapacitor. J. Alloys Compd. 2017, 726, 608–617. [Google Scholar] [CrossRef]
- He, X.; Li, R.; Liu, J.; Liu, Q.; Song, D.; Wang, J. Hierarchical FeCo2O4@ NiCo layered double hydroxide core/shell nanowires for high performance flexible all-solid-state asymmetric supercapacitors. Chem. Eng. J. 2018, 334, 1573–1583. [Google Scholar] [CrossRef]
- Cheng, F.; Shen, J.; Peng, B.; Pan, Y.; Tao, Z.; Chen, J. Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts. Nat. Chem. 2011, 3, 79–84. [Google Scholar] [CrossRef]
- Ju, Y.W.; Park, J.H.; Jung, H.R.; Cho, S.J.; Lee, W.J. Electrospun MnFe2O4 nanofibers: Preparation and morphology. Compos. Sci. Technol. 2008, 68, 1704–1709. [Google Scholar] [CrossRef]
- Rania, M.; Jabeena, M.; Batoola, K.; Mehmoodb, A.; Shafiquea, R.; Akhtara, N.; Yaqoob, T.; Maliha, Z.; Akram, M.; Umbreen, M.; et al. Synthesis and Characterization of MgCr2O4 Spinel Nanoparticles by Sol gel Method. Dig. J. Nanomater. Biostruct. 2021, 16, 951–958. [Google Scholar]
- Lim, D.W.; Kong, H.S.; Lim, C.W.; Kim, N.M.; Shim, S.E.; Baeck, S.H. Spinel-type NiCo2O4 with abundant oxygen vacancies as a high-performance catalyst for the oxygen reduction reaction. Int. J. Hydrogen Energy 2019, 44, 23775–23783. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, A.A.; Moreno-Trejo, M.B.; Meléndez-Zaragoza, M.J.; Collins-Martínez, V.; López-Ortiz, A.; Martínez-Guerra, E.; Sánchez-Domínguez, M. Spinel-type ferrite nanoparticles: Synthesis by the oil-in-water microemulsion reaction method and photocatalytic water-splitting evaluation. Int. J. Hydrogen Energy 2019, 44, 12421–12429. [Google Scholar] [CrossRef]
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M. Hydrothermal synthesis of nanomaterials. J. Nanomater. 2020, 2020, 8917013. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Xu, R. New materials in hydrothermal synthesis. Acc. Chem. Res. 2001, 34, 239–247. [Google Scholar] [CrossRef]
- Peng, J.; Hojamberdiev, M.; Xu, Y.; Cao, B.; Wang, J.; Wu, H. Hydrothermal synthesis and magnetic properties of gadolinium-doped CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 2011, 323, 133–137. [Google Scholar] [CrossRef]
- Kim, H.Y.; Shin, J.; Jang, I.C.; Ju, Y.W. Hydrothermal synthesis of three-dimensional perovskite NiMnO3 oxide and application in supercapacitor electrode. Energies 2019, 13, 36. [Google Scholar] [CrossRef] [Green Version]
- Sawabe, T.; Akiyoshi, M.; Yoshida, K.; Yano, T. Estimation of neutron-irradiation-induced defect in 3C-SiC from change in XRD peak shift and DFT study. J. Nucl. Mater. 2011, 417, 430–434. [Google Scholar] [CrossRef]
- Liu, H.L.; Nosheen, F.; Wang, X. Noble metal alloy complex nanostructures: Controllable synthesis and their electrochemical property. Chem. Soc. Rev. 2015, 44, 3056–3078. [Google Scholar] [CrossRef] [Green Version]
- Prieur, D.; Bonani, W.; Popa, K.; Walter, O.; Kriegsman, K.W.; Engelhard, M.H.; Guo, X.; Eloirdi, R.; Gouder, T.; Beck, A.; et al. Size Dependence of Lattice Parameter and Electronic Structure in CeO2 Nanoparticels. Inorg. Chem. 2020, 59, 5760–5767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chan, S.W.; Spanier, J.E.; Apak, E.; Jin, Q.; Robinson, R.D.; Herman, I.P. Cerium oxide nanoparticles: Size-selective formation and structure analysis. Appl. Phys. Lett. 2002, 80, 127–129. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Cai, Y.; Zhao, X.; Liang, Y.; Zheng, M.; Hu, H.; Dong, H.; Jiang, S.; Liu, Y.; Xiao, Y. Sulfur-doped nanoporous carbon spheres with ultrahigh specific surface area and high electrochemical activity for supercapacitor. J. Power Sources 2017, 360, 373–382. [Google Scholar] [CrossRef]
- Deng, Y.; Tian, X.; Shen, G.; Gao, Y.; Lin, C.; Ling, L.; Cheng, F.; Liao, S.; Zhang, S. Coupling hollow Fe3O4 nanoparticles with oxygen vacancy on mesoporous carbon as a high-efficiency ORR electrocatalyst for Zn-air battery. J. Colloid Interface Sci. 2020, 567, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Seal, S.; Guo, Y.; Schulte, A.; Norwood, J. Role of trivalent La and Nd dopants in lattice distortion and oxygen vacancy generation in cerium oxide nanoparticles. Appl. Phys. Lett. 2006, 88, 243110. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Lu, Z.; Tang, Y.; Li, T.; Tang, Z.; Yang, Z. Effect of lattice strain on the oxygen vacancy formation and hydrogen adsorption at CeO2(111) surface. Phys. Lett. A 2014, 378, 2570–2575. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Xia, J.; Pan, Z.; Wang, X.; Zhu, G.; Zheng, B.; Liu, G.; Lang, L. Reduced CoFe2O4/graphene composite with rich oxygen vacancies as a high efficient electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 11052–11061. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Fan, B.; Zhang, J.; Zhou, M.; Yang, W.; Hu, X.; Wang, H.; Pan, B.; Xie, Y. Ultrathin spinel-structured nanosheets rich in oxygen deficiencies for enhanced electrocatalytic water oxidation. Angew. Chem. 2015, 127, 7507–7512. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, C.; Zhang, Z.; Liu, D.; Chen, R.; Wang, S. In situ exfoliated, N-doped, and edge-rich ultrathin layered double hydroxides nanosheets for oxygen evolution reaction. Adv. Funct. Mater. 2018, 28, 1703363. [Google Scholar] [CrossRef]
- Kim, H.Y.; Ju, Y.W. Fabrication of Mn-N-C catalyst for oxygen reduction reactions using Mn-embedded carbon nanofiber. Energies 2020, 13, 2561. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Q.; Liu, X.; Shui, J. Temperature impacts on oxygen reduction reaction measured by the rotating disk electrode technique. J. Phys. Chem. C 2020, 124, 3069–3079. [Google Scholar] [CrossRef]
- Tomon, C.; Krittayavathananon, A.; Sarawutanukul, S.; Duangdangchote, S.; Phattharasupakun, N.; Homlamai, K.; Sawangphruk, M. Enhancing bifunctional electrocatalysts of hollow Co3O4 nanorods with oxygen vacancies towards ORR and OER for Li-O2 batteries. Electrochim. Acta 2021, 367, 137490. [Google Scholar] [CrossRef]
- Yuan, H.; Li, J.; Yang, W.; Zhuang, Z.; Zhao, Y.; He, L.; Xu, L.; Liao, X.; Zhu, R.; Mai, L. Oxygen vacancy-determined highly efficient oxygen reduction in NiCo2O4/Hollow carbon spheres. ACS Appl. Mater. Interfaces 2018, 10, 16410–16417. [Google Scholar] [CrossRef]
- Ma, T.Y.; Zheng, Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Mesoporous MnCo2O4 with abundant oxygen vacancy defects as high-performance oxygen reduction catalysts. J. Mater. Chem. A 2014, 2, 8676–8682. [Google Scholar] [CrossRef]
- Liu, Z.X.; Li, Z.P.; Qin, H.Y.; Liu, B.H. Oxygen reduction reaction via the 4-electron transfer pathway on transition metal hydroxides. J. Power Sources 2011, 196, 4972–4979. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Wang, Y.; Chen, Z.; Dong, L.; Cai, R.; Hong, M.; Long, X.; Yang, S. In situ templating synthesis of mesoporous Ni-Fe electrocatalyst for oxygen evolution reaction. RSC Adv. 2020, 10, 23321–23330. [Google Scholar] [CrossRef]
- Sakaushi, K.; Yang, S.J.; Fellinger, T.P.; Antonietti, M. Impact of large-scale meso-and macropore structures in adenosine-derived affordable noble carbon on efficient reversible oxygen electrocatalytic redox reactions. J. Mater. Chem. A 2015, 3, 11720–11724. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv. Mater. 2017, 29, 1606793. [Google Scholar] [CrossRef]


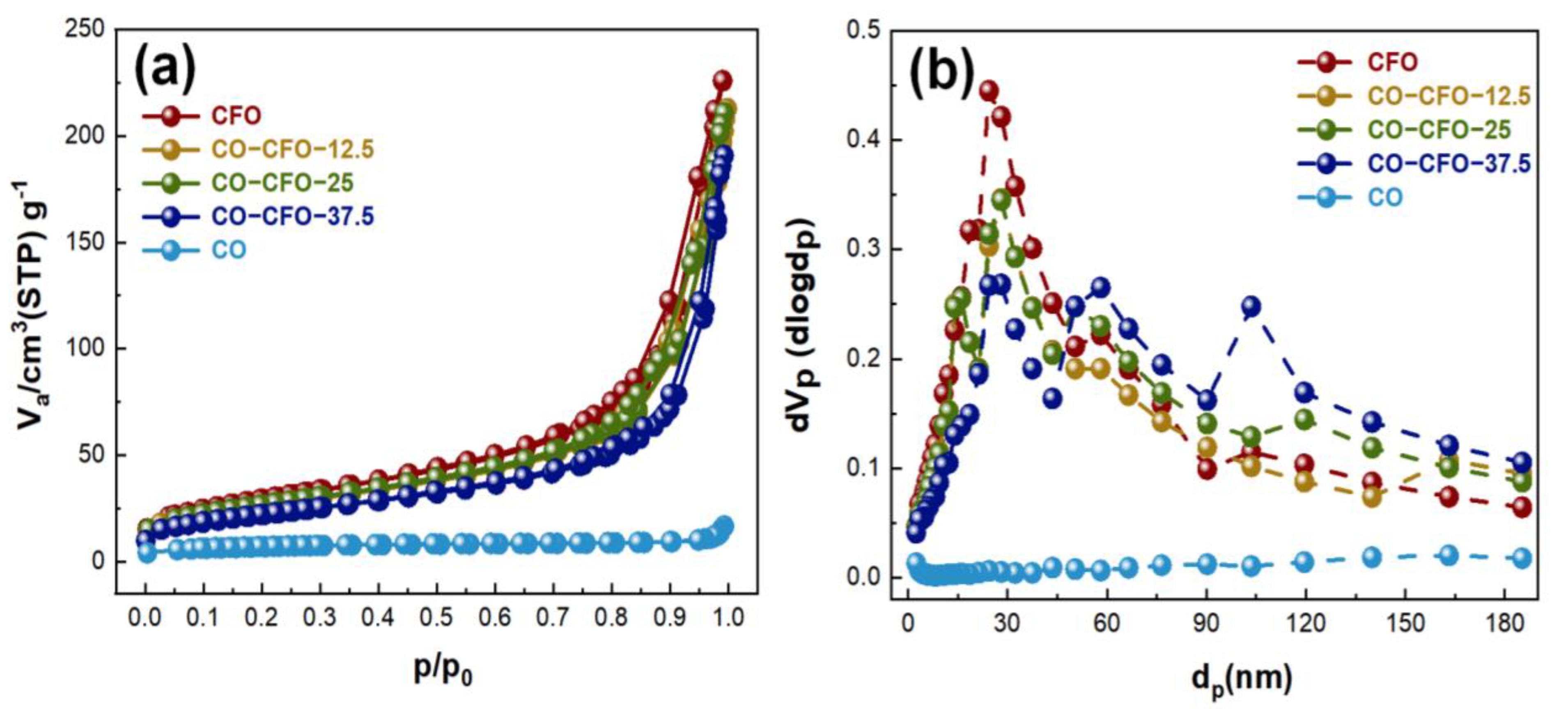

| Sample | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| CFO | 104.71 | 0.3483 | 24.41 |
| CO-CFO-12.5 | 93.54 | 0.2976 | 28.07 |
| CO-CFO-25 | 95.02 | 0.3168 | 28.07 |
| CO-CFO-37.5 | 79.70 | 0.2841 | 28.07 |
| CO | 24.92 | 0.0237 | 163.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-W.; Ju, Y.-W. Evaluation of Bi-Functional Electrochemical Catalytic Activity of Co3O4-CoFe2O4 Composite Spinel Oxide. Energies 2023, 16, 173. https://doi.org/10.3390/en16010173
Park J-W, Ju Y-W. Evaluation of Bi-Functional Electrochemical Catalytic Activity of Co3O4-CoFe2O4 Composite Spinel Oxide. Energies. 2023; 16(1):173. https://doi.org/10.3390/en16010173
Chicago/Turabian StylePark, Ji-Woo, and Young-Wan Ju. 2023. "Evaluation of Bi-Functional Electrochemical Catalytic Activity of Co3O4-CoFe2O4 Composite Spinel Oxide" Energies 16, no. 1: 173. https://doi.org/10.3390/en16010173
APA StylePark, J.-W., & Ju, Y.-W. (2023). Evaluation of Bi-Functional Electrochemical Catalytic Activity of Co3O4-CoFe2O4 Composite Spinel Oxide. Energies, 16(1), 173. https://doi.org/10.3390/en16010173







