Low-Energy Clay–Cement Slurries Find Application as Waterproofing Membranes for Limiting the Migration of Contaminants—Case Studies in Poland
Abstract
1. Introduction
- How do the different types of clay affect the rheological properties of the grout? Does fluidity have a direct bearing on the deep injection energy of the composite?
- Can local and waste raw materials, especially those produced by open-pit mining technology, be used to produce sealing clay–cement slurries?
- How can the rheological properties, especially the thixotropic properties of clay slurries with cementitious crosslinking components, be regulated?
- Can clay–cement slurries significantly reduce the migration of contaminants?
2. Properties of Clays
3. Cement Selection
4. Properties of Additives
5. Flowability of Clay–Cement Binders
6. Properties of Hardened Slurry
6.1. Filtration Properties
6.2. Binder Strength
6.3. Structure of Slurries on a Microscale
6.4. Reducing Migration of Pollutants
- -
- Interlayer position in clays;
- -
- Intermolecular bonds in cement;
- -
- Formation of other structures.
- Z—Ion charge:
- Δx—Electronegativity difference between ions and calcium;
- R—Ion radius;
- RCa2+—Calcium cation radius.
| Pb2+ | Zn2+ | Cr3+ | |
|---|---|---|---|
| Ionic radius (pm) | 132 | 74 | 64 |
| Coordination number | 4 | 4 | 6 |
| Electronegativity | 1.87 | 1.65 | 1.66 |
| Substitution pattern | Pb→Ca | Zn→Ca | Cr→Ca/Al |
| D factor | −1.32 | 0.328 | 0,7 |
- -
- Concentration of 20% of the following salts: PbNO3, ZnCl2 and CrCl3;
- -
- Sodium silicate Mk = 2.5;
- -
- Reagents in the ratio of 1:1.
- -
- Lead (II) silicate monohydrate PbO∙SiO2∙H2O (white crystals);
- -
- Zinc(II) silicate monohydrate ZnO∙SiO2∙H2O (blue gelatinous precipitate);
- -
- Chromium (III) silicate monohydrate Cr2O3∙SiO2∙H2O (pale green crystals).
7. Low-Energy Injection Methods
- -
- Fast working time of a few days, not months;
- -
- No need to lay out specialized working platforms;
- -
7.1. Vibration-Injected Slit Barrier Method
7.2. Deep Soil Mixing Method
7.3. Low-Pressure Injection Method
8. Recommendations for the Application of Clay–Cement Binders
- -
- Rheological properties that allow pumping over long distances;
- -
- Ability to penetrate cracks and voids;
- -
- Setting in a relatively short time;
- -
- Low filterability—filtration coefficient < 1∙10−8 m/s;
- -
- Mechanical strength > 300 kPa.
9. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Głąb, W.; Jawański, W.; Thiel, K. Badania nad Uszczelnianiem Podłoża Zapory Wodnej Zaczynami Cementowo-Iłowymi; IOMB: Warsaw, Poland, 1965; Volume 524. [Google Scholar]
- Wójcik, Ł.; Izak, P.; Mastalska-Popławska, J.; Gajek, M. Clay-cement suspensions-rheological and functional properties. J. Physics. Conf. Ser. 2017, 790, 1–7. [Google Scholar] [CrossRef]
- Michette, M.; Lorenz, R.; Ziegert, C. Clay barriers for protecting historic buildings from ground moisture intrusion. Herit. Sci. 2017, 5, 31. [Google Scholar] [CrossRef]
- Mousavi, S.S.; Bhojaraju, C.; Ouellet-Plamondon, C. Clay as a Sustainable Binder for Concrete—A Review. Constr. Mater. 2021, 1, 134–168. [Google Scholar] [CrossRef]
- Więcław, E.; Koda, E. Model przepływu wód podziemnych i transportu zanieczyszczeń dla składowiska z bentonitową przesłoną pionową. Przegląd Geol. 2005, 53, 770–775. [Google Scholar]
- Kouakou, C.; Morel, J.-C. Strength and elasto-plastic properties of non-industrial building materials manufactured with clay as anatural binder. Appl. Clay Sci. 2009, 44, 27–34. [Google Scholar] [CrossRef]
- Hanafi, M.; Ekinci, A.; Aydin, E. Engineering and Microstructural Properties of Alluvium Clay Stabilized with Portland Cement and Coal Bottom Ash for Sustainable Future. KSCE J. Civ. Eng. 2022, 26, 5049–5066. [Google Scholar] [CrossRef]
- Kumar, R.A.; Goyal, S.; Srivastava, A. A comprehensive study on the influence of supplementary cementitious materials on physico-mechanical, microstructural and durability properties of low carbon cement composites. Powder Technol. 2021, 394, 645–668. [Google Scholar] [CrossRef]
- García Calvo, J.L.; Pedrosa, F.; Carballosa, P.; Revuelta, D. Evaluation of the sealing effectiveness of expansive cement grouts through a novel water penetration test. Constr. Build. Mater. 2020, 251, 118974. [Google Scholar] [CrossRef]
- Słowikowski, D.; Kacprzak, G. Implementation of low pressure injection for soil reinforcement and sealing-selected application. Tech. Trans. Environ. Eng. 2013, 110, 111–119. [Google Scholar]
- Fu, J.; Wang, D.; Li, X.; Wang, Z.; Shang, Z.; Jiang, Z.; Wang, X.; Gao, X. Experimental Study on the Cement-Based Materials Used in Coal Mine Gas Extraction for Hole Sealing. ACS Omega 2021, 3, 21094–21103. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Wang, B.; Qu, L.; Liu, Q.; Zhu, D. Preparation and Performance Investigation of Optimized Cement-Based Sealing Materials Based on the Response Surface Methodology. ACS Omega 2022, 12, 25380–25393. [Google Scholar] [CrossRef]
- Reeves, G.M.; Sims, I.; Cripps, J.C. Clay Materials Used in Construction; Geological Society: London, UK, 2006. [Google Scholar]
- Kamon, M. Suitability assessment of bentonite-soil mixtures as the landfill bottom liner material. J. Soc. Mater. Sci. Japan 2002, 51, 36–41. [Google Scholar] [CrossRef]
- Stempkowska, A. The trial of immobilization amphoteric metals cations in clay-cement hydroisolation barriers. IOP Conf. Ser.: Mater. Sci. Eng 2019, 641, 1–8. [Google Scholar] [CrossRef]
- Stempkowska, A. The ability of clay-cement hydraulic binders to accumulate aliphatic and aromatic hydrocarbons. Cem. Wapno Beton Stow. Prod. Cem. I Wapna 2020, 52–60. [Google Scholar]
- Stempkowska, A. The mechanism of amphoteric metals cations immobilization into clay-cement mixtures. J. Pol. Miner. Eng. Soc. 2020, 22, 13–21. Available online: http://www.potopk.com.pl/Full_text/2020_v1_full/IM%201-2020-v1-a2.pdf (accessed on 25 January 2020).
- Stempkowska, A.; Wójcik, Ł.; Izak, P.; Staszewska, M.; Mastalska-Popławska, J. Investigation of post-industrial pollutions’ immobilization in a hydraulic self-solidifying clay-cement binder. IOP Conf. Ser.: Mater. Sci. Eng 2018, 427, 1–10. [Google Scholar] [CrossRef]
- Borys, M.; Rycharska, J. Parametry zawiesin twardniejących stosowanych do wykonywania przegród przeciwfiltracyjnych w wałach przeciwpowodziowych. Woda-Srodowisko-Obsz. Wiej. 2006, 6, 47–56. (In Polish) [Google Scholar]
- Falaciński, P.; Garbulewski, K.; Kledyński, Z.; Skutnik ZCiarkowska, K. Badania barier hydraulicznych z zawiesin cementowo-bentonitowych z dodatkiem popiołów fluidalnych, Przegląd Naukowy. Inżynieria I Kształtowanie Sr. 2004, 2, 202–215. (In Polish) [Google Scholar]
- Garzón, E.; Sánchez-Soto, P.; Romero, E. Physical and geotechnical properties of clay phyllites. Appl. Clay Sci. 2010, 48, 3. [Google Scholar] [CrossRef]
- Abd El Kader, M.M.; El Deeb, A.S. Preparation and characterization of low-cost waterproofing sheets from NR-loaded clay. HBRC J. 2020, 16, 1–15. [Google Scholar] [CrossRef]
- Duan, P.; Shui, Z.; Chen, W.; Shen, C. Effects of metakaolin, silica fume and slag on pore structure, interfacial transition zone and compressive strength of concrete. Constr. Build. Mater. 2013, 44, 1–6. [Google Scholar] [CrossRef]
- Cheng, S.; Shui, Z.; Sun, T.; Yu, R.; Zhang, G.; Ding, S. Effects of fly ash, blast furnace slag and metakaolin on mechanical properties and durability of coral sand concrete. Appl. Clay Sci. 2017, 141, 111–117. [Google Scholar] [CrossRef]
- Ponraj Sankar, L.; Sivasankar, S.; Shunmugasundaram, M.; Praveen Kumar, A. Investigation on binder and concrete with fine grinded fly ash and silica fume as pozzolanic combined replacement. Mater. Today: Proc. 2020, 27, 1157–1162. [Google Scholar] [CrossRef]
- Ponraj Sankar, L.; Aruna, G.; Krishna Rao, A.; Srinivas Kadrekar, K. Studies on drying shrinkage and water permeability of fine fly ash high performance concrete. Mater. Today: Proc. 2021, 46, 930–933. [Google Scholar] [CrossRef]
- Guggenheim, S.; Formoso, M.L.; Galan, E.; Bish, D.L. Definition of clay and clay mineral: Joint report of the AIPEA nomenclature and CMS nomenclature committees. Clay Clay Miner. 1995, 43, 255–256. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. Handbook of Clay Science 2013; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Kumari, N.; Mohan, C. Basics of Clay Minerals and Their Characteristic Properties; Nascimento, G.M.D., Ed.; Clay and Clay Minerals; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Murray, H.H. Structure and composition of clay minerals and their physical and chemical properties. Applied clay mineralogy-occurrence, processing and applications of Kaoline, Bentonite, Polygorskite-Sepiolite and common clays. Haydn H Murray 2006, 7–31. [Google Scholar] [CrossRef]
- Benjamin, K. The Clay Mineral-Structure. 2004. Available online: http://www.groundwaterresearch.com.au/reference_files/hydrology_and_the_clay_minerals/structure.htm (accessed on 23 July 2020).
- Andrade, F.A.; Al-Qureshi, H.A.; Hotza, D. Measuring and modeling the plasticity of clays. Mater. Res. 2010, 13. [Google Scholar] [CrossRef]
- Andrade, F.A.; Al-Qureshi, H.A.; Hotza, D. Measuring the plasticity of clays: A review. Appl. Clay Sci. 2011, 51, 1–7. [Google Scholar] [CrossRef]
- Pusch, R. Environmental Soil Properties and Behaviour, Swelling Clays, Chapter 4; CRC press, Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2012; pp. 133–161. [Google Scholar]
- Ren, Y.; Yang, S.; Andersen, K.H.; Yang, Q.; Wang, Y. Thixotropy of soft clay: A review. Eng. Geol. 2021, 287, 106097. [Google Scholar] [CrossRef]
- Shahriar, A.R.; Jadid, R. An experimental investigation on the effect of thixotropic aging on primary and secondary compression of reconstituted dredged clays. Appl. Clay Sci. 2018, 162, 524–533. [Google Scholar] [CrossRef]
- Kurdowski, W. Cement and Concrete Chemistry; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Saleh, H.M.; Eskander, S.B. Innovative Cement-Based Materials for Environmental Protection and Restoration, 2020 New Materials in Civil Engineering; Butterworth-Heinemann: Oxford, UK, 2020; pp. 613–641. [Google Scholar] [CrossRef]
- Damineli, B.L.; Pileggi, R.G.; John, V.M. Influence of packing and dispersion of particles on the cement content of concretes. Struct. Mater. J. 2017, 10. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Hu, Y.; Zhang, S.; Mei, C.; Wu, H.; Sun, X.; Fu, Y. Study on Dispersion of Cement Grout in Sand considering Filtration Effect through the EDTA Titration Test. Geofluids 2020, 2020, 6620979. [Google Scholar] [CrossRef]
- Mehairi, A.G.; Husein, M.M. Enhancement of cement properties by means of in situ grown nanoparticles. Constr. Build. Mater. 2020, 261, 120496. [Google Scholar] [CrossRef]
- Onaizi, A.M.; Huseien, G.F.; Lim, N.H.A.S.; Amran, M.; Samadi, M. Effect of nanomaterials inclusion on sustainability of cement-based concretes: A comprehensive review. Constr. Build. Mater. 2021, 306, 124850. [Google Scholar] [CrossRef]
- Lea, F.M. The Chemistry of Cement and Concrete; Chemical Publ. Co.: New York, NY, USA, 1971. [Google Scholar]
- Gartner, E.; Maruyama, I.; Chen, J. A new model for the C-S-H phase formed during the hydration of Portland cements. Cem. Concr. Res. 2017, 97, 95–106. [Google Scholar] [CrossRef]
- Duque-Redondo, E.; Bonnau, P.A.; Manzano, H. A comprehensive review of C-S-H empirical and computational models, their applications, and practical aspects. Cem. Concr. Res. 2022, 156, 106784. [Google Scholar] [CrossRef]
- Wójcik, Ł.; Izak, P.; Stempkowska, A. Pseudotiksotropowe właściwości zawiesin iłowo-cementowych—Pseudothixotropic properties of clay-cement slurries. Ceram. Mater. 2011, 6, 278–282. [Google Scholar]
- Szwabowski, J. Reologia Mieszanek na Spoiwach Cementowych; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 1999. [Google Scholar]
- Izak, P. Reologia w Ceramice; Wydawnictwa AGH: Kraków, Poland, 2017. [Google Scholar]
- Wójcik, Ł. Reologiczne właściwości zawiesin iłowo-cementowych. Ph.D. Thesis, AGH Kraków: Kraków, Poland, 2010. [Google Scholar]
- Litwinek, E. Lepkosprężyste właściwości zawiesin iłowo-cementowych. Ph.D. Thesis, AGH Kraków: Kraków, Poland, 2021. [Google Scholar]
- Haha, M.B.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Berodier, E.; Scrivener, K. Understanding the filler effect on the nucleation and growth of C-S-H. J. Am. Ceram. Soc. 2014, 97, 3764–3773. [Google Scholar] [CrossRef]
- Bergold, S.T.; Goetz-Neunhoeffer, F.; Neubauer, J. Interaction of silicate and aluminate reaction in a synthetic cement system: Implications for the process of alite hydration. Cem. Concr. Res. 2017, 93, 32–44. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. The influence of sulfate addition on hydration kinetics and C-S-H morphology of C3S and C3S/C3A systems. Cem. Concr. Res. 2022, 160, 106930. [Google Scholar] [CrossRef]
- Litwinek-Rozbicka, E.; Izak, O.; Wójcik, Ł.; Mastalska-Popławska, J. Clay-cement suspensions based on Belchatow and Patoka polymineral clays–rheological characteristics. J. Physics. Conf. Ser. 2020, 1527, 1–7. [Google Scholar] [CrossRef]
- Darcy, H. Les Fontaines Publiques de la Ville de Dijon; Gallica: Paris, France, 1856. [Google Scholar]
- Brylska, E. Modelowanie Filtracji Wód Podziemnych w Rejonach Ujęć Wodnych; Wydawnictwo Geologiczne: Warszawa, Poland, 1972. [Google Scholar]
- Dowgiałło, J.; Kleczkowski, A.S.; Maciaszczyk, T.; Różkowski, A. Słownik Hydrogeologiczny; PIG: Warszawa, Poland, 2002. [Google Scholar]
- Ferré, T.P.A.; Warrick, A.W. Infiltration. Encycl. Soils Environ. 2005. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P. Principles of Hydrogeology, Groundwater Remediation and Treatment Technologies; William Andrew Publishing: Norwich, NY, USA, 1997; pp. 85–126. [Google Scholar] [CrossRef]
- Rakowska, J.; Radwan, K.; Ślosorz, Z.; Pietraszak, E.; Łudzik, M.; Suchorab, P. Usuwanie Substancji Ropopochodnych z Dróg i Gruntów; Państwowy Instytut Badawczy: Józefów, Poland, 2012. [Google Scholar]
- Cebula, J.; Rajca, M. Oczyszczanie Gleb i Gruntów; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2014. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry. Risks Best Available Strateg. Remediat. SRN Ecol. 2011, 402647. [Google Scholar] [CrossRef]
- Andersen, S.; Ødegård, S.; Seip, M.H. Background levels of heavy metals in Polish forest soils. Ecol. Eng. 1994, 3, 245–253. [Google Scholar] [CrossRef]
- Seip, H.M.; Pawałowski, L.; Sulliven, T. Environmental degradation dur to heavy metals and acidifying deposition—A Polish-Scandinavian workshop. Ecol. Eng. 1994, 3, 205–206. [Google Scholar] [CrossRef]
- Zhang, H.; He, P.J.; Shao, L.M.; Li, X.J. Leaching behavior of heavy metals from municipal solid waste incineration bottom ash and its geochemical modelling. J. Mater. Cycles. Waste Manag. 2008. [CrossRef]
- Wang, D.; Liu, D.; Tao, L.; Li, Z. The impact on the effects of leachate concentrates recirculation for different fill age waste. J. Mater. Cycles. Waste Manag. 2017, 19, 1211–1219. [Google Scholar] [CrossRef]
- Bashir, M.J.K.; Aziz, H.A.; Yusoff, M.S. New sequential treatment for mature landfill leachate by cationic/anionic and anionic/cationic processes: Optimization and comparative study. J. Hazard Mater. 2011, 186, 92–102. [Google Scholar] [CrossRef]
- Lafhaj, Z.; Samara, M.; Agostini, F.; Boucard, L.; Skoczylas, F.; Depelsenaire, G. Polluted river sediments from the North region of France: Treatment with Novosol process and valorization in clay bricks. Constr. Build Mater. 2007, 148, 606–612. [Google Scholar] [CrossRef]
- Varshney, S.; Jain, P.; Srivastava, S.J. Application of ameliorated wood pulp to recover Cd(II), Pb(II), and Ni(II) from e-waste. Mater Cycles Waste Manag. 2017, 19, 1446–1456. [Google Scholar] [CrossRef]
- Zmijowa, D.; Koliba, M.; Raclavski, K. Human Health Risk Assessment of Heavy Meatls Bound on Particulate Matter. J. Pol. Miner. Eng. Soc. 2018, 1, 93–98. [Google Scholar] [CrossRef]
- Whitworth, T.M. Clay minerals: Ion exchange. In Geochemistry; Encyclopedia of Earth Science; Springer: Dordrecht, The Netherlands, 1998. [Google Scholar] [CrossRef]
- Zou, Y.C.; Mogg, L.; Clark, N.; Bacaksiz, C.; Milovanovic, S.; Sreepal, V.; Hao, G.P.; Wang, Y.C.; Hopkinson, D.G.; Gorbachev, R.; et al. Ion exchange in atomically thin clays and micas. Nat. Mater. 2021, 20, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.; Cecilia, J.A.; Lopez-Moreno, C.; Hernandez, V.; Pozo, M.; Bentabol, J.M.; Franco, F. Influence of the Structure and Experimental Surfaces Modifications of 2:1 Clay Minerals on the Adsorption Properties of Methylene Blue. Minerals 2018, 8, 359. [Google Scholar] [CrossRef]
- Esaifan, M.; Warr, L.; Grathoff, G.; Meyer, T.; Schafmeister, M.; Kurt, A.; Testrich, H. Synthesis of Hydroxy-Sodalite/Cancrinite Zeolites from Calcite-Bearing Kaolin for the Removal of Heavy Metal Ions in Aqueous Media. Minerals 2019, 9, 484. [Google Scholar] [CrossRef]
- Carroll, D. Ion Exchange in clays and other minerals. GSA Bull. 1959, 70, 749–779. [Google Scholar] [CrossRef]
- Sparks, D.L. Ion Exchange Processes, Environmental Soil Chemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 187–205. [Google Scholar] [CrossRef]
- Whittaker, M.L.; Lammers, L.N.; Carrero, S.; Gilbert, B.; Banfield, J.F. Ion exchange selectivity in clay is controlled by nanoscale chemical–mechanical coupling. Earth Atmos. Planet. Sci. 2021, 116, 22052–22057. [Google Scholar] [CrossRef]
- Horst, M.L.; Wensheng, Z. Research review of cement clinker chemistry. Cem. Concr. Res. 2015, 78, 24–37. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, X.; Ouyang, S. Development on ion substitution effect on the crystal structure and properties of tricalcium silikat. J. Chin. Ceram. Soc. 2011, 39, 1666–1672. [Google Scholar]
- Kowacki, M. Przesłony przeciwfiltracyjne Nowoczesne Budownictwo Inżynieryjne. 2015, 6, 68‖72. Available online: http://www.nbi.com.pl/assets/NBI-pdf/2015/6_63_2015/full/nb_63_web.pdf (accessed on 1 December 2015).
- Egorova, A.; Rybak, J.; Stefaniuk, D.; Zajączkowski, P. Basic Aspects of Deep Soil Mixing Technology Control. IOP Conf. Ser. Mater. Sci. Eng. 2017, 245, 022019. [Google Scholar] [CrossRef]
- Alhamdi, M.K.; Albusoda, B.S. A Review on Deep mixing method for soil improvement. IOP Conf. Ser.: Mater. Sci. Eng. 2021, 1105, 012110. [Google Scholar] [CrossRef]
- Kuś, R.; Janik, G.; Izak, P.; Wójcik, Ł.; Słowikowski, D. Hydraulic mixture for placing wastes dangerous to the environment in the waste disposal grounds PL 386030 A1 Granted 2010. Available online: http://patenty.bg.agh.edu.pl/pelneteksty/PL386030A1.pdf (accessed on 30 May 2014).
- Izak, P.; Wójcik, Ł.; Gajek, M.; Mastalska-Popławska, J.; Stempkowska, A.; Kuś, R. Hydroinsulating binder for making anti-filtration partitions PL 229788 B1; Granted 9 January 2018. Available online: http://patenty.bg.agh.edu.pl/pelneteksty/PL229788B1.pdf (accessed on 31 August 2018).
- Izak, P.; Wójcik, Ł.; Gajek, M.; Mastalska-Popławka, J.; Stempkowska, A.; Litwinek, E.; Kuś, R.; Janik, G.; Kuś, M. Clay-cement binder with increased resistance to excessive loss of moisture PL 238570 B1 Granted 7 June 2021. Available online: http://patenty.bg.agh.edu.pl/pelneteksty/PL238570B1.pdf (accessed on 1 July 2019).

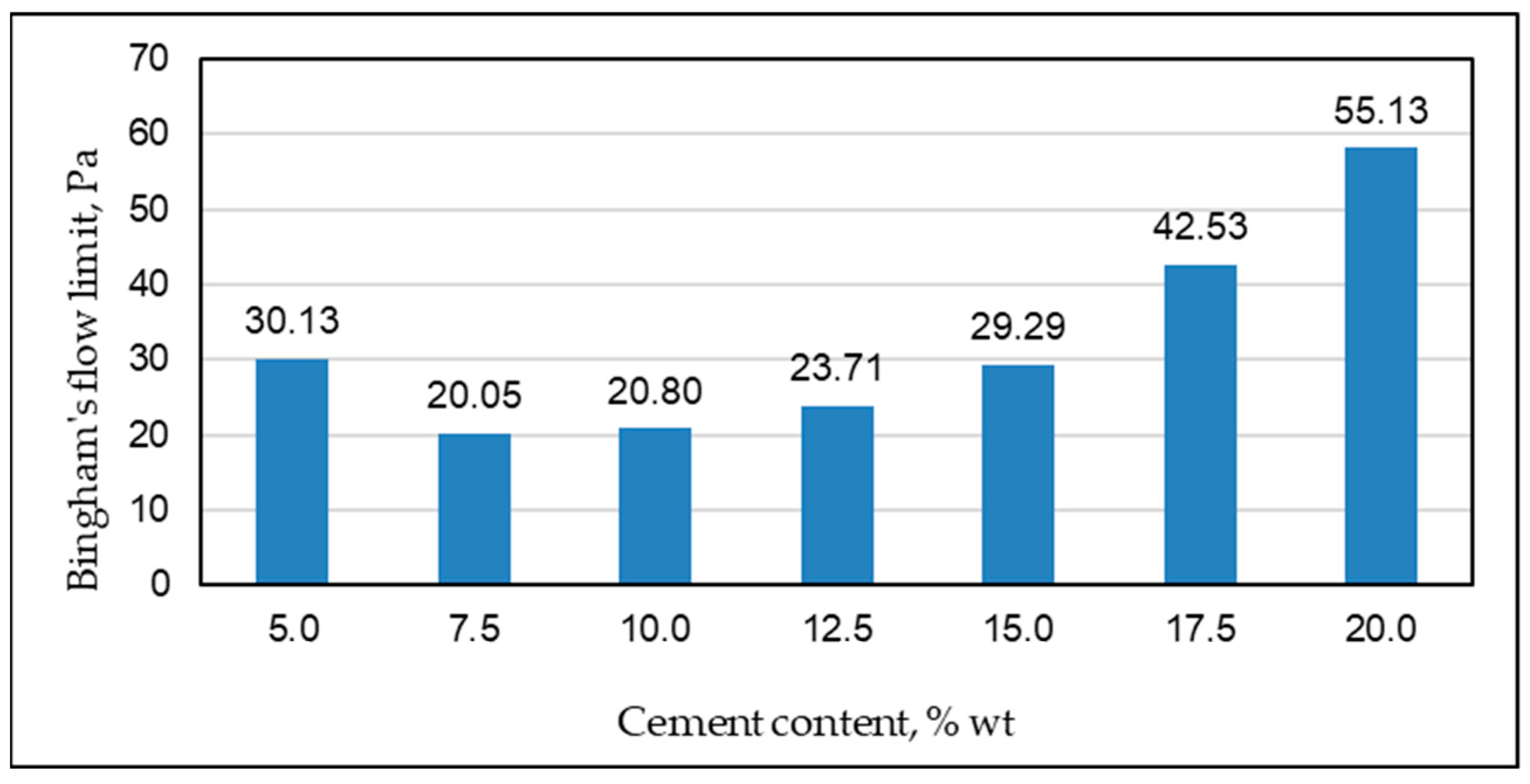
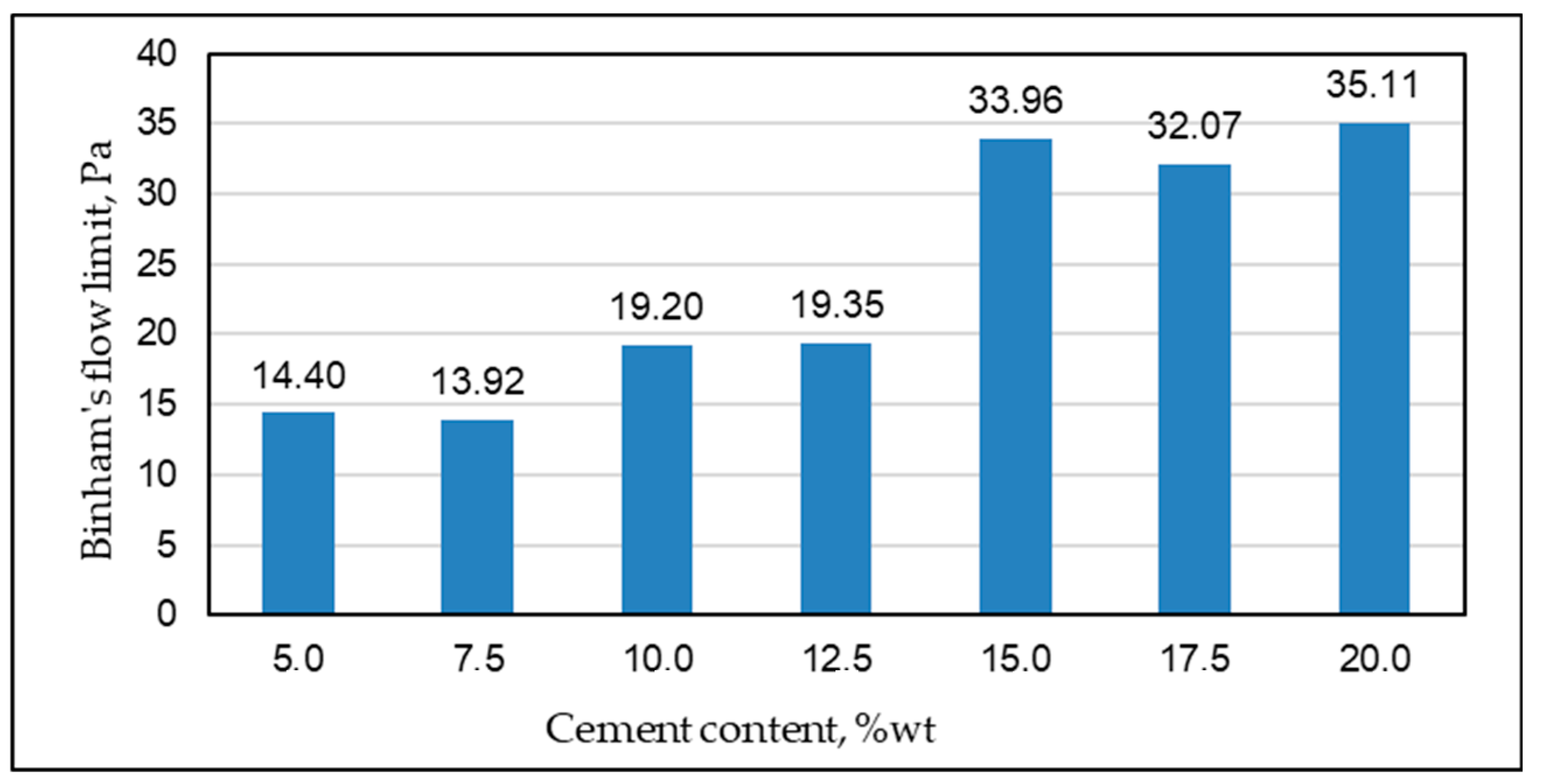

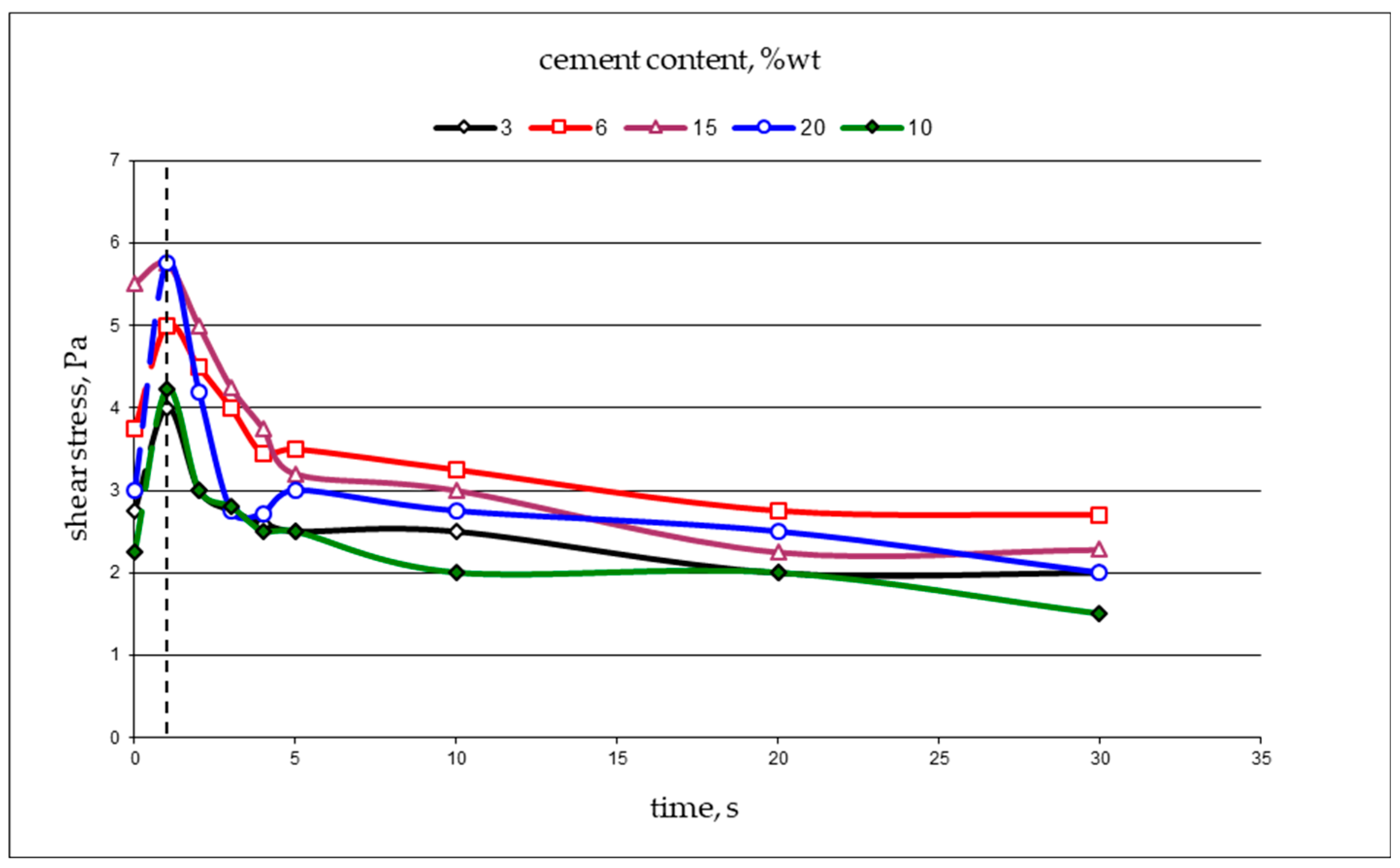

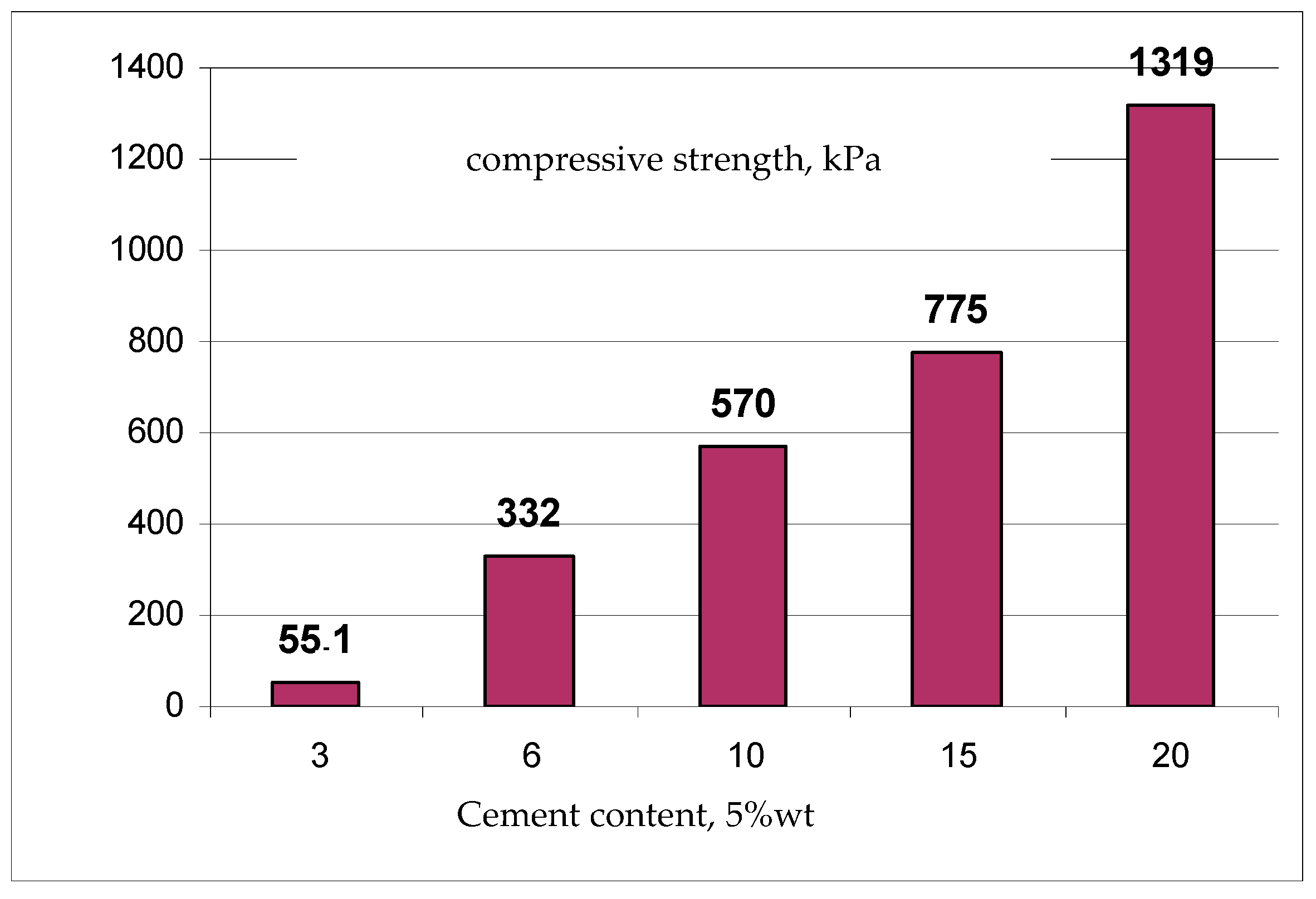
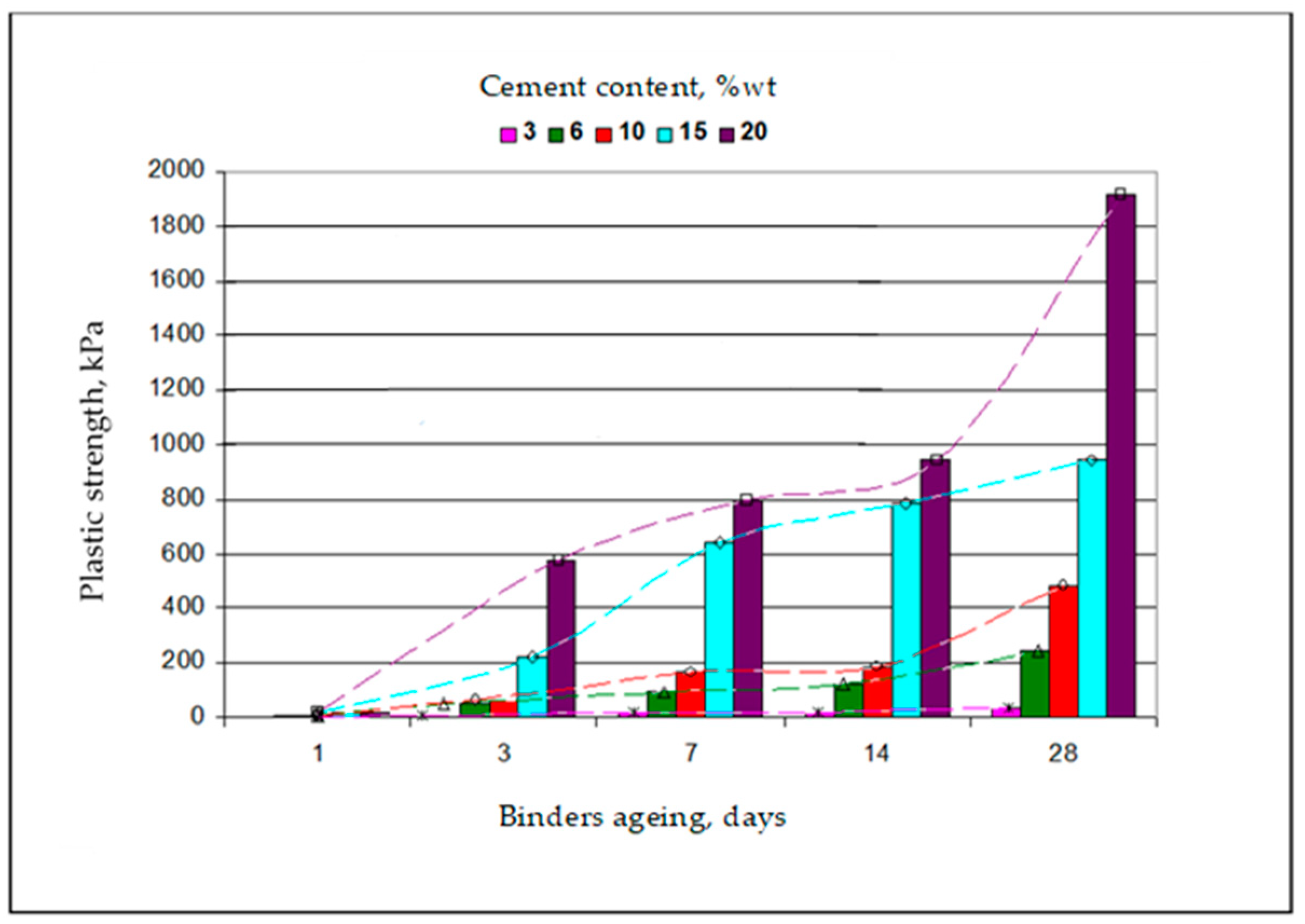






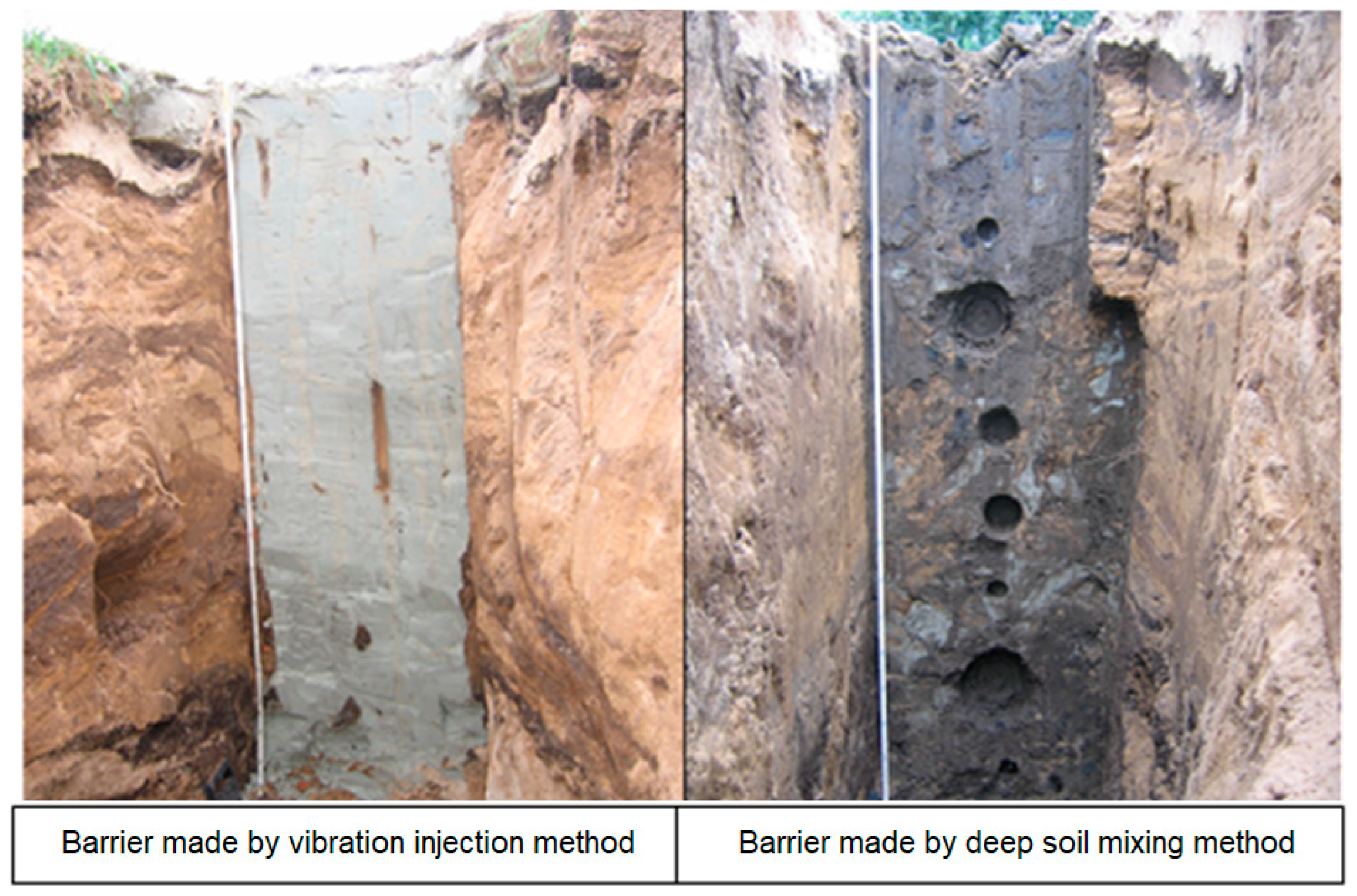
| Permeability | Filtration Coefficient (m/s) | Rock Examples |
|---|---|---|
| Very good | >10−3 | rubble, gravel, coarse-grained and even-grained sands, massive rocks with a very dense network of small gaps |
| Good | 10−3–10−4 | coarse-grained sands, slightly loamy sands, mixed-grained and medium-grained sands, poorly bonded coarse-grained sandstones, massive rocks with a dense network of gaps |
| Average | 10−4–10−5 | fine-grained sands, evenly-grained sands, loess |
| Low | 10−5–10−6 | dusty and clay sands, silt, sandstones, massive rocks with a rare network of fine cracks |
| Semi-permeable | 10−6–10−8 | clays, silt, siltstones, sandy clays |
| Impermeable | <10−8 | clays, clay shale, hard clay, clay marls, unbroken massive rocks |
| Sample | Aliphatic Hydrocarbon Content before Filtration (mg/kg) | Aliphatic Hydrocarbons Content in the Filtrate (mg/l) | Accumulation of Impurities in the Binder (%) |
|---|---|---|---|
| 40/10 | 27.78 | 5.415 | 80.507 |
| 40/15 | 28.12 | 5.180 | 81.579 |
| 40/20 | 25.87 | 4.670 | 81.948 |
| 50/10 | 22.76 | 3.169 | 86.076 |
| 50/15 | 23.29 | 4.470 | 80.911 |
| 50/20 | 21.17 | 4.903 | 76.840 |
| 60/10 | 18.09 | 5.010 | 72.305 |
| 60/15 | 18.20 | 3.698 | 79.681 |
| 60/20 | 17.61 | 3.475 | 80.607 |
| 70/10 | 14.59 | 3.326 | 77.203 |
| 70/15 | 14.45 | 3.321 | 77.017 |
| 70/20 | 13.02 | 2.159 | 83.417 |
| 80/10 | 9.91 | 2.514 | 74.631 |
| 80/15 | 9.34 | 2.403 | 74.272 |
| 80/20 | 8.87 | 3.930 | 55.693 |
| Filtration Coefficient (m/s) | Method by which the Screen Was Made | |
|---|---|---|
| Embankment before Making the Screen | Embankment after Making the Screen | |
| 2.9∙10−5–6.0∙10−6 | 6.0·10−10 | Vibration-injected |
| 4.0·10−8 | Deep soil mixing | |
| 7.5·10−10 | Low-pressure injection | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stempkowska, A.; Wójcik, Ł.; Ostrowski, K.A.; Gawenda, T. Low-Energy Clay–Cement Slurries Find Application as Waterproofing Membranes for Limiting the Migration of Contaminants—Case Studies in Poland. Energies 2023, 16, 230. https://doi.org/10.3390/en16010230
Stempkowska A, Wójcik Ł, Ostrowski KA, Gawenda T. Low-Energy Clay–Cement Slurries Find Application as Waterproofing Membranes for Limiting the Migration of Contaminants—Case Studies in Poland. Energies. 2023; 16(1):230. https://doi.org/10.3390/en16010230
Chicago/Turabian StyleStempkowska, Agata, Łukasz Wójcik, Krzysztof Adam Ostrowski, and Tomasz Gawenda. 2023. "Low-Energy Clay–Cement Slurries Find Application as Waterproofing Membranes for Limiting the Migration of Contaminants—Case Studies in Poland" Energies 16, no. 1: 230. https://doi.org/10.3390/en16010230
APA StyleStempkowska, A., Wójcik, Ł., Ostrowski, K. A., & Gawenda, T. (2023). Low-Energy Clay–Cement Slurries Find Application as Waterproofing Membranes for Limiting the Migration of Contaminants—Case Studies in Poland. Energies, 16(1), 230. https://doi.org/10.3390/en16010230











