1. Introduction
Conditioning and processing of natural gas in cryogenic gas processing plants usually require cooling of the feed gas in refrigeration systems to separate the heavy hydrocarbons (HHC). This is required to meet the delivery hydrocarbon dew-point pipeline specifications of the sales gas and/or to separate the natural gas liquids (NGL), C2+, for further fractionation to make NGL products. Production of Liquefied Natural Gas (LNG) requires deep cooling of the gas to condense it to a liquid state.
The feed to gas processing and liquefaction plants may contain trace amounts of heavy hydrocarbons such as BTEX compounds (benzene, toluene, ethylbenzene, and xylenes) and heavier hydrocarbon components, which have high melting points [
1]. These components may freeze out during the cooling process of the gas and precipitate in the small flow channels of the heat exchangers, control valves, and process strainers. The precipitation of these solids may cause partial blockage of the process equipment leading to unplanned shutdowns, production loss and costly downtime required to defrost the clogged process systems [
2]. To reduce the level of contaminants, such as acid gases, moisture, and heavy hydrocarbons to acceptable levels before liquefaction, feed natural gas is subjected to a variety of treatment operations, as shown in
Figure 1.
BTEX and HHC compounds can precipitate at the very low temperatures in the cold section of the LNG production process when excessive concentrations of these undesired heavy components slip into the liquefaction exchangers during process upsets.
Table 1 shows the relatively high triple point temperatures of BTEX and HHC. These components are typically removed in the Heavies Removal Unit, which consists mainly of a refluxed cryogenic distillation column (called Scrub Column or NGL Absorber) where BTEX and HHC are removed to acceptable and safe limits along with the NGL.
The gas analysis for designing new gas processing and liquefaction plants is usually detailed for the light hydrocarbons from methane to pentanes and also typically shows the BTEX composition. However, the heavier hydrocarbons, including those with high melting points, are typically lumped in pseudo components (usually C6+) according to their normal boiling points (NBP). For the operating gas processing and LNG plants, the routine gas analysis used to monitor the process usually has a similar limited resolution. This resolution needs to provide the required information about the compositions and the molecular type; paraffinic, iso-paraffinic, naphthenic, and aromatic (PINA) distribution of the heavy hydrocarbons lumped in the pseudo components. This information is required to provide a robust process design for the new gas processing and LNG plants. It is also required to effectively troubleshoot the freeze-out problems that may occur in the operating plants.
Chen and Ott [
4] reported that lean feed gases are becoming increasingly common as more LNG is based on unconventional gas resources, such as shale gas, tight sands gas, coal-bed methane, and domestic pipelines. Both also reported that the feed gas to some plants operating LNG plants had been gradually replaced by newer sources, such as gas produced from wells in deep water. These new feed gas sources often contain more methane and considerably fewer natural gas liquid (NGL) components than conventional feed gas sources while also having heavy paraffinic and aromatic hydrocarbon content comparable to conventional feed gas sources. This trend has brought a new challenge to the design and operation of these liquefaction units. Additionally, Chen and Ott [
4] reported that many lean feed gas sources contain disproportionately large amounts of HHCs. These heavy components can exceed the maximum amount allowed to avoid freezing in the liquefaction process. Another characteristic of some unconventional gas sources is the significant variation in feed composition over a relatively short period, which can occur when the feed gas is supplied from multiple sources.
The presence of the long-chain HHC in the feed gas to LNG plants, even in trace amounts, increases the risk of freeze-out of these complex components at the Precooling Section of the LNG plant shown in
Figure 2, which has a typical lower-limit temperature of 238.7 K (−30 °F) [
1,
5]. The higher risk is caused by their higher melting points, as shown in
Table 2 and
Table 3. These tables show that the characterization of HHC should not be assumed to be 100% n-paraffins due to the increased risk of heavy aromatic isomers freeze-out in the Precooling Section. Rather an accurate estimate of the molecule class (PINA distribution) of these HHC components is crucial for the robust design of new LNG plants and the effective troubleshooting of HHC freeze-out in actively operating LNG plants.
Miethe et al. [
6] reported that the light hydrocarbons up to n-pentane are generally detailed in the design basis of LNG, NGL or ethane separation plants. However, the HHCs that might foul the pipes at lower temperatures are often lumped into pseudocomponents according to their boiling point. They also proposed a new method developed to improve the calculation of solids precipitation in natural gases using correlations endorsed by the American Petroleum Institute (API), as described in Riazi and Daubert [
7]. Their method consists of two steps:
Splitting the pseudo-components into four sub-fractions each: n-paraffin (P), branched paraffins (I), naphthenes (N), and aromatics (A).
Representing the fusion properties of each sub-fraction with a discrete component, where no additional physical properties need to be generated for the pseudo component (PC).
To validate their approach, henceforth referred to as PINA
API, they compare the melting points and precipitation curves of a fully characterized mixture up to C
10+, where the fusion properties are estimated with multiple approaches, as described in
Table 4. These approaches are accompanied by a characterization of the gas mixture into a Lump component list containing discrete single carbon number (SCN) groups of HHCs heavier than isopentane up to C
10+. In their comparison of the Full methods with various Lump + Freeze-out approaches, they showcased that the Lump + PINA
API predicted the melting point of the mixture within 1 °C of the Full method with a very similar precipitation curve when aromatics were considered explicitly. However, there has been no application of the Lump + PINA
API approach to defined mixtures that are known to have heavy HHCs up to C
14.. Additionally, Miethe et al. [
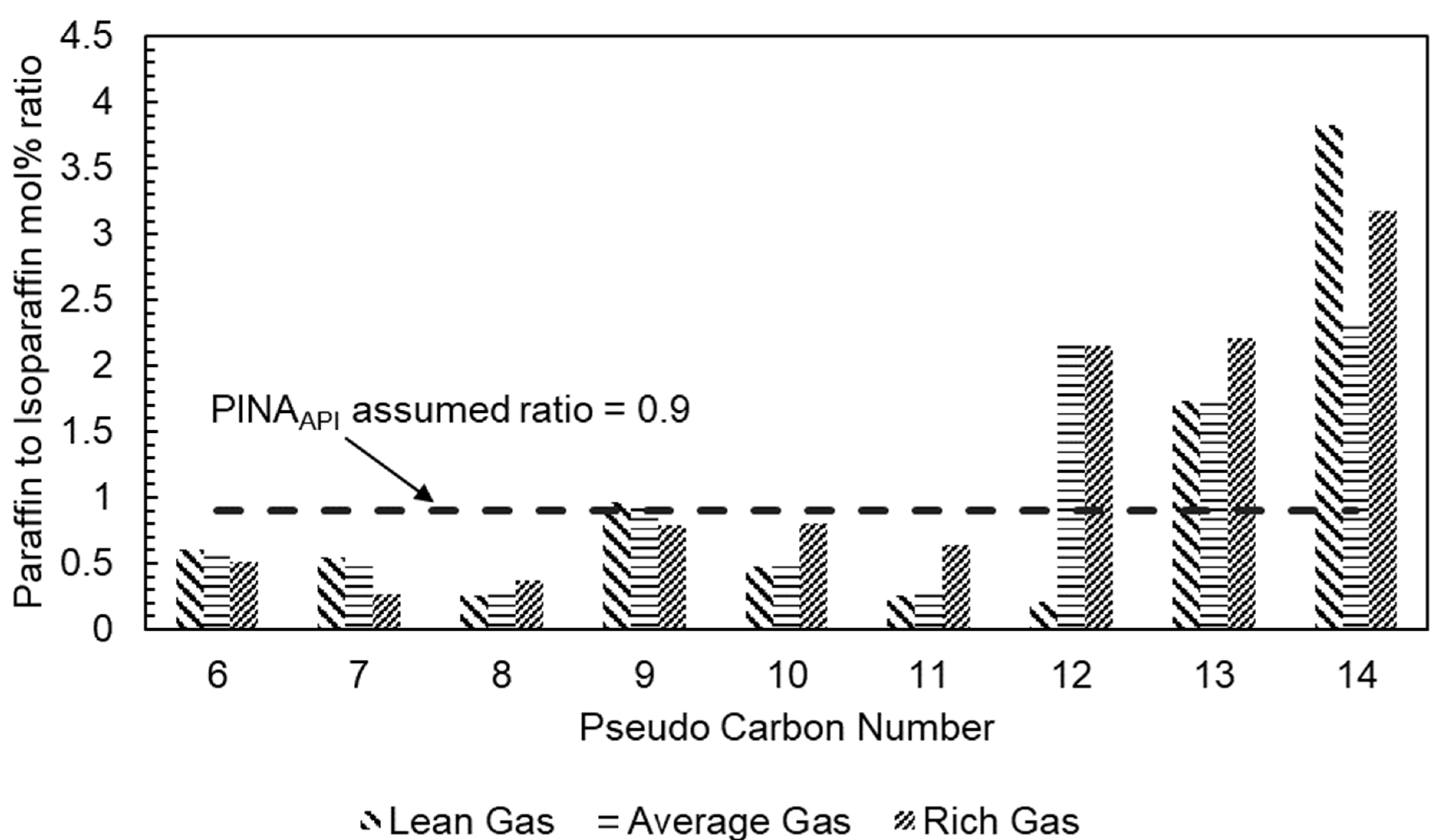
6] make an unvalidated assumption that the ratio of paraffinic (
to isoparaffinic (
content is
. Therefore, it is unlikely that the estimated PINA splits will reflect the actual PINA split of HHCs of LNG feed gas sources with high methane content and fewer NGL components.
Recently, Attalla et al. [
8] demonstrated that the approach developed by Miethe et al. [
6] was inadequate for multiple reasons. First, their Lump characterization does not extend to C
14 compounds and second, the methods for estimating the PINA splits were developed for petroleum fractions and are not necessarily applicable to high-methane content gas condensates. Attalla et al. [
8] demonstrated that for natural gas mixtures known to freeze out in LNG production, Lump + Freeze-out methods underpredict melting temperatures by more than 85 K relative to the melting temperatures determined from a full compositional characterization of the gas mixture. Their work demonstrated the necessity for either a complete characterization of natural gas mixtures up to C
14+ compounds to accurately predict freeze-out conditions in LNG plants and/or the development of an improved approach to predicting freeze-out when only limited compositional information is available.
This work builds upon the approach of Miethe et al. [
6] by incorporating more robust methods suited for estimating the PINA splits for natural gas mixtures based on experimental measurements of natural gas samples. This work also extends the use of representative compounds in the Lump + PINA
API method to consider up to C
14+ compounds. Representative compounds are enhanced to include properties relevant to both the fluid and solid phases in contrast to previous Lump approaches where only properties relevant to solid formation by the representative compounds were used. The efficacy of this new approach, labelled PINA
Attalla, is evaluated using predictions of the melting temperature against the PINA
API method from Miethe et al. [
6], and the full characterization of three different gas samples.
3. Results and Discussion
The melting temperatures based on the complete characterizations for Sample 1 (lean gas), Sample 2 (rich gas), and Sample 3 (average gas) indicate that the freeze-out can occur at temperatures around 263.2 K (14.1 °F), 260.1 K (8.5 °F), and 248.3 K (−12.8 °F), respectively. These temperatures are well within the cooling curve of a typical LNG production plant’s Precooling Section and are consistent with the freeze-out observed in some of operating LNG facilities.
Figure 4,
Figure 5 and
Figure 6 compare the melting temperatures predicted using the various PINA methods with those determined from the Full method for the lean gas, average gas and rich gas samples, respectively. Across all three samples, the new PINA
Attalla method predicts melting temperatures within 1 K of those determined from the full compositional characterization. Full compositional characterizations with the resolution presented here for Samples 1–3 are uncommon and often unavailable during the design or normal operation of an LNG plant. While actual natural gas compositions are inevitably more complex, the correlations in the PINA
Attalla method deliver a significant improvement in the ability to predict heavy hydrocarbon freeze out in LNG production, which is currently a significant operational problem for plants on the US Gulf Coast, from more standard, lower resolution compositional analyses. This improved performance reflects the fact that representative compounds and the PINA distributions used by the correlations are based on those measured for 46 actual pipeline gas samples collected from the US Gulf Coast.
The lower estimations of the PINA
Equal and PINA
API methods highlight the importance of accurately characterizing the PINA distribution of each SCN fraction for predicting the melting point accurately for natural gas mixtures. In particular, as the melting point is influenced by the composition of the heaviest components considered, achieving an accurate estimate of the PINA-split in the C
14+ pseudocomponent for the paraffin component is vital for accurate results. Despite the poorer performance of the PINA
API and PINA
Equal methods relative to the new PINA
Attalla approach, the melting points predicted are only about 20 K below the temperature determined using the Full method. This is a significant improvement relative to the predictions made using the Lump + PINA
Equal and Lump + PINA
API methods for the lean gas and rich gas samples detailed by Attalla et al. [
8], which were low by around 100 K. By describing the properties of all phases using the representative compounds from the PINA-only methods (i.e., without using any lumped pseudocomponents to describe fluid-phase properties), the predicted melting temperature is at least above the lowest temperature of an LNG plant’s precooling section. These comparisons underscore the improvement brought by characterizing gas mixtures with pseudocomponents up to at least C
14+ and robust PINA distributions coupled to representative discrete compounds.
4. Conclusions
This paper showcases the new PINAAttalla method for estimating the PINA distribution of pseudocomponents within natural gas mixtures classed according to the molecular weight of their C6+ fractions. New correlations were established based on 46 samples of natural gas from the US Gulf Coast, grouped into three classes of mixture: lean gas (C6+ MW < 92 g/mol), rich gas (C6+ MW > 100 g/mol) and average gas. Each class is accompanied by a distinct characterization of PINA distributions for each pseudocomponent up to C14+ together with discrete compounds that should be used to represent each SCN sub fraction. Representative samples of each gas type were fully compositionally characterized to enable determination of the HHC melting temperatures: 263.2 K (14.1 °F), 260.1 K (8.5 °F) and 248.3 K (−12.8 °F) for the lean, rich and average gas samples, respectively. The results of the melting point predictions made using the PINAAttalla method are within 1–2 K of the melting temperature obtained from the full method, while the PINAAPI and PINAEqual methods lead to under estimates of 10 to 20 K. Importantly, this work also shows that PINA-only methods provide superior HHC melting temperature predictions relative to Lump-PINA methods where correlations are used to estimate the thermodynamic properties of the pseudocomponent in the fluid phase.
Thus, this work demonstrates the use of the PINAAttalla method as a robust alternative to the complete characterization of natural gas mixtures to predict freeze-out conditions in cryogenic natural gas processing. This method can be directly applied to the design of new cryogenic gas plants, including LNG plants. Additionally, in operating cryogenic plants the PINAAttalla method could help with the design of process system modifications needed to ensure the safe removal of HHCs at a point upstream of where they would otherwise freeze-out.
The new PINAAttalla method was developed from the detailed compositional analysis of 46 natural gas samples from the US Gulf Coast, and accordingly is likely to be most accurate when applied to LNG plants operating in this region. Future work should explore the efficacy of the PINAAttalla method for natural gas feeding LNG plants in other regions, such as Qatar or Australia, where the PINA distribution within a SCN fraction might be different. However, other features of the PINAAttalla method—namely using representative discrete compounds for SCNs up to C14 in both the solid and fluid phases—suggest that it is likely to still be more accurate than other correlation-based approaches derived from basin-specific data. Exploring the viability of using representative compounds for SCN fractions beyond C14+ to predict freeze-out of HHCs in different hydrocarbon mixture types, such as waxes from crude oils and petroleum fractions might also be worthy of further investigation.