1. Introduction
In 2019, in the EU, transport accounted for almost 30% of total GHG emissions, 72% of which came from road transport [
1]. The European Commission has implemented several mechanisms to reduce global GHG emissions by 55% by 2030, with the aim of becoming climate neutral and achieving net zero GHG emissions by 2050 [
2,
3]. The EU has established a plan to transform European transport for a sustainable future, with the aim of reducing emissions from this sector by 90% by 2050. Directive 2014/94/EU, adopted in 2014 by the EU and the Council, aimed to support the use of alternative fuels in transport to reduce GHG emissions and to reduce the EU’s dependence on oil imports. In Poland, in 2018, the then Ministry of Energy, referring to Directive 2014/94/EU, published the document
Law on Electromobility and Alternative Fuels [
4]. Currently in Polish transport, as a substitute for conventional fuels, mainly Liquefied Petroleum Gas, natural gas in the form of CNG and LNG and electricity are present. However, according to [
4], synthetic and paraffinic fuels, biofuels, and hydrogen are also considered as alternative fuels.
The interest in hydrogen as an energy carrier and the efforts to replace fossil fuels is due to its favorable characteristics: reacting with oxygen releases a large amount of energy (−143.1 MJ/kg), and the only reaction product is water [
5]. Currently, hydrogen is mainly produced from fossil fuels [
6,
7], with about three-quarters of the world’s production coming from natural gas. The second most important raw material for hydrogen production is coal. Approximately 107 Mt of coal is used per year to produce hydrogen, which represents about 2% of its world’s consumption [
8]. The most widely used technologies for producing hydrogen include: the conversion of natural gas and light hydrocarbons [
9,
10], water electrolysis [
11], biomass gasification [
12] and coal gasification [
13] where coal gasification systems can be integrated to produce both electricity and chemical products, mainly liquid motor fuels, methanol or just hydrogen [
14]. According to [
15] and a comparative analysis, biomass hydrogen production provides approximately the same efficiency as water-based hydrogen production technologies, but has the advantages of lower operating costs and higher energy efficiency. Sustainable energy production using renewable feedstocks to produce hydrogen, as opposed to conventional fossil fuel-based feedstocks, will achieve the goal of a green hydrogen economy. When one looks at the coal utilization market, China is the biggest leader in this issue, as well as in the chemical feedstock processing technologies themselves. A high-efficiency industrial pulverized coal boiler is currently being investigated, with thermal efficiencies above 90% and emission levels close to the standard of a natural gas boiler [
16]. Coal conversion technologies such as coal gasification, coal-to-liquid (CTL) and coal-to-oil transition (CTO) are being rapidly developed in China. Direct coal liquefaction (DCL) technology is being developed with the capacity to convert one million tons of coal into oil [
17]. Carbon Capture Utilization and Sequestration (CCUS) technologies are in the start-up phase. Several years of industrial research has been conducted on aspects such as CO
2 capture and use, oxygen-enriched combustion, geological sequestration of high-purity CO
2, enhanced oil recovery (EOR), and enhanced coal bed methane extraction [
18].
Hydrogen for transport is seen as the low-carbon fuel of the future. Hydrogen FCEVs will credibly minimize local air pollution since, similarly to BEVs, they have no exhaust emissions. BEVs are still recognized as zero-emission vehicles by European legislation, although their indirect emissions, that is, vehicle and battery production or disposal and recycling, can be significant [
19]. An FCEV, similar to a BEV, is powered by an electric motor, but the difference lies in the way energy is stored and extracted [
20,
21]. In FCEVs, batteries are replaced by hydrogen tanks, which are converted to electricity and steam through a chemical reaction in the fuel cells. In theory, this is the ideal solution to power a car, but the potential of hydrogen technology is hampered by the high production costs of FCEVs [
22] and the poor infrastructure [
23]. There are currently around 34,400 FCEVs in circulation worldwide, mainly in Korea, the USA, and Japan [
24]. In Europe, FCEVs can be found mainly in Germany and France [
8]. The number of hydrogen refueling stations is small. At the beginning of 2020, hydrogen refueling stations, for road, public, and private transport, represented 432 points on a global scale [
25], including 53 registered in Poland. Poland, on a European scale, produces significant amounts of hydrogen—1 million Mg per year of H
2 (which is 10–15% of European production), but this is used only for chemical synthesis [
26]. The number of electric FCEVs should and can grow steadily, but the demand for these vehicles depends on a number of factors, including the purchase subsidy scheme for FCEVs, the provision of charging infrastructure, and the production and availability of hydrogen fuel. Poland, Germany and the Czech Republic, in the so-called European coal triangle, consume the most coal in the whole EU. Currently, the activities of Polish institutions do not aim at resigning and abandoning coal, but rather at a more rational use of coal, taking into account the entire potential of perspective and alternative products contained in it. Between 2010 and 2015, a scientific and industrial consortium with a budget of 20 million EUR was active in the country, addressing the topic of developing coal gasification technology for highly efficient fuel and energy production [
27]. In Poland, for many years, in accordance with global trends, advanced research on the production and storage of hydrogen has been and is being conducted.
During the design phase of technological processes such as gasification, in addition to technical and economic considerations, environmental aspects must also be taken into account [
28,
29]. LCA is a technique that illustrates the complex interactions that occur between a product or technology and the environment, where the main categories of environmental impact consider the effects on human health, the use of natural resources, and the impact on ecosystem quality [
30,
31]. LCA is recognized as one of the best environmental management tools for comparing the impacts of different alternative products or process systems [
32,
33].
The results of LCA studies on the environmental impacts of UCG show that groundwater is mainly contaminated by phenols and other aromatic compounds, heavy metals, ammonia and cyanides [
34,
35]. Similar results were obtained for UCG simulations in an ex situ reactor [
36]. Hyder et al. [
37] showed that GHG emissions from UCG are about 28% lower than those from conventional power plants. Burchart et al. [
38] showed that, during energy production through UCG, direct CO
2 emissions from gas combustion and indirect emissions from domestic electricity consumption have the greatest impact on the damage categories. Śliwińska et al. [
39] evaluated the LCA of methanol and electricity production in the vicinity of a co-production system based on above-ground coal gasification technology. The analysis was for a polygeneration technology where two products are produced—methanol and electricity—so an allocation procedure was used. Cetinkaya [
40] presented a comprehensive LCA assessment for coal gasification and four other hydrogen production methods, i.e., natural gas steam reforming, wind-based water electrolysis, solar electrolysis and thermochemical water splitting of the Cu-Cl cycle. Natural gas steam reforming, coal gasification and thermochemical water splitting of the Cu-Cl cycle were shown to be preferred over renewable methods in terms of hydrogen production efficiency. [
41] analyzed the LCA for the power generation technology based on above-ground coal gasification in terms of greenhouse gas emissions, mainly CO
2. A total of two approaches to LCA calculation were compared for the construction and operation of integrated gasification combined cycle power plants—IGCC operating with coal gasification. IGCC with 90% CO
2 capture was shown to have lower life cycle GHG emissions than combined cycle natural gas, NGCC and photovoltaic systems. [
42] analyzed energy consumption and GHG emissions between underground hydrogen-oriented coal gasification, UCG-H2 and conventional surface coal gasification (SCG) based on hydrogen production (SCG-H2). Energy consumption in the hydrogen industry has been shown to decrease by 38.8% when hydrogen production is replaced by UCG with CCS to fully meet the demand of 21 Mt in 2030. In [
43], the authors simulated a coal-to-hydrogen conversion process based on gasification of the agglomerating fluidized bed (AFB). A life cycle, primary fossil energy consumption (PFEC) and GHG emissions analysis was conducted to provide theoretical guidance for the development of a coal-to-hydrogen conversion process. The results indicate that the PFEC of the scenario with CCS is 2.32% higher than the corresponding value of the scenario without CCS, while the GHG emissions of the scenario with CCS are 81.72% lower than the corresponding value of the scenario without CCS.
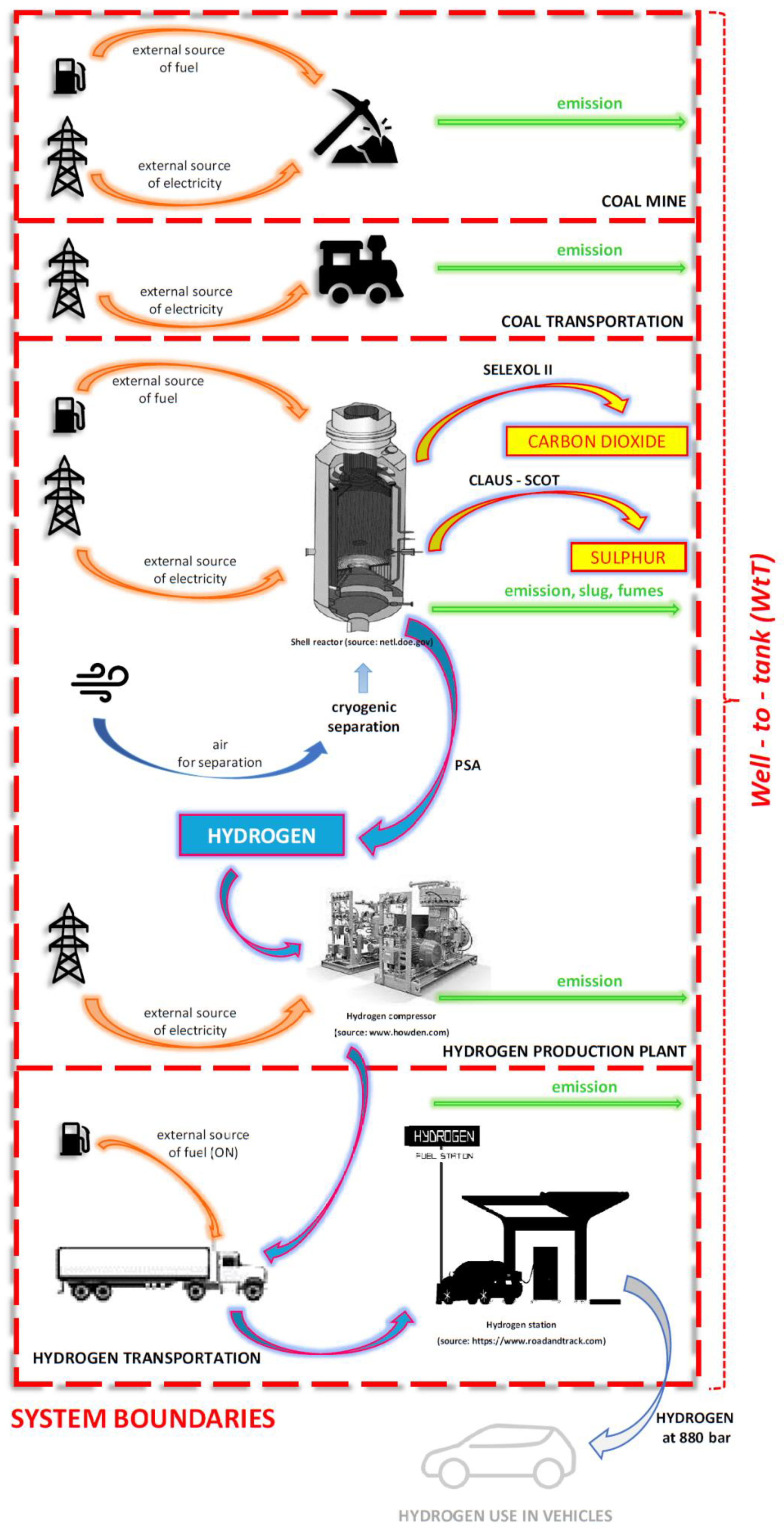
In the literature on LCAs of hydrogen production, little work is focused on coal gasification, in contrast to the numerous comprehensive analyses of other processes for producing this fuel. This article presents a life cycle analysis of hydrogen produced by coal gasification with Shell technology. The LCA was performed within the system boundaries under the weel-to-tank assumption, taking into account the comprehensiveness of the various categories of environmental impacts recommended by the Guidance Document for the performance of LCAs in fuel cells and H₂ Technologies and the CML baseline method. The originality of the work is mainly due to the use of process assumptions, real data and balance streams (energy and mass) derived from Shell’s own studies and process modelling for Shell’s hydrogen-focused technology in the LCA. This type of study has not been published to date. Currently, the scientific literature on the topic focuses mainly on databases. This thesis fills a gap in LCA studies of hydrogen production technology by coal gasification for fossil systems. The work presented here is a critical environmental LCA of the production of gray hydrogen as a fuel for FCEVs, which is extremely important, especially for the countries of the so-called European Carbon Triangle. Poland, the Czech Republic and Germany need gray hydrogen from coal for a fair and sustainable energy transition to green hydrogen in order to become independent of oil and gas imports. The results of the comprehensive LCA presented in this paper allow for a substantive discussion of the topic of the impact of coal gasification technology aimed at hydrogen production, which so far has mainly been considered through the prism of green-use gases. This paper presents the results for the following LCIA impact categories: global warming potential, GHG; acidification, AP; abiotic resource depletion, ADP in [MJ] and [kg]; eutrophication potential, EP; and optional indicators: stratospheric ozone depletion, ODP; human toxicity potential, HTP; inland water toxicity potential, FAETP; aquatic toxicity potential, MAETP; soil toxicity potential, TETP and photo-oxidant formation, POCP.
The purpose of this paper is a comprehensive LCA of hydrogen production as an alternative fuel by coal gasification. The analysis includes hydrogen production through Shell coal gasification technology and its compression and transportation to the distribution point. The analysis was performed for two variants of the hydrogen production—with and without the integration of the system with a CO
2 capture and compression. The choice of the Shell technology as an object of the LCA study was guided by earlier results of the authors [
44], who, in a study concerning the carbon footprint of lignite and hard coal gasification by Shell and Texaco technologies, found that Shell technology is the most beneficial in terms of GHG emissions and hydrogen yield. Furthermore, none of the results of the LCA cited in the literature, in contrast to this publication, used the guidelines contained in the FC-Hy Guide.
3. Results and Discussion
The results of the LCA of hydrogen production from coal gasification and its application to FCEVs in terms of FU are shown in
Figure 4,
Figure 5 and
Figure 6 and
Table 7.
Figure 4 compares the effect of using H
2 seq./non-seq. on the relative change in environmental indicators captured by the CML method. According to the FC-Hy Guide for Fuel Cells and H₂ Technologies LCA guide, it should be mandatory to use 4 of the 11 midpoint impact categories when conducting the LCA, viz., GHG, AP, ADP and EP. The additional impacts used in this study are optional. The absolute values of the indicators presented are collected in
Table 7. GHG is a generally and globally accepted impact category to describe climate change. This category shows a significant advantage of H
2 seq. production technology, which reduces GHG emissions by about 44% from 34.8 kgCO
2(eq)/FU to only 19.5 kgCO
2(eq)/FU.
Figure 5 shows the structure of the GHG categories for H
2 seq. technology, showing the four main dominant causes of emissions: direct emissions at the gasification installation (residual gases); indirect emissions resulting from the consumption of electric energy during extraction and enrichment operations and those at the gasification installation; and the cryogenic air separation operation necessary to obtain oxygen for gasifying the raw material.
Figure 6 shows the same impact categories, but excluding sequestration operations (non-seq.). The dominant contribution, over 60%, to GHG here is direct CO
2 emissions from residual gases that are not directed to the SELEXOL process. Equivalent CO
2 emissions, direct and indirect, from coal mining or the use of electricity at the gasification plant contribute approximately 30% to the GHG structure.
When planning an LCA, a technology based on a non-renewable raw material, it is necessary to consider nature conservation and biodiversity in terms of acidification and eutrophication. In this context, the AP and EP impact categories are most relevant. The key pollutants in the AP impact category are SO
X, NH
3 and NO
X, which are major sources of forest and soil damage. In the CML method used in this study, the characterization parameter for the effect of acidification is the potential calculated for air emissions according to the RAINS 10 model, which describes the accumulation of acidifying substances. AP is expressed in kg of SO
2 equivalent emitted. In the AP category, H
2 seq. technology shows a higher environmental impact. The AP values for H
2 seq. and non-seq. technologies are 3.78∙10
−2 and 3.64∙10
−2 kg of SO
2(eq) per FU, respectively (
Table 7). Despite small absolute differences in the AP values of the two technologies, the structure for both technology variants (
Figure 5 and
Figure 6) indicates that the main unit operations that have an impact on this category are: oxygen production by cryogenic air separation, electric energy production and oil combustion for drying raw materials. The difference in AP structure between the considered seq./non-seq. options lies in the appearance of an additional component, resulting from energy consumption for sequestration operations.
EP is the characterization parameter for the eutrophication impact category, which is expressed in general units: kg PO
43−eq. This impact represents the overfertilization of the marine environment and is particularly detrimental to the marine biota.
Figure 4 shows the higher value in the EP category for the H
2 seq technology. In absolute values, these parameters are 5.18∙10
−2 and 5.57∙10
−2 kg PO
43− per FU for H
2 non-seq and seq, respectively. Carbon dioxide sequestration does not affect the structure of the EP (
Figure 5 and
Figure 6). Its value consists mainly of coal mining, electricity production, oxygen generation operation and the energy required to compress hydrogen for transport and distribution.
The abiotic resource depletion index, ADP, is related to the protection of living standards, human health and ecosystem quality. The ADP is related to mineral and energy extraction and is determined for each type of mineral and solid fuel extraction in units of kg of Sb equivalent/kg of metal or MJ. Similar to the acidification and eutrophication indices, several percent higher ADP values are observed when seq. is applied. In terms of energy depletion, the ADP value increases from 405 to 414 MJ/FU, while, in terms of mineral depletion, from 1.54∙10−5 to 1.61∙10−5 kg of Sb(eq)/FU. The main contribution to the value of this indicator (about 80%) in terms of energy depletion is the use of hard coal, while in terms of the depletion of mineral resources, it is the production of oxygen and electricity.
The presented analysis shows that the use of carbon dioxide sequestration in hydrogen production results in a reduction in the global warming impact of this technology by almost half, but at the same time results in an increase in other environmental impact indicators, with increments of less than 10%. The GHG emission factors available in the literature for hydrogen production from coal gasification range from 0.1129 through 11.29 kg CO
2-eq/kg H
2 for SCG [
40], to as high as 18.00 kg CO
2-eq/kg H
2 for UCG technologies with seq. and non-seq. [
83]. The GHG emissions factors of the present study are higher than those from the literature review. The compared GHG emissions quantities differ in the system assumptions of the LCA and, first of all, in the different H
2 production technologies. According to [
46], the results obtained for both UCG and SCG technologies were comparable and accounted for 207.582 and 197.419 kg CO
2-eq, respectively, for an assumed FU of 13.393 kg H
2 using sequestration. GHG emissions for SCG and UCG had a similar structure, where the dominant component was emissions from the primary hydrogen production stage (installation), accounting for 92.6% and 99.7% of total emissions, respectively. The authors of the study demonstrated the relevance of CO
2 sequestration. GHG emissions for SCG and UCG accounted for 416.362 and 547.717 kg CO
2-eq, respectively, without sequestration operations. Comparing the results of the LCA presented by [
42] with the results of this analysis, it is correct to make a comparison only for the SCG technology using sequestration. The LCA presented by [
42] focuses only on the GHG impact category, and, relative to this category, the Shell technology (of the present analysis) incorporating CO
2 sequestration is not very different. By omitting the sequestration operation during hydrogen production, Shell technology is about 50% more carbon-intensive compared to SCG analyzed by [
42]. The analysis performed by [
42] included: (i) coal extraction and cleaning; (ii) raw material transport; and (iii) hydrogen production. In the case of the present analysis, these boundaries were extended to include fuel compression, its transport to dispensers (trucks back and forth) and fuel cooling processes, which may have influenced the differences in GHG emissions of the compared analyses. Furthermore, GHG emissions are also influenced by coal composition (Shell-Europe; SCG-Asia), gasification technological assumptions, syngas composition for simulated H
2 production from reactors and emission factors for national energy mixes.
LCAs presented by [
84] indicate the environmental advantage in terms of GHG emissions from biomass gasification, as a source of hydrogen for FCEVs, over methane steam reforming and wind energy technologies. The GHG emissions for each technology were 0.46; 1.17 and 11.51 kg CO
2-eq./kg H
2, respectively. For the hydrogen produced by Shell technology, in this study, the values of this indicator were 19.5 kg CO
2-eq./kg H
2 for seq. and 34.8 kg CO
2-eq./kg H
2 for non-seq. The environmental impact of the AP of the analysis [
84] is of the order of 2.03∙10
−2; 1.61∙10
−2 and 1.13∙10
−2 kg SO
2 eq for the methane steam reforming, biomass gasification and wind energy technologies, respectively, and they are comparable with results obtained in the presented analysis. Valente et al. [
84] adopted wider LCA system boundaries than the present ones: hydrogen fuel production, vehicle production and vehicle operation (i.e., the use phase of both fuel and vehicle, including maintenance and servicing). The differences in AP and GHG for the compared methane steam reforming and Shell technologies may be due to both the width of the system boundaries, the difference in fossil fuel, the difference in hydrogen production technology and the input/output data used for the LCA. The work of [
84] carried out analyses based on databases only, while, in the present LCA, we acted on real industrial data and our own data from balances and process streams modelled in ChemCad. An LCA study by [
85], in Germany, assessed the costs and environmental impacts of battery electric vehicles and FCEVs, assuming that hydrogen is compressed to the 70.0 MPa pressure needed to reach a fuel temperature of −40 °C. Although the work focused on H
2 of water electrolysis origin, such a comparison (with the Shell technology of this study) may also be valuable. The similarity in the analyses compared was solely due to the use of two identical categories of GHG and HTTP impact, modelled, however, using different methods: ReCiPe for [
85] and CML baseline for the present analysis. The authors of the cited work assumed broad system boundaries compared to the present work, considering the manufacture and disposal of cells and vehicles. GHG emissions for the FCEVs at an FU of 1 km, being 0.168 kg CO
2-eq. from [
85]
, is not significantly different from the lowest GHG value in this study for sequestration at an FU recalculated to 1 km (0.185 kg CO
2-eq.). When comparing the GHG factor presented by [
85] with the GHG results of the present study, one sees emission differences of 1.7 kg CO
2eq. to the disadvantage of the Shell technology. The HTTP impact category (in kg 1.4-DCB eq) for (Bekel and Pauliuk, 2019a) was 0.683 for an FU of 1 km, while, in the present analysis, it was at 6.1∙10
−2 and 6.48∙10
−2 for non-seq. and seq. for the same FU. The limitations and assumptions of the compared LCA methods should be kept in mind, but the impact of the HTTP category of the Shell technology seems to have more favorable environmental values.
4. Conclusions
The LCA of hydrogen fuel production by the hard coal gasification process in the Shell reactor showed that the implementation of carbon dioxide sequestration causes a significant reduction in GHG emissions and thus reduces the global carbon footprint of this energy carrier. However, carbon dioxide sequestration simultaneously increases the values of other environmental impact indicators. By applying sequestration, it is possible to reduce the GHG emissions index for the assumed FU by approximately 44%, from 34.8 kg CO2-eq to 19.5 kg CO2-eq, but this involves a concomitant increase in the acidification potential index, AP, by approximately 4% from 3.64∙10−2 to 3.78∙10−2 kg SO2-eq, the eutrophication potential index, EP, by 7.5% from 5.18∙10−2 kg to 5.57∙10−2 kg PO3−4-eq and the ADP depletion index by approximately 2% from 405 MJ to 414 MJ and by about 5% from 1.54∙10−5 to 1.61∙10−5 kg Sbeq. The increase in environmental impact category indicators is related to the increased consumption of electricity for the compression and injection of carbon dioxide, which in Poland is produced mainly based on fossil fuels. However, considering the high weight of the categories of greenhouse gases in relation to other categories of environmental impact and the high absolute reduction in greenhouse gas emissions, it can be concluded that the application of carbon dioxide sequestration technology in the production of hydrogen by Shell coal gasification may have measurable environmental effects, reducing the greenhouse gas emissions of this energy carrier by about 44%. A comparison of the obtained results with LCA studies of hydrogen production available in the literature is not possible because few authors have used the CML method recommended by the FC-Hy Guide. In addition, few works deal with the gasification of fossil fuels, which are so ecologically unpopular and crucial for the rational economic and energy transition of the European countries of the carbon triangle. The production of hydrogen discussed in this LCA, commonly categorized as gray because production is based on the use of fossil fuels, is considered an environmentally unfavorable method of obtaining the fuel. The ongoing scientific and political discussions about hydrogen colors are very interesting but can be equally confusing. In fact, strategic or legislative decisions should not be guided by hydrogen colors, but by the level of CO2 emissions from its production, as well as other environmental impact factors gleaned from comprehensive LCA, preferably on real data. In countries whose power industry is based on coal, as is the case in Poland, the production of hydrogen, as well as any other technology, will be burdened with high values of indirect GHG emissions, related primarily to the production of electricity and/or heat necessary for the process. Therefore, increasing emphasis should be placed on expanding environmental analyses to include impact categories other than GHG, as is the case in this LCA.