Cistus ladanifer as a Potential Feedstock for Biorefineries: A Review
Abstract
1. Introduction
2. Traditional Products from Rockrose
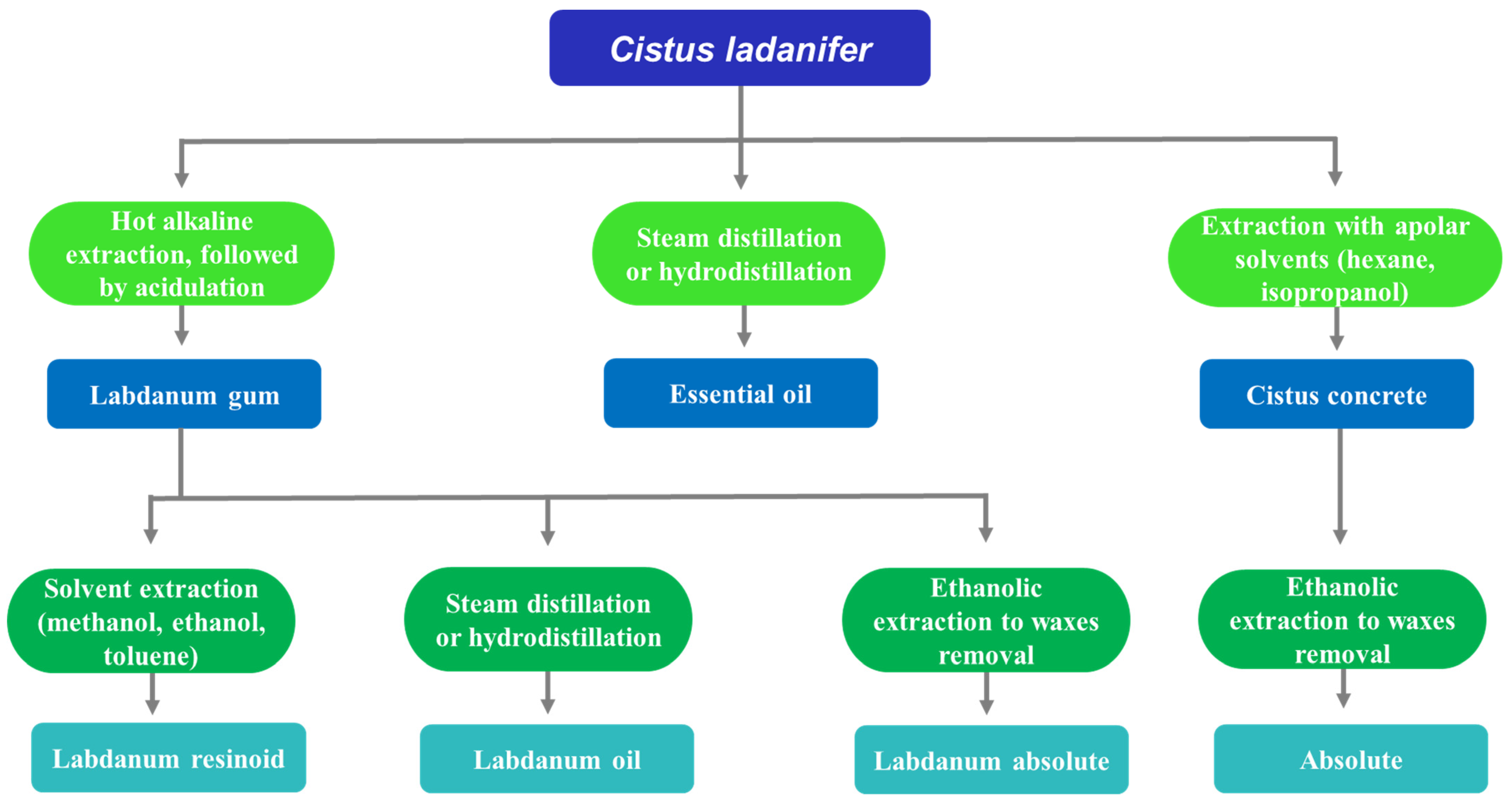
2.1. Aromatic Extracts
2.1.1. Labdanum Gum
2.1.2. Concrete
2.1.3. Essential Oils
2.2. Extractives
3. Bioactivity
3.1. Antimicrobial Activity
3.2. Antioxidant Activity
3.3. Allelopathic Activity
3.4. Antihypertensive Activity
3.5. Antitumoral Activity
3.6. Hypoglycemic and Hypolipidemic Activities
4. Novel and Potential Applications
4.1. Phytoremediation
4.2. Animal Feed
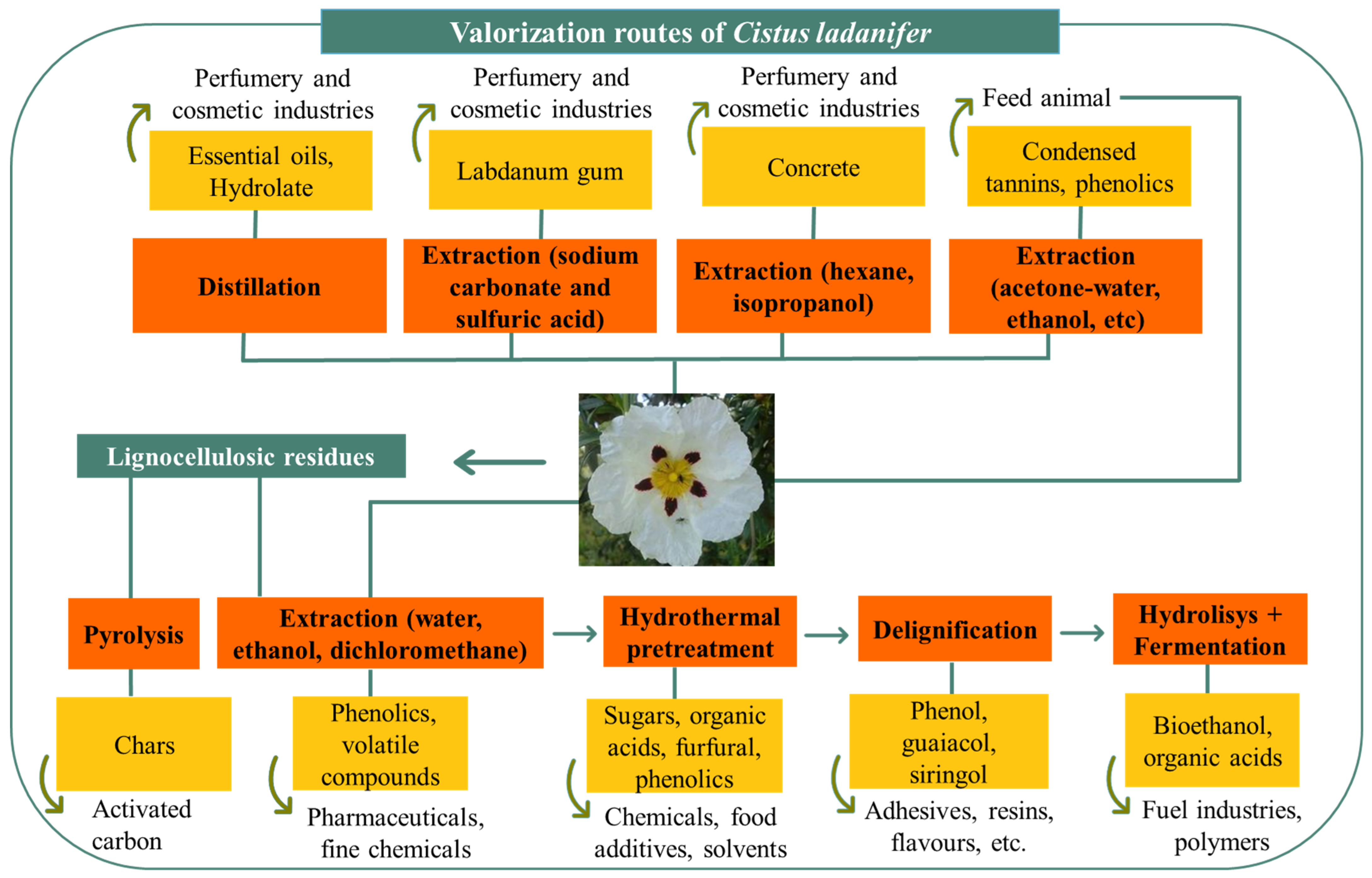
4.3. Added-Value Products from Lignocellulosic Material
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heywood, V.H. (Ed.) Las Plantas Con Flores; Reverté: Barcelona, Spain, 1985. [Google Scholar]
- Weyerstahl, P.; Marschall, H.; Weirauch, M.; Thefeld, K.; Surburg, H. Constituents of Commercial Labdanum Oil. Flavour Fragr. J. 1998, 13, 295–318. [Google Scholar] [CrossRef]
- Ferreira, S.; Duarte, A.P.; Queiroz, J.A.; Domingues, F.C. Experimental Design for Enzymatic Hydrolysis of Cistus ladanifer. J. Biotechnol. 2008, 136, S274–S275. [Google Scholar] [CrossRef]
- Tomas-Menor, L.; Morales-Soto, A.; Barrajon-Catalan, E.; Roldan-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the Antibacterial Activity and the Composition of Extracts Derived from Various Spanish Cistus Species. Food Chem. Toxicol. 2013, 55, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Talavera, S.; Gibbs, P.E.; Herrera, J. Reproductive Biology of Cistus ladanifer (Cistaceae). Plant Syst. Evol. 1993, 186, 123–134. [Google Scholar] [CrossRef]
- Morales-Soto, A.; Oruna-Concha, M.J.; Elmore, J.S.; Barrajón-Catalán, E.; Micol, V.; Roldán, C.; Segura-Carretero, A. Volatile Profile of Spanish Cistus Plants as Sources of Antimicrobials for Industrial Applications. Ind. Crops Prod. 2015, 74, 425–433. [Google Scholar] [CrossRef]
- Guzmán, B.; Vargas, P. Long-Distance Colonization of the Western Mediterranean by Cistus ladanifer (Cistaceae) despite the Absence of Special Dispersal Mechanisms. J. Biogeogr. 2009, 36, 954–968. [Google Scholar] [CrossRef]
- Ferreira, M.R.; Almeida, A.M.; Quintela-Sabarís, C.; Roque, N.; Fernandez, P.; Ribeiro, M.M. The Role of Littoral Cliffs in the Niche Delimitation on a Microendemic Plant Facing Climate Change. PLoS ONE 2021, 16, e0258976. [Google Scholar] [CrossRef]
- Carlier, J.; LeitÃo, J.; Fonseca, F. Population Genetic Structure of Cistus ladanifer L. (Cistaceae) and Genetic Differentiation from Co-Occurring Cistus Species. Plant Species Biol. 2008, 23, 141–151. [Google Scholar] [CrossRef]
- Demoly, J.-P.; Montserrat, P. Cistus; Castroviejo, S., Aedo, C., Laínz, M., Muñoz Garmendia, F., Nieto Feliner, G., Paiva, J., Benedí, C., Eds.; Flora iber; CSIC: Madrid, Spain, 1993.
- Borges, A.E.L. Contribuição Para o Estudo Da Anatomia Da Folha e Caule de Cistus ladanifer L. In Proceedings of the I Jornadas Ibericas de Plantas Medicinales, Aromaticas y de Aceites Esenciales, Madrid, Spain, 12–14 July 1989; pp. 119–128. [Google Scholar]
- Alías, J.C.; Sosa, T.; Escudero, J.C.; Chaves, N. Autotoxicity Against Germination and Seedling Emergence in Cistus ladanifer L. Plant Soil 2006, 282, 327–332. [Google Scholar] [CrossRef]
- Brickell, C. Gardeners’ Encyclopedia of Plants and Flowers; Dorling Kindersley: London, UK, 1989. [Google Scholar]
- Raimundo, J.R.; Frazão, D.F.; Domingues, J.L.; Quintela-Sabarís, C.; Dentinho, T.P.; Anjos, O.; Alves, M.; Delgado, F. Neglected Mediterranean Plant Species Are Valuable Resources: The Example of Cistus ladanifer. Planta 2018, 248, 1351–1364. [Google Scholar] [CrossRef]
- Iriondo, J.M.; Moreno, C.; Pérez, C. Micropropagation of Six Rockrose (Cistus) Species. Hortscience 1995, 30, 1080–1081. [Google Scholar] [CrossRef]
- Kidd, P.S.; Díez, J.; Monterroso Martínez, C. Tolerance and Bioaccumulation of Heavy Metals in Five Populations of Cistus ladanifer L. Subsp. Ladanifer. Plant Soil 2004, 258, 189–205. [Google Scholar] [CrossRef]
- Rossini-Oliva, S.; Mingorance, M.D.; Monaci, F.; Valdés, B. Ecophysiological Indicators of Native Cistus ladanifer L. at Riotinto Mine Tailings (SW Spain) for Assessing Its Potential Use for Rehabilitation. Ecol. Eng. 2016, 91, 93–100. [Google Scholar] [CrossRef]
- Simões, M.P.; Madeira, M.; Gazarini, L. The Role of Phenology, Growth and Nutrient Retention during Leaf Fall in the Competitive Potential of Two Species of Mediterranean Shrubs in the Context of Global Climate Changes. Flora Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 578–589. [Google Scholar] [CrossRef]
- Santos, E.; Abreu, M.M.; Nabais, C.; Saraiva, J. Antioxidant Enzymes Activity of Cistus ladanifer L. from Areas Non-Contaminated in Trace Elements. Rev. Ciênc. Agrár. 2011, 34, 32–43. [Google Scholar]
- Pang, J.; Chan, G.S.Y.; Zhang, J.; Liang, J.; Wong, M.H. Physiological Aspects of Vetiver Grass for Rehabilitation in Abandoned Metalliferous Mine Wastes. Chemosphere 2003, 52, 1559–1570. [Google Scholar] [CrossRef]
- Calvo, L.; Tárrega, R.; Luis, E.; Valbuena, L.; Marcos, E. Recovery after Experimental Cutting and Burning in Three Shrub Communities with Different Dominant Species. Plant Ecol. 2005, 180, 175–185. [Google Scholar] [CrossRef]
- Tárrega, R.; Luis-Calabuig, E.; Valbuena, L. Eleven Years of Recovery Dynamic after Experimental Burning and Cutting in Two Cistus Communities. Acta Oecol. 2001, 22, 277–283. [Google Scholar] [CrossRef]
- Pérez-García, F. Germination of Cistus ladanifer in Relation to Parent Material. Plant Ecol. 1997, 133, 57–62. [Google Scholar] [CrossRef]
- Nuñez, E. Ecología Del Jaral de Cistus ladanifer L.; Universidad de Extremadura: Badajoz, Spain, 1989. [Google Scholar]
- Gallego, J.C.A. Influencia de Los Factores Climáticos En La Síntesis y Actividad de Compuestos Fitotóxicos Secretados Por Cistus ladanifer L.; Universidad de Extremadura: Badajoz, Spain, 2006. [Google Scholar]
- Delgado, J.A.; Serrano, J.M.; López, F.; Acosta, F.J. Seed Size and Seed Germination in the Mediterranean Fire-Prone Shrub Cistus Ladanifer. Plant Ecol. 2007, 197, 269–276. [Google Scholar] [CrossRef]
- Narbona, E.; Guzmán, B.; Arroyo, J.; Vargas, P. Why Are Fruit Traits of Cistus ladanifer (Cistaceae) so Variable: A Multi-Level Study across the Western Mediterranean Region. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 305–315. [Google Scholar] [CrossRef]
- Greche, H.; Mrabet, N.; Zrira, S.; Ismaïli-Alaoui, M.; Benjilali, B.; Boukir, A. The Volatiles of the Leaf Oil of Cistus ladanifer L. Var. Albiflorus and Labdanum Extracts of Moroccan Origin and Their Antimicrobial Activities. J. Essent. Oil Res. 2009, 21, 166–173. [Google Scholar] [CrossRef]
- Gomes, P.B.; Mata, V.G.; Rodrigues, A.E. Characterization of the Portuguese-Grown Cistus ladanifer Essential Oil. J. Essent. Oil Res. 2005, 17, 160–165. [Google Scholar] [CrossRef]
- Teixido, A.L.; Méndez, M.; Valladares, F. Flower Size and Longevity Influence Florivory in the Large-Flowered Shrub Cistus ladanifer. Acta Oecol. 2011, 37, 418–421. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.; de-Miguel, S.; Pukkala, T.; Oria-de-Rueda, J.A.; Martín-Pinto, P. Climate-Sensitive Models for Mushroom Yields and Diversity in Cistus ladanifer Scrublands. Agric. For. Meteorol. 2015, 213, 173–182. [Google Scholar] [CrossRef]
- Santos, E.S.; Balseiro-Romero, M.; Abreu, M.M.; Macías, F. Bioextracts of Cistus ladanifer L. Growing in São Domingos Mine as Source of Valuable Compounds. J. Geochem. Explor. 2016, 174, 84–90. [Google Scholar] [CrossRef]
- Pérez, P.; Saúl, L.; Ciria, M.P. Distribución Geográfica, Caracterización Ecológica y Evaluación de Cistus laurifolius y Cistus ladanifer. Estudios Sobre El Matorral Como Recurso Energético. Agroenerg. Biomassa Vida Rural 2011, 331, 66–70. [Google Scholar]
- Lourenço, K.R.; Costa, M.C.; Palma, M.L. Possibilidades Terapêuticas da Esteva (Cistus ladanifer L.). Rev. Fitoter. 2015, 15, 115–126. [Google Scholar]
- Morgado, J.M.; Tapias, R.; Alesso, P. Producción de Goma Bruta de Jara (Cistus ladanifer L.) En El Suroeste de La Península Ibérica. In Proceedings of the Actas 4 Congreso Forestal Español, Zaragoza, Spain, 26–30 September 2005. [Google Scholar]
- Barrajón-Catalán, E.; Tomás-Menor, L.; Morales-Soto, A.; Bruñá, N.M.; López, D.S.; Segura-Carretero, A.; Micol, V. Rockroses (Cistus sp.) Oils In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780124166417. [Google Scholar]
- Biolandes Cistus Labdanum in Andalusia. Available online: https://www.biolandes.com/catalogue-extraits-naturels/ (accessed on 1 February 2022).
- De Andrés, A.I.; Gómez-Serranillos, M.P.; Iglesias, I.; Villar, A.M. Effects of Extract of Cistus populifolius L. on the Central Nervous System. Phytother. Res. 1999, 13, 575–579. [Google Scholar] [CrossRef]
- Stübing, G.; Peris, J.B. Plantas Medicinales de La Comunidad Valenciana; Generalitat Valenciana, Conselleria de Medio Ambiente: Valencia, Spain, 1998.
- Nunes, M.C.S.; Vasconcelos, M.J.; Pereira, J.M.C.; Dasgupta, N.; Alldredge, R.J.; Rego, F.C. Land Cover Type and Fire in Portugal: Do Fires Burn Land Cover Selectively? Landsc. Ecol. 2005, 20, 661–673. [Google Scholar] [CrossRef]
- Castro, H.; Freitas, H. Above-Ground Biomass and Productivity in the Montado: From Herbaceous to Shrub Dominated Communities. J. Arid Environ. 2009, 73, 506–511. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.; Oria-de-Rueda, J.A.; Martín-Pinto, P. Post-Fire Fungal Succession in a Mediterranean Ecosystem Dominated by Cistus ladanifer L. For. Ecol. Manag. 2013, 289, 48–57. [Google Scholar] [CrossRef]
- Deforce, K. The Historical Use of Ladanum. Palynological Evidence from 15th and 16th Century Cesspits in Northern Belgium. Veg. Hist. Archaeobot. 2005, 15, 145–148. [Google Scholar] [CrossRef]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A Model for Exploring Labdane-Type Diterpenes’ Biosynthesis and a Natural Source of High Value Products with Biological, Aromatic, and Pharmacological Properties. Front. Chem. 2014, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Frazão, D.F.; Martins-Gomes, C.; Steck, J.L.; Keller, J.; Delgado, F.; Gonçalves, J.C.; Bunzel, M.; Pintado, C.M.B.S.; Díaz, T.S.; Silva, A.M. Labdanum Resin from Cistus ladanifer L.: A Natural and Sustainable Ingredient for Skin Care Cosmetics with Relevant Cosmeceutical Bioactivities. Plants 2022, 11, 1477. [Google Scholar] [CrossRef]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 5th ed.; Surburg, H., Panten, J., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2006; ISBN 3-527-31315-X. [Google Scholar]
- Pereira, C.G.; Meireles, M.A.A. Supercritical Fluid Extraction of Bioactive Compounds: Fundamentals, Applications and Economic Perspectives. Food Bioprocess Technol. 2009, 3, 340–372. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils-A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Grand View Research Essential Oils Market-Market Analysis, 2017–2028; Grand View Research, Inc.: San Francisco, CA, USA, 2021.
- Adam, K.L. Lavender Production, Products, Markets, and Entertainment Farms. Natl. Sustain. Agric. Inf. Serv. 2006, 5, 2006. [Google Scholar]
- Teixeira, S.; Mendes, A.; Alves, A.; Santos, L. Simultaneous Distillation-Extraction of High-Value Volatile Compounds from Cistus ladanifer L. Anal. Chim. Acta 2007, 584, 439–446. [Google Scholar] [CrossRef]
- Verdeguer, M.; Blazquez, M.A.; Boira, H. Chemical Composition and Herbicidal Activity of the Essential Oil from a Cistus ladanifer L. Population from Spain. Nat. Prod. Res. 2012, 26, 1602–1609. [Google Scholar] [CrossRef]
- Zidane, H.; Elmiz, M.; Aouinti, F.; Tahani, A.; Wathelet, J.; Sindic, M.; Elbachiri1, A. Chemical Composition and Antioxidant Activity of Essential Oil, Various Organic Extracts of Cistus ladanifer and Cistus libanotis Growing in Eastern Morocco. Afr. J. Biotechnol. 2013, 12, 5314–5320. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Sendra, E.; Alvarez, J.A.P.; Fernández-López, J.; Amensour, M.; Abrini, J. Identification of Flavonoid Content and Chemical Composition of the Essential Oils of Moroccan Herbs: Myrtle (Myrtus communis L.), Rockrose (Cistus ladanifer L.) and Montpellier Cistus (Cistus monspeliensis L.). J. Essent. Oil Res. 2011, 23, 1–9. [Google Scholar] [CrossRef]
- Mariotti, J.P.; Tomi, F.; Casanova, J.; Costa, J.; Bernardini, A.F. Composition of the Essential Oil of Cistus ladaniferus L. Cultivated in Corsica (France). Flavour Fragr. J. 1997, 12, 147–151. [Google Scholar] [CrossRef]
- Robles, C.; Bousquet-Mélou, A.; Garzino, S.; Bonin, G. Comparison of Essential Oil Composition of Two Varieties of Cistus ladanifer. Biochem. Syst. Ecol. 2003, 31, 339–343. [Google Scholar] [CrossRef]
- Peakall, D.B. Phthalate Esters: Occurrence and Biological Effects. In Residue Reviews; Gunther, F.A., Gunther, J.D., Eds.; Springer: New York, NY, USA, 1975; pp. 1–41. [Google Scholar]
- Manayi, A.; Kurepaz-Mahmoodabadi, M.; Gohari, A.R.; Ajani, Y.; Saeidnia, S. Presence of Phthalate Derivatives in the Essential Oils of a Medicinal Plant Achillea tenuifolia. DARU J. Pharm. Sci. 2014, 22, 78. [Google Scholar] [CrossRef]
- Fromme, H.; Küchler, T.; Otto, T.; Pilz, K.; Müller, J.; Wenzel, A. Occurrence of Phthalates and Bisphenol A and F in the Environment. Water Res. 2002, 36, 1429–1438. [Google Scholar] [CrossRef]
- Tümay Özer, E.; Osman, B.; Kara, A.; Beşirli, N.; Gücer, Ş.; Sözeri, H. Removal of Diethyl Phthalate from Aqueous Phase Using Magnetic Poly(EGDMA-VP) Beads. J. Hazard. Mater. 2012, 229–230, 20–28. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhao, Z.; Zhu, L.; Chen, Y.; Ji, H.; Yang, D. Removal of Three Kinds of Phthalates from Sweet Orange Oil by Molecular Distillation. LWT Food Sci. Technol. 2013, 53, 487–491. [Google Scholar] [CrossRef]
- Anitescu, G.; Doneanu, C.; Radulescu, V. Isolation of Coriander Oil: Comparison between Steam Distillation and Supercritical CO2 Extraction. Flavour Fragr. J. 1997, 12, 173–176. [Google Scholar] [CrossRef]
- Ferraz, C.A.; Sousa, A.C.A.; Caramelo, D.; Delgado, F.; de Oliveira, A.P.; Pastorinho, M.R. Chemical Profile and Eco-Safety Evaluation of Essential Oils and Hydrolates from Cistus ladanifer, Helichrysum italicum, Ocimum basilicum and Thymbra capitata. Ind. Crops Prod. 2022, 175, 114232. [Google Scholar] [CrossRef]
- European Chemicals Agency-ECHA Essential Oil of Cistus ladaniferus L. (Cistaceae) Obtained from Stems and Leaves by Distillation. Available online: https://www.echa.europa.eu/web/guest/registration-dossier/-/registered-dossier/21887/6/2/4 (accessed on 5 June 2022).
- Tavares, C.S.; Martins, A.; Faleiro, M.L.; Miguel, M.G.; Duarte, L.C.; Gameiro, J.A.; Roseiro, L.B.; Figueiredo, A.C. Bioproducts from Forest Biomass: Essential Oils and Hydrolates from Wastes of Cupressus lusitanica Mill. and Cistus ladanifer L. Ind. Crops Prod. 2020, 144, 112034. [Google Scholar] [CrossRef]
- Pereira, H.; Graça, J.; Rodrigues, J.C. Wood Chemistry in Relation to Quality. In Wood Quality and Its Biological Basis; Barnett, J.R., Jeronimidis, G., Eds.; Blackwell Publishing: Oxford, UK, 2003; ISBN 1-84127-319-8. [Google Scholar]
- Rowe, J.W.; Conner, A.H. Extractives in Eastern Hardwoods-A Review; U.S. Department of Agriculture: Madison, WI, USA, 1979.
- Brusotti, G.; Cesari, I.; Dentamaro, A.; Caccialanza, G.; Massolini, G. Isolation and Characterization of Bioactive Compounds from Plant Resources: The Role of Analysis in the Ethnopharmacological Approach. J. Pharm. Biomed. Anal. 2014, 87, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, P.S.; de Freitas, V.A.P.; Macedo, A.; Silva, G.; Silva, A.M.S. Volatile Components Of Cistus ladanifer Leaves. Flavour Fragr. J. 1999, 14, 300–302. [Google Scholar] [CrossRef]
- Chaves, N.; Ríos, J.J.; Gutierrez, C.; Escudero, J.C.; Olías, J.M. Analysis of Secreted Flavonoids of Cistus ladanifer L. by High-Performance Liquid Chromatography–Particle Beam Mass Spectrometry. J. Chromatogr. A 1998, 799, 111–115. [Google Scholar] [CrossRef]
- Fernandez-Arroyo, S.; Barrajon-Catalan, E.; Micol, V.; Segura-Carretero, A.; Fernandez-Gutierrez, A. High-Performance Liquid Chromatography with Diode Array Detection Coupled to Electrospray Time-of-Flight and Ion-Trap Tandem Mass Spectrometry to Identify Phenolic Compounds from a Cistus ladanifer Aqueous Extract. Phytochem. Anal. 2010, 21, 307–313. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Alves, C.T.; Silva, S.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C. Antifungal Activity and Detailed Chemical Characterization of Cistus ladanifer Phenolic Extracts. Ind. Crops Prod. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. Phenolic Compd. Biol. Act. 2017, 8, 1–24. [Google Scholar] [CrossRef]
- Sosa, T.; Alías, J.C.; Escudero, J.C.; Chaves, N. Interpopulational Variation in the Flavonoid Composition of Cistus ladanifer L. Exudate. Biochem. Syst. Ecol. 2005, 33, 353–364. [Google Scholar] [CrossRef]
- Herranz, J.M.; Ferrandis, P.; Copete, M.A.; Duro, E.M.; Zalacaín, A. Effect of Allelopathic Compounds Produced by Cistus ladanifer on Germination of 20 Mediterranean Taxa. Plant Ecol. 2005, 184, 259–272. [Google Scholar] [CrossRef]
- Barrajon-Catalan, E.; Fernandez-Arroyo, S.; Saura, D.; Guillen, E.; Fernandez-Gutierrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae Aqueous Extracts Containing Ellagitannins Show Antioxidant and Antimicrobial Capacity, and Cytotoxic Activity against Human Cancer Cells. Food Chem. Toxicol. 2010, 48, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Alías, J.C.; Sosa, T.; Valares, C.; Escudero, J.C.; Chaves, N. Seasonal Variation of Cistus ladanifer L. Diterpenes. Plants 2012, 1, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Escudero, J.C.; Gutierrez-Merino, C. Role of Ecological Variables in the Seasonal Variation of Flavonoid Content of Cistus ladanifer Exudate. J. Chem. Ecol. 1997, 23, 579–603. [Google Scholar] [CrossRef]
- Guerreiro, O.; Dentinho, M.T.P.; Moreira, O.C.; Guerra, A.R.; Ramos, P.A.B.; Bessa, R.J.B.; Duarte, M.F.; Jerónimo, E. Potential of Cistus ladanifer L. (Rockrose) in Small Ruminant Diets–Effect of Season and Plant Age on Chemical Composition, in Vitro Digestibility and Antioxidant Activity. Grass Forage Sci. 2016, 71, 437–447. [Google Scholar] [CrossRef]
- Micco, V.; Aronne, G. Anatomical Features, Monomer Lignin Composition and Accumulation of Phenolics in 1-year-old Branches of the Mediterranean Cistus ladanifer L. Bot. J. Linn. Soc. 2007, 155, 361–371. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Sousa, M.J.; Morais, J.S.; Ferreira, I.C. Aromatic Plants as a Source of Important Phytochemicals: Vitamins, Sugars and Fatty Acids in Cistus ladanifer, Cupressus lusitanica and Eucalyptus gunnii Leaves. Ind. Crops Prod. 2009, 30, 427–430. [Google Scholar] [CrossRef]
- Guerreiro, O.; Alves, S.P.; Duarte, M.F.; Bessa, R.J.B.; Jerónimo, E. Cistus ladanifer L. Shrub Is Rich in Saturated and Branched Chain Fatty Acids and Their Concentration Increases in the Mediterranean Dry Season. Lipids 2015, 50, 493–501. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Lee, S.; Kang, S.S.; Shin, H.S. Selected Commercial Plants: A Review of Extraction and Isolation of Bioactive Compounds and Their Pharmacological Market Value. Trends Food Sci. Technol. 2018, 82, 89–109. [Google Scholar] [CrossRef]
- Andrade, D.; Gil, C.; Breitenfeld, L.; Domingues, F.; Duarte, A.P. Bioactive Extracts from Cistus ladanifer and Arbutus unedo L. Ind. Crops Prod. 2009, 30, 165–167. [Google Scholar] [CrossRef]
- Belmokhtar, M.; Bouanani, N.E.; Ziyyat, A.; Mekhfi, H.; Bnouham, M.; Aziz, M.; Matéo, P.; Fischmeister, R.; Legssyer, A. Antihypertensive and Endothelium-Dependent Vasodilator Effects of Aqueous Extract of Cistus ladaniferus. Biochem. Biophys. Res. Commun. 2009, 389, 145–149. [Google Scholar] [CrossRef]
- Chaves, N.; Sosa, T.; Escudero, J.C. Plant Growth Inhibiting Flavonoids in Exudate of Cistus ladanifer and in Associated Soils. J. Chem. Ecol. 2001, 27, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.S.; Moreira, I. Interaction between Water Soluble and Volatile Compounds of Cistus ladanifer L. Chemoecology 2002, 12, 77–82. [Google Scholar] [CrossRef]
- Sánchez-Hernández, M.E.; Gutiérrez-García, J.; Trapero-Casas, A. Botryosphaeria canker of Cistus ladanifer. Plant Pathol. 2002, 51, 365–373. [Google Scholar] [CrossRef]
- El Karkouri, J.; Bouhrim, M.; Al Kamaly, O.M.; Mechchate, H.; Kchibale, A.; Adadi, I.; Amine, S.; Ismaili, S.A.; Zair, T. Chemical Composition, Antibacterial and Antifungal Activity of the Essential Oil from Cistus ladanifer L. Plants 2021, 10, 2068. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Sousa, M.J.; Ferreira, I.C.F.R. Contribution of Essential Oils and Phenolics to the Antioxidant Properties of Aromatic Plants. Ind. Crops Prod. 2010, 32, 152–156. [Google Scholar] [CrossRef]
- Gallego, J.C.A.; Masa, C.V.; Díaz, T.S.; Lobón, N.C. Estudio de La Persistencia de Sustancias Alelopáticas En Suelos de Ecosistemas Mediterráneos. Cuad. Soc. Esp. Ciencias For. 2008, 25, 41–45. [Google Scholar]
- Tena, C.; Santiago, A.D.R.; Osuna, D.; Sosa, T. Phytotoxic Activity of P-Cresol, 2-Phenylethanol and 3-Phenyl-1-Propanol, Phenolic Compounds Present in Cistus ladanifer L. Plants 2021, 10, 1136. [Google Scholar] [CrossRef]
- Lobón, N.C.; De La Cruz, I.F.; Gallego, J.C.A. Autotoxicity of Diterpenes Present in Leaves of Cistus ladanifer L. Plants 2019, 8, 27. [Google Scholar] [CrossRef]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising Anticancer Agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- El Kabbaoui, M.; Chda, A.; Azdad, O.; Mejrhit, N.; Aarab, L.; Bencheikh, R.; Tazi, A. Evaluation of Hypoglycemic and Hypolipidemic Activities of Aqueous Extract of Cistus ladaniferus in Streptozotocin-Induced Diabetic Rats. Asian Pac. J. Trop. Biomed. 2016, 6, 1044–1049. [Google Scholar] [CrossRef]
- Hooda, V. Phytoremediation of Toxic Metals from Soil and Waste Water. J. Environ. Biol. 2007, 28, 367–376. [Google Scholar] [PubMed]
- Alvarenga, P.M.; Araújo, M.F.; Silva, J.A.L. Elemental Uptake and Root-Leaves Transfer in Cistus ladanifer L. Growing in a Contaminated Pyrite Mining Area (Aljustrel-Portugal). Water Air Soil Pollut. 2004, 152, 81–96. [Google Scholar] [CrossRef]
- Abreu, M.M.; Santos, E.; Fernandes, E.; Joao, M.; Ferreira, M. Acumulação e Translocação de Elementos Vestigiais Em Cistus ladanifer L. de Áreas Mineiras Da FPI Portuguesa. Rev. Ciênc. Agrár. 2011, 34, 44–56. [Google Scholar]
- Santos, E.S.; Abreu, M.M.; Nabais, C.; Magalhães, M.C.F. Trace Element Distribution in Soils Developed on Gossan Mine Wastes and Cistus ladanifer L. Tolerance and Bioaccumulation. J. Geochem. Explor. 2012, 123, 45–51. [Google Scholar] [CrossRef]
- Abreu, M.M.; Magalhães, M.C. Phytostabilization of Soils in Mining Areas. Case Studies from Portugal. In Soil Remediation; Aachen, L., Eichmann, P., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2009; pp. 297–344. [Google Scholar]
- Jerónimo, E.; Alves, S.P.; Dentinho, M.T.; Martins, S.V.; Prates, J.A.; Vasta, V.; Santos-Silva, J.; Bessa, R.J. Effect of Grape Seed Extract, Cistus ladanifer L., and Vegetable Oil Supplementation on Fatty Acid Composition of Abomasal Digesta and Intramuscular Fat of Lambs. J. Agric. Food Chem. 2010, 58, 10710–10721. [Google Scholar] [CrossRef]
- Jerónimo, E.; Alfaia, C.M.; Alves, S.P.; Dentinho, M.T.; Prates, J.A.; Vasta, V.; Santos-Silva, J.; Bessa, R.J. Effect of Dietary Grape Seed Extract and Cistus ladanifer L. in Combination with Vegetable Oil Supplementation on Lamb Meat Quality. Meat Sci. 2012, 92, 841–847. [Google Scholar] [CrossRef]
- Zamora-Lozano, M.; Mata-Moreno, C.; Martínez-Teruel, A.; Gómez-Castro, A.G.; Peinado Lucena, E.; Medina-Blanco, M. Utilización de Cistus ladanifer (L.) En Piensos Para Conejos. Arch. Zootec. 1984, 33, 295–300. [Google Scholar]
- Dentinho, M.T.P.; Belo, A.T.; Bessa, R.J.B. Digestion, Ruminal Fermentation and Microbial Nitrogen Supply in Sheep Fed Soybean Meal Treated with Cistus ladanifer L. Tannins. Small Rumin. Res. 2014, 119, 57–64. [Google Scholar] [CrossRef]
- Dentinho, M.T.P.; Paulos, K.; Francisco, A.; Belo, A.T.; Jerónimo, E.; Almeida, J.; Bessa, R.J.B.; Santos-Silva, J. Effect of Soybean Meal Treatment with Cistus ladanifer Condensed Tannins in Growth Performance, Carcass and Meat Quality of Lambs. Livest. Sci. 2020, 236, 104021. [Google Scholar] [CrossRef]
- Guerreiro, O.; Alves, S.P.; Soldado, D.; Cachucho, L.; Almeida, J.M.; Francisco, A.; Santos-Silva, J.; Bessa, R.J.B.; Jerónimo, E. Inclusion of the Aerial Part and Condensed Tannin Extract from Cistus ladanifer L. in Lamb Diets–Effects on Growth Performance, Carcass and Meat Quality and Fatty Acid Composition of Intramuscular and Subcutaneous Fat. Meat Sci. 2020, 160, 107945. [Google Scholar] [CrossRef] [PubMed]
- Jerónimo, E.; Soldado, D.; Sengo, S.; Francisco, A.; Fernandes, F.; Portugal, A.P.V.; Alves, S.P.; Santos-Silva, J.; Bessa, R.J.B. Increasing the α-Tocopherol Content and Lipid Oxidative Stability of Meat through Dietary Cistus ladanifer L. in Lamb Fed Increasing Levels of Polyunsaturated Fatty Acid Rich Vegetable Oils. Meat Sci. 2020, 164, 108092. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.M. Hemicellulose Biorefineries: A Review on Biomass Pretreatments. J. Sci. Ind. Res. 2008, 67, 849–864. [Google Scholar]
- Patel, S.; Goyal, A. Functional Oligosaccharides: Production, Properties and Applications. World J. Microbiol. Biotechnol. 2011, 27, 1119–1128. [Google Scholar] [CrossRef]
- Lopes, T.F.; Carvalheiro, F.; Duarte, L.C.; Gírio, F.; Quintero, J.A.; Aroca, G. Techno-Economic and Life-Cycle Assessments of Small-Scale Biorefineries for Isobutene and Xylo-Oligosaccharides Production: A Comparative Study in Portugal and Chile. Biofuels Bioprod. Biorefining 2019, 13, 1321–1332. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Duarte, L.C.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Hydrothermal Treatments of Cistus ladanifer Industrial Residues Obtained from Essential Oil Distilleries. Waste Biomass Valorization 2019, 10, 1303–1310. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Duarte, L.C.; Lourenço, A.; Roseiro, L.B.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Distillery Residues from Cistus ladanifer (Rockrose) as Feedstock for the Production of Added-Value Phenolic Compounds and Hemicellulosic Oligosaccharides. Bioenergy Res. 2019, 12, 347–358. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Miranda, I.; Duarte, L.C.; Roseiro, L.B.; Lourenço, A.; Quilhó, T.; Cardoso, S.; Fernandes, M.C.; Carvalheiro, F.; Pereira, H. Cistus ladanifer as a Source of Chemicals: Structural and Chemical Characterization. Biomass Convers. Biorefinery 2020, 10, 325–337. [Google Scholar] [CrossRef]
- Ferro, M.D.; Fernandes, M.C.; Paulino, A.F.C.; Prozil, S.O.; Gravitis, J.; Evtuguin, D.V.; Xavier, A.M. Bioethanol Production from Steam Explosion Pretreated and Alkali Extracted Cistus ladanifer (Rockrose). Biochem. Eng. J. 2015, 104, 98–105. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent Updates on Different Methods of Pretreatment of Lignocellulosic Feedstocks: A Review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef]
- Balat, M. Production of Bioethanol from Lignocellulosic Materials via the Biochemical Pathway: A Review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Gil, N.; Domingues, F.C.; Amaral, M.E.; Duarte, A.P. Optimization of Diluted Acid Pretreatment of Cytisus striatus and Cistus ladanifer for Bioethanol Production. J. Biobased Mater. Bioenergy 2012, 6, 292–298. [Google Scholar] [CrossRef]
- Ferreira, S.; Duarte, A.P.; Ribeiro, M.H.L.; Queiroz, J.A.; Domingues, F.C. Response Surface Optimization of Enzymatic Hydrolysis of Cistus ladanifer and Cytisus striatus for Bioethanol Production. Biochem. Eng. J. 2009, 45, 192–200. [Google Scholar] [CrossRef]
- Pontes, R.; Romaní, A.; Michelin, M.; Domingues, L.; Teixeira, J.; Nunes, J. Comparative Autohydrolysis Study of Two Mixtures of Forest and Marginal Land Resources for Co-Production of Biofuels and Value-Added Compounds. Renew. Energy 2018, 128, 20–29. [Google Scholar] [CrossRef]
- Pontes, R.; Romaní, A.; Michelin, M.; Domingues, L.; Teixeira, J.; Nunes, J. L-Lactic Acid Production from Multi-Supply Autohydrolyzed Economically Unexploited Lignocellulosic Biomass. Ind. Crops Prod. 2021, 170, 113775. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Lourenço, A.; Morgado, F.; Duarte, L.C.; Roseiro, L.B.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Delignification of Cistus ladanifer Biomass by Organosolv and Alkali Processes. Energies 2021, 14, 1127. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Alves-Ferreira, J.; Duarte, L.C.; Pereira, H.; Carvalheiro, F.; Martínez, A. D-Lactic Acid Production from Hydrothermally Pretreated, Alkali Delignified and Enzymatically Saccharified Rockrose with the Metabolic Engineered Escherichia coli Strain JU15. Biomass Convers. Biorefin. 2022, 1–10. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Carvalheiro, F.; Duarte, L.C.; Ferreira, A.R.P.; Martinez, A.; Pereira, H.; Fernandes, M.C. D-Lactic Acid Production from Cistus Ladanifer Residues: Co-Fermentation of Pentoses and Hexoses by Escherichia coli JU15. Ind. Crops Prod. 2022, 177, 114519. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Nogales-Delgado, S. Catalyzed Steam Gasification of Biomass. Catalysts 2020, 10, 1430. [Google Scholar] [CrossRef]
- Pastor-Villegas, J.; Gómez-Serrano, V.; Durán-Valle, C.J.; Higes-Rolando, F.J. Chemical Study of Extracted Rockrose and of Chars and Activated Carbons Prepared at Different Temperatures. J. Anal. Appl. Pyrolysis 1999, 50, 1–16. [Google Scholar] [CrossRef]
- Pastor-Villegas, J.; Durán-Valle, C.J. Pore Structure of Activated Carbons Prepared by Carbon Dioxide and Steam Activation at Different Temperatures from Extracted Rockrose. Carbon N. Y. 2002, 40, 397–402. [Google Scholar] [CrossRef]
- Rodrigues, A.E.; de Pinto, P.C.O.R.; Barreiro, M.F.; Esteves da Costa, C.A.; Ferreira da Mota, M.I.; Fernandes, I. Chemical Pulp Mills as Biorefineries; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-99312-6. [Google Scholar]
- Moniz, P.; Serralheiro, C.; Matos, C.T.; Boeriu, C.G.; Frissen, A.E.; Duarte, L.C.; Roseiro, L.B.; Pereira, H.; Carvalheiro, F. Membrane Separation and Characterisation of Lignin and Its Derived Products Obtained by a Mild Ethanol Organosolv Treatment of Rice Straw. Process Biochem. 2018, 65, 136–145. [Google Scholar] [CrossRef]
- Sridach, W. The Environmentally Benign Pulping Process of Non-Wood Fibers. Suranaree J. Sci. Technol. 2010, 17, 105–123. [Google Scholar]
- Carvalheiro, F.; Duarte, L.C.; Bogel-lukasik, R.; Moniz, P. Métodos de Fraccionamento de Biomassa para as Biorrefinarias. Bol. Biotecnol. 2013, 3, 7–10. [Google Scholar]

| Component | Centre Interior of Portugal a | Central Spain a | Corsica (Spanish Origin) b | Eastern Morocco a |
|---|---|---|---|---|
| Monoterpene hydrocarbons | ||||
| Tricyclene | - | - | - | 2.7 |
| α-Pinene | 2.1 | 4.70 | 39 | 4.2 |
| Camphene | 0.3 | 0.64 | 2.1 | 15.5 |
| Pinocarvone | 1.1 | - | 0.9 | - |
| Limonene | - | 0.37 | 1.7 | - |
| γ-Terpinene | - | 0.10 | 0.4 | 3.8 |
| α-Terpinene | - | - | 0.1 | 1.8 |
| p-cymenene | - | 1.17 | 1.7 | 2.3 |
| Oxygenated monoterpenes | ||||
| Bornyl acetate | 1.6 | 7.03 | 3.1 | |
| Terpinen-4-ol | 1.0 | 6.37 | 1.1 | 6.3 |
| α-Terpineol | - | 2.20 | - | 1.2 |
| trans-pinocarveol | 2.1 | 20.00 | 1.9 | |
| Borneol | 0.7 | - | 0.8 | 11.1 |
| Myrtenal | 0.7 | 2.26 | 0.5 | - |
| cis-Pinocamphone | - | 3.84 | - | - |
| 2 (10)-Pinen-3-one | - | 5.05 | - | - |
| Verbonene | - | 0.85 | 0.3 | 0.8 |
| Camphor | - | 0.86 | - | 1.5 |
| p-Mentha-1,5-dien-8-ol | - | 4.78 | - | - |
| Sesquiterpene hydrocarbons | ||||
| Viridiflorene | 1.3 | 0.41 | - | - |
| C15H26O sesquiterpene alcohol | 6.0 | - | - | - |
| Cyclosativene | - | 0.70 | 0.7 | 0.6 |
| Aromadendrene | - | 1.77 | - | - |
| Allo-aromadendrene | 0.8 | 1.9 | - | |
| α-Copaene | - | 0.62 | 0.8 | - |
| α-Cubebene | - | - | - | 2.2 |
| δ-cadinene | 1.0 | - | 0.8 | 6.4 |
| Oxygenated sesquiterpenes | ||||
| Viridiflorol | 17.4 | 13.59 | 11.8 | 2.8 |
| Spathulenol | 0.8 | 0.53 | 0.5 | - |
| Globulol | 5.0 | - | 0.3 | - |
| Ledol | - | 4.36 | 3.3 | - |
| Caryophyllene oxide | 1.8 | - | - | - |
| Palustrol | - | 0.50 | - | - |
| Others | ||||
| 2,2,6-trimethylcyclohexanone | 2.8 | - | 0.9 | 7.3 |
| Phthalates | ||||
| Diethyl phthalate | - | - | - | 2.9 |
| Bis (2-ethylhexyl) phthalate | - | - | - | 0.2 |
| Material used for hydrodistillation | dry leaves and small branches | fresh leaves | leaves and stems | dry leaves |
| Compound | Chemical Group | Part of the Plant | References |
|---|---|---|---|
| Volatile compounds | |||
| α-Pinene | Monoterpene hydrocarbons | Aerial part; shoots | [6,32] |
| Camphene | Aerial part; shoots | [6,32] | |
| Pinocarvone | Aerial part; shoots | [6,32] | |
| Limonene | Aerial part | [6] | |
| α-Phellandrene | Leaves | [69] | |
| γ-Terpinene | Aerial part | [6] | |
| α-Thujene | Aerial part | [6] | |
| p-cymene | Aerial part | [6] | |
| Bornyl acetate | Oxygenated monoterpenes | Aerial part; shoots | [6,32] |
| Terpinen-4-ol | Aerial part; shoots | [6,32] | |
| α-Campholenal | Aerial part | [6] | |
| trans-pinocarveol | Aerial part | [6] | |
| Borneol | Leaves; aerial part | [6,69] | |
| Myrtenal | Aerial part | [6] | |
| (cis)-Verbenol | Leaves; shoots | [32,69] | |
| Verbonene | Leaves; aerial part; shoots | [6,32,69] | |
| Camphor | Leaves; shoots | [32,69] | |
| Viridiflorol | Shoots | [32] | |
| Globulol | Oxygenated sesquiterpenes | Shoots | [32] |
| Ledol | Leaves | [69] | |
| Caryophyllene oxide | Shoots | [32] | |
| Eugenol | Phenylpropene | Leaves | [69] |
| Benzenepropanoic acid | Phenylpropanoid | Shoots | [32] |
| 2-Phenylethanol | Alcohol | Leaves; aerial part | [6,69] |
| Acetophenone | Aromatic ketone | Leaves | [69] |
| Thuja-2,4(10)-diene | Others | Aerial part | [6] |
| Rhododendrol | Shoots | [32] | |
| 2,2,6-trimethylcyclohexanone | Leaves; aerial part; shoots | [6,32,69] | |
| Soluble compounds (phenolics) | |||
| Apigenin | Flavonoids | Leaves; aerial part; whole plant | [4,70,71] |
| Apigenin-6-C-glucose-8-C-glucose | Leaves | [72] | |
| Apigenin methylether | Whole plant | [4] | |
| Kaempferol dimethylether | Aerial part; leaves; whole plant | [4,71,72] | |
| Kaempferol diglycoside | Whole plant | [4] | |
| 4′(o)methyl-apigenin | Leaves | [70] | |
| 7(o)methyl-apigenin | Leaves | [70] | |
| 3-methyl-kaempferol | Leaves | [70] | |
| 4′-dimethyl-kaempferol | Leaves | [70] | |
| 3,7-dimethyl-kaempferol | Leaves | [70] | |
| 3,7,4′-trimethyl-kaempferol | Leaves | [70] | |
| Kaempferol methylether | Aerial part; leaves | [71,72] | |
| Quercetin-O-hexoside-Ohexoside | leaves | [72] | |
| Epigallocatechin | Aerial part; leaves | [71,72] | |
| Gallic acid | Phenolic acids and derivatives | Aerial part | [71] |
| Glucogallin (isomer) | Aerial part | [71] | |
| Gentisoyl glucoside | Aerial part; whole plant | [4,71] | |
| Digaloil-β-D-glucopiranose | Aerial part | [71] | |
| Galloyl glucose | Leaves | [72] | |
| Mirciaphenone B | Aerial part | [71] | |
| Punicalagin isomer 1 | Ellagic acid and derivatives | Aerial part, leaves | [71,72] |
| Punicalagin isomer 2 | Aerial part, leaves | [71,72] | |
| Punicalagin gallate 1 | Leaves | [72] | |
| Punicalagin gallate 2 | Leaves | [72] | |
| Punicalin | Aerial part; whole plant | [4,71] | |
| Cornusiin | Aerial part | [71] | |
| Ellagic acid-7-xyloside | Aerial part | [71] | |
| Ellagic acid | Aerial part | [71] | |
| Ducheside A | Aerial part | [71] | |
| Shikimic acid | Others | Aerial part Whole plant | [4,71] |
| Quinic acid | Aerial part Whole plant | [4,71] | |
| Hexahydroxydiphenoyl-D-glucose (isomer) | Aerial part | [71] | |
| Phenethyl-β-primeveroside | Aerial part | [71] | |
| Plant Part | Type of Extract | Biological Activities | References |
|---|---|---|---|
| Fresh leaves from flowering stems | Methanol/water extract | Antifungal | [72] |
| Leaves | Aqueous extract | Autotoxicity | [12] |
| Wood/stalks, bark, and leaves | Ethanol extract and Acetone extract | Antioxidant | [85] |
| Leaves | Aqueous extract | Allelopathic | [76] |
| Aerial parts | Aqueous extract | Antihypertensive | [86] |
| Whole plant | Hydroalcoholic and spray-dried/spray-dried aqueous extract | Antibacterial | [4] |
| Leaves | Aqueous extract | Antioxidant Antimicrobial, Cytotoxic activity against human cancer cells | [77] |
| Leaves | Flavonoids extract | Allelopathic | [87] |
| Shoots | Water-soluble and volatile compounds | Phytotoxic | [88] |
| Leaves and small branches Whole plant | Essential oil Labdanum extracts | Antitungical, Antibacterial | [28] |
| Aerial parts | Essential oil | Herbicidal activity | [52] |
| Fruits, stems, flowers, and leaves | Essential oil water, ethanol, ethanol: water (50:50), methanol, methanol: water (50:50), acetonitrile | Antioxidant | [53] |
| Aerial parts | Hydrolates volatiles Essential oil | Antioxidant Anti-inflammatory Antimicrobial | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves-Ferreira, J.; Duarte, L.C.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Cistus ladanifer as a Potential Feedstock for Biorefineries: A Review. Energies 2023, 16, 391. https://doi.org/10.3390/en16010391
Alves-Ferreira J, Duarte LC, Fernandes MC, Pereira H, Carvalheiro F. Cistus ladanifer as a Potential Feedstock for Biorefineries: A Review. Energies. 2023; 16(1):391. https://doi.org/10.3390/en16010391
Chicago/Turabian StyleAlves-Ferreira, Júnia, Luís C. Duarte, Maria C. Fernandes, Helena Pereira, and Florbela Carvalheiro. 2023. "Cistus ladanifer as a Potential Feedstock for Biorefineries: A Review" Energies 16, no. 1: 391. https://doi.org/10.3390/en16010391
APA StyleAlves-Ferreira, J., Duarte, L. C., Fernandes, M. C., Pereira, H., & Carvalheiro, F. (2023). Cistus ladanifer as a Potential Feedstock for Biorefineries: A Review. Energies, 16(1), 391. https://doi.org/10.3390/en16010391










