Experimental Study on Utilizing Silica Gel with Ethanol and Water for Adsorption Heat Storage
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Materials Characterization
3. Results and Discussion
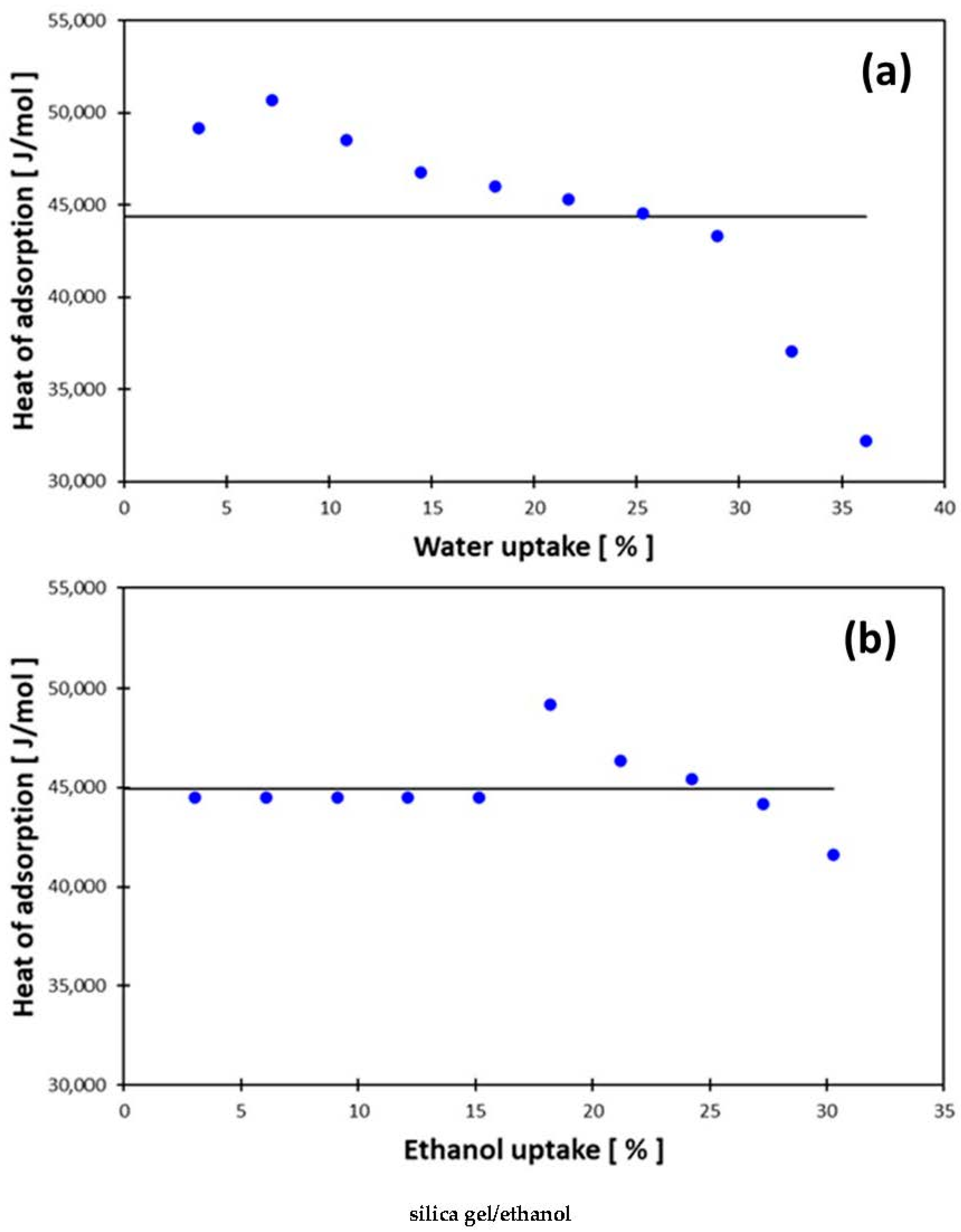
3.1. Heat of Adsorption
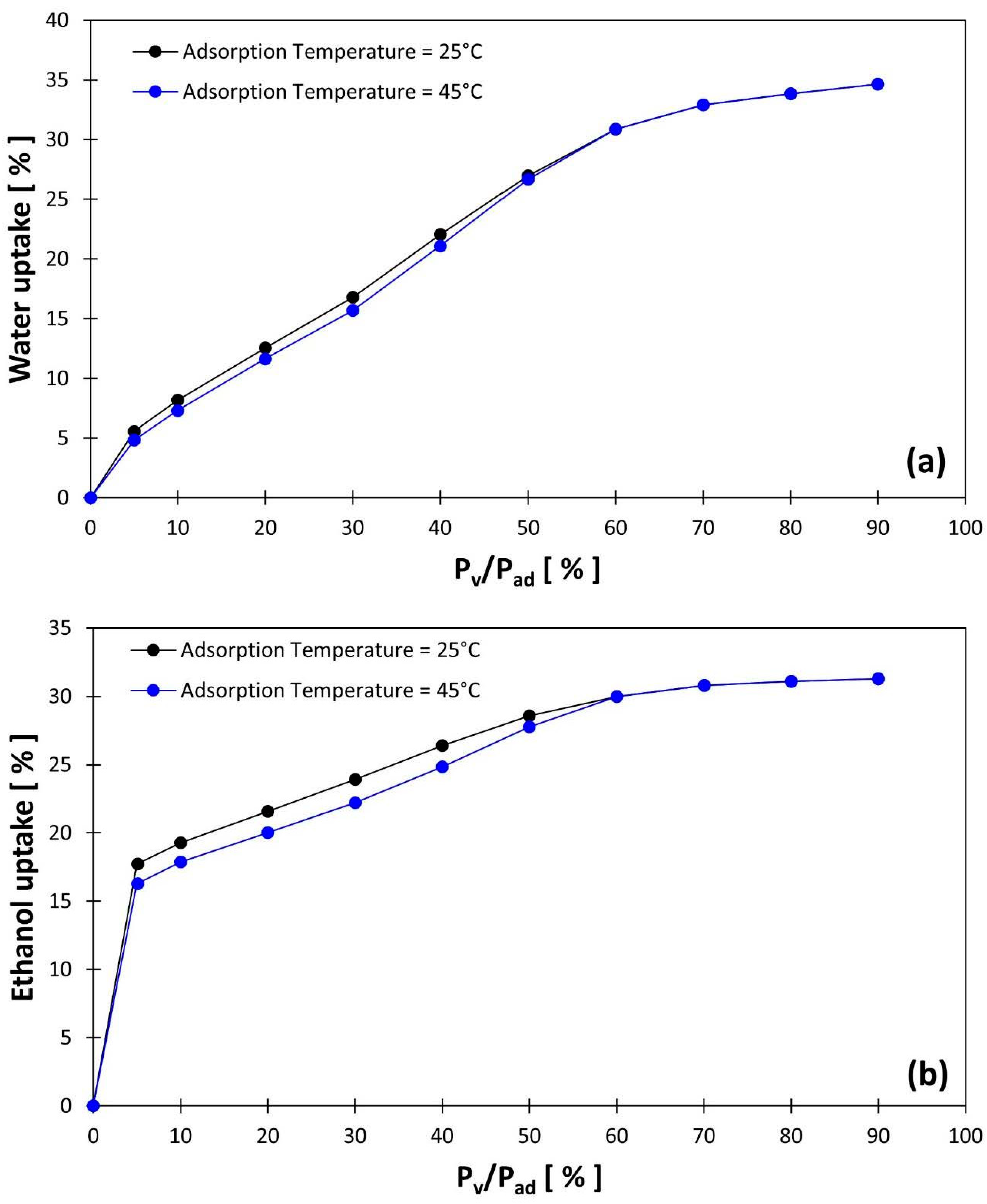
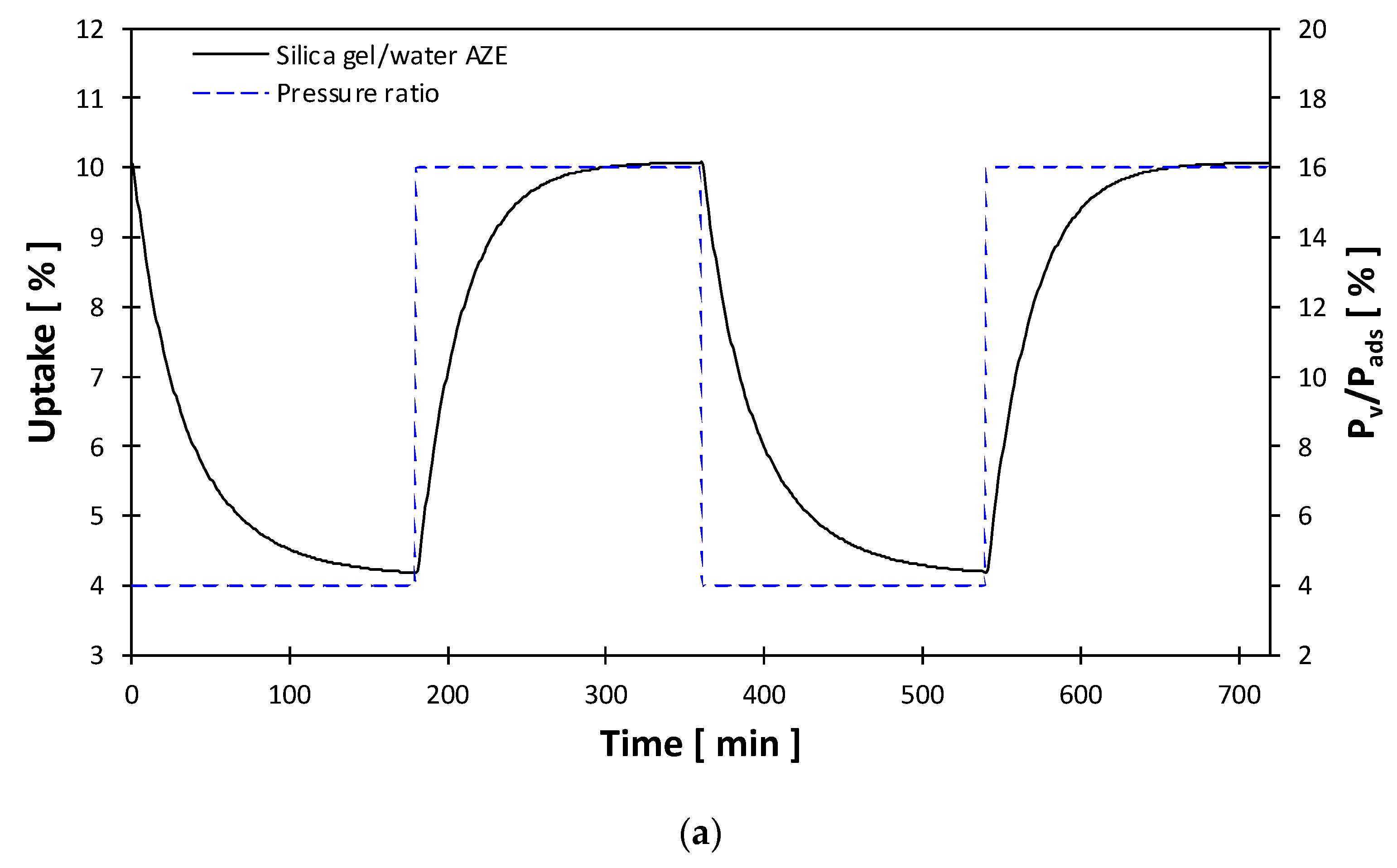
3.2. Heat Storage Capacity
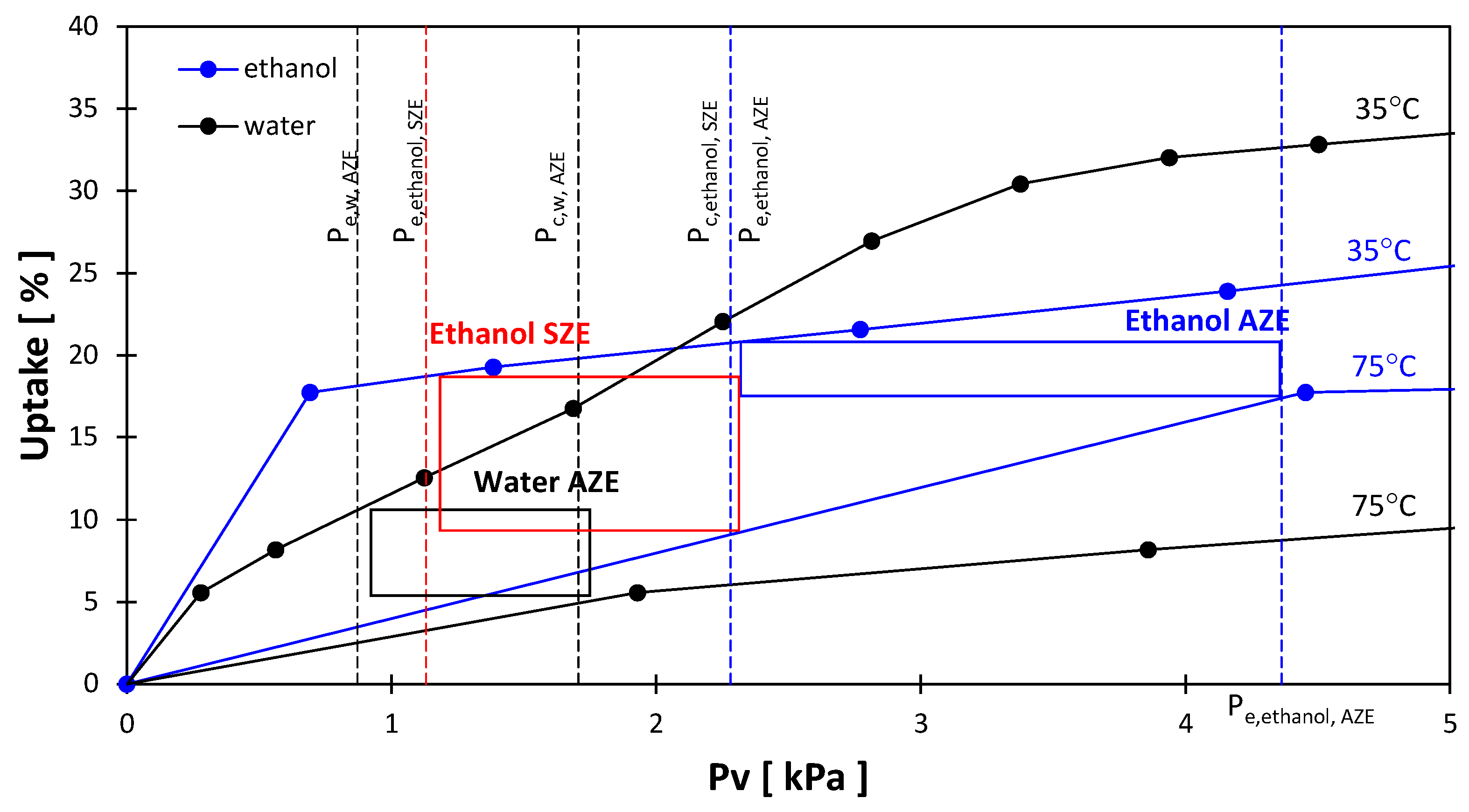
3.3. Exergy Analysis
4. Conclusions and Prospects
- Ethanol is a viable working fluid for adsorption heat storage to utilize the sub-zero ambient conditions without needing an underground heat source for the evaporator.
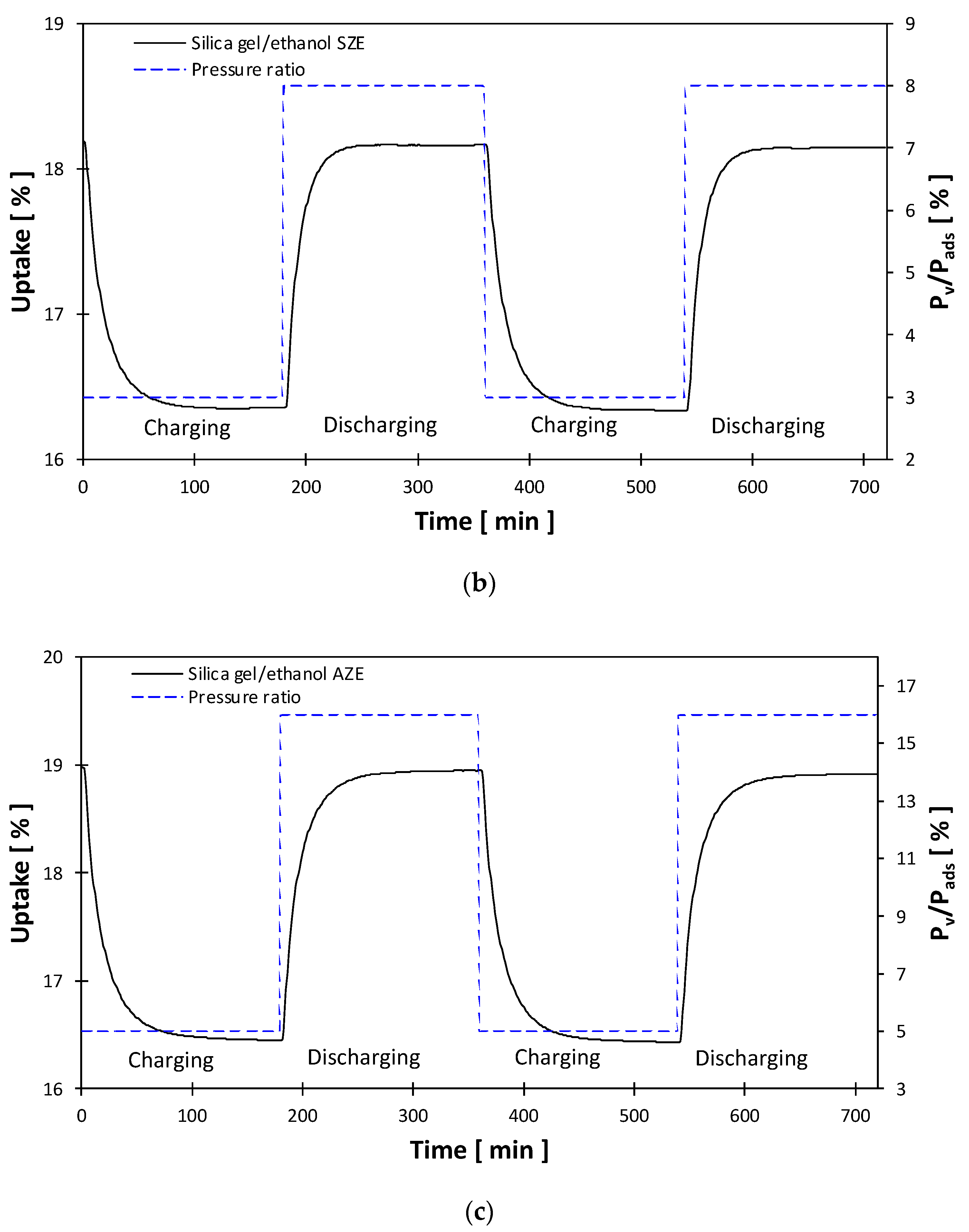
- Silica, gel/ethanol under SZE, showed the most significant net equilibrium cyclic uptake of double the equilibrium for silica gel/water and more than three times for silica gel/ethanol under AZE. On the other hand, a degree of non-desorbed ethanol during the charging process occurred, which affected the quasi-realistic cyclic uptake hence the heat storage capacity of the silica gel/ethanol pair. Such an effect stemmed from the large molecular size of ethanol for the high microporosity level of the investigated silica gel.
- Ethanol adsorbate has a high affinity towards silica gel, resulting in faster adsorption/desorption process and hence faster discharging/charging processes, which flags that utilizing silica gel of other porous structures of more accessible sites for ethanol molecules can enable faster heat charging/discharging rate along with the magnitude of heat storage capacity.
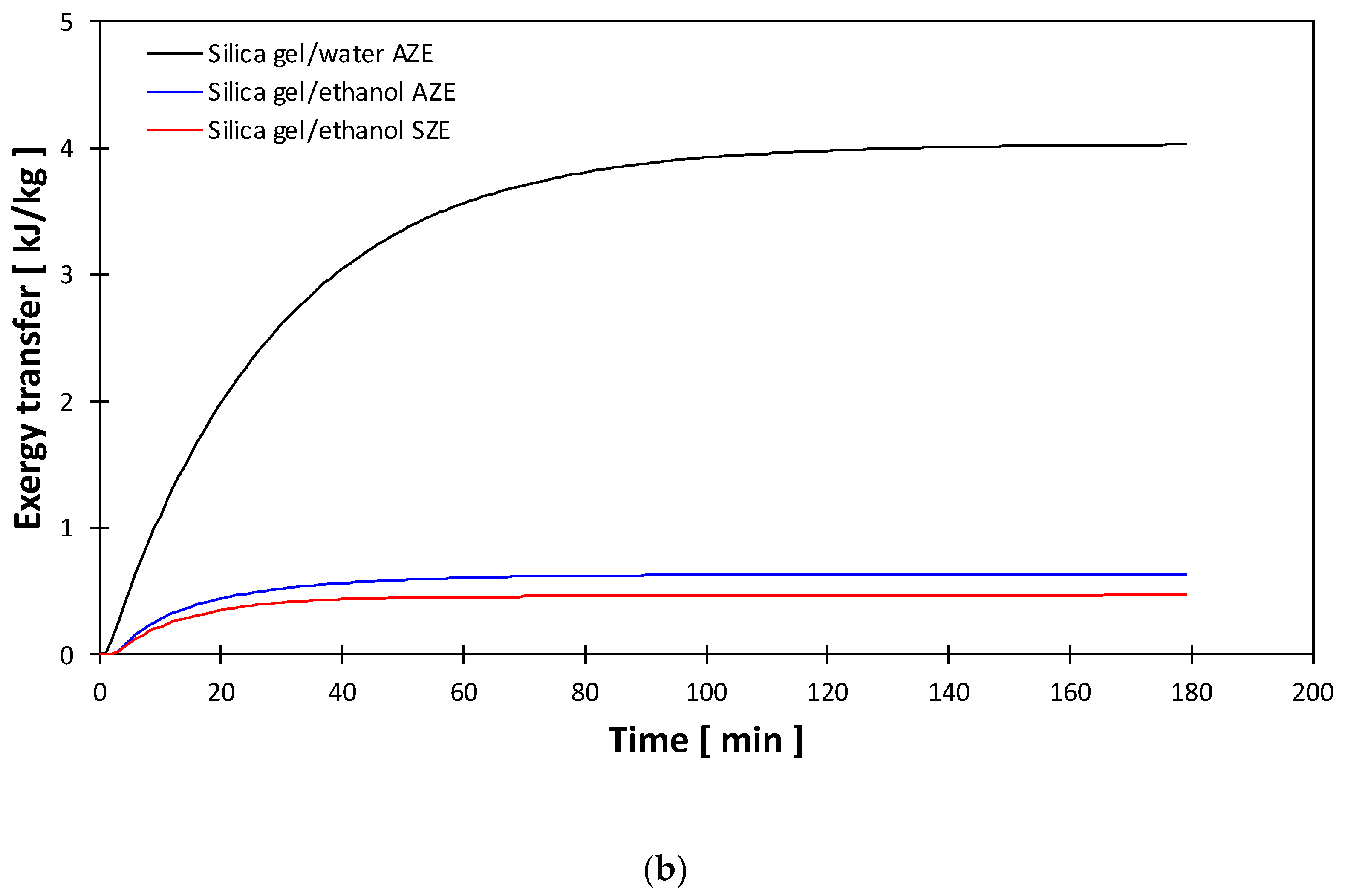
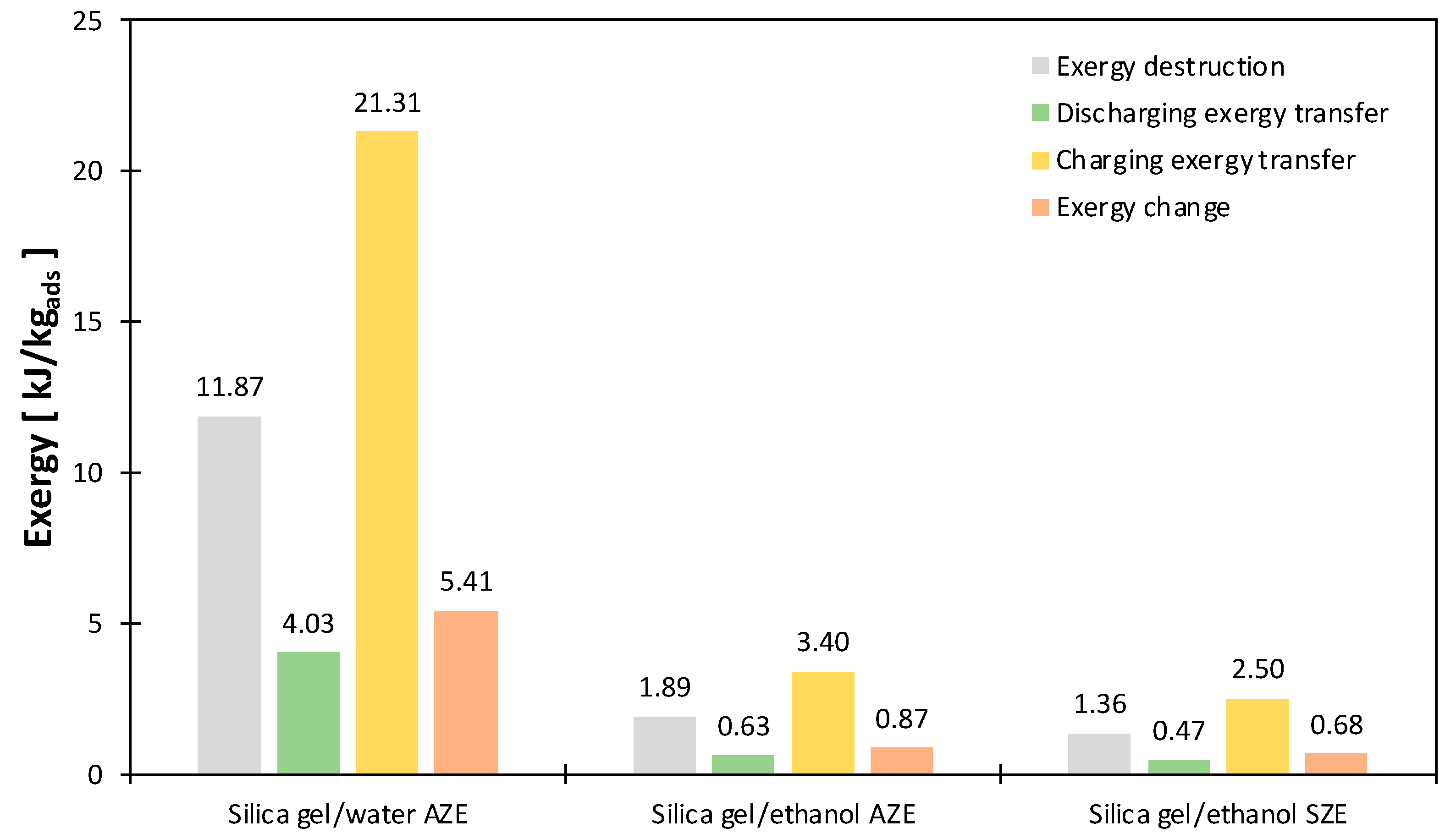
- The exergy transfer during the heat charging process was the primary contributor to the exergy destruction due to the high-temperature level and heat quantities. On the contrary, the exergy transfer during heat discharging was the minor contributor to the exergy destruction, albeit the amount of heat discharging was equal to that for charging. Therefore, it is concluded that the degree of heat (i.e., temperature) dominates the exergy transfer contribution.
- The entropy change during the charging and discharging processes was the main contributor to the breakup of exergy change.
- Given the potential of utilizing ethanol as a working fluid for adsorption heat storage, future work will revolve around pairing ethanol with porous materials of various structures aiming to fast charging/discharging responses simultaneously with heat storage quantities.
Author Contributions
Funding
Conflicts of Interest
References
- Olabi, A.G.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Maghrabie, H.M.; Abdelkareem, M.A. Large scale application of carbon capture to process industries—A review. J. Clean. Prod. 2022, 362, 132300. [Google Scholar] [CrossRef]
- Rehman, A.u.; Lal, B. Gas Hydrate-Based CO2 Capture: A Journey from Batch to Continuous. Energies 2022, 15, 8309. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Maghrabie, H.M.; Sayed, E.T.; Kais, E.-C.A.; Abo-Khalil, A.G.; Radi, M.A.; Baroutaji, A.; Olabi, A.G. Heat pipe-based waste heat recovery systems: Background and applications. Therm. Sci. Eng. Prog. 2022, 29, 101221. [Google Scholar] [CrossRef]
- Elahi, M.A.E.; Mahmud, T.; Alam, M.M.; Hossain, M.J.; Biswas, B.N. Exergy Analysis of Organic Rankine Cycle for Waste Heat Recovery Using Low GWP Refrigerants. Int. J. 2022, 16, 100243. [Google Scholar] [CrossRef]
- Dai, R.; Chen, G.; Wei, K.; Chen, Z.; Lv, X.; Liu, G.; Li, Y.; Geng, S. Effect of chemical reactions between electrolyte and lithium compounds on the electrochemical performance of the ceramic fuel cells. Carbon Resour. Convers. 2022, 5, 131–138. [Google Scholar] [CrossRef]
- Evangelisti, L.; De Lieto Vollaro, R.; Asdrubali, F. Latest advances on solar thermal collectors: A comprehensive review. Renew. Sustain. Energy Rev. 2019, 114, 109318. [Google Scholar] [CrossRef]
- Ravi Kumar, K.; Krishna Chaitanya, N.V.V.; Sendhil Kumar, N. Solar thermal energy technologies and its applications for process heating and power generation—A review. J. Clean. Prod. 2021, 282, 125296. [Google Scholar] [CrossRef]
- Awasthi, A.; Shukla, A.K.; Manohar, S.R.M.; Dondariya, C.; Shukla, K.N.; Porwal, D.; Richhariya, G. Review on sun tracking technology in solar PV system. Energy Rep. 2020, 6, 392–405. [Google Scholar] [CrossRef]
- Lund, J.W.; Toth, A.N. Direct utilization of geothermal energy 2020 worldwide review. Geothermics 2021, 90, 101915. [Google Scholar] [CrossRef]
- Soltani, M.; Moradi Kashkooli, F.; Souri, M.; Rafiei, B.; Jabarifar, M.; Gharali, K.; Nathwani, J.S. Environmental, economic, and social impacts of geothermal energy systems. Renew. Sustain. Energy Rev. 2021, 140, 110750. [Google Scholar] [CrossRef]
- Ahmed, S.D.; Al-Ismail, F.S.M.; Shafiullah, M.; Al-Sulaiman, F.A.; El-Amin, I.M. Grid Integration Challenges of Wind Energy: A Review. IEEE Access 2020, 8, 10857–10878. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, Y.C.; Liu, W.; Ma, Z.W.; Wang, R.Q.; Zhang, X.J.; Roskilly, A.P. Thermophysical characterization of magnesium chloride and its application in open sorption thermal energy storage system. Sol. Energy Mater. Sol. Cells 2022, 236, 111528. [Google Scholar] [CrossRef]
- Nienborg, B.; Helling, T.; Fröhlich, D.; Horn, R.; Munz, G.; Schossig, P. Closed Adsorption Heat Storage—A Life Cycle Assessment on Material and Component Levels. Energies 2018, 11, 3421. [Google Scholar] [CrossRef] [Green Version]
- Gao, N.; Deng, L.; Li, J.; Huang, H.; Zhou, B.; Zhou, Y. Multi-form heat storage performance of expanded graphite based CaCl2 composites for low-grade heat source. Energy Rep. 2022, 8, 12117–12125. [Google Scholar] [CrossRef]
- Wolf, V.; Bertrand, A.; Leyer, S. Analysis of the thermodynamic performance of transcritical CO2 power cycle configurations for low grade waste heat recovery. Energy Rep. 2022, 8, 4196–4208. [Google Scholar] [CrossRef]
- Yu, N.; Wang, R.Z.; Wang, L.W. Sorption thermal storage for solar energy. Prog. Energy Combust. Sci. 2013, 39, 489–514. [Google Scholar] [CrossRef]
- Banaei, A.; Zanj, A. A Review on the Challenges of Using Zeolite 13X as Heat Storage Systems for the Residential Sector. Eneriges 2021, 14, 8062. [Google Scholar] [CrossRef]
- Elias, C.N.; Stathopoulos, V.N. A comprehensive review of recent advances in materials aspects of phase change materials in thermal energy storage. Energy Procedia 2019, 161, 385–394. [Google Scholar] [CrossRef]
- Zahid, M.S.; Ahmed, N.; Qaisrani, M.A.; Mahmood, M.; Ali, M.; Waqas, A.; Assadi, M. Charging and discharging characterization of a novel combined sensible-latent heat thermal energy storage system by experimental investigations for medium temperature applications. J. Energy Storage 2022, 55, 105612. [Google Scholar] [CrossRef]
- El-Kaddadi, L.; Asbik, M.; Zari, N.; Zeghmati, B. Experimental study of the sensible heat storage in the water/TiO2 nanofluid enclosed in an annular space. Appl. Therm. Eng. 2017, 122, 673–684. [Google Scholar] [CrossRef]
- Olabi, A.G.; Elsaid, K.; Sayed, E.T.; Mahmoud, M.S.; Wilberforce, T.; Hassiba, R.J.; Abdelkareem, M.A. Application of nanofluids for enhanced waste heat recovery: A review. Nano Energy 2021, 84, 105871. [Google Scholar] [CrossRef]
- Pinel, P.; Cruickshank, C.A.; Beausoleil-Morrison, I.; Wills, A. A review of available methods for seasonal storage of solar thermal energy in residential applications. Renew. Sustain. Energy Rev. 2011, 15, 3341–3359. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sustain. Energy Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Solé, A.; Barreneche, C. Review on sorption materials and technologies for heat pumps and thermal energy storage. Renew. Energy 2017, 110, 3–39. [Google Scholar] [CrossRef] [Green Version]
- Abohamzeh, E.; Frey, G. Numerical Investigation of the Adsorption Process of Zeolite/Water in a Thermochemical Reactor for Seasonal Heat Storage. Energies 2022, 15, 5944. [Google Scholar] [CrossRef]
- Schreiber, H.; Lanzerath, F.; Bardow, A. Predicting performance of adsorption thermal energy storage: From experiments to validated dynamic models. Appl. Therm. Eng. 2018, 141, 548–557. [Google Scholar] [CrossRef]
- Zbair, M.; Bennici, S. Survey Summary on Salts Hydrates and Composites Used in Thermochemical Sorption Heat Storage: A Review. Energies 2021, 14, 3105. [Google Scholar] [CrossRef]
- Courbon, E.; D’Ans, P.; Skrylnyk, O.; Frère, M. New prominent lithium bromide-based composites for thermal energy storage. J. Energy Storage 2020, 32, 101699. [Google Scholar] [CrossRef]
- D’Ans, P.; Courbon, E.; Permyakova, A.; Nouar, F.; Simonnet-Jégat, C.; Bourdreux, F.; Malet, L.; Serre, C.; Frère, M.; Steunou, N. A new strontium bromide MOF composite with improved performance for solar energy storage application. J. Energy Storage 2019, 25, 100881. [Google Scholar] [CrossRef]
- Clark, R.-J.; Farid, M. Experimental investigation into the performance of novel SrCl2-based composite material for thermochemical energy storage. J. Energy Storage 2021, 36, 102390. [Google Scholar] [CrossRef]
- Grekova, A.; Gordeeva, L.; Aristov, Y. Composite sorbents “Li/Ca halogenides inside Multi-wall Carbon Nano-tubes” for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2016, 155, 176–183. [Google Scholar] [CrossRef]
- Rezk, A.; Gediz Ilis, G.; Demir, H. Experimental study on silica gel/ethanol adsorption characteristics for low-grade thermal driven adsorption refrigeration systems. Therm. Sci. Eng. Prog. 2022, 34, 101429. [Google Scholar] [CrossRef]
- Rezk, A.; Al-Dadah, R.; Mahmoud, S.; Elsayed, A. Investigation of Ethanol/metal organic frameworks for low temperature adsorption cooling applications. Appl. Energy 2013, 112, 1025–1031. [Google Scholar] [CrossRef]
- Yunus, C.; Boles, M.; Kanoglu, M. Thermodynamic and Engineering Approach; MC Graw Hill Education: New York, NY, USA, 2015. [Google Scholar]




| Cycle | Tch [°C] | Tc [°C] | Tdis [°C] | Te [°C] | Tamb [°C] | Adsorbate |
|---|---|---|---|---|---|---|
| AZE | 75 | 15 | 35 | 5 | 10 | Water |
| Ethanol | ||||||
| SZE | 75 | 5 | 35 | −5 | 0 | Ethanol |
| Pair | Heat of Adsorption (J/mol) | Standard Deviation (J/mol) |
|---|---|---|
| Silica gel/water | 4.43 × 104 | 5.70 ×103 |
| Silica gel/ethanol | 4.49 × 104 | 1.92 × 103 |
| [kJ/kgads] | [%] | [kJ/kgads] | [%] | |
| Silica gel/water AZE | 1.08 | 20% | 4.33 | 80% |
| Silica gel/ethanol AZE | 0.39 | 45% | 0.48 | 55% |
| Silica gel/ethanol SZE | 0.28 | 41% | 0.40 | 59% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezk, A.; Olabi, A.G.; Alami, A.H.; Radwan, A.; Demir, H.; Rahman, S.M.A.; Shah, S.K.; Abdelkareem, M.A. Experimental Study on Utilizing Silica Gel with Ethanol and Water for Adsorption Heat Storage. Energies 2023, 16, 444. https://doi.org/10.3390/en16010444
Rezk A, Olabi AG, Alami AH, Radwan A, Demir H, Rahman SMA, Shah SK, Abdelkareem MA. Experimental Study on Utilizing Silica Gel with Ethanol and Water for Adsorption Heat Storage. Energies. 2023; 16(1):444. https://doi.org/10.3390/en16010444
Chicago/Turabian StyleRezk, Ahmed, Abdul Ghani Olabi, Abdul Hai Alami, Ali Radwan, Hasan Demir, Shek Mohammod Atiqure Rahman, Sheikh Khaleduzzaman Shah, and Mohammad Ali Abdelkareem. 2023. "Experimental Study on Utilizing Silica Gel with Ethanol and Water for Adsorption Heat Storage" Energies 16, no. 1: 444. https://doi.org/10.3390/en16010444
APA StyleRezk, A., Olabi, A. G., Alami, A. H., Radwan, A., Demir, H., Rahman, S. M. A., Shah, S. K., & Abdelkareem, M. A. (2023). Experimental Study on Utilizing Silica Gel with Ethanol and Water for Adsorption Heat Storage. Energies, 16(1), 444. https://doi.org/10.3390/en16010444














