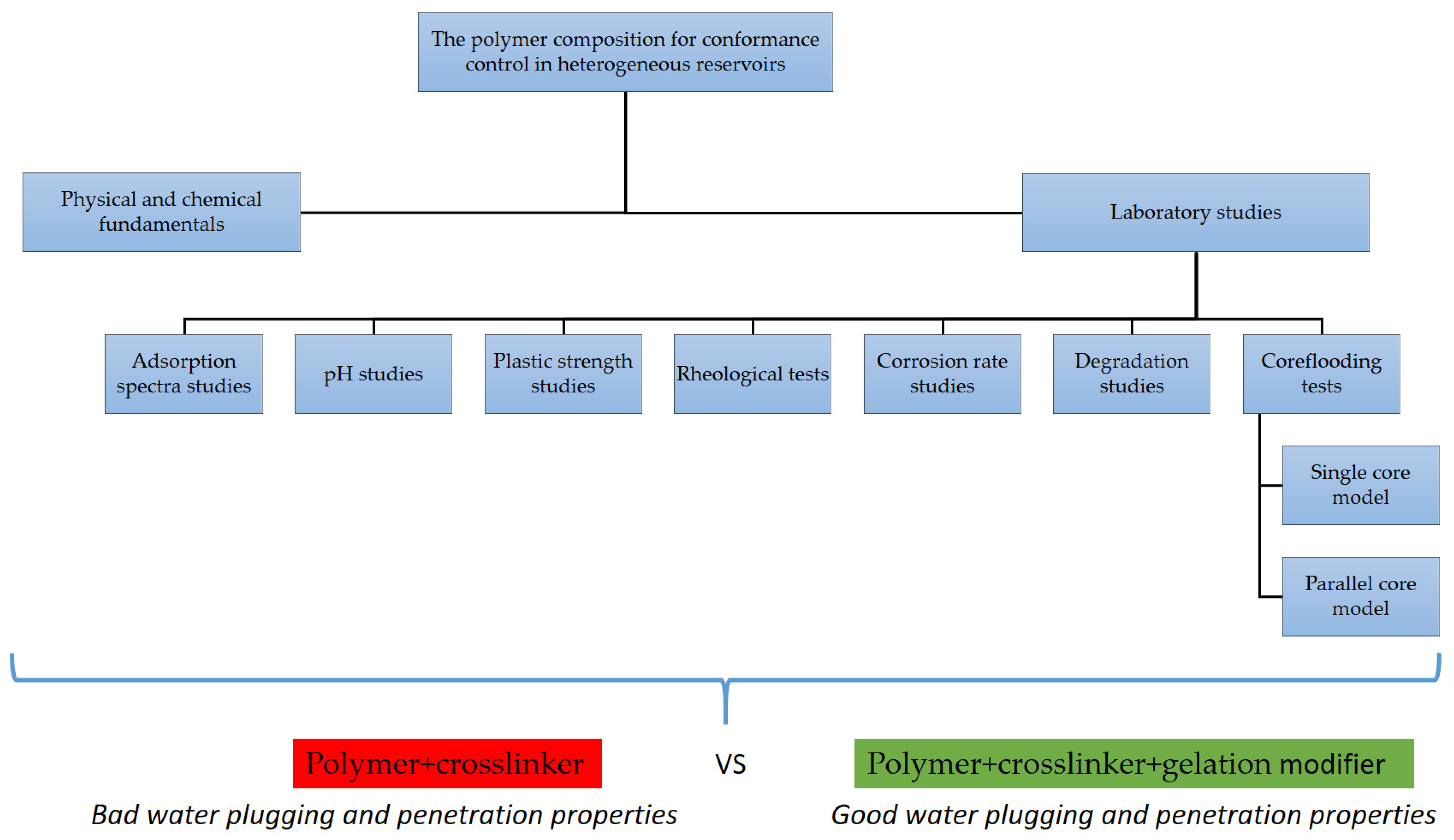
Design of a Polymer Composition for the Conformance Control in Heterogeneous Reservoirs
Abstract
:1. Introduction
- -
- a decrease in the water cut, due to selective properties;
- -
- an increase in the oil production, due to the water flow redirection into previously unswept oil-saturated zones with a low permeability.
2. Physical and Chemical Fundamentals
- -
- a molecular weight reduction of PAMs. The optimal molecular weight is considered to be in the range of 12 to 15 MDa. However, the reduced molecular weight can lead to the increased PAM concentration in the solution, to obtain the desired physicochemical and mechanical properties of the polymer composition;
- -
- an increase in the salinity. In high-salinity water, the residual resistance factor decreases because of the polymer degradation [16];
- -
- a decrease in the concentration of chemical reagents. As noticed above, a decrease in the concentration of the polymer causes a decrease in its strength and gel degradation;
- -
- adding a gelation inhibitor. It is considered one of the most promising ways to reduce the viscosity and improve the physicochemical and mechanical properties of the polymer composition;
- -
- dynamic injection. The selection of the optimal injection rate for the polymer composition was based on determining the range of the shear rates at which the effective viscosity of the water plugging composition would be the lowest [17]. An increase in the shear rate leads to an increase in the elastic deformation and may also lead to an increase in the complex viscosity. The upper limit of the injection rate value also depends on the fracturing pressure;
- -
- a decrease in temperature. The only effect here is an increase in the gelation time.
- -
- the use of the organic acids as gelation delayers reduces the viscosity and increases the gelation time while decreasing the plastic strength;
- -
- the use of water repellents reduces both the critical shear stress and the adhesion of the polymer composition to the hydrophilic rock, which can make the gel too mobile and cause it to be removed from the formation;
- -
- the exposure of the polymer molecules to radiation is a promising method that allows for the synthesis of the crosslinked polymer particles with sizes up to 100 nm, which can increase the depth of the polymer penetration into the formation. However, this method is technologically sophisticated, laborious, and energy-intensive.
- -
- when a strong monobasic acid (such as hydrochloric acid) is added to water, HCl dissociates into the H+ cation and the Cl− anion, resulting in an excess of H+ protons in the system and a decrease in pH:
- -
- the addition of HPAN to the HCl solution causes polyacrylonitrile to hydrolyze, resulting in the formation of a carboxyl group and its reaction products (ammonium hydroxide):
 + H+ + H2O →
+ H+ + H2O →  + NH4OH
+ NH4OH
- -
- due to the fact that NH4OH is an unstable compound, it decomposes into ammonia and water. This becomes evident from the pungent smell of ammonia:
- -
- since the crosslinker in an acidic medium dissociates into the cation Al3+ and the anion Cl-, the precipitate Al(OH)3↓ is not observed. This allows the Al3+ cations to interact with the polymer molecules. However, due to the fact that the polymer molecules are in a compressed state, the cations of the polyvalent metals are blocked from entering the coil. So, the crosslinking process occurs only on its surface. This is the reason why there is no sharp increase in viscosity that is characteristic of the polymer crosslinking process. Instead of the Al3+ cations, the Cr3+ or Fe3+ ions can be used.
 + Al3+ + Cl− + Cl− + Cl− →
+ Al3+ + Cl− + Cl− + Cl− →  + 3HCl
+ 3HCl
3. Material and Methods
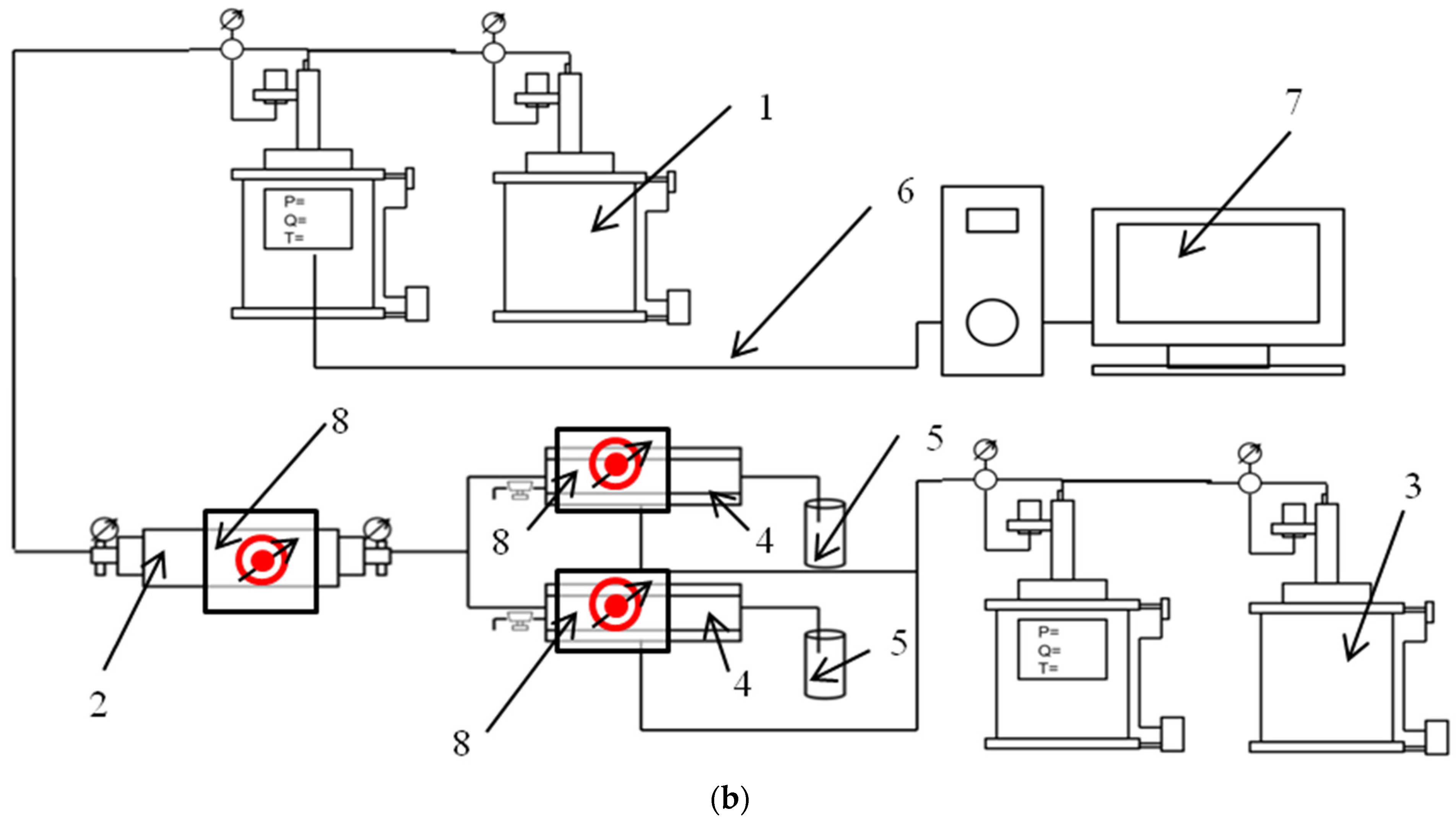
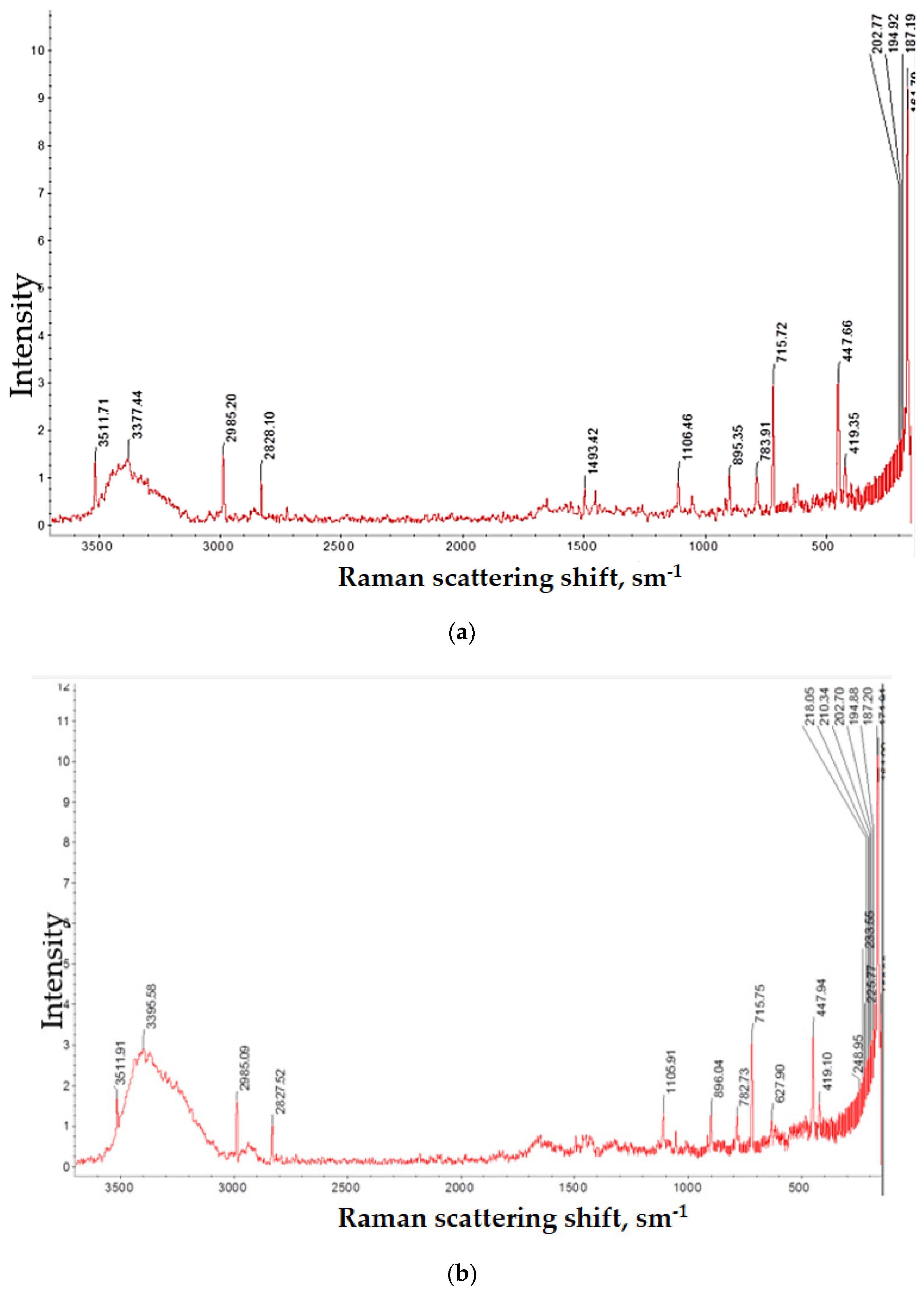
3.1. Absorption Spectra Study
3.2. Hydrogen Index Study
3.3. Plastic Strength Research Method
- Ps is plastic strength, Pa;
- α is the angle of the axial section of the cone at the apex, equal to 60°;
- Ka is a coefficient depending on the angle of the axial section of the cone at the apex, unit fraction;
- F is the weight of the submerged system, N;
- h is the depth of immersion of the cone in the gel, m.
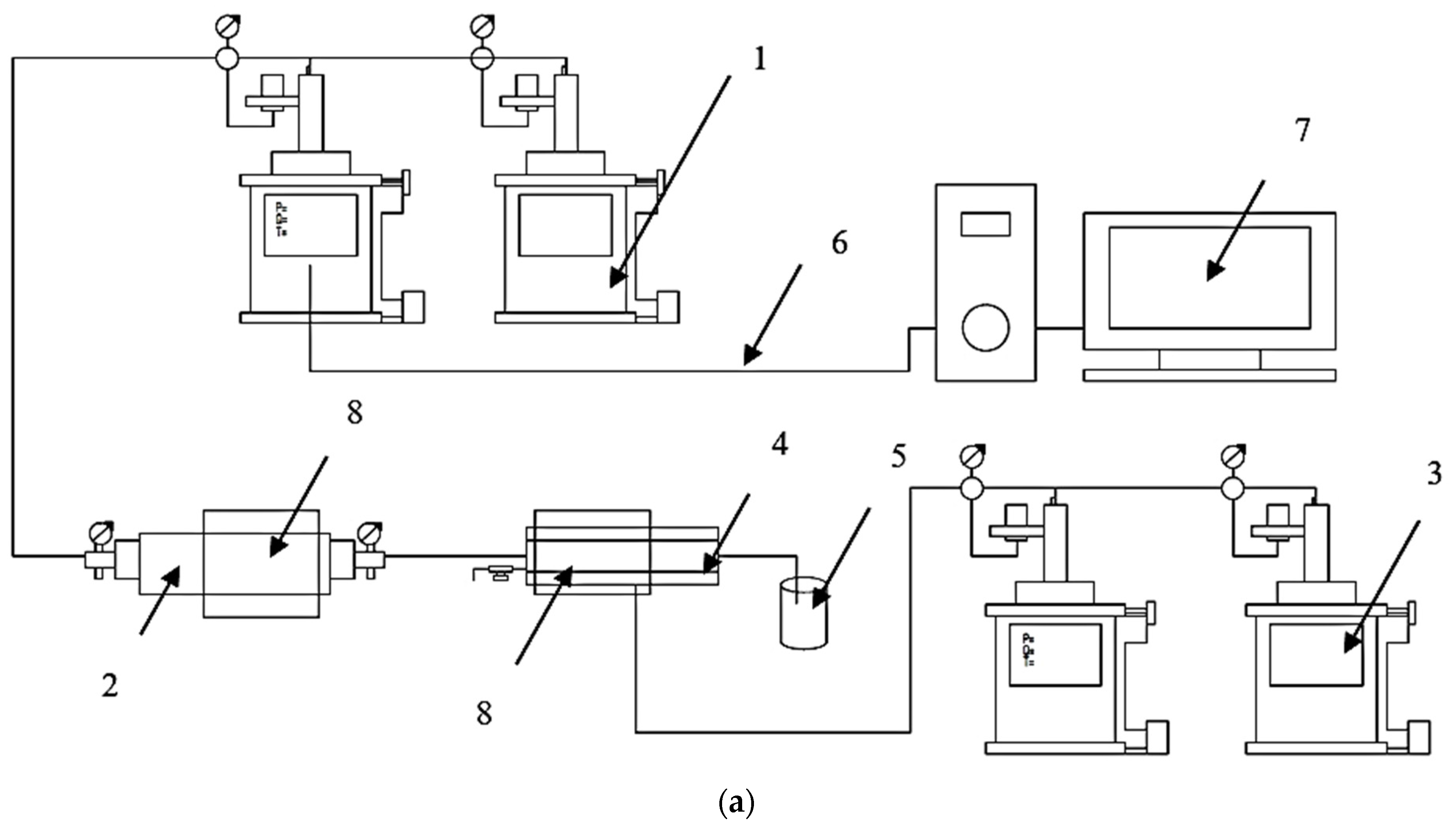
3.4. Rheological Study
3.5. Corrosion Rate Study
3.6. Degradation Study
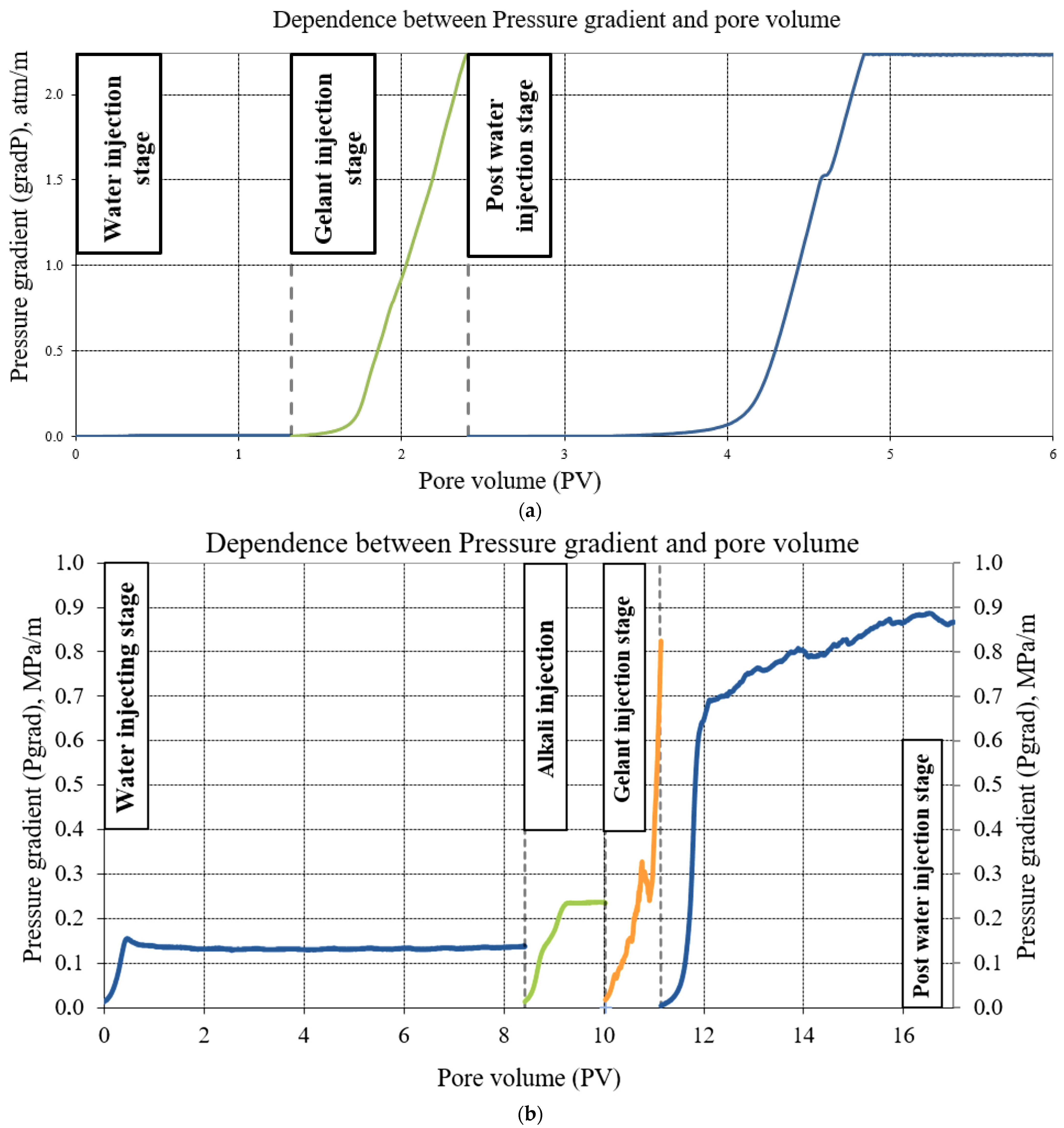
3.7. Plugging Efficency Evaluation through the Core Flooding Test
3.7.1. Single Core Model Test
3.7.2. Parallel Core Model Test
- -
- Likewise, before the main part of the test, residual water saturation and residual oil saturation were designed. At this stage, the oil volume that was the saturated cores was fixed (Vio);
- -
- Then, the model of the reservoir water was injected into the parallel core unit before a 100% water cut was reached. At this stage, which was called the water injecting stage, there were estimated: the displacement coefficient (DC) before treatment, the effective permeability of water, volume of displaced oil (Vdow1), and the amount of water through each core;
- -
- In the follow-up step, the polymer composition was injected at a constant flow rate (Q = 0.5 mL/min) in the amount of 1 PV. The volume of displaced oil (Vdop) was measured. The flooding test was carried out in the “forward” direction. This stage was called the gelant injecting stage;
- -
- Previously, there was injected the alkaline solution at a constant flow rate (Q = 0.5 mL/min) in the amount of 1 PV into the core samples No. 1A and 1B. The effective permeability of the alkaline solution was measured. The flooding was carried out in the “forward” direction;
- -
- Once the polymer composition was injected, the core samples were aged for 24 h;
- -
- Then, the water was injected into the simulated reservoir, in order to define the oil displacement coefficient after the treatment. The volume of the displaced oil was also measured for each sample (Vdow2). The flooding test was carried out in the “forward” direction;
- -
- The next step was to estimate the selectivity coefficient (SC). SC represents which zone (oil- or water-saturated) of the formation is plugged by the polymer composition. The polymer composition with a higher selectivity coefficient has penetrated less into the water-saturated zones. The next equation was used to define the selectivity coefficient:
- —volume of water, that was flooded into the high-permeable sample, mL;
- —the total volume of water, that was injected into the simulated reservoir, mL.
4. Results and Discussion
4.1. Absorption Spectra Study: Results
4.2. Hydrogen Index Study: Results
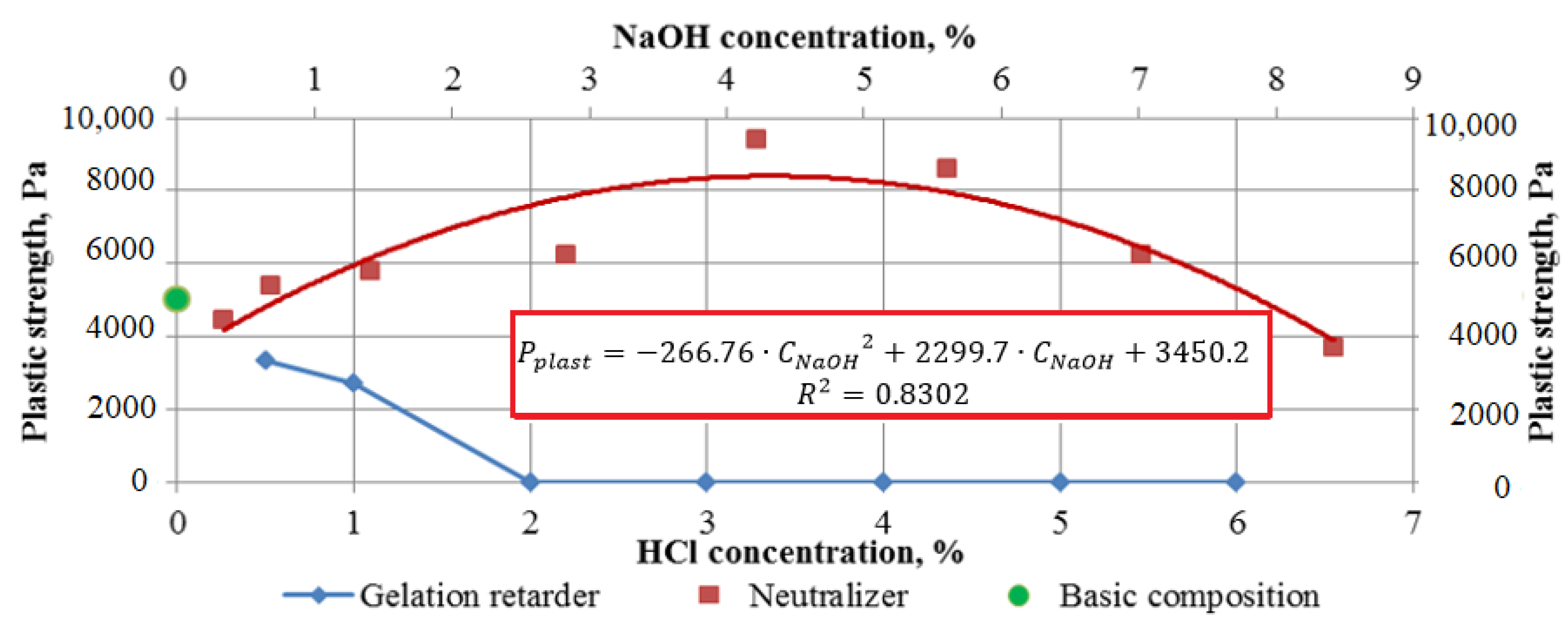
4.3. Plastic Strength Study: Results
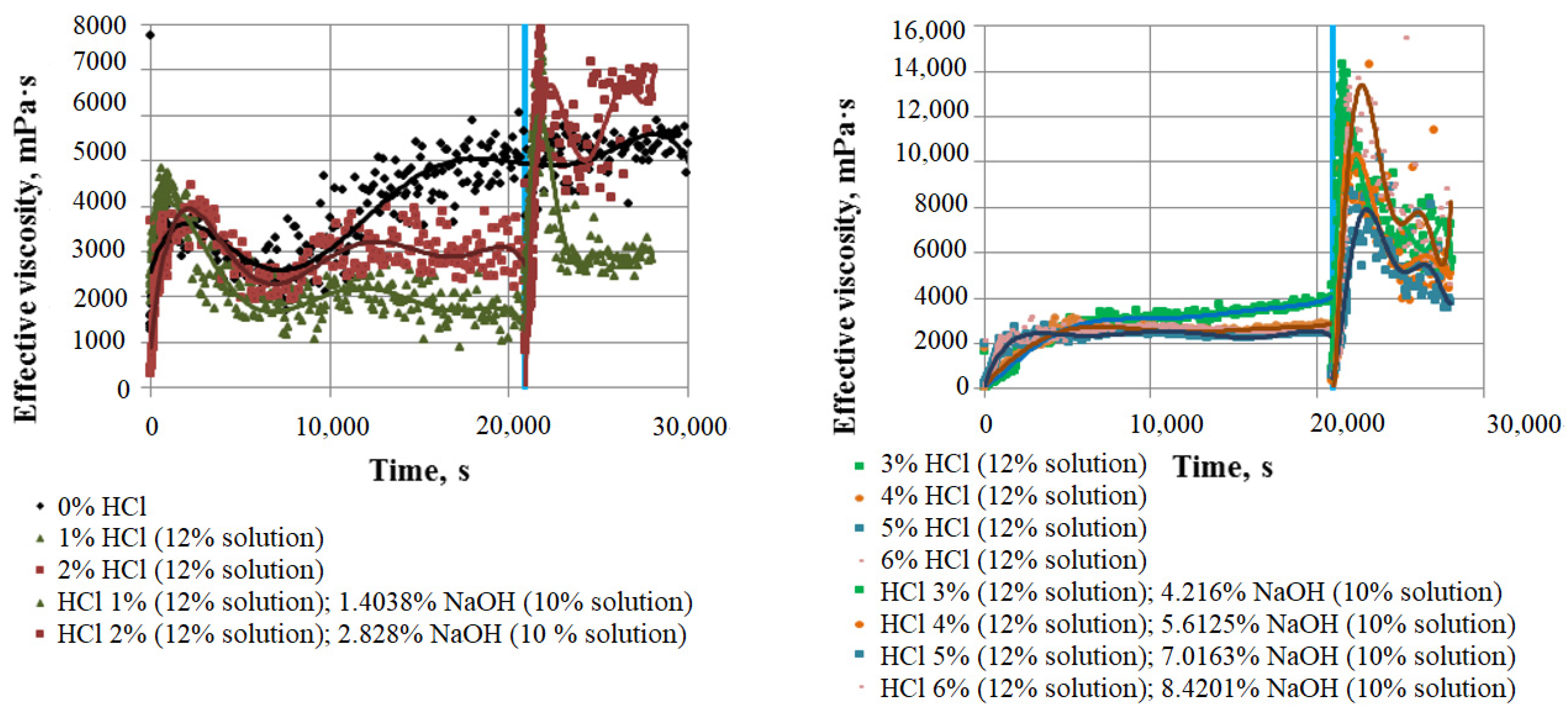
4.4. Rheological Study: Results
4.5. Corrosion Rate Study: Results
4.6. Degradation Study: Results
4.7. Plugging the Efficency Evaluation through the Core Flooding Tests: Results
4.7.1. Single Core Model Test: Results
4.7.2. Parallel Core Model Test: Results
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Averyanova, O.Y. Domanik type petroleum systems in Timan-Pechora sedimentary basin. Neftegazov. Geol. Teor. I Prakt. 2016, 11, 1–30. [Google Scholar] [CrossRef]
- Buslaev, G.; Tsvetkov, P.; Lavrik, A.; Kunshin, A.; Loseva, E.; Sidorov, D. Ensuring the Sustainability of Arctic Industrial Facilities under Conditions of Global Climate Change. Resources 2021, 10, 128. [Google Scholar] [CrossRef]
- Loseva, E.; Lozovsky, I.; Zhostkov, R.; Syasko, V. Wavelet Analysis for Evaluating the Length of Precast Spliced Piles Using Low Strain Integrity Testing. Appl. Sci. 2022, 12, 10901. [Google Scholar] [CrossRef]
- Gizatullin, R.R.; Budovskaya, M.E.; Dvoynikov, M.V. Development of Detergent for Drilling Muds While Directional Drilling. In Advances in Raw Material Industries for Sustainable Development Goals; CRC Press: London, UK, 2020; pp. 309–313. [Google Scholar]
- Prishepa, O.M.; Nefedov, Y.V.; Kochineva, O.E. Raw material base of hard-to-extract oil reserves of Russia. Periódico Tchê Química 2020, 17, 915–924. [Google Scholar] [CrossRef]
- Filimonova, I.V.; Nemov, V.Y.; Prompt, I.V.; Mishenin, M.V.; Komarova, A.V.; Zemnukhova, E.A.; Shumilova, S.I.; Kozhevin, V.D.; Nikiforenko, E.K.-M.; Kontorovich, A.E. Oil industry-2020: Long-term trends and current state. In Oil and Gas Complex of Russia; Publishing House of INGG SB RAS: Novosibirsk, Russia, 2021; p. 88. ISBN 978-5-4437-1222-2, 978-5-4437-1221-5. [Google Scholar]
- Palyanitsina, A.; Safiullina, E.; Byazrov, R.; Podoprigora, D.; Alekseenko, A. Environmentally Safe Technology to Increase Efficiency of High-Viscosity Oil Production for the Objects with Advanced Water Cut. Energies 2022, 15, 753. [Google Scholar] [CrossRef]
- Zhu, D.; Bai, B.; Hou, J. Polymer Gel Systems for Water Management in High-Temperature Petroleum Reservoirs: A Chemical Review. Energy Fuels 2017, 31, 13063–13087. [Google Scholar] [CrossRef]
- Shagiakhmetov, A.; Yushchenko, S. Substantiation of In Situ Water Shut-Off Technology in Carbonate Oil Reservoirs. Energies 2022, 15, 5059. [Google Scholar] [CrossRef]
- Mardashov, D.V.; Limanov, M.N. Improving the efficiency of oil well killing at the fields of the volga-ural oil and gas province with abnormally low reservoir pressure. Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2022, 333, 185–194. [Google Scholar] [CrossRef]
- Islamov, S.R.; Bondarenko, A.V.; Gabibov, A.F.; Mardashov, D.V. Polymer compositions for well killing operation in fractured reservoirs. In Advances in Raw Material Industries for Sustainable Development Goals; CRC Press: London, UK, 2020; pp. 343–351. [Google Scholar]
- Rogachev, M.K.; Mukhametshin, V.V.; Kuleshova, L.S. Improving the efficiency of using resource base of liquid hydrocarbons in Jurassic deposits of Western Siberia. J. Min. Inst. 2019, 240, 711–715. [Google Scholar] [CrossRef] [Green Version]
- Leusheva, E.; Morenov, V.; Liu, T. Dependence of the Equivalent Circulation Density of Formate Drilling Fluids on the Molecular Mass of the Polymer Reagent. Energies 2021, 14, 7639. [Google Scholar] [CrossRef]
- Mardashov, D. Development of blocking compositions with a bridging agent for oil well killing in conditions of abnormally low formation pressure and carbonate reservoir rocks. J. Min. Inst. 2021, 251, 617–626. [Google Scholar] [CrossRef]
- Rellegadla, S.; Prajapat, G.; Agrawal, A. Polymers for enhanced oil recovery: Fundamentals and selection criteria. Appl. Microbiol. Biotechnol. 2017, 101, 4387–4402. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, P.; Bai, B.; Xiao, L.; Liu, L. Using Associated Polymer Gels to Control Conformance for High Temperature and High Salinity Reservoirs. J. Can. Pet. Technol. 2006, 45, 49–54. [Google Scholar] [CrossRef]
- Krevelen, D.W.V. Properties of polymers: Their Correlation with Chemical Structure, Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: New York, NY, USA, 1997; ISBN 044482877X. [Google Scholar]
- Procedure for Treatment of Underground Reservoir Using Rheological Model for Optimisation of Fluid Medium. Available online: https://www.fips.ru/cdfi/fips.dll/ru?ty=29&docid=2424428 (accessed on 11 July 2022).
- Method for Water Inflow Isolation in High-Temperature Reservoirs. Available online: https://patents.s3.yandex.net/RU2272891C1_20060327.pdf (accessed on 11 July 2022).
- Sydansk, R.D. Polymers, Gels, Foams and Resins. In Petroleum Engineering Handbook; Society of Petroleum Engineers: Houston, TX, USA, 2007; pp. 1219–1224. ISBN 978-1-55563-120-8. [Google Scholar]
- Ghosh, P.; Metidji, M.O.; Dupuis, G.; Wilton, R.; Ravikiran, R.; Bowers, A.; Seright, R. Pushing the Envelope of Polymer Injectivity in Low Permeability Sandstones. In Proceedings of the IOR 2021–21st European Symposium on Improved Oil Recovery; EAGE, Online Event, 19–22 April 2021; p. 21. [Google Scholar]
- Bershtein, V.A.; Ryzhov, V.A. Far infrared spectroscopy of polymers. In Polymer Analysis and Characterization; Springer: Berlin/Heidelberg, Germany, 1994; pp. 43–121. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry; John Wiley & Sons: New York, NY, USA, 2009; ISBN 978-0-471-74493-1. [Google Scholar]
- Mardashov, D.; Duryagin, V.; Islamov, S. Technology for Improving the Efficiency of Fractured Reservoir Development Using Gel-Forming Compositions. Energies 2021, 14, 8254. [Google Scholar] [CrossRef]
- Sultanbekov, R.R.; Terekhin, R.D.; Nazarova, M.N. Stress-stain state of a vertical steel tank affected by bottom sediments inconditions of extreme temperature differences. In Advances in Raw Material Industries for Sustainable Development Goals; Taylor & Francis: London, UK, 2021. [Google Scholar]
- Martyushev, D.A.; Govindarajan, S.K. Development and study of a visco-elastic gel with controlled destruction times for killing oil wells. J. King Saud Univ.-Eng. Sci. 2021, 34, 408–415. [Google Scholar] [CrossRef]
| No. | Concentration of 12% Solution HCl CHCl (%) | Solution pH | Concentration of 10% Solution NaOH CNaOH (%) | Solution pH |
|---|---|---|---|---|
| 1 | 0.2300 | 3.57 | 0.3424 | 7.58 |
| 2 | 0.4900 | 3.34 | 0.6848 | 7.58 |
| 3 | 1.0090 | 3.13 | 1.4038 | 7.30 |
| 4 | 2.0121 | 2.96 | 2.8280 | 7.75 |
| 5 | 3.0120 | 2.84 | 4.2160 | 7.44 |
| 6 | 4.0067 | 2.78 | 5.6125 | 7.35 |
| 7 | 5.0056 | 2.72 | 7.0163 | 7.57 |
| No. | Polymer Concentration Cpol, % | Crosslinker Concentration Ccrsl, % (Chromium Acetate) | Gelation Time, min. | Plastic Strength, Pa | ||
|---|---|---|---|---|---|---|
| After 24 h | After 48 h | After 72 h | ||||
| 1 | 0.5 | 0.1 | Did not crosslink (thickened composition) | Not strong | Not strong | Not strong |
| 2 | 1 | 0.1 | Did not crosslink (thickened composition) | Not strong | Not strong | Not strong |
| 3 | 2 | 0.2 | Did not crosslink (thickened composition) | Not strong | Not strong | Not strong |
| 4 | 3 | 0.3 | 1140 | Not strong | 343.60 | 1747 |
| 5 | 4 | 0.4 | 150 | 5049 | 5796 | 5796 |
| 6 | 5 | 0.5 | 105 | 6722 | 5796 | 5796 |
| No. | Concentration of 12% Solution CHCl, % | Concentration of 10% Solution CNaOH, % | Gelation Time, min | Plastic Strength, Pa | ||
|---|---|---|---|---|---|---|
| After 24 h | After 48 h | After 72 h | ||||
| 1 | 0.25 | 0.34 | 24 | 4438 | 5049 | 5796 |
| 2 | 0.5 | 0.68 | 24 | 5403 | 6233 | 6233 |
| 3 | 1 | 1.40 | 28 | 5796 | 6722 | 6722 |
| 4 | 2 | 2.83 | 26 | 6233 | 8590 | 8590 |
| 5 | 3 | 4.22 | 28 | 9389 | 9389 | 9389 |
| 6 | 4 | 5.61 | 23 | 8590 | 8590 | 10,304 |
| 7 | 5 | 7.02 | 26 | 3931 | 7889 | 9389 |
| 8 | 6 | 8.42 | 20 | 3709 | 7270 | 7270 |
| No. | Time, min. | Effective Viscosity, mPas | ||||||
|---|---|---|---|---|---|---|---|---|
| HCl content, % of weight | ||||||||
| 0% (Initial) | 1% | 2% | 3% | 4% | 5% | 6% | ||
| 1 | 10 | 3770 | 3940 | 2600 | 340 | 555 | 1080 | |
| 2 | 60 | 3310 | 1740 | 3920 | 2130 | 2110 | 2800 | 2640 |
| 3 | 120 | 2360 | 1760 | 2030 | 3130 | 2500 | 2520 | 2580 |
| 4 | 300 | 5900 | 1600 | 2910 | 3420 | 2620 | 2390 | 2540 |
| NaOH content, % of weight | ||||||||
| 0% (Initial) | 1.4038% | 2.828% | 4.216% | 5.6125% | 7.0163% | 8.4201% | ||
| 5 | 10 | 3770 | 6710 | 6120 | 13,100 | 7290 | 2800 | 5450 |
| 6 | 60 | 3310 | 2830 | 4980 | 8210 | 6480 | 8880 | 8600 |
| 7 | 120 | 2360 | 2820 | 7010 | 5680 | 5110 | 3850 | 5500 |
| No. | Temperature, °C | Corrosion Rate, mm/Year | |
|---|---|---|---|
| (Initial) | (Proposed) | ||
| 1 | 20 | 0.0611 | 0.0627 |
| 2 | 105 | 0.0962 | 0.0973 |
| No. | Gel-degrading Fluid Name | Polymer Mass Before, g | Polymer Mass After, g | Relative Mass Change, % |
|---|---|---|---|---|
| 1 | H2O | 4.787 | 15.383 | 321.3 |
| 2 | HCl (12% solution) | 4.818 | 3.617 | 75.1 |
| 3 | HNO3 (12% solution) | 4.873 | 6.396 | 131.3 |
| 4 | CH3COOH (12% solution) | 5.007 | 6.464 | 129.1 |
| 5 | HCOOH (12% solution) | 4.740 | 6.160 | 130.0 |
| No. | Sample Number | Kabs, 10−3 μm2 | m, % | gradP at 1 PV MPa/m | gradPs, MPa/m | RRF | RF |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 18.5 | 27 | 0.03 | 1.17 | 87 | 90 |
| 2 | 2 | 20 | 29 | 2.19 | 2.19 | 182 | 182 |
| 3 | 3 | 30 | 30 | 0.12 | 0.85 | 198 | 198 |
| 4 | 7 | 1.25 | 26 | 2.3 | 2.81 | 15 | 15 |
| 5 | 8 | 8.11 | 29 | 2.57 | 2.58 | 87 | 87 |
| No. | Sample Number | Kabs, 10−3 μm2 | m, % | gradP at 1 PV MPa/m | gradPs, MPa/m | RRF | RF |
|---|---|---|---|---|---|---|---|
| 1 | 4 | 16.8 | 30 | 0.072 | 1.93 | 136 | 136 |
| 2 | 10 | 17.6 | 27 | 0.6 | 2.24 | 165 | 166 |
| 3 | 14 | 19.34 | 30 | 0.98 | 2.24 | 182 | 182 |
| 4 | 9 | 31 | 31 | 0.0118 | 2.15 | 285 | 286 |
| 5 | 13 | 6.65 | 28 | 0.023 | 2.24 | 62 | 63 |
| No. | Sample Number | m, % | Vio, mL | Kabs, 10−3 μm2 | Water Injection Stage | Gelant Injection Stage | Post Water Injection Stage | DC Before Treatment | DC After Treatment | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WF, % | Vdow1, mL | Vdop, mL | WF, % | Vdow2, mL | ||||||||
| 1 | High-permeability sample 2C | 33 | 7.9 | 30.3 | 100 | 4.8 | 1.8 | 0 | 0 | 0.61 | 0.23 | |
| 2 | Low-Permeability sample 2D | 31 | 6.3 | 0.99 | 0 | 0 | 1.8 | 0 | 0 | 0 | 0.29 | |
| 3 | Entire model | 14.2 | Before treatment | After treatment | 4.8 | 3.6 | 0 | 0.34 | 0.25 | |||
| 24 | 0.1 | |||||||||||
| No. | Sample Number | m, % | Vio, mL | Kabs, 10−3 μm2 | Water Injection Stage | Gelant Injection Stage | Post Water Injection Stage | DC Before Treatment | DC After Treatment | SC | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WF, % | Vdow1, mL | Vdop, mL | WF, % | Vdow2, mL | ||||||||
| 1 | High-permeability sample 1A | 31 | 6.6 | 3.7 | 100 | 5.6 | 0.6 | 21 | 0 | 0.85 | 0.09 | 0.21 |
| 2 | Low-permeability sample 2B | 28 | 6.8 | 1.1 | 0 | 0.4 | 3.2 | 79 | 0.4 | 0.06 | 0.59 | 0.79 |
| 3 | Entire model | 13.4 | 6 | 3.8 | 0.4 | 0.45 | 0.34 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raupov, I.; Rogachev, M.; Sytnik, J. Design of a Polymer Composition for the Conformance Control in Heterogeneous Reservoirs. Energies 2023, 16, 515. https://doi.org/10.3390/en16010515
Raupov I, Rogachev M, Sytnik J. Design of a Polymer Composition for the Conformance Control in Heterogeneous Reservoirs. Energies. 2023; 16(1):515. https://doi.org/10.3390/en16010515
Chicago/Turabian StyleRaupov, Inzir, Mikhail Rogachev, and Julia Sytnik. 2023. "Design of a Polymer Composition for the Conformance Control in Heterogeneous Reservoirs" Energies 16, no. 1: 515. https://doi.org/10.3390/en16010515






