The Effect of C45 Carbon Black-Phosphomolybdic Acid Nanocomposite on Hydrogenation and Corrosion Resistance of La2Ni9Co Hydrogen Storage Alloy
Abstract
:1. Introduction
2. Research Material and Methodology
3. Research Results
4. Conclusions
- -
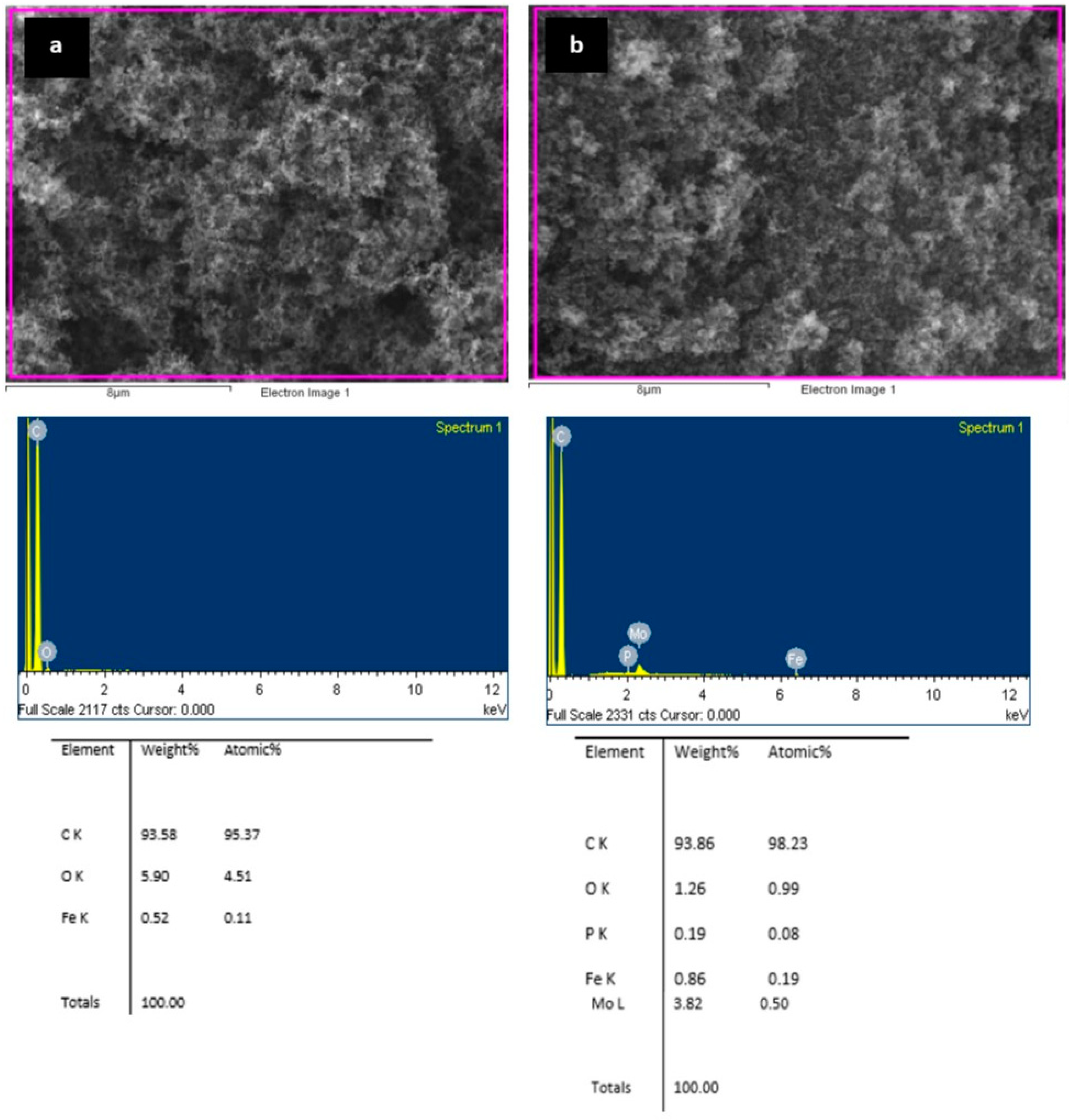
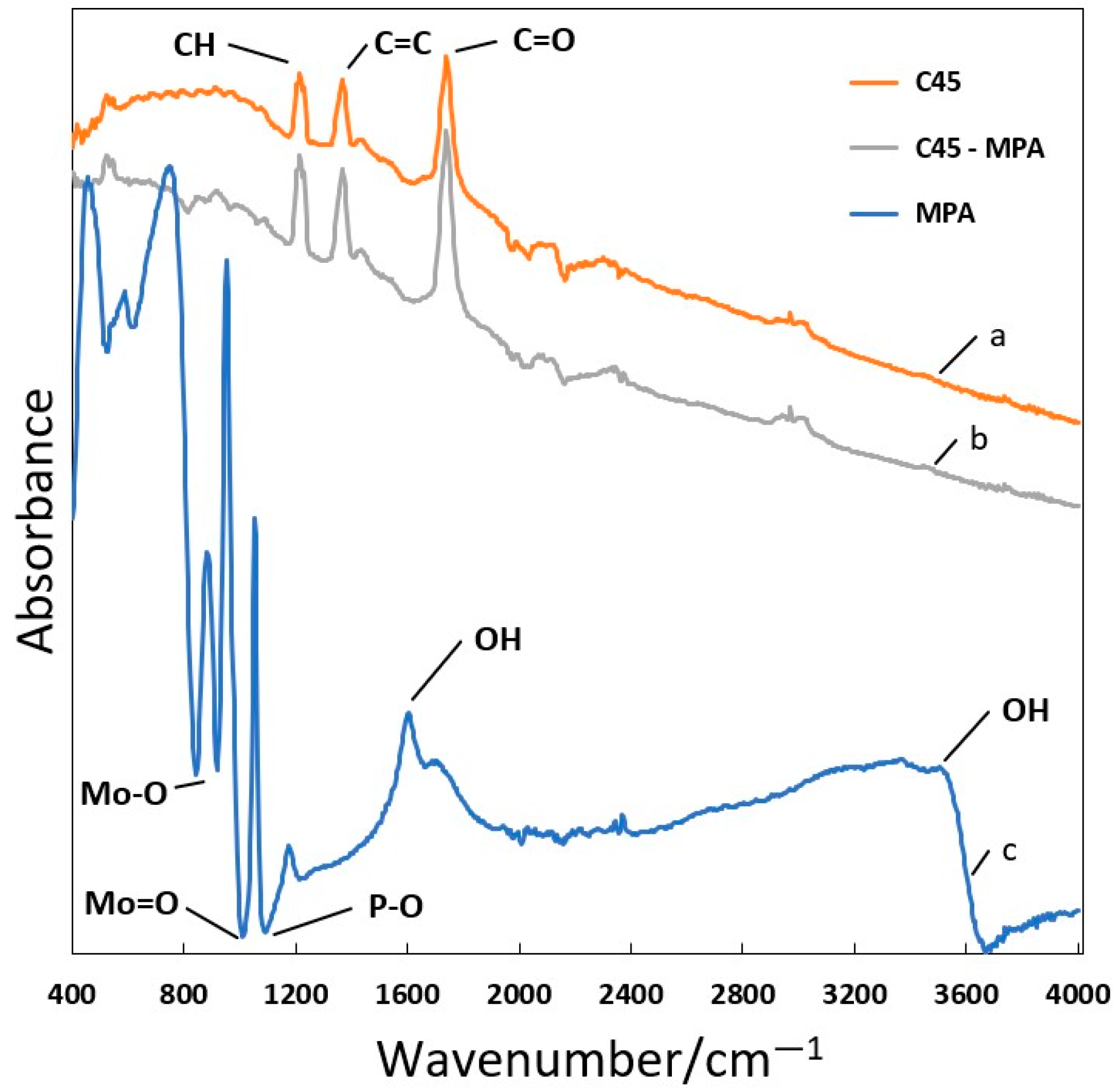
- FTIR analysis of the C45 carbon black conducted before and after the chemical modification confirmed the integration of the Keggin structure of the PMo12O403− anion with the carbon matrix in the C45-MPA nanocomposite.
- -
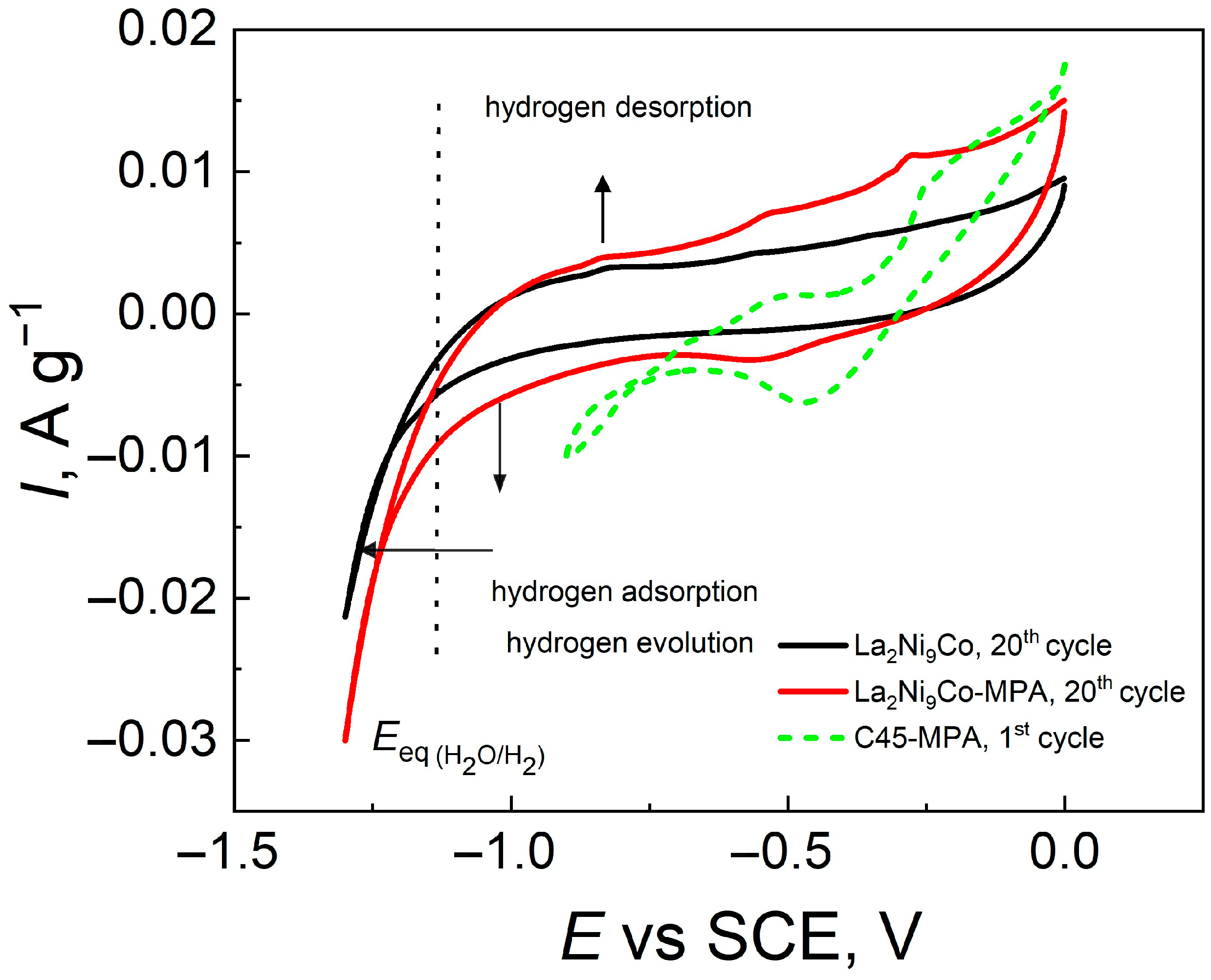
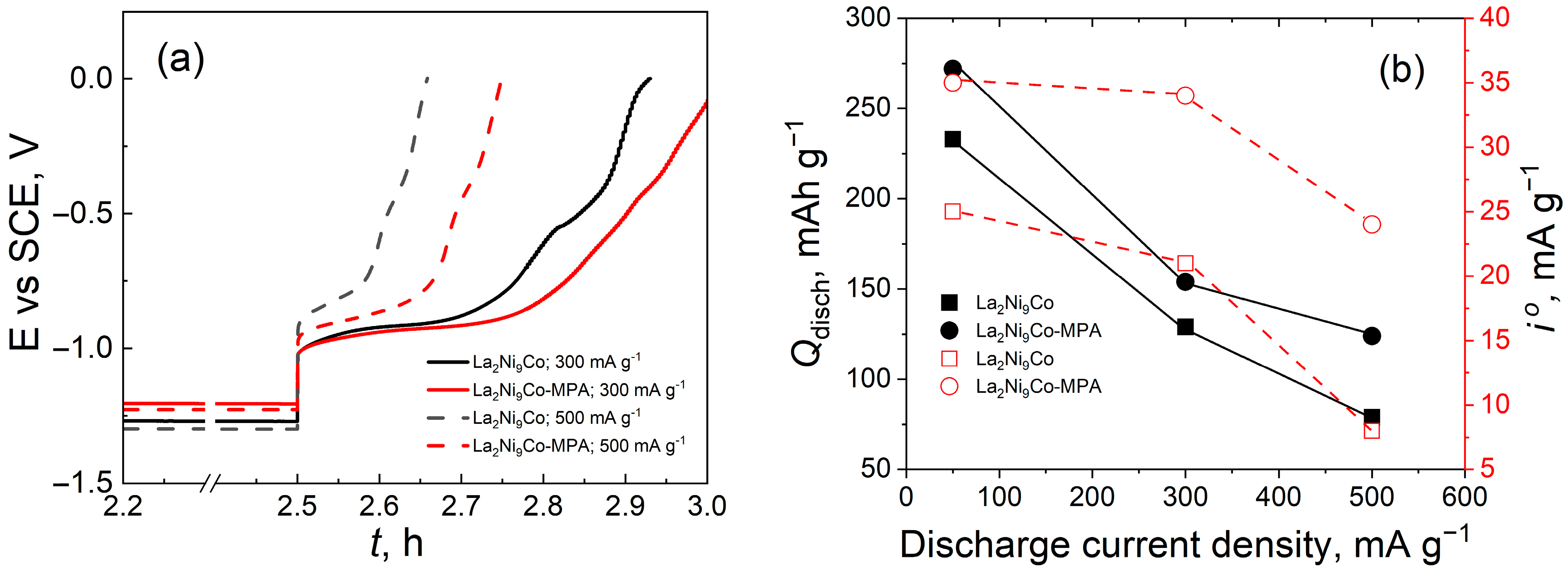
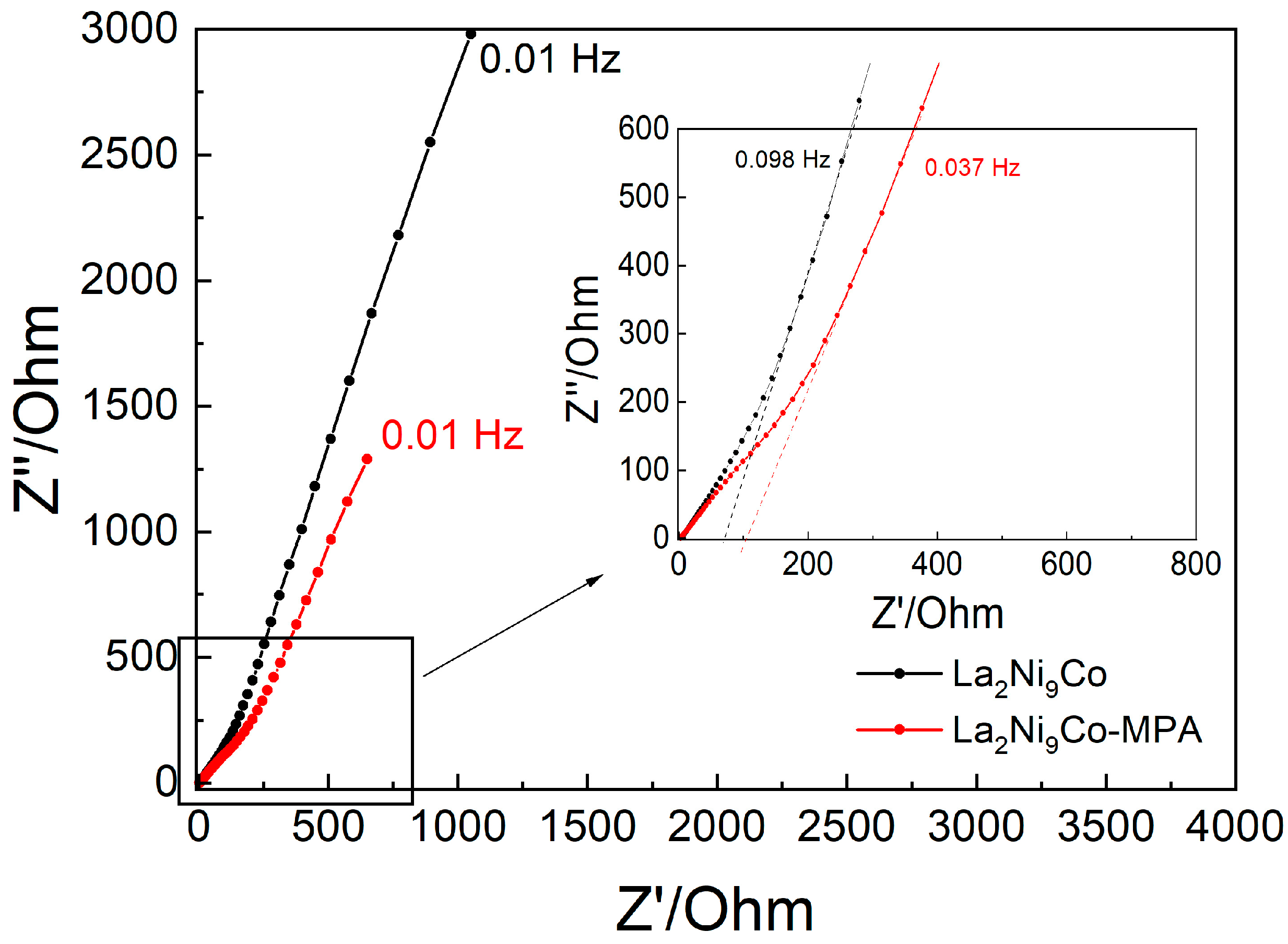
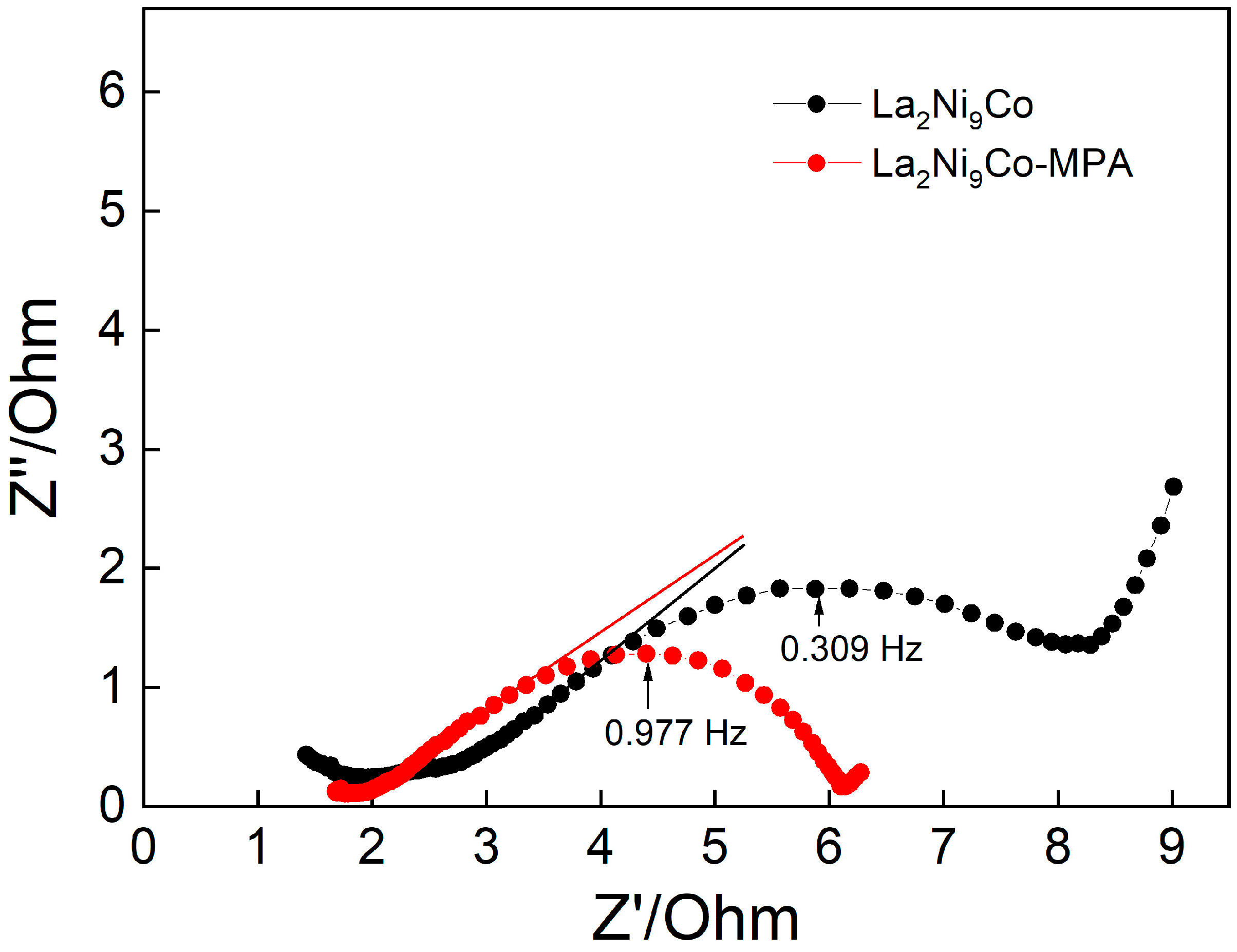
- The addition of the C45-MPA carbon black nanocomposite leads to better capacitive and kinetic parameters of the studied composite hydride electrodes.
- -
- The presence of a conductive C45-PVDF matrix and mixed Mo(VI,V) valence sites facilitates electron transfer and charge accumulation.
- -
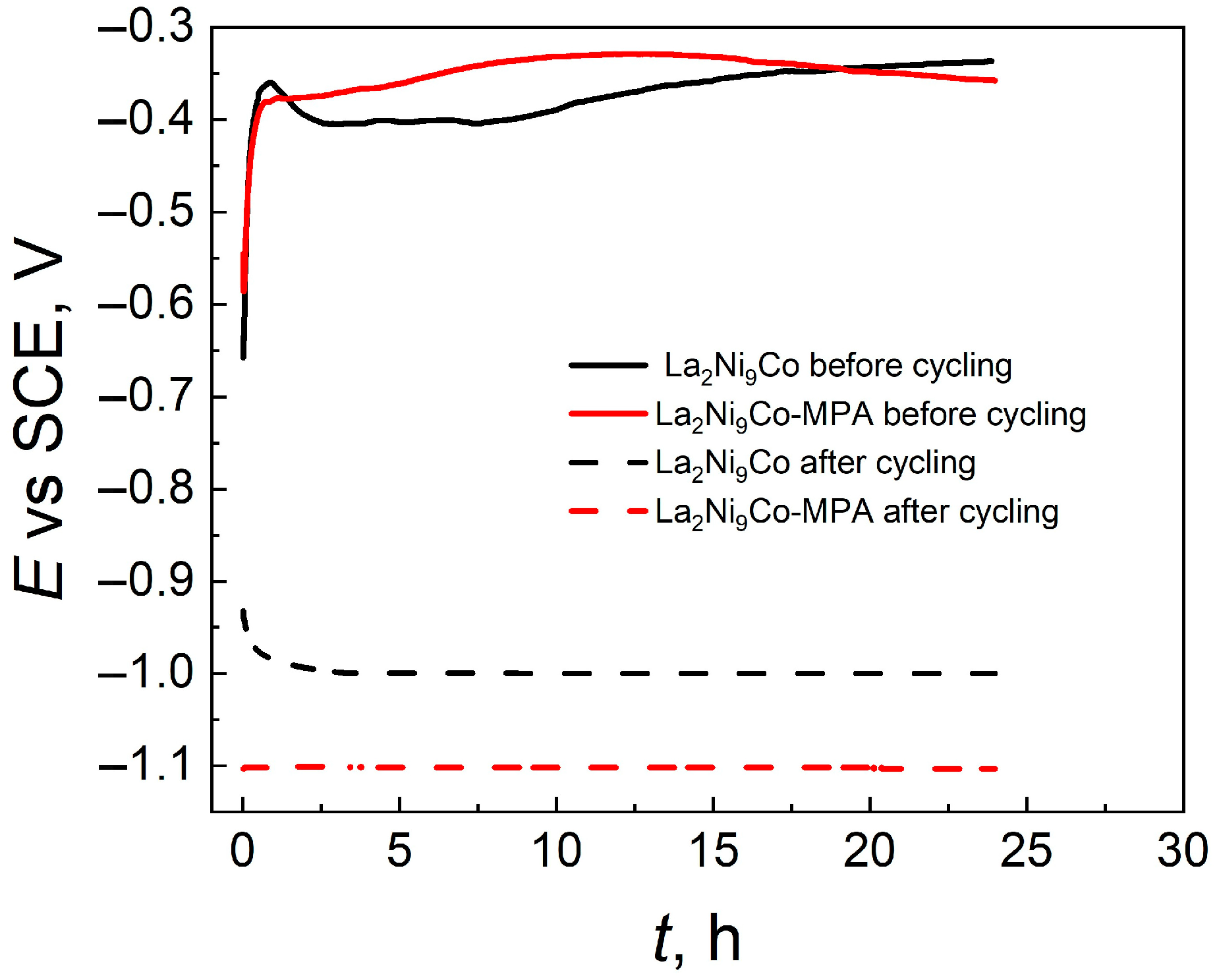
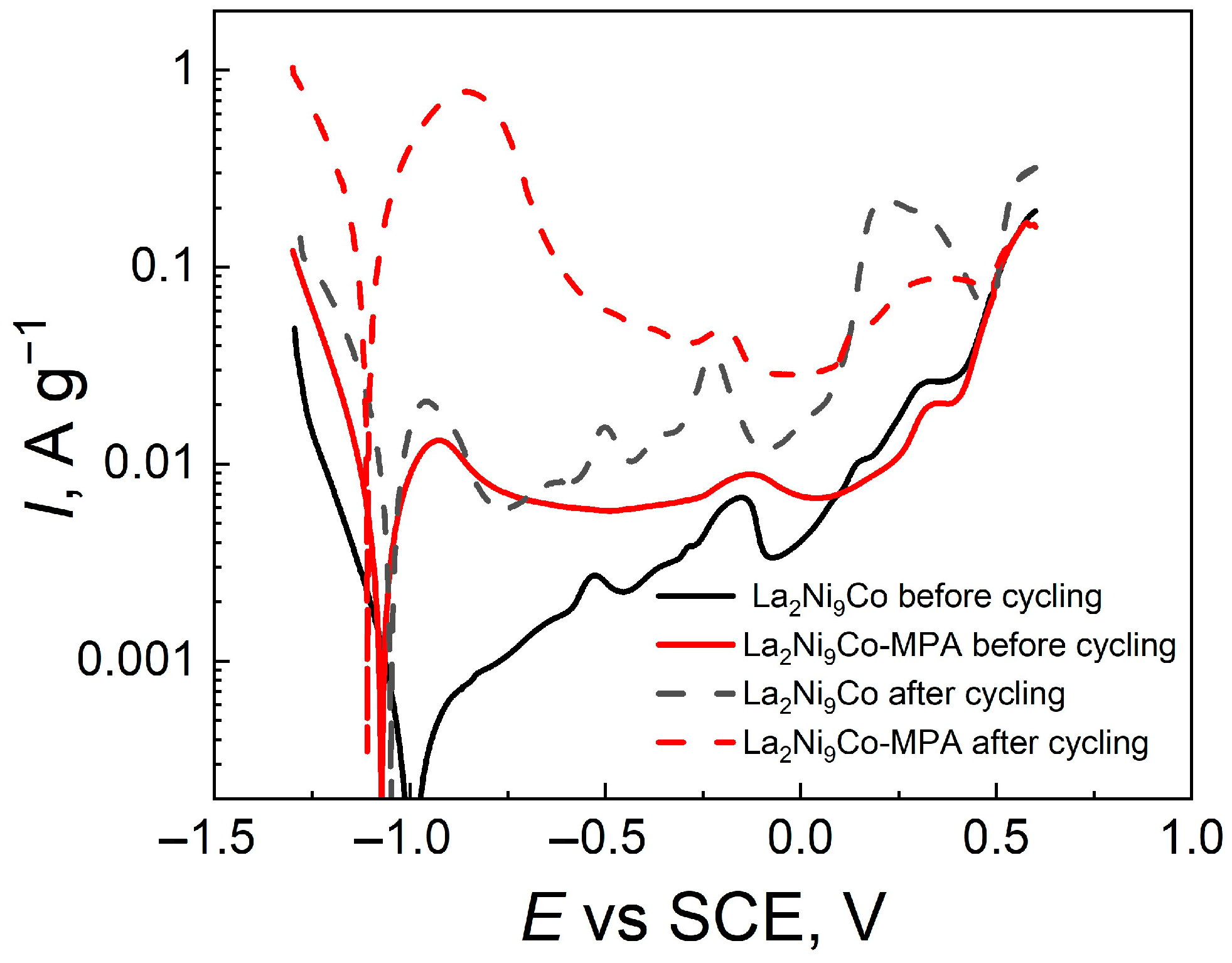
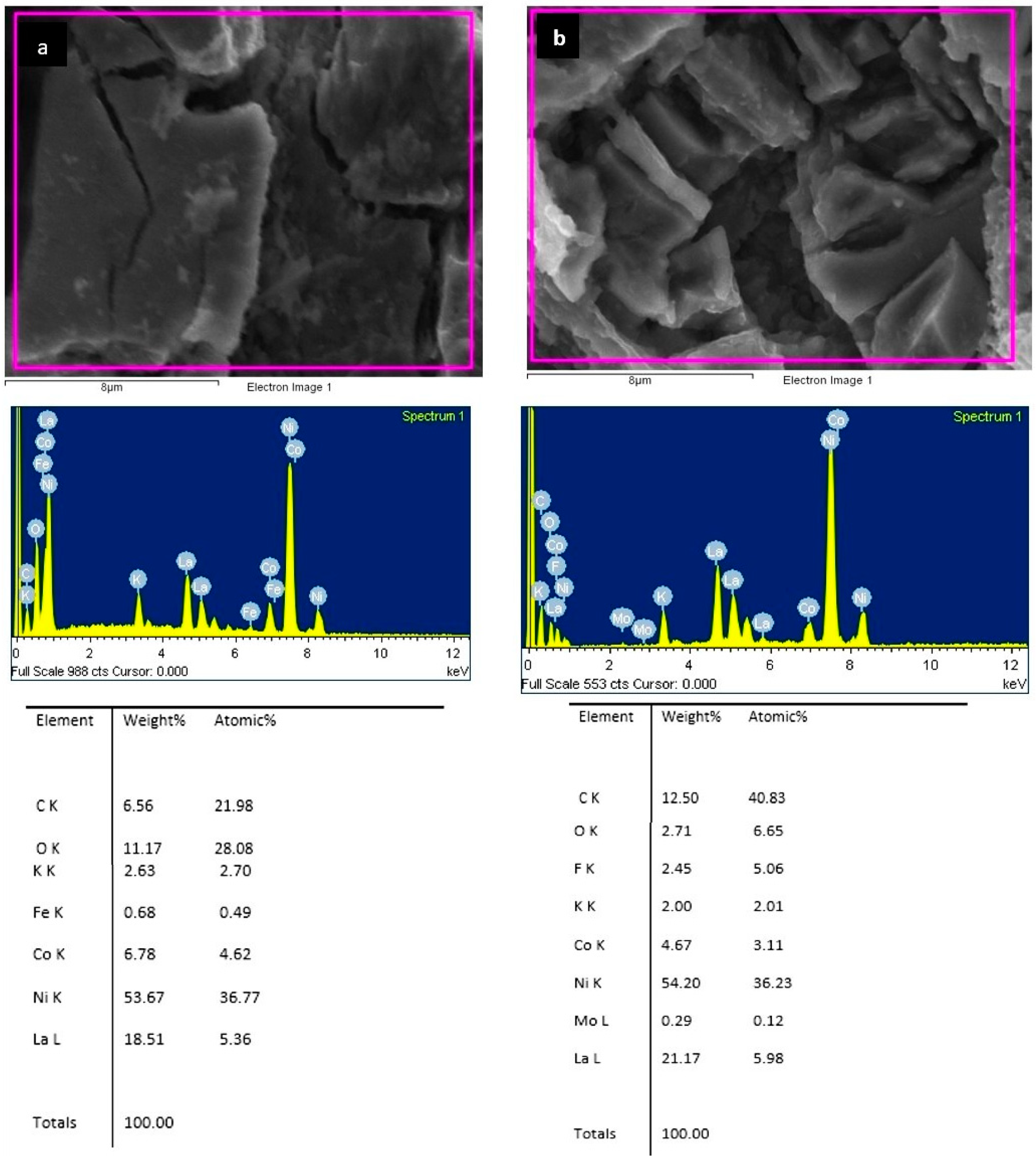
- The OCP values and the EDS analysis of the electrode surface after the charging/discharging processes indicate that the unmodified electrode oxidizes much more strongly, and the C45-MPA nanocomposite can be a protective barrier for the La2Ni9Co alloy against chemical degradation.
- -
- Higher anode currents in the passive range of the modified electrodes are the result of their much larger effective surface in relation to the active surface of the reference and their greater hydrogen capacity.
- -
- The use of H3PMo12O40 may be important in the design of new anode materials for NiMH batteries, but further work is needed to optimize the composition of the C45-MPA nanocomposite.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baykara, S.Z. Hydrogen: A brief overview on its sources, production and environmental impact. Int. J. Hydrogen Energy 2018, 43, 10605–10614. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; De, M. Scope of doped mesoporous (<10 nm) surfactant-modified alumina templated carbons for hydrogen storage applications. Int. J. Energy Res. 2019, 43, 4264–4280. [Google Scholar] [CrossRef]
- Pasquini, L.; Sakaki, S.; Akiba, E.; Allendorf, M.D.; Cho, Y.W.; Alvares, E.; Ares, J.D.; Badai, D.; Bricco, M.; von Colbe, J.B.; et al. Magnesium- and Intermetallic Alloys-Based Hydrides for Energy Storage: Modelling, Synthesis and Properties. Prog. Energy 2022, 4, 032007. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Fursikov, P.V.; Volodin, A.A.; Bocharnikov, M.S.; Shimkus, Y.Y.; Kashin, A.M.; Lototskyy, M.V. Metal hydride hydrogen storage and compression systems for energy storage technologies. Int. J. Hydrogen Energy 2021, 46, 13647–13657. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Linkov, V.; Pasupathi, S.; Davids, M.W.; Tolj, I.; Radica, G.; Denys, R.V.; Eriksen, J.; Taube, K.; et al. HYDRIDE4MOBILITY: An EU HORIZON 2020 project on hydrogen powered fuel cell utility vehicles using metal hydrides in hydrogen storage and refuelling systems. Int. J. Hydrogen Energy 2021, 46, 35896–35909. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Bellosta von Colbe, J.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for Hydrogen-Based Energy Storage Past, Recent Progress and Future Outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Z.; Zheng, J.; Li, X.; Akiba, E.; Li, H.W. Perspectives and challenges of hydrogen storage in solid-state hydrides. Chin. J. Chem. Eng. 2021, 29, 1–12. [Google Scholar] [CrossRef]
- Fukumoto, M.; Nakajima, K.; Takahashi, H. Formation of LaNi5 hydrogen storage alloy by electrodeposition of La using molten salt. Coatings 2022, 12, 1268. [Google Scholar] [CrossRef]
- Todorova, S.; Abrashev, B.; Rangelova, V.; Vassileva, E.; Spassov, T. Effect of low Al content on the electrode performance of LaNi5-xAlx hydrogen storage alloys. J. Chem. Technol. Metall. 2023, 58, 200–207. [Google Scholar]
- Sato, T.; Saitoh, H.; Utsumi, R.; Ito, J.; Nakahira, Y.; Obana, K.; Takagi, S.; Orimo, S.I. Hydrogen absorption reactions of hydrogen storage alloy LaNi5 under high pressure. Molecules 2023, 28, 1256. [Google Scholar] [CrossRef] [PubMed]
- Giza, K.; Iwasieczko, W.; Pavlyuk, V.V.; Bala, H.; Drulis, H. Thermodynamical properties of La–Ni–T (T = Mg, Bi and Sb) hydrogen storage systems. J. Power Sources 2008, 181, 38–40. [Google Scholar] [CrossRef]
- Todorova, S.; Abrashev, B.; Rangelova, V.; Mihaylov, L.; Vassileva, E.; Petrov, K.; Spassov, T. Hydrogen gas phase and electrochemical hydriding of LaNi5− xMx (M = Sn, Co, Al) alloys. Materials 2020, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Giza, K.; Owczarek, E. Electrochemical Hydrogenation and Corrosion Behaviour of LaNi5-xGex (x = 0.3 and 0.6) Alloys. Energies 2021, 14, 5285. [Google Scholar] [CrossRef]
- Giza, K.; Iwasieczko, W.; Pavlyuk, V.V.; Bala, H.; Drulis, H.; Adamczyk, L. Hydrogen absorption and corrosion resistance of LaNi4.8Al0.2 and LaNi4.8Al0.1Li0.1 alloys. J. Alloys Compd. 2007, 429, 352–356. [Google Scholar] [CrossRef]
- Stetskiv, A.; Rożdżyńska-Kiełbik, B.; Kowalczyk, G.; Prochwicz, W.; Siemion, P.; Pavlyuk, V. The structural and thermal stability; electrochemical hydrogenation and corrosion behaviour of LaT5-xMx (T = Co, Ni and M = Al, Ge, Li) phases. Solid State Sci. 2014, 38, 35–41. [Google Scholar] [CrossRef]
- Giza, K. Electrochemical studies of LaNi4.3Co0.4Al0.3 hydrogen storage alloy. Intermetallics 2013, 34, 128–131. [Google Scholar] [CrossRef]
- Pęska, M.; Dworecka-Wójcik, J.; Płociński, T.; Polański, M. The influence of cerium on the hydrogen storage properties of La1-xCexNi5 Alloys. Energies 2020, 13, 1437. [Google Scholar] [CrossRef]
- Joubert, J.M.; Paul-Boncour, V.; Cuevas, F.; Zhang, J.; Latroche, M. LaNi5 related AB5 compounds: Structure, properties and applications. J. Alloys Compd. 2021, 862, 158163. [Google Scholar] [CrossRef]
- Ouyang, L.; Huang, J.; Wang, H.; Liu, J.; Zhu, M. Progress of hydrogen storage alloys for Ni-MH rechargeable power batteries in electric vehicles: A review. Mater. Chem. Phys. 2017, 200, 164–178. [Google Scholar] [CrossRef]
- Poblano-Salas, C.A.; Sotelo-Mazón, O.; Henao, J.; Corona-Castuera, J.; Martinez, G.; Casales-Diaz, M.; Porcayo-Calderón, J.; Tathagata, K.; Navarro, M.; Kesarla, M.K. Flame sprayed LaNi5-based mischmetal alloy: Building-up negative electrodes for potential application in Ni-based batteries. J. Therm. Spray Technol. 2021, 30, 1940–1956. [Google Scholar] [CrossRef]
- Hubkowska, K.; Soszko, M.; Krajewski, M.; Czerwiński, A. Enhanced kinetics of hydrogen electrosorption in AB5 hydrogen storage alloy decorated with Pd nanoparticles. Electrochem. Commun. 2019, 100, 100–103. [Google Scholar] [CrossRef]
- Xie, D. Effect of surface coating on electrochemical properties of rare earth-based AB5-type hydrogen storage alloys. Int. J. Electrochem. Sci. 2016, 11, 9153–9163. [Google Scholar] [CrossRef]
- Williams, M.; Lototsky, M.; Nechaev, A.; Yartys, V.; Solberg, J.K.; Denys, R.V.; Linkov, V.M. Palladium mixed-metal surface-modified AB5-type intermetallides enhance hydrogen sorption kinetics. S. Afr. J. Sci. 2010, 106, 1–6. [Google Scholar] [CrossRef]
- Modibane, K.D.; Lototskyy, M.; Davids, M.W.; Williams, M.; Hato, M.J.; Molapo, K.M. Influence of co-milling with palladium black on hydrogen sorption performance and poisoning tolerance of surface modified AB5-type hydrogen storage alloy. J. Alloys Compd. 2018, 750, 523–529. [Google Scholar] [CrossRef]
- Ambrosio, R.C.; Ticianelli, E.A. Studies on the influence of palladium coatings on the electrochemical and structural properties of a metal hydride alloy. Surf. Coat. Technol. 2005, 197, 215–222. [Google Scholar] [CrossRef]
- Visintin, A.; Castro, E.B.; Real, S.G.; Triaca, W.E.; Wang, C.; Soriaga, M.P. Electrochemical activation and electrocatalytic enhancement of a hydride-forming metal alloy modified with palladium, platinum and nickel. Electrochim. Acta 2006, 51, 3658–3667. [Google Scholar] [CrossRef]
- Prigent, J.; Joubert, J.M.; Gupta, M. Modification of the hydrogenation properties of LaNi5 upon Ni substitution by Rh, Ir, Pt or Au. J. Alloys Compd. 2012, 511, 95–100. [Google Scholar] [CrossRef]
- Karwowska, M.; Fijalkowski, K.J.; Czerwiński, A. Corrosion of hydrogen storage metal alloy LaMm-Ni4.1Al0.3Mn0.4Co0.45 in the aqueous solutions of alkali metal hydroxides. Materials 2018, 11, 2423. [Google Scholar] [CrossRef]
- Giza, K.; Owczarek, E. Microstructure and corrosion resistance of LaNi5-xMgx alloys. Micromachines 2022, 13, 1192. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Li, S.L.; Su, Z.M.; Lan, Y.Q. Polyoxometalate-based materials for sustainable and clean energy conversion and storage. EnergyChem 2019, 1, 100021. [Google Scholar] [CrossRef]
- Genovese, M.; Foong, Y.W.; Lian, K. Designing polyoxometalate based layer-by-layer thin films on carbon nanomaterials for pseudocapacitive electrodes. J. Electrochem. Soc. 2015, 162, A5041. [Google Scholar] [CrossRef]
- Skunik, M.; Chojak, M.; Rutkowska, I.A.; Kulesza, P.J. Improved capacitance characteristics during electrochemical charging of carbon nanotubes modified with polyoxometallate monolayers. Electrochim. Acta 2008, 53, 3862–3869. [Google Scholar] [CrossRef]
- Horn, M.R.; Singh, A.; Alomari, S.; Goberna-Ferrón, S.; Benages-Vilau, R.; Chodankar, N.; Motta, N.; Ostrikov, K.; MacLeod, J.; Sonar, P.; et al. Polyoxometalates (POMs): From electroactive clusters to energy materials. Energy Environ. Sci. 2021, 14, 1652–1700. [Google Scholar] [CrossRef]
- Cuentas-Gallegos, A.K.; Martínez-Rosales, R.; Baibarac, M.; Gomez-Romero, P.; Rincon, M.E. Electrochemical supercapacitors based on novel hybrid materials made of carbon nanotubes and polyoxometalates. Electrochem. Commun. 2007, 9, 2088–2092. [Google Scholar] [CrossRef]
- Bonastre, J.; Garcés, P.; Huerta, F.; Quijada, C.; Andión, L.G.; Cases, F. Electrochemical study of polypyrrole/PW12O403-coatings on carbon steel electrodes as protection against corrosion in chloride aqueous solutions. Corros. Sci. 2006, 48, 1122–1136. [Google Scholar] [CrossRef]
- Giza, K.; Adamczyk, L. Influence of H3PW12O40 on electrochemical properties of LaCo4.8Bi0.2 alloy. Cent. Eur. J. Chem. 2013, 11, 330–334. [Google Scholar] [CrossRef]
- Ernst, A.Z.; Zoladek, S.; Wiaderek, K.; Cox, J.A.; Kolary-Zurowska, A.; Miecznikowski, K.; Kulesza, P.J. Network films of conducting polymer-linked polyoxometalate-modified gold nanoparticles: Preparation and electrochemical characterization. Electrochim. Acta 2008, 53, 3924–3931. [Google Scholar] [CrossRef]
- Karnicka, K.; Chojak, M.; Miecznikowski, K.; Skunik, M.; Baranowska, B.; Kolary, A.; Piranska, A.; Palys, B.; Adamczyk, L.; Kulesza, P.J. Polyoxometallates as inorganic templates for electrocatalytic network films of ultra-thin conducting polymers and platinum nanoparticles. Bioelectrochemistry 2005, 66, 79–87. [Google Scholar] [CrossRef]
- Kulesza, P.J.; Skunik, M.; Baranowska, B.; Miecznikowski, K.; Chojak, M.; Karnicka, K.; Ernst, A. Fabrication of network films of conducting polymer-linked polyoxometallate-stabilized carbon nanostructures. Electrochim. Acta 2006, 51, 2373–2379. [Google Scholar] [CrossRef]
- Giza, K. Communication—A New Catalytic Application of H3PMo12O40 in the Performance of Hydride Electrode for Ni-MH Battery. J. Electrochem. Soc. 2019, 166, A3332–A3334. [Google Scholar] [CrossRef]
- Giza, K. Electrochemical characterization of C45 carbon black-H3PMo12O40-polyvinylidene fluoride system. Ochr. Przed Korozją 2022, 65, 340–343. [Google Scholar] [CrossRef]
- Bala, H.; Dymek, M.; Drulis, H. Development of metal hydride material efficient surface in conditions of galvanostatic charge/discharge cycling. Mat. Chem. Phys. 2014, 148, 1008–1012. [Google Scholar] [CrossRef]
- Rozdzynska-Kielbik, B.; Stetskiv, I.; Pavlyuk, V.; Stetskiv, A. Significant improvement of electrochemical hydrogenation, corrosion protection and thermal stability of LaNi4.6Zn0.4−xLix (x ≤ 0.2) solid solution phases due to Li-doping. Solid State Sci. 2021, 113, 106552. [Google Scholar] [CrossRef]
- Maksimovskaya, R. Molybdophosphate heteropoly blues: Electron-transfer reactions in aqueous solutions as studied by NMR. Polyhedron 2013, 65, 54–59. [Google Scholar] [CrossRef]
- Giza, K.; Musiał-Gładysz, A. Evaluation of the influence of Cu2O addition on electrochemical properties of LaNi5 hydrogen storage alloy. Ochr. Przed Korozją 2018, 61, 114–118. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Rompel, A. Synthesis, structures and applications of electron-rich polyoxometalates. Nat. Rev. Chem. 2018, 2, 0112. [Google Scholar] [CrossRef]
- Kulesza, P.J.; Pieta, I.S.; Rutkowska, I.A.; Wadas, A.; Marks, D.; Klak, K.; Stobinski, L.; Cox, J.A. Electrocatalytic oxidation of small organic molecules in acid medium: Enhancement of activity of noble metal nanoparticles and their alloys by supporting or modifying them with metal oxides. Electrochim. Acta 2013, 110, 474–483. [Google Scholar] [CrossRef]
- Giza, K. Electrochemical properties of LaNi4.2Co0.4Zn0.1Al0.3 and LaNi4.3Co0.4Zn0.1Al0.2 alloys as anode materials for Ni-MH batteries. Mater. Test. 2017, 59, 598–601. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press: Oxford, UK, 1996. [Google Scholar]
- Dymek, M.; Bala, H.; Drulis, H.; Hackemer, A. Hydrogenation and corrosion properties of LaNi4.5Co0.5-based alloy doped with 1.7 at% Sn. Solid State Phenom. 2015, 227, 263–266. [Google Scholar] [CrossRef]
- Bala, H.; Gęga, J.; Dymek, M.; Pawlik, P. Activation of superstoichiometric hydride alloy by its selective leaching in 6 M KOH solution. Ochr. Przed Koroz. 2019, 62, 124. [Google Scholar]
- Ogihara, N.; Itou, Y.; Sasaki, T.; Takeuchi, Y. Impedance spectroscopy characterization of porous electrodes under different electrode thickness using a symmetric cell for high-performance lithium-ion batteries. J. Phys. Chem. C 2015, 119, 4612–4619. [Google Scholar] [CrossRef]
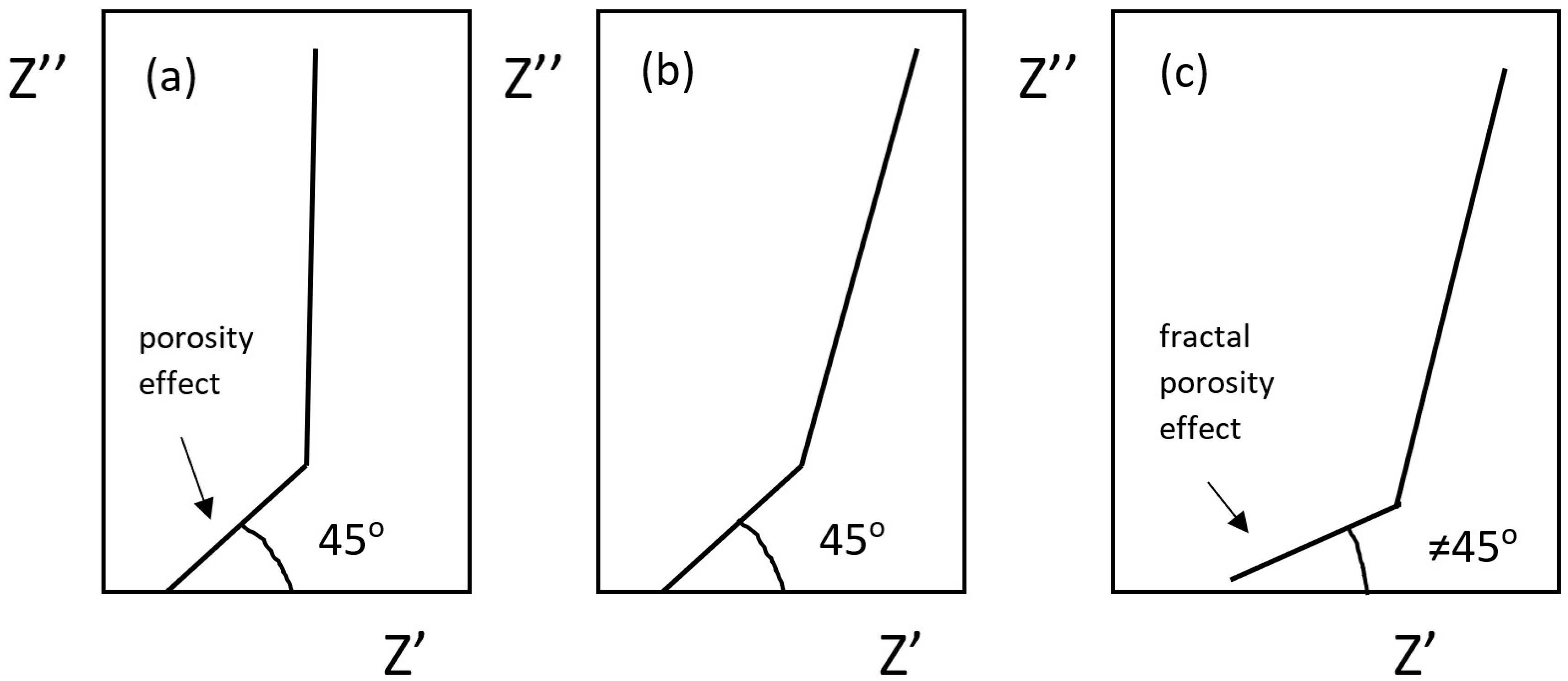
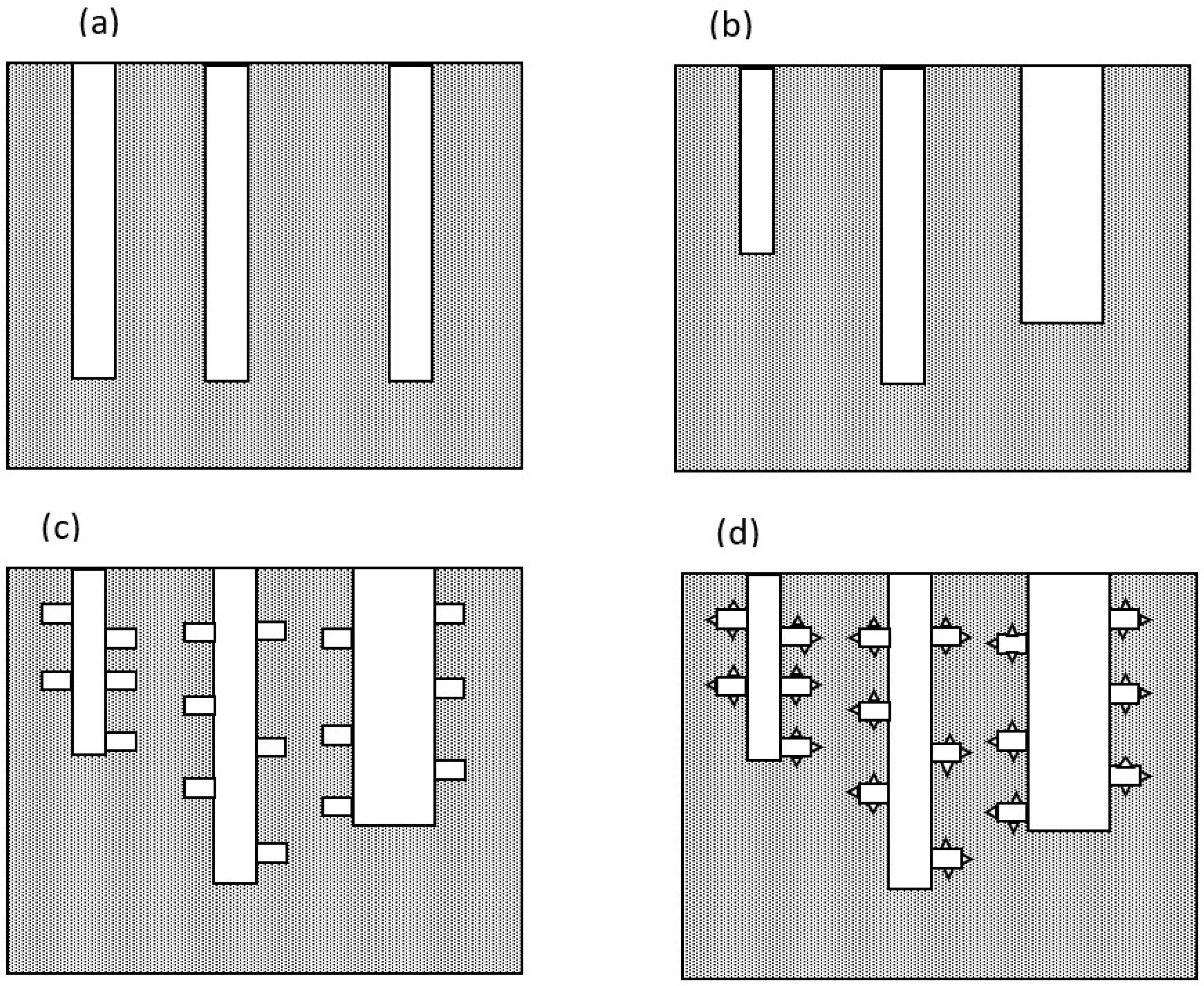
- Itagaki, M.; Hatada, Y.; Shitanda, I.; Watanabe, K. Complex impedance spectra of porous electrode with fractal structure. Electrochim. Acta 2010, 55, 6255–6262. [Google Scholar] [CrossRef]
- De Levie, R. On porous electrodes in electrolyte solutions. Electrochim. Acta 1963, 8, 751–780. [Google Scholar] [CrossRef]
- Song, H.K.; Hwang, H.Y.; Lee, K.H.; Dao, L.H. The effect of pore distribution on the frequency dispersion of porous electrodes. Electrochim. Acta 2000, 45, 2241–2257. [Google Scholar] [CrossRef]
- Cericola, D.; Spahr, M.E. Impedance spectroscopic studies of the porous structure of electrodes containing graphite materials with different particle size and shape. Electrochim. Acta 2016, 191, 558–566. [Google Scholar] [CrossRef]
- Gourbeyre, Y.; Tribollet, B.; Dagbert, C.; Hyspecka, L. A physical model for anticorrosion behaviour of duplex coatings. J. Electrochem. Soc. 2006, 153, B162–B168. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giza, K.; Owczarek, E.; Miszczyk, A. The Effect of C45 Carbon Black-Phosphomolybdic Acid Nanocomposite on Hydrogenation and Corrosion Resistance of La2Ni9Co Hydrogen Storage Alloy. Energies 2023, 16, 4002. https://doi.org/10.3390/en16104002
Giza K, Owczarek E, Miszczyk A. The Effect of C45 Carbon Black-Phosphomolybdic Acid Nanocomposite on Hydrogenation and Corrosion Resistance of La2Ni9Co Hydrogen Storage Alloy. Energies. 2023; 16(10):4002. https://doi.org/10.3390/en16104002
Chicago/Turabian StyleGiza, Krystyna, Edyta Owczarek, and Andrzej Miszczyk. 2023. "The Effect of C45 Carbon Black-Phosphomolybdic Acid Nanocomposite on Hydrogenation and Corrosion Resistance of La2Ni9Co Hydrogen Storage Alloy" Energies 16, no. 10: 4002. https://doi.org/10.3390/en16104002




