Modelling Self-Heating and Self-Ignition Processes during Biomass Storage
Abstract
:1. Introduction
2. Modelling and Methods
2.1. Models and Its Numerical Solution
2.2. Methods for Determining the Low-Temperature Oxidation Kinetics
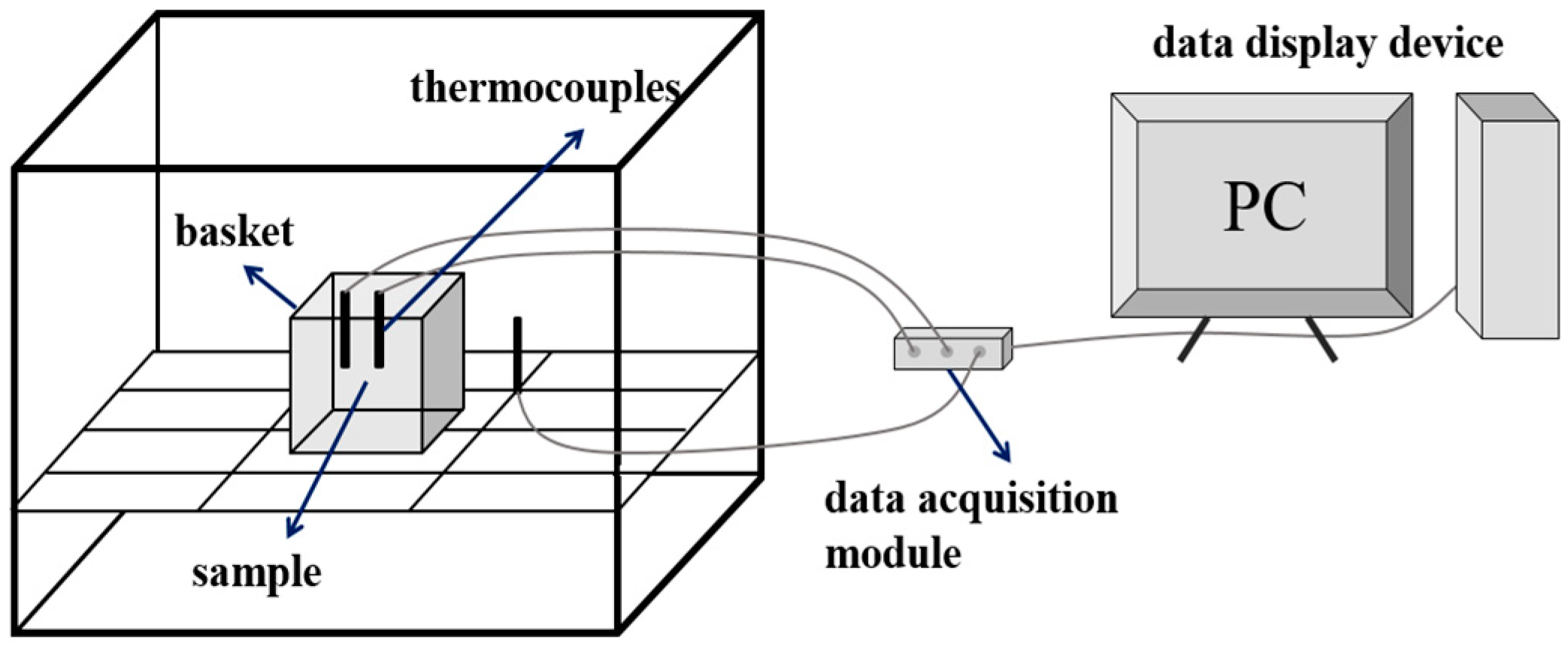
2.3. Experiments on the Self-Heating Process of Biomass Pellets
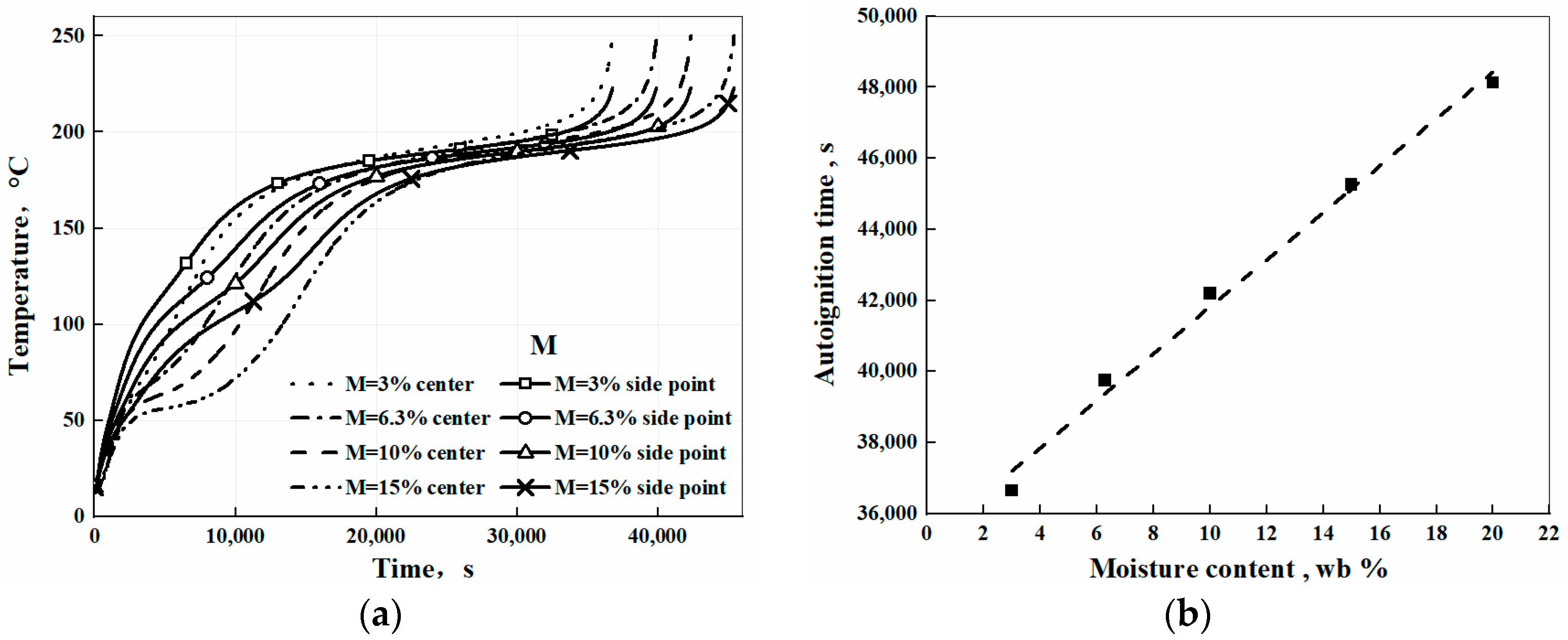
3. Results and Discussion
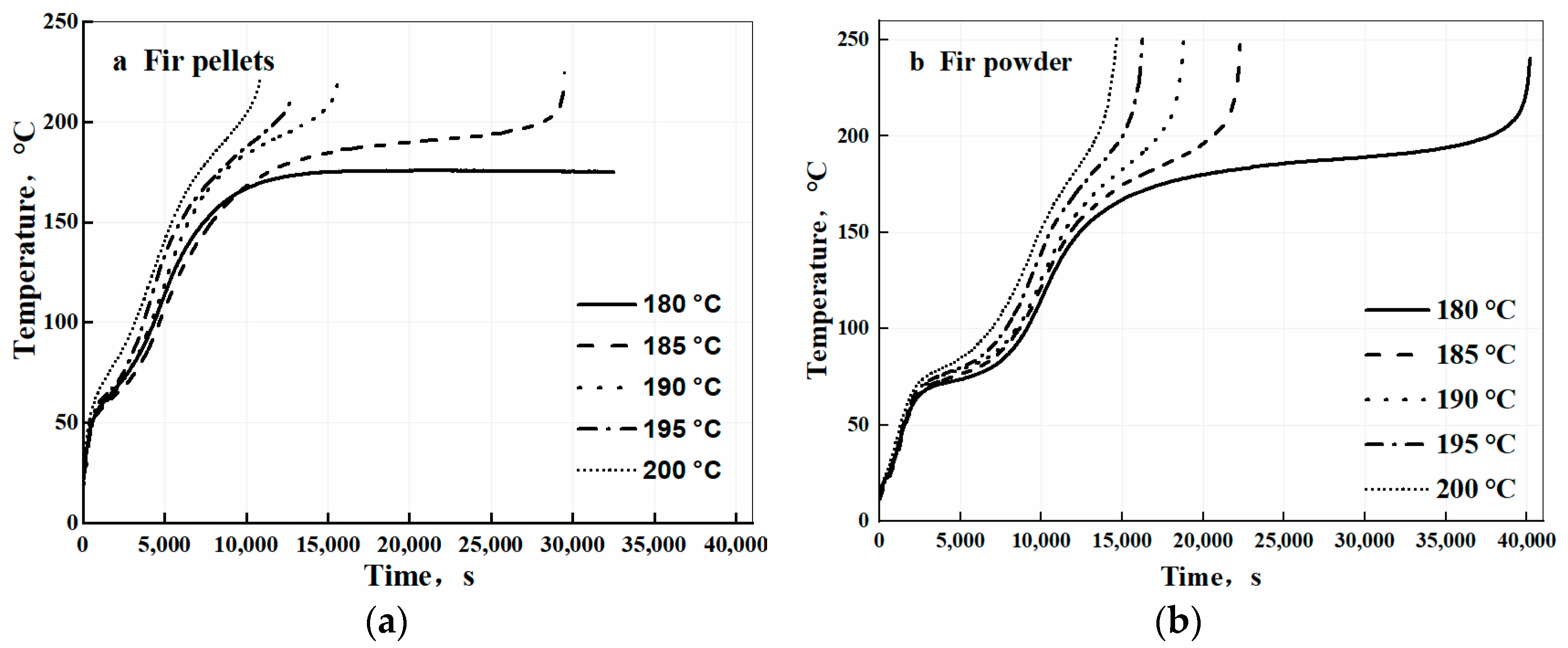
3.1. Temperature Evolution from Self-Heating to Spontaneous Combustion Process
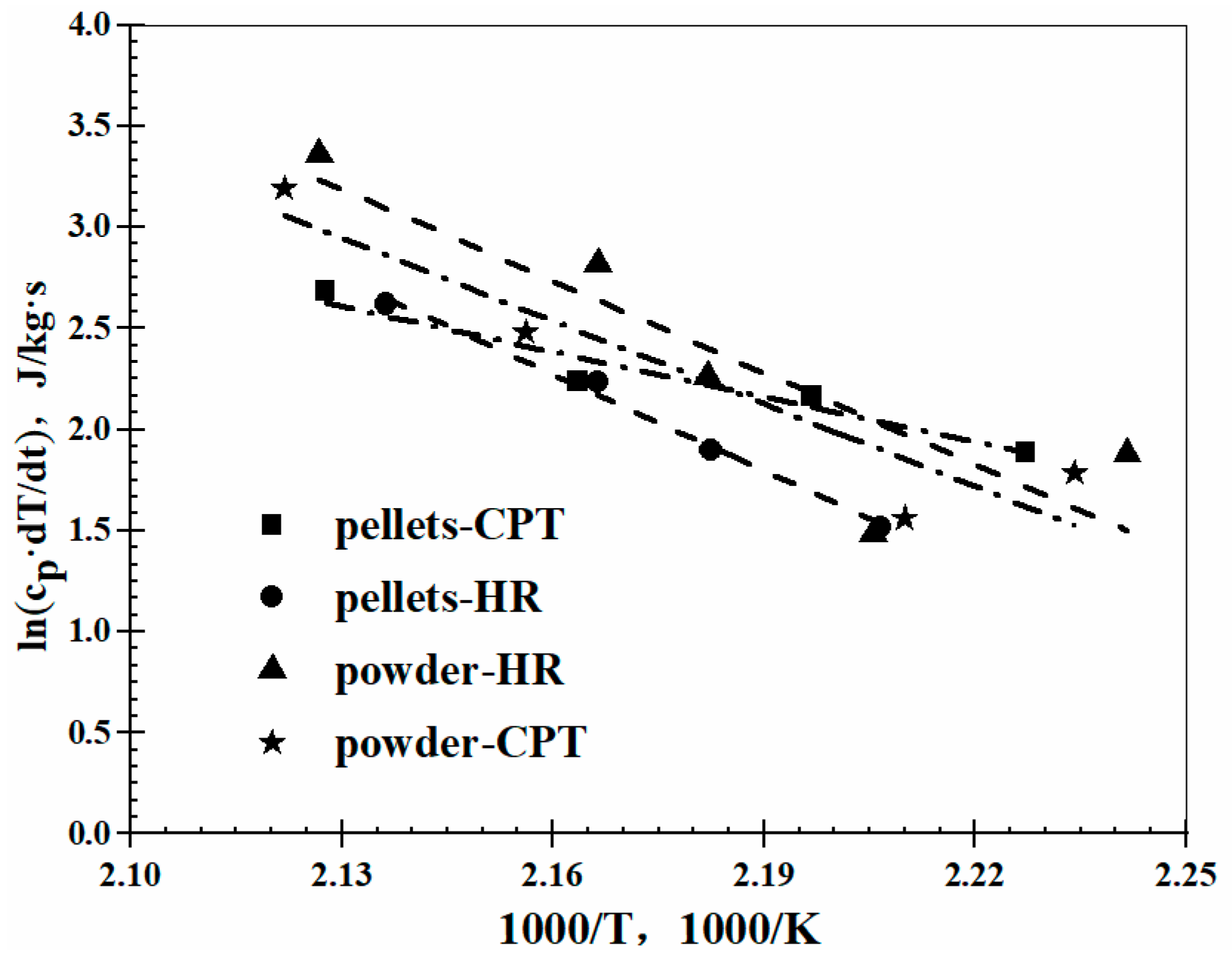
3.2. Determination of Low-Temperature Oxidation Kinetics
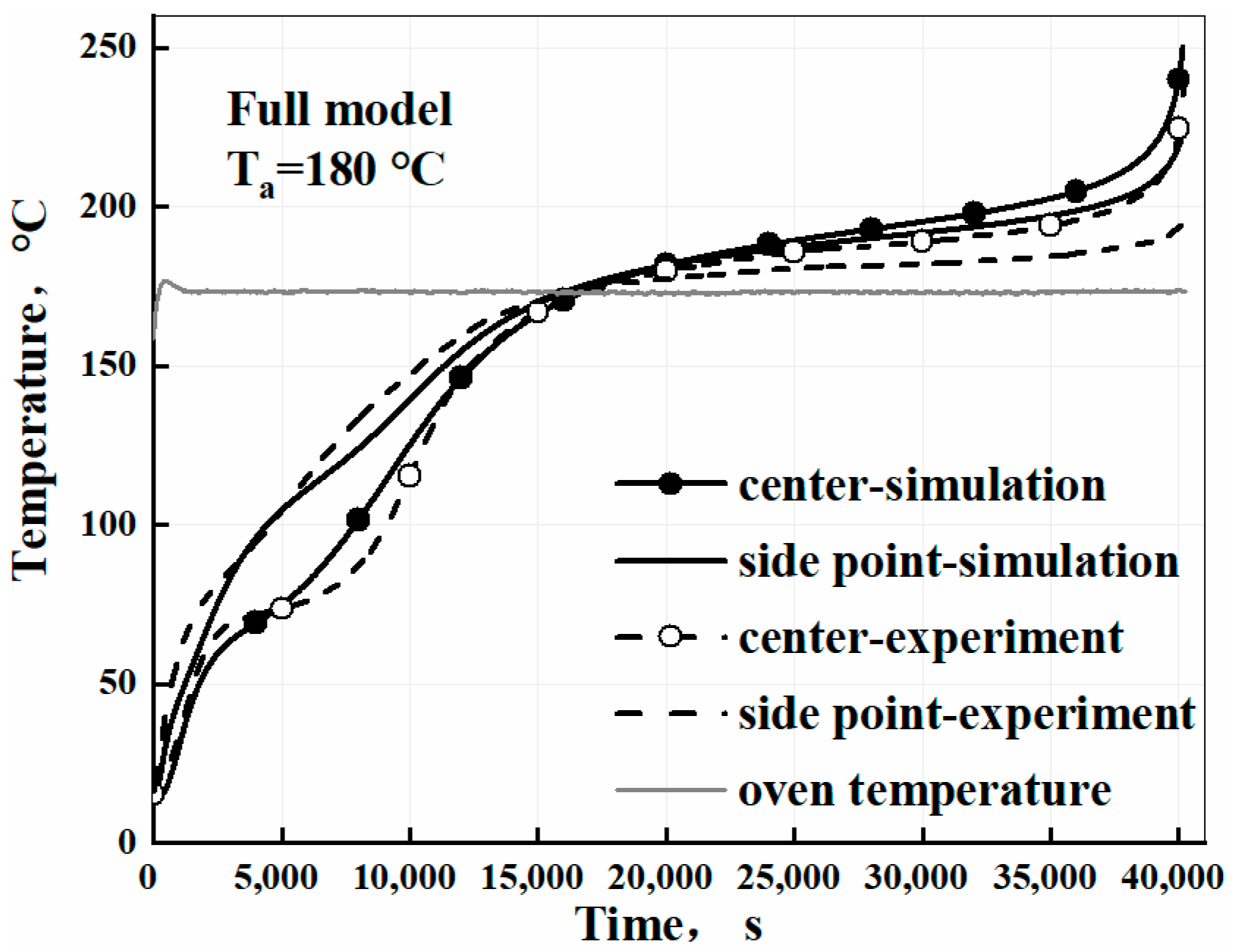
3.3. Validation of the Self-Heating Model
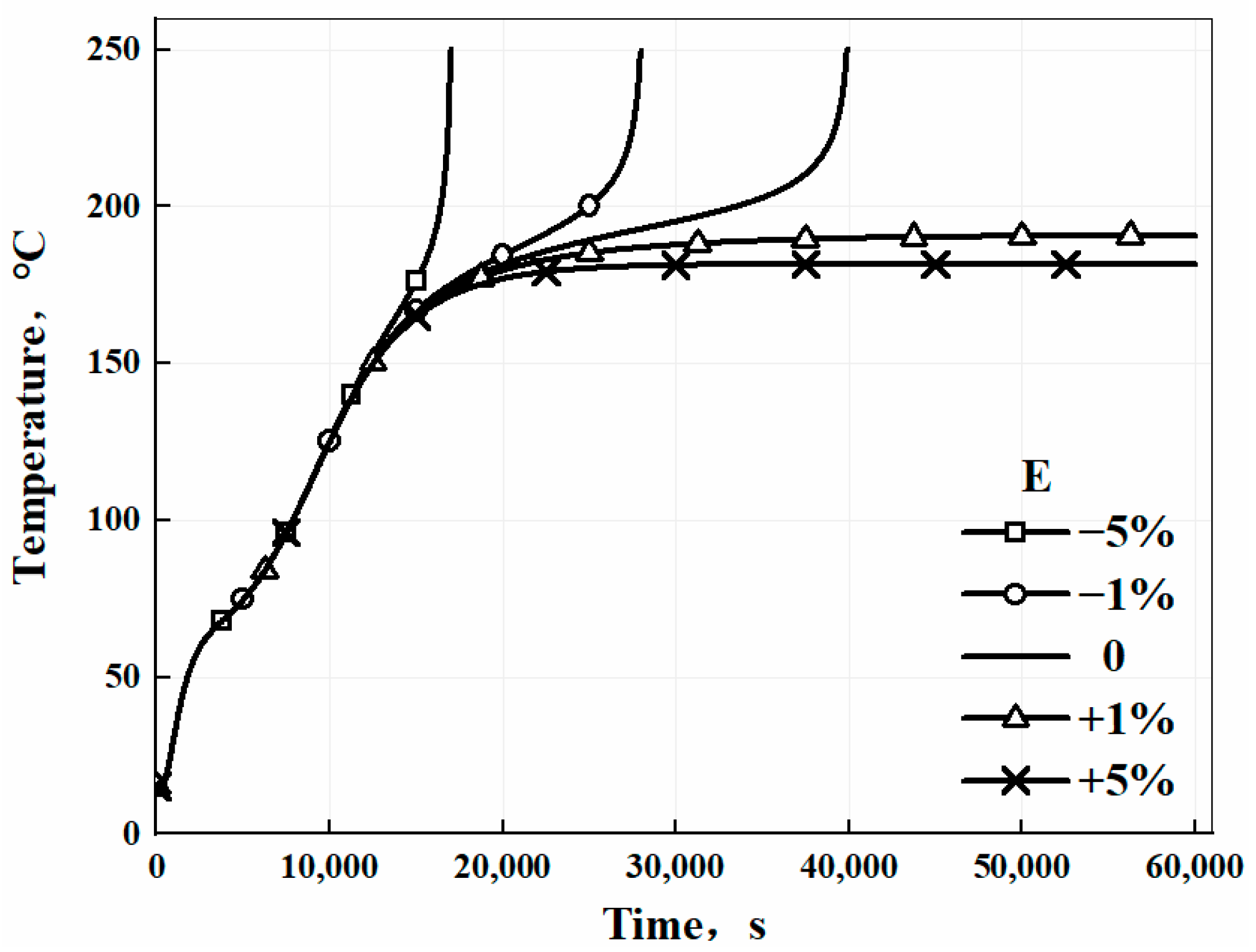
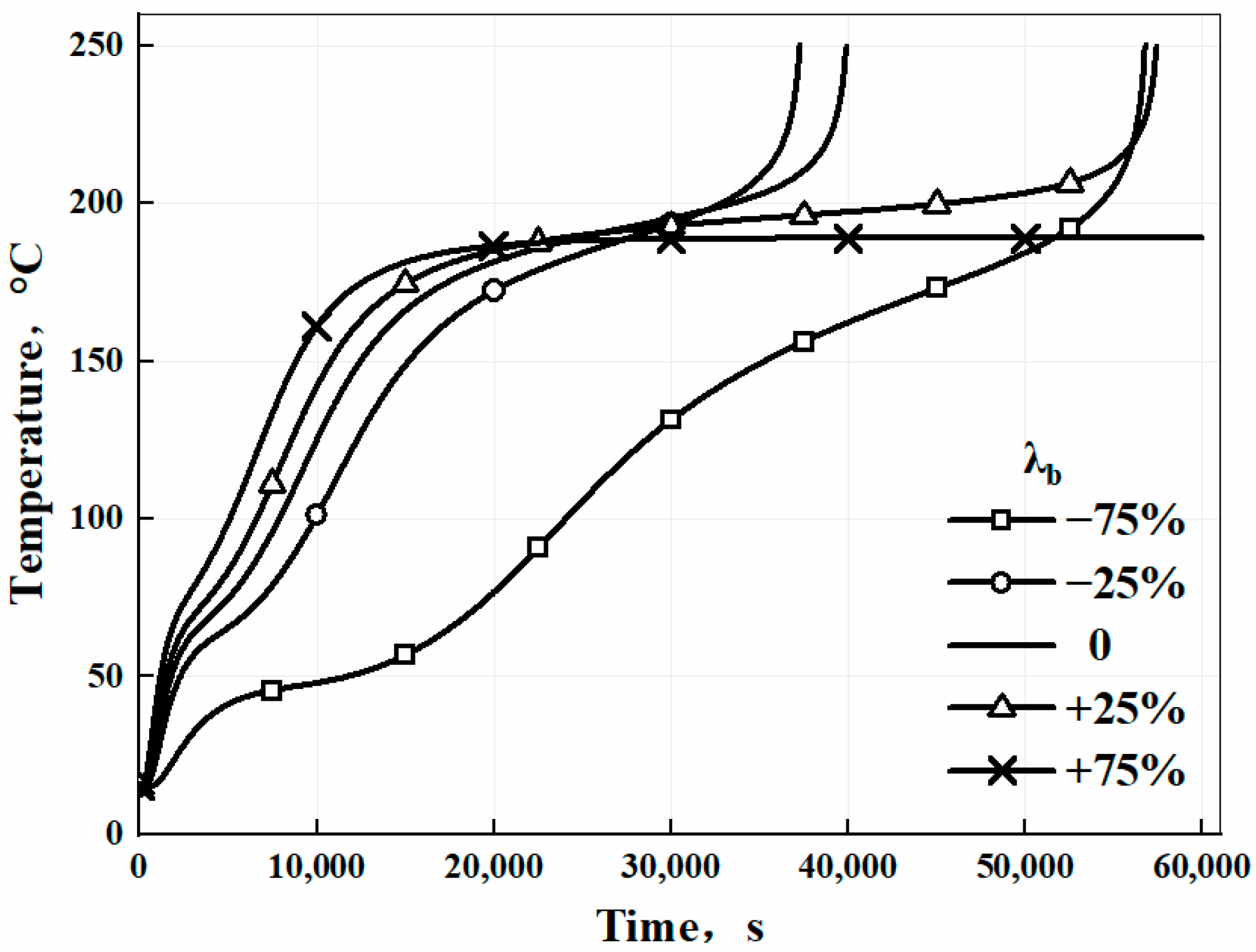
3.4. Numerical Study and Sensitive Analysis of the Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martinez-Valencia, L.; Camenzind, D.; Wigmosta, M.; Garcia-Perez, M.; Wolcott, M. Biomass Supply Chain Equipment for Renewable Fuels Production: A Review. Biomass Bioenergy 2021, 148, 106054. [Google Scholar] [CrossRef]
- Judl, J.; Koskela, S.; Korpela, T.; Karvosenoja, N.; Häyrinen, A.; Rantsi, J. Net Environmental Impacts of Low-Share Wood Pellet Co-Combustion in an Existing Coal-Fired Chp (Combined Heat and Power) Production in Helsinki, Finland. Energy 2014, 77, 844–851. [Google Scholar] [CrossRef]
- Sheng, C.; Yao, C. Review on Self-Heating of Biomass Materials: Understanding and Description. Energy Fuels 2022, 36, 731–761. [Google Scholar] [CrossRef]
- Ferrero, F.; Lohrer, C.; Schmidt, B.M.; Noll, M.; Malow, M. A Mathematical Model to Predict the Heating-up of Large-Scale Wood Piles. J. Loss Prev. Process. Ind. 2009, 22, 439–448. [Google Scholar] [CrossRef]
- Hogland, W.; Marques, M. Physical, Biological and Chemical Processes during Storage and Spontaneous Combustion of Waste Fuel. Resour. Conserv. Recycl. 2003, 40, 53–69. [Google Scholar] [CrossRef]
- Murasawa, N.; Koseki, H. Investigation of Heat Generation from Biomass Fuels. Energies 2015, 8, 5143–5158. [Google Scholar] [CrossRef]
- Fan, P.; Fan, S.; Sheng, C. Low Temperature Oxidation and Its Kinetics of Cornstalk Chars. Fuel 2016, 184, 915–921. [Google Scholar] [CrossRef]
- Krigstin, S.; Wetzel, S.; Jayabala, N.; Helmeste, C.; Madrali, S.; Agnew, J.; Volpe, S. Recent Health and Safety Incident Trends Related to the Storage of Woody Biomass: A Need for Improved Monitoring Strategies. Forests 2018, 9, 538. [Google Scholar] [CrossRef]
- Krigstin, S.; Wetzel, S. A Review of Mechanisms Responsible for Changes to Stored Woody Biomass Fuels. Fuel 2016, 175, 75–86. [Google Scholar] [CrossRef]
- Alakoski, E.; Jämsén, M.; Agar, D.; Tampio, E.; Wihersaari, M. From Wood Pellets to Wood Chips, Risks of Degradation and Emissions from the Storage of Woody Biomass—A Short Review. Renew. Sust. Energ. Rev. 2016, 54, 376–383. [Google Scholar] [CrossRef]
- Koppejan, J.; Loennermark, A.; Persson, H.; Larsson, I.; Blomqvist, P.; Arshadi, M.; Valencia-Reyes, E.; Melin, S.; Howes, P.; Wheeler, P.; et al. Health and Safety Aspects of Solid Biomass Storage, Transportation and Feeding; Medium, Ed.; IEA Bioenergy: Utrecht, The Netherlands, 2013; p. 100. [Google Scholar]
- Gauthier, S.; Grass, H.; Lory, M.; Krämer, T.; Thali, M.; Bartsch, C. Lethal Carbon Monoxide Poisoning in Wood Pellet Storerooms—Two Cases and a Review of the Literature. Ann. Occup. Hyg. 2012, 56, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.H.; Lestander, T.A.; Crompton, D.; Melin, S.; Sokhansanj, S. Temperature Patterns in Large Scale Wood Pellet Silo Storage. Appl. Energy 2012, 92, 322–327. [Google Scholar] [CrossRef]
- Ashman, J.M.; Jones, J.M.; Williams, A. Some Characteristics of the Self-Heating of the Large Scale Storage of Biomass. Fuel Process. Technol. 2018, 174, 1–8. [Google Scholar] [CrossRef]
- Pauner, M.A.; Bygbjerg, H. Spontaneous Ignition in Storage and Production Lines: Investigation on Wood Pellets and Protein Powders. Fire Mater. 2007, 31, 477–494. [Google Scholar] [CrossRef]
- Krause, U.; Schmidt, M.; Ferrero, F. Investigation of the Development of Conflagration of Solid Material Via Analysis of Coupled Heat, Mass and Momentum Transport. Chem. Eng. Technol. 2009, 32, 292–305. [Google Scholar] [CrossRef]
- Nelson, M.I.; Balakrishnan, E.; Chen, X.D. A Semenov Model of Self-Heating in Compost Piles. Process. Saf. Env. Prot. 2003, 81, 375–383. [Google Scholar] [CrossRef]
- Chen, X.D.; Sidhu, H.; Nelson, M. A Linear Relationship between Dimensionless Crossing-Point-Temperature and Frank–Kamenetskii Reactivity Parameter in Self-Heating Test at Infinite Biot Number for Slab Geometry. Fire Saf. J. 2013, 61, 138–143. [Google Scholar] [CrossRef]
- Guo, W.; Bi, X.; Lim, C.J.; Sokhansanj, S. Investigation of Wood Pellets Self-Heating Kinetics and Thermal Runaway Phenomena Using Lab Scale Experiments. Can. J. Chem. Eng. 2019, 98, 127–137. [Google Scholar] [CrossRef]
- Sidhu, H.S.; Nelson, M.I.; Chen, X.D. A Simple Spatial Model for Self-Heating Compost Piles. ANZIAM J. 2007, 48, 135–150. [Google Scholar] [CrossRef]
- Fu, S.; Chen, H.; Watt, S.D.; Sidhu, H.S.; Luangwilai, T.; Shu, Y. Numerical Study on Effect of Ambient Humidity Variation on Self-Heating and Spontaneous Ignition of the Eucalyptus Bark Pile. Fire Technol. 2021, 57, 1803–1825. [Google Scholar] [CrossRef]
- Gray, B.F.; Sexton, M.J.; Halliburton, B.; Macaskill, C. Wetting-Induced Ignition in Cellulosic Materials. Fire Saf. J. 2002, 37, 465–479. [Google Scholar] [CrossRef]
- Krigstin, S.; Helmeste, C.; Jia, H.; Johnson, K.E.; Wetzel, S.; Volpe, S.; Faizal, W.; Ferrero, F. Comparative Analysis of Bark and Woodchip Biomass Piles for Enhancing Predictability of Self-Heating. Fuel 2019, 242, 699–709. [Google Scholar] [CrossRef]
- Sexton, M.J.; Macaskill, C.; Gray, B.F. Self-Heating and Drying in Two-Dimensional Bagasse Piles. Combust. Theory Model. 2007, 5, 517–536. [Google Scholar] [CrossRef]
- Luangwilai, T.; Sidhu, H.S.; Nelson, M.I. A Two Dimensional, Reaction-Diffusion Model of Compost Piles. ANZIAM J. 2012, 52, 34–52. [Google Scholar] [CrossRef]
- Luangwilai, T.; Sidhu, H.S.; Nelson, M.I. One-Dimensional Spatial Model for Self-Heating in Compost Piles: Investigating Effects of Moisture and Air Flow. Food Bioprod. Process. 2018, 108, 18–26. [Google Scholar] [CrossRef]
- Kubler, H.; Wang, Y.R.; Barkalow, D. Generation of Heat in Wood between 80 and 130 °C. Holzforschung 1985, 39, 85–89. [Google Scholar] [CrossRef]
- Patankar, S.V. Numerical Heat Transfer and Fluid Flow; Hemisphere Pub. Corp.: Washington, DC, USA, 1980. [Google Scholar]
- Garcia-Torrent, J.; Ramirez-Gomez, A.; Querol-Aragon, E.; Grima-Olmedo, C.; Medic-Pejic, L. Determination of the Risk of Self-Ignition of Coals and Biomass Materials. J. Hazard. Mater. 2012, 213–214, 230–235. [Google Scholar] [CrossRef]
- Chen, X.D.; Chong, L.V. Some Characteristics of Tranisent Self-Heating inside an Exothermically Reactive Porous Solid Slab. Process. Saf. Environ. Prot. 1995, 73, 101–107. [Google Scholar]
- Jones, J.C.; Chiz, P.S.; Koh, R.; Matthew, J. Kinetic Parameters of Oxidation of Bituminous Coals from Heat-Release Rate Measurements. Fuel 1996, 75, 1755–1757. [Google Scholar] [CrossRef]
- EN 15188:2020; Determination of the Spontaneous Ignition Behaviour of Dust Accumulations. BSI Standards Institution: Brussels, Belgium, 2020.
- Ferreira, T.; Marques, E.; Paiva, J.M.; Pinho, C. A Study on the Spontaneous Ignition of Some Ligneous Pellets. Fire 2023, 6, 153. [Google Scholar] [CrossRef]
- Restuccia, F.; Fernandez-Anez, N.; Rein, G. Experimental Measurement of Particle Size Effects on the Self-Heating Ignition of Biomass Piles: Homogeneous Samples of Dust and Pellets. Fuel 2019, 256, 115838. [Google Scholar] [CrossRef]
- Sjöström, J.; Blomqvist, P. Direct Measurements of Thermal Properties of Wood Pellets: Elevated Temperatures, Fine Fractions and Moisture Content. Fuel 2014, 134, 460–466. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, X.; Fan, L.; Hu, Y.; Cen, K. Experimental Measurements of Thermal Conductivity of Wood Species in China: Effects of Density, Temperature, and Moisture Content. For. Prod. J. 2011, 61, 130–135. [Google Scholar] [CrossRef]
- Guo, W.; Lim, C.J.; Bi, X.; Sokhansanj, S.; Melin, S. Determination of Effective Thermal Conductivity and Specific Heat Capacity of Wood Pellets. Fuel 2013, 103, 347–355. [Google Scholar] [CrossRef]
- Mason, P.E.; Darvell, L.I.; Jones, J.M.; Williams, A. Comparative Study of the Thermal Conductivity of Solid Biomass Fuels. Energy Fuels 2016, 30, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Chiriac, R.; Gauthier, G.; Toche, F. Heat Capacity Measurements of Various Biomass Types and Pyrolysis Residues. Fuel 2014, 115, 644–651. [Google Scholar] [CrossRef]

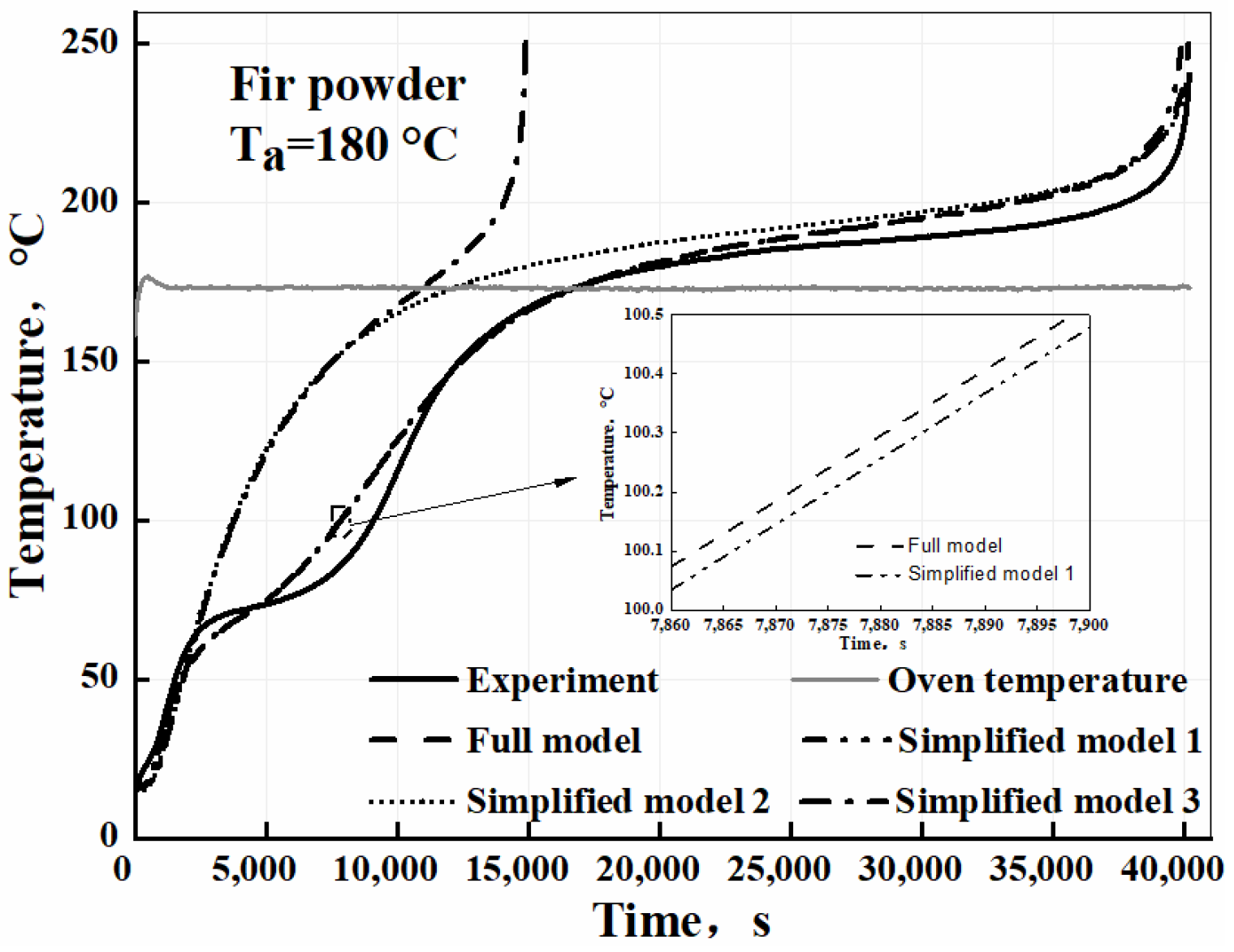
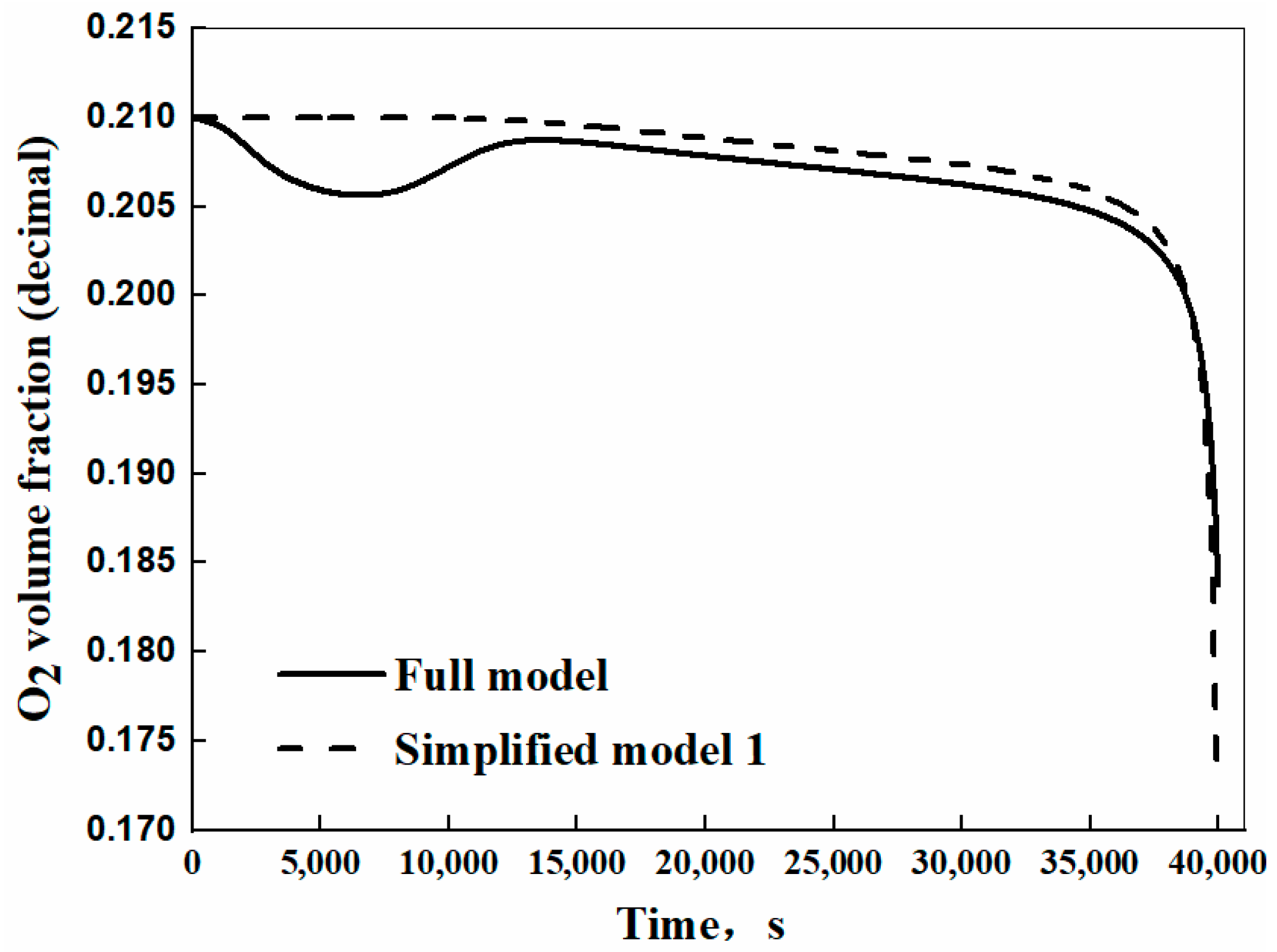
| The full model | |||||
| Simplified model 1 | |||||
| Simplified model 2 | - | - | - | ||
| Simplified model 3 | - | - | - | - |
| CPT | HR | CPT | HR | |
|---|---|---|---|---|
| E kJ/mol | QA J/(kg·s) | E kJ/mol | QA J/(kg·s) | |
| Fir pellets | 62 | 1.01 × 108 | 132 | 7.71 × 1015 |
| Fir powder | 113 | 7.94 × 1013 | 126 | 2.40 × 1015 |
| Parameters | Description | Unit | Data |
|---|---|---|---|
| A | Pre-exponential factor of low-temperature chemical oxidation | s−1 or m3/kg s | 1.0 × 108 |
| CO2,0 | Ambient oxygen concentration | - | 0.21 |
| Cv,0 | Initial vapour concentration | mol/m3 | 1.5 |
| CW,0 | Initial moisture concentration in biomass material | mol/m3 | 1300 |
| CD | Pre-exponential factor of water vapour condensation process | s−1 | 4.7 |
| cp,a | Heat capacity of air | J/kg K | 1006 |
| cp,b | Heat capacity of fir powder | J/kg K | 1350 |
| Do | Diffusivity of oxygen | m2/s | 2.5 × 10−5 |
| Dv | Diffusivity of vapour | m2/s | 7.5 × 10−6 |
| E | Apparent activation energy | J/mol | 1.26 × 105 |
| EV | Pre-exponential factor of water vapour evaporation process | s−1 | 3.41 × 104 |
| h | Convective heat transfer coefficient | W/m2 K | 25 |
| l | Half-length of the basket | m | 0.05 |
| Lv | Latent heat of water vapour condensation | J/mol | 4.2 × 104 |
| Q | Calorific value of the biomass | J/kg | 2.4 × 107 |
| R | universal gas constant | J/mol K | 8.314 |
| T0 | Initial temperature of the biomass | K | 285.9 |
| Ta | Oven temperature | K | 453 |
| λa | Thermal conductivity of air | W/m K | 0.023 |
| λb | Thermal conductivity of fir powder | W/m K | 0.18 |
| ρa | Air density | kg/m3 | 1.29 |
| Ρb | Fir powder bulk density | kg/m3 | 370 |
| ε | Porosity | - | 0.3 |
| Parameter | Symbol | Increase/ Decrease | Effect on | |
|---|---|---|---|---|
| Time to Reach Ta | Autoignition Time | |||
| Porosity | ε | −75% | −8.6% | +0.7% |
| −25% | −2.7% | −1.5% | ||
| +25% | +2.3% | +1.9% | ||
| +75% | +7.3% | +6.3% | ||
| Bulk density | ρb | −75% | −48.4% | - |
| −25% | −14% | - | ||
| +25% | +13.3% | −9.1% | ||
| +75% | +40.5% | −5.7% | ||
| Thermal conductivity | λb | −75% | +168.6% | +42.6% |
| −25% | +20.3% | −6.6% | ||
| +25% | −12.8% | +44.1% | ||
| +75% | −28.4% | - | ||
| Specific heat capacity | cp,b | −75% | −50% | −63.8% |
| −25% | −14.7% | −20.5% | ||
| +25% | +14.1% | +20.7% | ||
| +75% | +50% | - | ||
| Oxygen diffusivity | Do | −75% | 0% | +7.7% |
| −25% | 0% | +0.8% | ||
| +25% | 0% | −0.5% | ||
| +75% | 0% | −1% | ||
| Vapour diffusivity | Dv | −75% | +30.4% | +16% |
| −25% | +3.7% | +1.7% | ||
| +25% | −2.6% | −1% | ||
| +75% | −6.1% | −2.3% | ||
| Pre-exponential factor of evaporation process | EV | −75% | +12.1% | +5.9% |
| −25% | +1.4% | +0.8% | ||
| +25% | −1.7% | −0.6% | ||
| +75% | −3.1% | −1.3% | ||
| Pre-exponential factor of condensation process | CD | −75% | −11.1% | −5.1% |
| −25% | −3.4% | −1.6% | ||
| +25% | +3.2% | +1.6% | ||
| +75% | +9.6% | +4.6% | ||
| Pre-exponential factor of low-temperature chemical oxidation | QA or A | −25% | +1.1% | - |
| −10% | +0.2% | +28.4% | ||
| −5% | +0.06% | +10.9% | ||
| +25% | −1.4% | −24.1% | ||
| +75% | −3.3% | −39.3% | ||
| Activation energy | E | −5% | −12.2% | −57.3% |
| −1% | −2.3% | −29.9% | ||
| +1% | +1.2% | - | ||
| +5% | +4.5% | - | ||
| Moisture content | M | 3% | −17.7% | −7.8% |
| 10% | +14% | +6.2% | ||
| 15% | +32.5% | +13.9% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Yao, C.; Sheng, C. Modelling Self-Heating and Self-Ignition Processes during Biomass Storage. Energies 2023, 16, 4048. https://doi.org/10.3390/en16104048
Wei J, Yao C, Sheng C. Modelling Self-Heating and Self-Ignition Processes during Biomass Storage. Energies. 2023; 16(10):4048. https://doi.org/10.3390/en16104048
Chicago/Turabian StyleWei, Jiayu, Can Yao, and Changdong Sheng. 2023. "Modelling Self-Heating and Self-Ignition Processes during Biomass Storage" Energies 16, no. 10: 4048. https://doi.org/10.3390/en16104048




