Effect of a Cu-Ferrite Catalyzed DPF on the Ultrafine Particle Emissions from a Light-Duty Diesel Engine
Abstract
1. Introduction
- i.
- ii.
- iii.
- Composite regeneration, which consists of a combination of the other two [23].
- i.
- A decreased filtration efficiency may be due to the particles being taken away from the cake layer and from inside the porous walls;
- ii.
- The increased temperature due to active regeneration has been suggested to lead to expansion of the porous wall, resulting in a further lower filtration efficiency;
- iii.
- The particle flow into the exhaust system may be changed due to different operating conditions in the engine in the case of active regeneration; fuel injection into the engine cylinder towards the end of the expansion stroke could change it significantly;
- iv.
- The injection of extra fuel into the tailpipe may lead to the formation of additional particles upstream of the inlet of the DPF (the main hypothesis is that the trapped particles may fragment during rapid oxidation events, thereby escaping the DPF, resulting in particle breakthrough phenomena);
- v.
- Active regeneration may be characterized by the emission of semi-volatile particles in addition to solid ones.
- i.
- At the DPF inlet, during the accumulation phase, a strong bimodality with sub-10 nm particles dominating the PN concentration is present;
- ii.
- At the DPF outlet, a filtration efficiency higher than 90% was detected, demonstrating that the wall-flow filter can also remove the sub-23 nm particles. In particular, an efficiency value of about 99% has been detected in the range of 20–40 nm [43].
2. Materials and Methods
2.1. The Monoliths
2.2. Catalytic Filter Preparation
2.3. Characterization of the Catalytic Filters
- i.
- Scanning electron microscopy (SEM), using a Philips Mod.XL30 coupled to an energy dispersive X-ray spectrometer (EDS; Oxford, UK) for the surface analysis;
- ii.
- Hg porosimetry tests, with N2 adsorption at −196 °C (to calculate the samples’ specific surface areas (SSAs) through BET);
- iii.
- Ultrasound adherence test (exposure of the monoliths to ultrasound according to an optimized experimental procedure [44]).
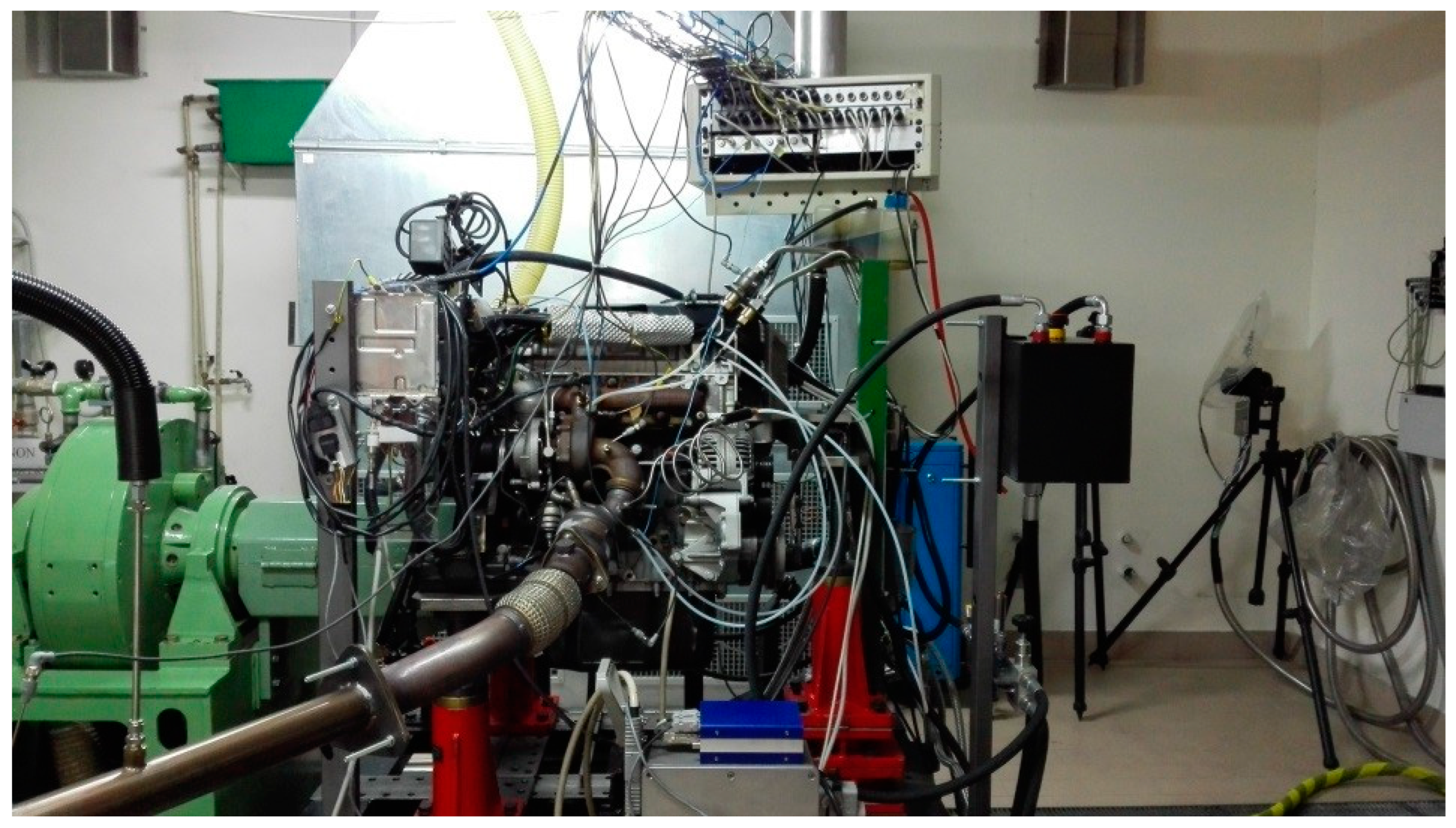
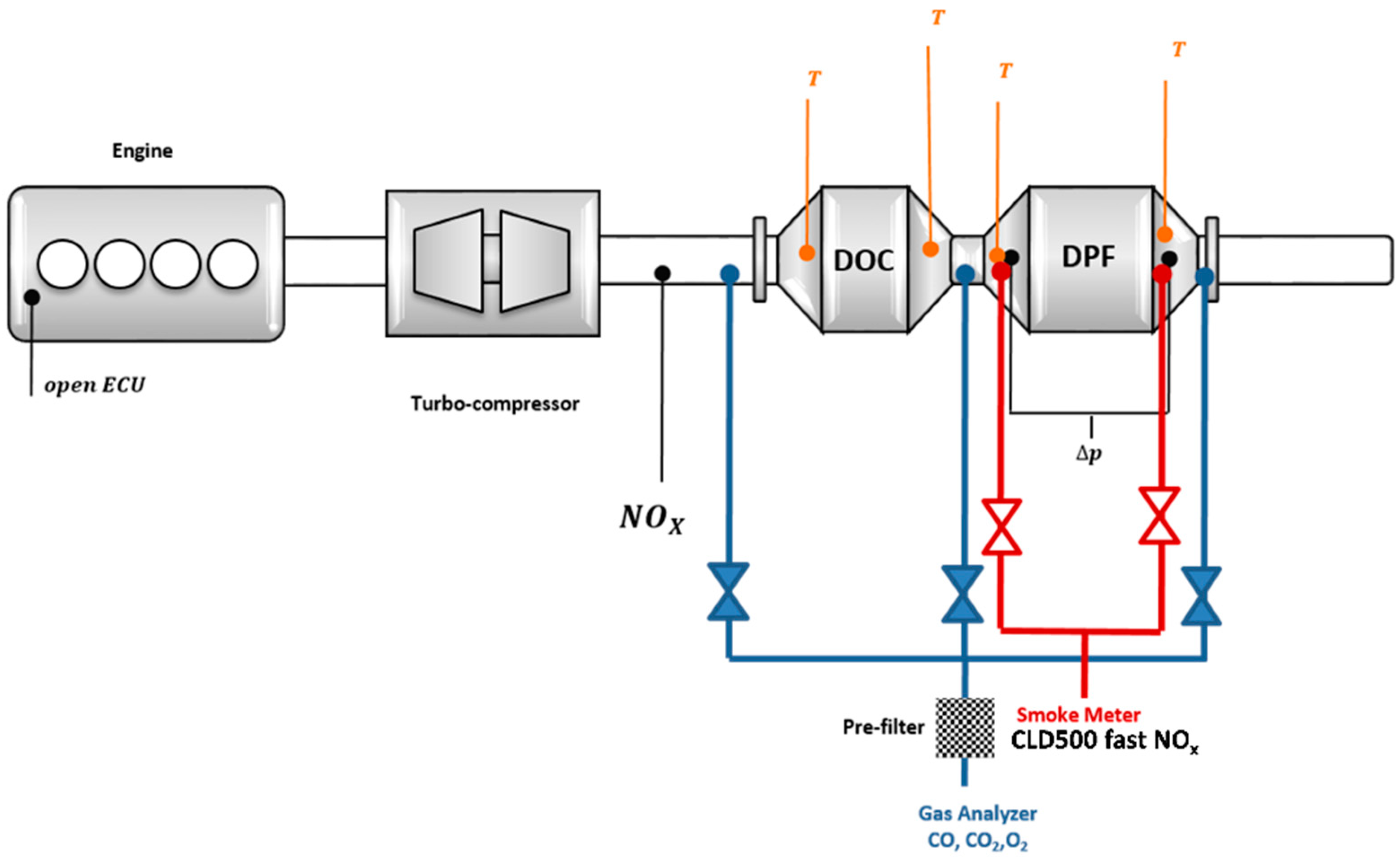
2.4. Experimental Layout and Instruments
- i.
- The AVL microIFEM for the management of the engine-dyno system by means of Puma open software;
- ii.
- The AVL Indimicro to monitor and process the in-cylinder data by means of Indicom software;
- iii.
- The control unit ETAS ES592.1 to manage the engine control unit (ECU) by means of INCA v7.1. The latter allows the visualization, processing and handling of the measurement signals and the control settings of the ECU, thus enabling engine operating modes different from the basic ECU calibration. In particular, it is possible to handle the combustion control variables, such as rail pressure, injection pattern, boost pressure and exhaust gas recirculation (EGR), to investigate specific engine operating conditions.
3. Results and Discussion
3.1. SiC Monoliths before Catalyst Deposition
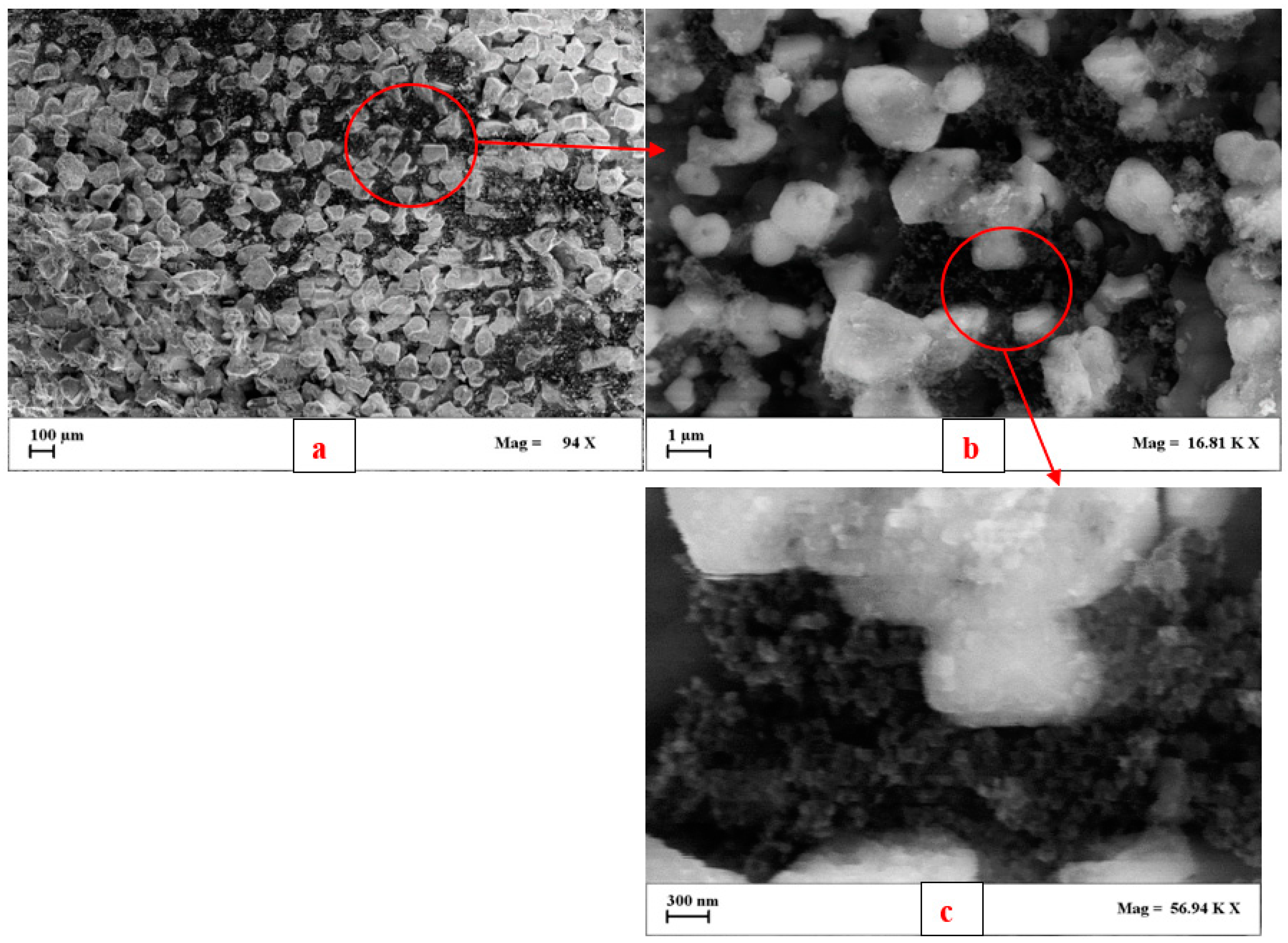
3.2. Soot Emitted by the Diesel Engine
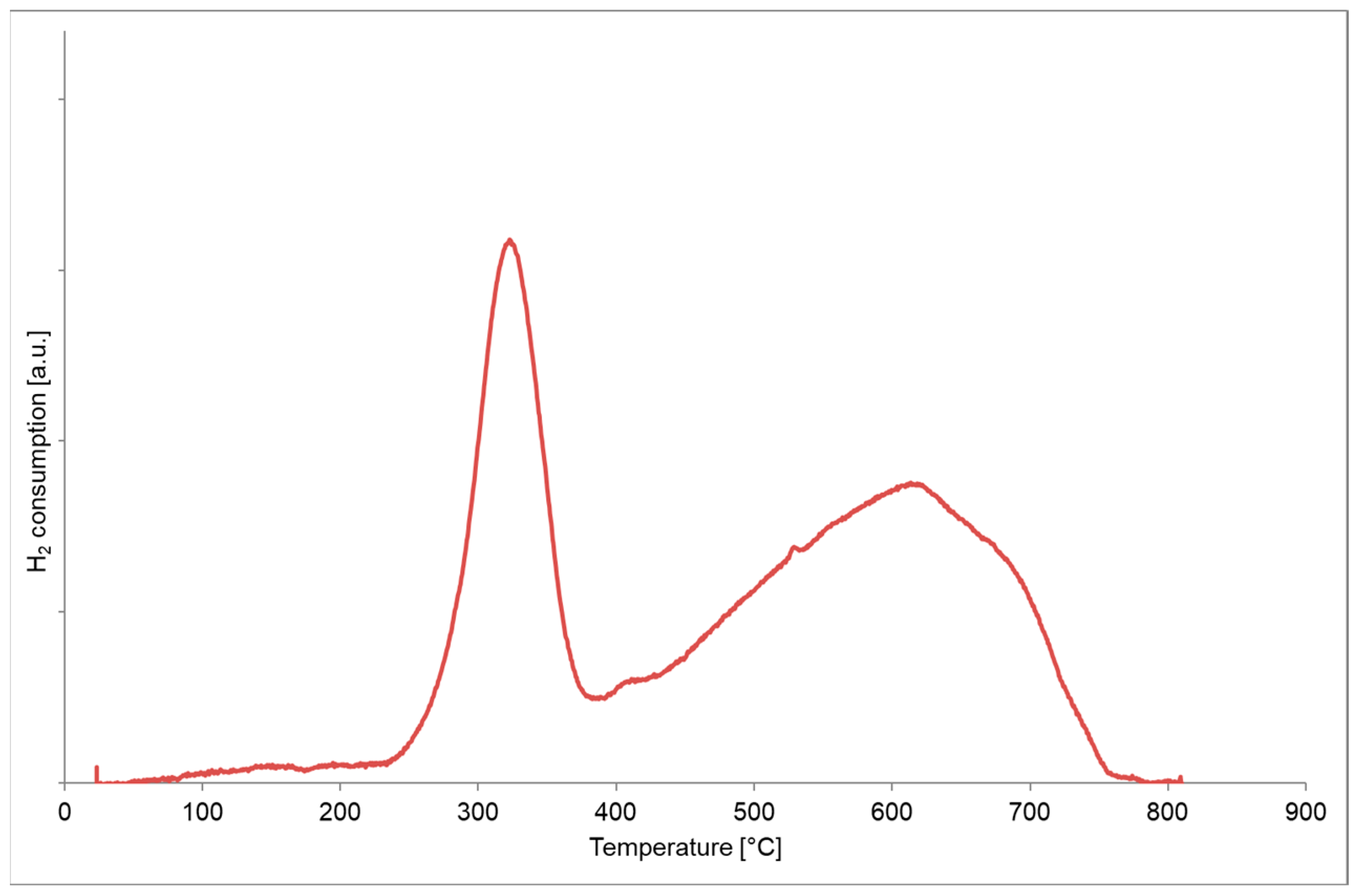
3.3. Fresh Catalytic Samples
- i.
- Is consistent with the amount necessary to obtain the complete reduction of CuFe2O4 to Cu and Fe following the overall reaction (Equation (1)); and
- ii.
- Corresponds to a load of CuFe2O4 equal to about 30.01 %wt, which is in very good agreement with the estimated 30 %wt of CuFe2O4 on the catalytic sample.
3.4. Experimental Tests in the Engine Test Cell
- i.
- ΔPfilter, due to the presence of the clean filter in the exhaust pipe, depending on both gas flow parameters (volumetric flow rate, viscosity and density) and filter geometry (volume, filter cell size, wall thickness and filter length); and
- ii.
- ΔPsoot, related to the soot accumulation in the DPF, mainly in terms of soot cake properties [54].
- i.
- Start-up (0–200 s), in which any soot oxidation occurs;
- ii.
- Soot combustion (200–450 s);
- iii.
- End of regeneration, in which constant DP (after 450 s) and constant differences between temperatures across the CDPF are present.
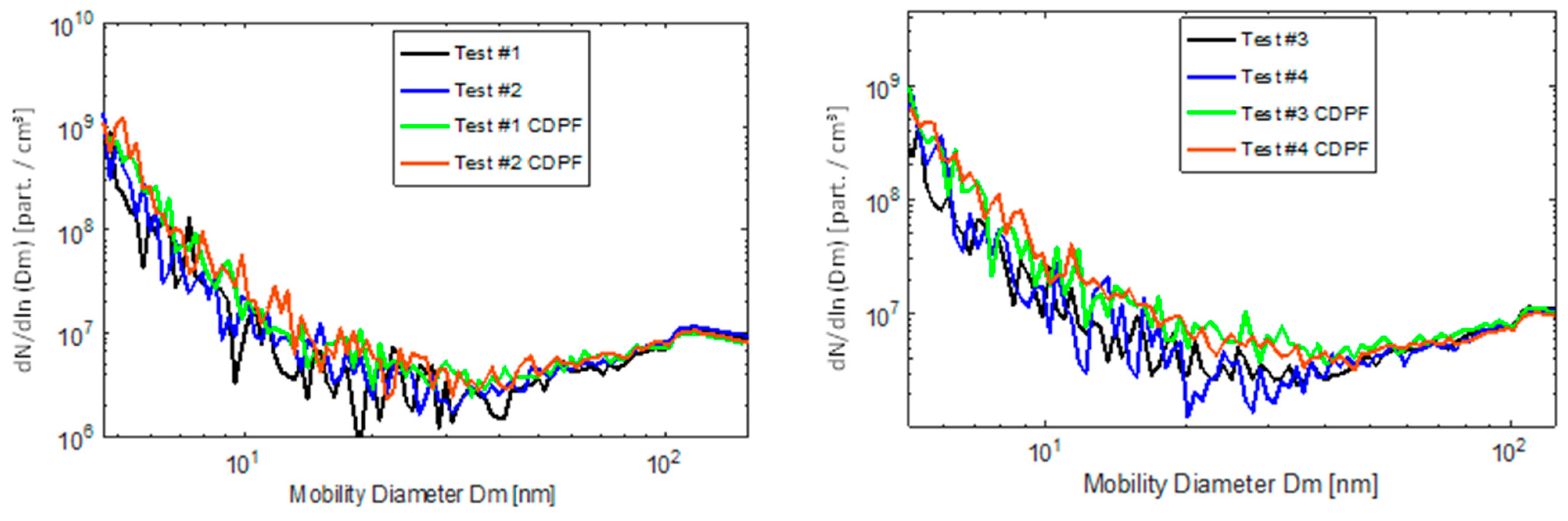
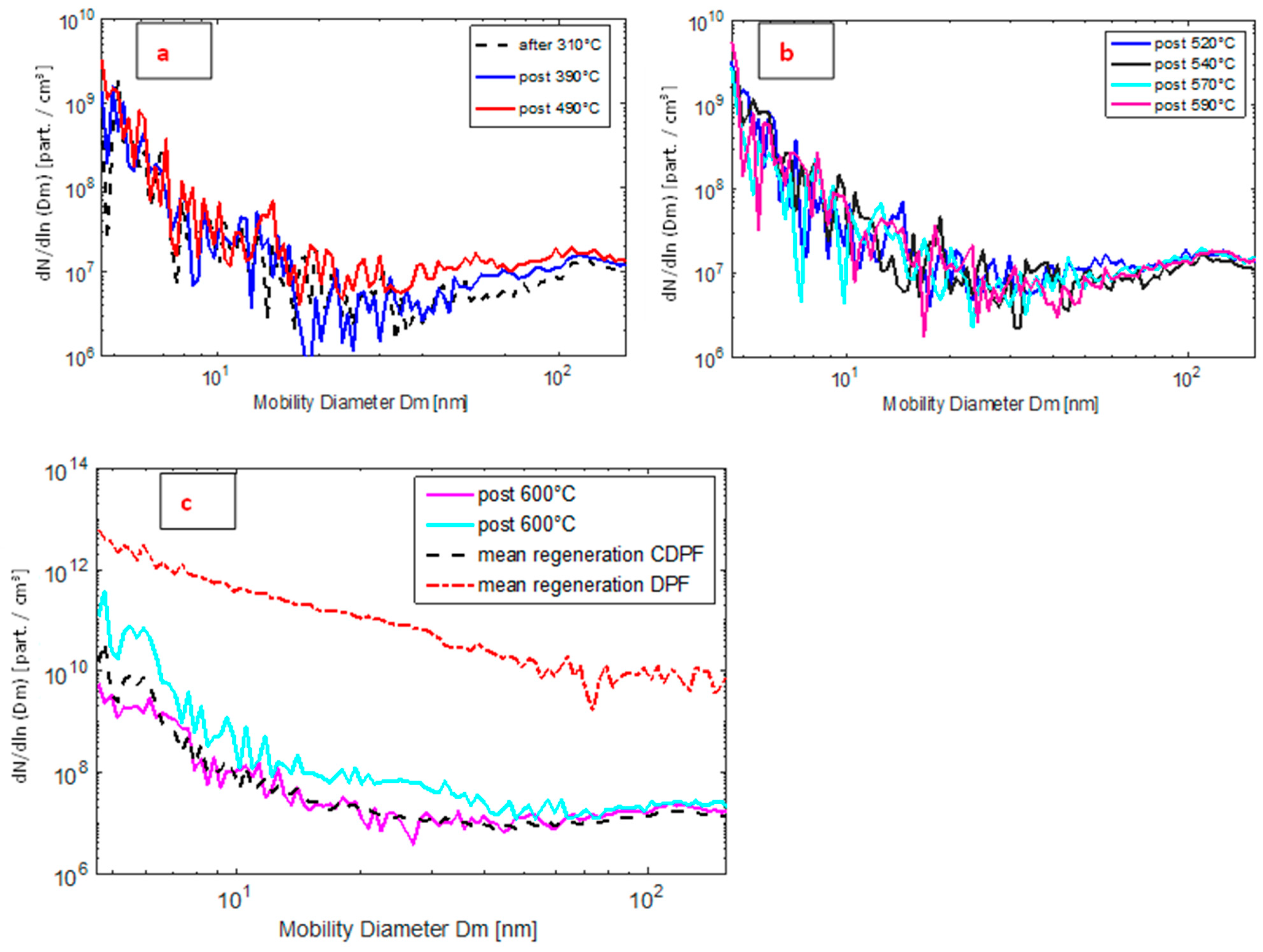
Particle Emission Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Beatrice, C.; Di Iorio, S.; Guido, C.; Napolitano, P. Detailed characterization of particulate emissions of an automotive catalyzed DPF using actual regeneration strategies. Exp. Therm. Fluid Sci. 2012, 39, 45–53. [Google Scholar] [CrossRef]
- Alexandrino, K. Comprehensive Review of the Impact of 2,5-Dimethylfuran and 2-Methylfuran on Soot Emissions: Experiments in Diesel Engines and at Laboratory-Scale. Energy Fuels 2020, 34, 6598–6623. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, C.; Liu, D.; Zhu, Y.; Feng, Y. Experimental investigation of the high-pressure SCR reactor impact on a marine two-stroke diesel engine. Fuel 2023, 335, 127064. [Google Scholar] [CrossRef]
- Pacino, A.; La Porta, C.; La Rocca, A.; Cairns, A. Copper leaching effects on combustion characteristics and particulate emissions of a direct injection high pressure common rail diesel engine. Fuel 2023, 340, 127536. [Google Scholar] [CrossRef]
- Apicella, B.; Mancaruso, E.; Russo, C.; Tregrossi, A.; Oliano, M.M.; Ciajolo, A.; Vaglieco, B.M. Effect of after-treatment systems on particulate matter emissions in diesel engine exhaust. Exp. Therm. Fluid Sci. 2020, 116, 110107. [Google Scholar] [CrossRef]
- Bagi, S.; Kamp, C.J.; Sharma, V.; Aswath, P.B. Multiscale characterization of exhaust and crankcase soot extracted from heavy-duty diesel engine and implications for DPF ash. Fuel 2020, 282, 118878. [Google Scholar] [CrossRef]
- Meloni, E.; Palma, V. Most Recent Advances in Diesel Engine Catalytic Soot Abatement: Structured Catalysts and Alternative Approaches. Catalysts 2020, 10, 745. [Google Scholar] [CrossRef]
- Gren, L.; Malmborg, V.B.; Jacobsen, N.R.; Shukla, P.C.; Bendtsen, K.M.; Eriksson, A.C.; Essig, Y.J.; Krais, A.M.; Loeschner, K.; Shamun, S.; et al. Effect of Renewable Fuels and Intake O2 Concentration on Diesel Engine Emission Characteristics and Reactive Oxygen Species (ROS) Formation. Atmosphere 2020, 11, 641. [Google Scholar] [CrossRef]
- Fang, J.; Meng, Z.; Li, J.; Du, Y.; Qin, Y.; Jiang, Y.; Bai, W.; Chase, G.G. The effect of operating parameters on regeneration characteristics and particulate emission characteristics of diesel particulate filters. Appl. Therm. Eng. 2019, 148, 860–867. [Google Scholar] [CrossRef]
- Meloni, E.; Palma, V. Low Temperature Microwave Regeneration of Catalytic Diesel Particulate Filter. Chem. Eng. Trans. 2018, 70, 721–726. [Google Scholar] [CrossRef]
- Saffaripour, M.; Chan, T.W.; Liu, F.; Thomson, K.A.; Smallwood, G.J.; Kubsh, J.; Brezny, R. Effect of Drive Cycle and Gasoline Particulate Filter on the Size and Morphology of Soot Particles Emitted from a Gasoline-Direct-Injection Vehicle. Environ. Sci. Technol. 2015, 49, 11950–11958. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Li, Z.; Shen, B.; Wu, Y.; Zhang, Y.; Cai, D. Simulation of flow and soot particle distribution in wall-flow DPF based on lattice Boltzmann method. Chem. Eng. Sci. 2019, 202, 169–185. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nakamura, M.; Yane, H.; Yamashita, H. Simulation on catalytic reaction in diesel particulate filter. Catal. Today 2010, 153, 118–124. [Google Scholar] [CrossRef]
- Yamamoto, K.; Oohori, S.; Yamashita, H.; Daido, S. Simulation on soot deposition and combustion in diesel particulate filter. Proc. Combust. Inst. 2009, 32, 1965–1972. [Google Scholar] [CrossRef]
- Oi-Uchisawa, J.; Obuchi, A.; Wang, S.; Nanba, T.; Ohi, A. Catalytic performance of Pt/MOx loaded over SiC-DPF for soot oxidation. Appl. Catal. B Environ. 2003, 43, 117–129. [Google Scholar] [CrossRef]
- Zhang, M.; Ge, Y.; Wang, X.; Peng, Z.; Tan, J.; Hao, L.; Lv, L.; Wang, C. An investigation into the impact of burning diesel/lubricant oil mixtures on the nature of particulate emissions: Implications for DPF ash-loading acceleration method. J. Energy Inst. 2020, 93, 1207–1215. [Google Scholar] [CrossRef]
- Giechaskiel, B. Particle Number Emissions of a Diesel Vehicle during and between Regeneration Events. Catalysts 2020, 10, 587. [Google Scholar] [CrossRef]
- Jiaqiang, E.; Zhao, M.; Zuo, Q.; Zhang, B.; Zhang, Z.; Peng, Q.; Han, D.; Zhao, X.; Deng, Y. Effects analysis on diesel soot continuous regeneration performance of a rotary microwave-assisted regeneration diesel particulate filter. Fuel 2020, 260, 116353. [Google Scholar] [CrossRef]
- Kurien, C.; Srivastava, A.K.; Lesbats, S. Experimental and computational study on the microwave energy based regeneration in diesel particulate filter for exhaust emission control. J. Energy Inst. 2020, 93, 2133–2147. [Google Scholar] [CrossRef]
- Kurien, C.; Srivastava, A.K.; Gandigudi, N.; Anand, K. Soot deposition effects and microwave regeneration modelling of diesel particulate filtration system. J. Energy Inst. 2020, 93, 463–473. [Google Scholar] [CrossRef]
- Rossomando, B.; Arsie, I.; Meloni, E.; Palma, V.; Pianese, C. Experimental Test on the Feasibility of Passive Regeneration in a Catalytic DPF at the Exhaust of a Light-Duty Diesel Engine; SAE Technical Paper 2019-24-0045; SAE International (Europe): London, UK, 2019. [Google Scholar]
- Rossomando, B.; Arsie, I.; Meloni, E.; Palma, V.; Pianese, C. Experimental Testing of a Low Temperature Regenerating Catalytic DPF at the Exhaust of a Light-Duty Diesel Engine; SAE Technical Paper 2018-01-0351; SAE International (Europe): London, UK, 2018. [Google Scholar]
- Meloni, E.; Palma, V.; Vaiano, V. Optimized microwave susceptible catalytic diesel soot trap. Fuel 2017, 205, 142–152. [Google Scholar] [CrossRef]
- Leach, F.C.; Davy, M.; Terry, B. Combustion and emissions from cerium oxide nanoparticle dosed diesel fuel in a high speed diesel research engine under low temperature combustion (LTC) conditions. Fuel 2021, 288, 119636. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Balasubramanian, R. Effects of Cerium Oxide and Ferrocene Nanoparticles Addition as Fuel-Borne Catalysts on Diesel Engine Particulate Emissions: Environmental and Health Implications. Environ. Sci. Technol. 2017, 51, 4248–4258. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yao, P.; Qiu, J.; Zhang, H.; Jiao, Y.; Wang, J.; Chen, Y. Enhancement effect of oxygen mobility over Ce0.5Zr0.5O2 catalysts doped by multivalent metal oxides for soot combustion. Fuel 2021, 286, 119359. [Google Scholar] [CrossRef]
- Andana, T.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Heterogeneous mechanism of NOx-assisted soot oxidation in the passive regeneration of a bench-scale diesel particulate filter catalyzed with nanostructured equimolar ceria-praseodymia. Appl. Catal. A Gen. 2019, 583, 117136. [Google Scholar] [CrossRef]
- Mori, K.; Jida, H.; Kuwahara, Y.; Yamashita, H. CoOx-decorated CeO2 heterostructures: Effects of morphology on their catalytic properties in diesel soot combustion. Nanoscale 2020, 12, 1779–1789. [Google Scholar] [CrossRef]
- Serhan, N.; Tsolakis, A.; Wahbi, A.; Martos, F.; Golunski, S. Modifying catalytically the soot morphology and nanostructure in diesel exhaust: Influence of silver De-NOx catalyst (Ag/Al2O3). Appl. Catal. B Environ. 2019, 241, 471–482. [Google Scholar] [CrossRef]
- Ji, F.; Men, Y.; Wang, J.; Sun, Y.; Wang, Z.; Zhao, B.; Tao, X.; Xu, G. Promoting diesel soot combustion efficiency by tailoring the shapes and crystal facets of nanoscale Mn3O4. Appl. Catal. B Environ. 2019, 242, 227–237. [Google Scholar] [CrossRef]
- Singer, C.; Kureti, S. Soot oxidation in diesel exhaust on manganese oxide catalyst prepared by flame spray pyrolysis. Appl. Catal. B Environ. 2020, 272, 118961. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Fujibayashi, A.; Uehara, H.; Mori, K.; Yamashita, H. Catalytic combustion of diesel soot over Fe and Ag-doped manganese oxides: Role of heteroatoms in the catalytic performances. Catal. Sci. Technol. 2018, 8, 1905–1914. [Google Scholar] [CrossRef]
- Di Sarli, V.; Landi, G.; Lisi, L.; Saliva, A.; Di Benedetto, A. Catalytic diesel particulate filters with highly dispersed ceria: Effect of the soot-catalyst contact on the regeneration performance. Appl. Catal. B Environ. 2016, 197, 116–124. [Google Scholar] [CrossRef]
- Jeftić, M.; Yu, S.; Han, X.; Reader, G.T.; Wang, M.; Zheng, M. Effects of Postinjection Application with Late Partially Premixed Combustion on Power Production and Diesel Exhaust Gas Conditioning. J. Combust. 2011, 2011, 891096. [Google Scholar] [CrossRef][Green Version]
- D’Aniello, F.; Rossomando, B.; Arsie, I.; Pianese, C. Development and Experimental Validation of a Control Oriented Model of a Catalytic DPF; SAE Technical Paper 2019-01-0985; SAE International (Europe): London, UK, 2019. [Google Scholar]
- Lao, C.T.; Akroyd, J.; Eaves, N.; Smith, A.; Morgan, N.; Bhave, A.; Kraft, M. Modelling particle mass and particle number emissions during the active regeneration of diesel particulate filters. Proc. Combust. Inst. 2019, 37, 4831–4838. [Google Scholar] [CrossRef][Green Version]
- Wang, D.-Y.; Tan, P.-Q.; Zhu, L.; Wang, Y.-H.; Hu, Z.-Y.; Lou, D.-M. Novel soot loading prediction model of diesel particulate filter based on collection mechanism and equivalent permeability. Fuel 2021, 286, 119409. [Google Scholar] [CrossRef]
- Dwyer, H.; Ayala, A.; Zhang, S.; Collins, J.; Huai, T.; Herner, J.; Chau, W. Emissions from a diesel car during regeneration of an active diesel particulate filter. J. Aerosol Sci. 2010, 41, 541–552. [Google Scholar] [CrossRef]
- Meng, Z.; Chen, C.; Li, J.; Fang, J.; Tan, J.; Qin, Y.; Jiang, Y.; Qin, Z.; Bai, W.; Liang, K. Particle emission characteristics of DPF regeneration from DPF regeneration bench and diesel engine bench measurements. Fuel 2020, 262, 116589. [Google Scholar] [CrossRef]
- Wihersaari, H.; Pirjola, L.; Karjalainen, P.; Saukko, E.; Kuuluvainen, H.; Kulmala, K.; Keskinen, J.; Rönkkö, T. Particulate emissions of a modern diesel passenger car under laboratory and real-world transient driving conditions. Environ. Pollut. 2020, 265, 114948. [Google Scholar] [CrossRef]
- Liati, A.; Schreiber, D.; Dasilva, Y.A.R.; Eggenschwiler, P.D. Ultrafine particle emissions from modern Gasoline and Diesel vehicles: An electron microscopic perspective. Environ. Pollut. 2018, 239, 661–669. [Google Scholar] [CrossRef]
- Meng, Z.; Li, J.; Fang, J.; Tan, J.; Qin, Y.; Jiang, Y.; Qin, Z.; Bai, W.; Liang, K. Experimental study on regeneration performance and particle emission characteristics of DPF with different inlet transition sections lengths. Fuel 2020, 262, 116487. [Google Scholar] [CrossRef]
- Rossomando, B.; Meloni, E.; De Falco, G.; Sirignano, M.; Arsie, I.; Palma, V. Experimental characterization of ultrafine particle emissions from a light-duty diesel engine equipped with a standard DPF. Proc. Combust. Inst. 2021, 38, 5695–5702. [Google Scholar] [CrossRef]
- Palma, V.; Ciambelli, P.; Meloni, E.; Sin, A. Optimal CuFe2O4 load for MW susceptible catalysed DPF. Chem. Eng. Trans. 2013, 35, 727–732. [Google Scholar] [CrossRef]
- R’mili, B.; Boréave, A.; Meme, A.; Vernoux, P.; Leblanc, M.; Noël, L.; Raux, S.; D’anna, B. Physico-Chemical Characterization of Fine and Ultrafine Particles Emitted during Diesel Particulate Filter Active Regeneration of Euro5 Diesel Vehicles. Environ. Sci. Technol. 2018, 52, 3312–3319. [Google Scholar] [CrossRef] [PubMed]
- Salenbauch, S.; Sirignano, M.; Pollack, M.; D’anna, A.; Hasse, C. Detailed modeling of soot particle formation and comparison to optical diagnostics and size distribution measurements in premixed flames using a method of moments. Fuel 2018, 222, 287–293. [Google Scholar] [CrossRef]
- Sgro, L.A.; Sementa, P.; Vaglieco, B.M.; Rusciano, G.; D’anna, A.; Minutolo, P. Investigating the origin of nuclei particles in GDI engine exhausts. Combust. Flame 2012, 159, 1687–1692. [Google Scholar] [CrossRef]
- De Filippo, A.; Maricq, M.M. Diesel Nucleation Mode Particles: Semivolatile or Solid? Environ. Sci. Technol. 2008, 42, 7957–7962. [Google Scholar] [CrossRef]
- Burton, J.C.; Sun, L.; Pophristic, M.; Lukacs, S.J.; Long, F.H.; Feng, Z.C.; Ferguson, I.T. Spatial characterization of doped SiC wafers by Raman spectroscopy. J. Appl. Phys. 1998, 84, 6268–6273. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Giani, L.; Cristiani, C.; Groppi, G.; Tronconi, E. Washcoating methods for Pd/gamma-Al2O3 deposition on metallic foams. Appl. Catal. B Environ. 2006, 62, 121–131. [Google Scholar] [CrossRef]
- Palma, V.; Ciambelli, P.; Meloni, E.; Sin, A. Catalytic DPF microwave assisted active regeneration. Fuel 2015, 140, 50–61. [Google Scholar] [CrossRef]
- Kameoka, S.; Tanabe, T.; Tsai, A.P. Self-assembled porous nano-composite with high catalytic performance by reduction of tetragonal spinel CuFe2O4. Appl. Catal. A Gen. 2010, 375, 163–171. [Google Scholar] [CrossRef]
- Choi, S.; Oh, K.-C.; Lee, C.-B. The effects of filter porosity and flow conditions on soot deposition/oxidation and pressure drop in particulate filters. Energy 2014, 77, 327–337. [Google Scholar] [CrossRef][Green Version]
- Haralampous, O.A.; Kandylas, I.P.; Koltsakis, G.C.; Samaras, Z.C. Diesel particulate filter pressure drop Part 1: Modelling and experimental validation. Int. J. Engine Res. 2004, 5, 149–162. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernández, J.; Oliva, F. Effect of soot accumulation in a diesel particle filter on the combustion process and gaseous emissions. Energy 2012, 47, 543–552. [Google Scholar] [CrossRef]
- Yamada, H.; Inomata, S.; Tanimoto, H. Mechanisms of Increased Particle and VOC Emissions during DPF Active Regeneration and Practical Emissions Considering Regeneration. Environ. Sci. Technol. 2017, 51, 2914–2923. [Google Scholar] [CrossRef]

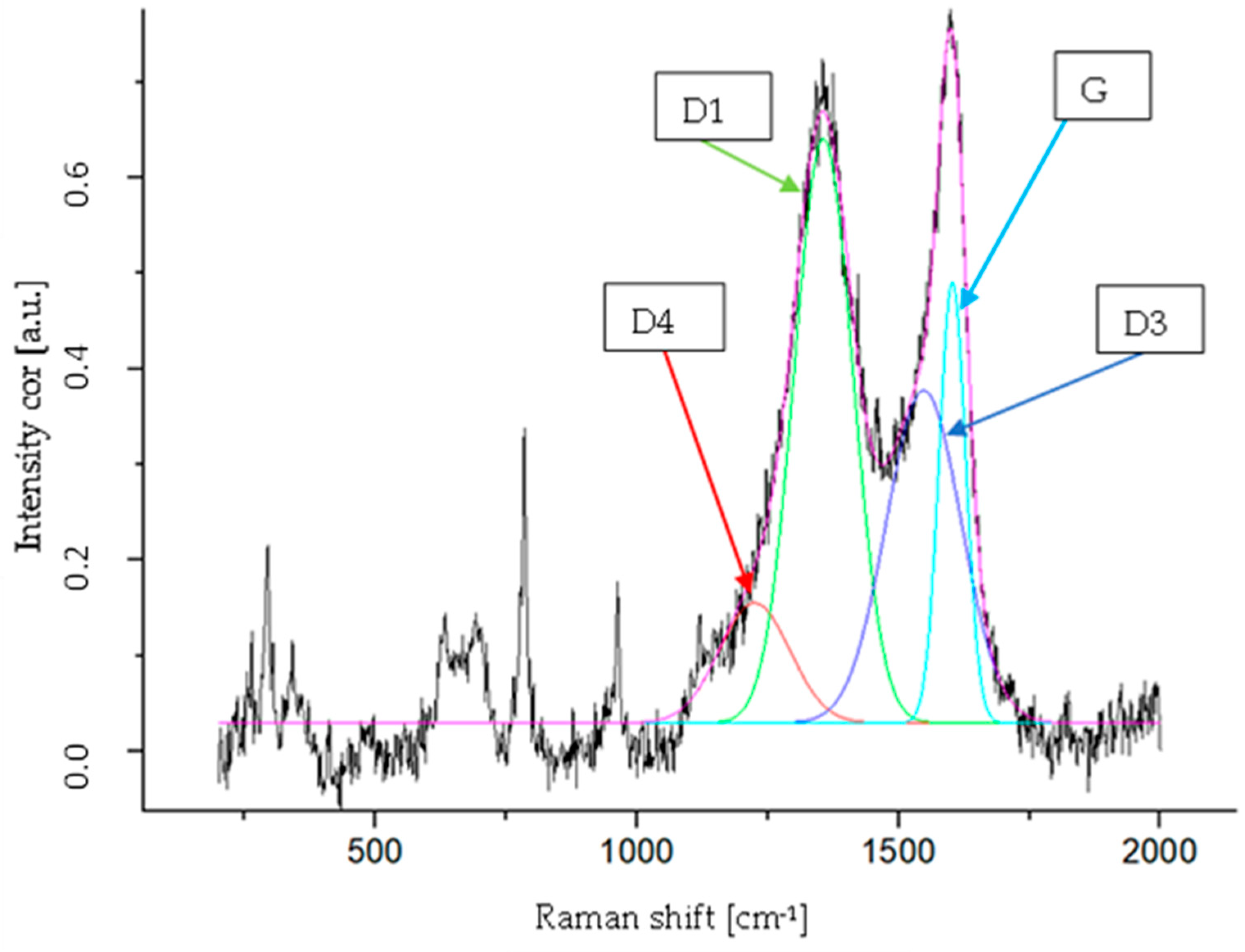




| Band | Raman Shift (cm−1) | Vibration Mode [50] |
|---|---|---|
| G | 1602 | Ideal graphitic lattice (E2g-symmetry) |
| D1 | 1356 | Disordered graphitic lattice (graphene layer edges, A1g symmetry) |
| D3 | 1548 | Amorphous carbon (Gaussian line shape) |
| D4 | 1224 | Disordered graphitic lattice (A1g symmetry) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meloni, E.; Rossomando, B.; De Falco, G.; Sirignano, M.; Arsie, I.; Palma, V. Effect of a Cu-Ferrite Catalyzed DPF on the Ultrafine Particle Emissions from a Light-Duty Diesel Engine. Energies 2023, 16, 4071. https://doi.org/10.3390/en16104071
Meloni E, Rossomando B, De Falco G, Sirignano M, Arsie I, Palma V. Effect of a Cu-Ferrite Catalyzed DPF on the Ultrafine Particle Emissions from a Light-Duty Diesel Engine. Energies. 2023; 16(10):4071. https://doi.org/10.3390/en16104071
Chicago/Turabian StyleMeloni, Eugenio, Bruno Rossomando, Gianluigi De Falco, Mariano Sirignano, Ivan Arsie, and Vincenzo Palma. 2023. "Effect of a Cu-Ferrite Catalyzed DPF on the Ultrafine Particle Emissions from a Light-Duty Diesel Engine" Energies 16, no. 10: 4071. https://doi.org/10.3390/en16104071
APA StyleMeloni, E., Rossomando, B., De Falco, G., Sirignano, M., Arsie, I., & Palma, V. (2023). Effect of a Cu-Ferrite Catalyzed DPF on the Ultrafine Particle Emissions from a Light-Duty Diesel Engine. Energies, 16(10), 4071. https://doi.org/10.3390/en16104071











