The Growth and Evolution of Biomass Soot in Partial Oxidation-Assisted Hot Gas Filtration
Abstract
:1. Introduction
2. Models
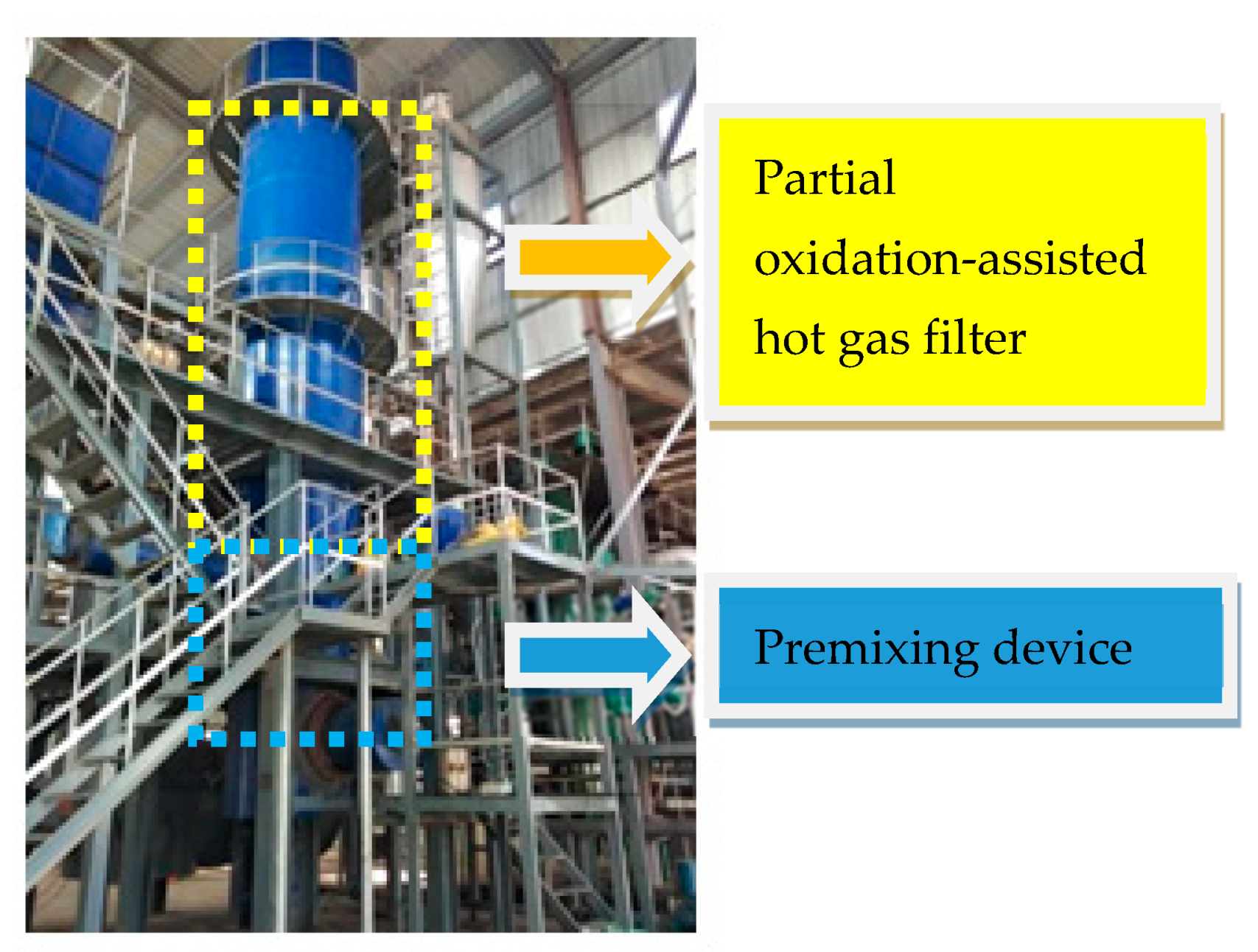
2.1. Physical Models
2.2. DPM and Chemical Reaction Model
2.3. Semi-Physical Boundary Conditions
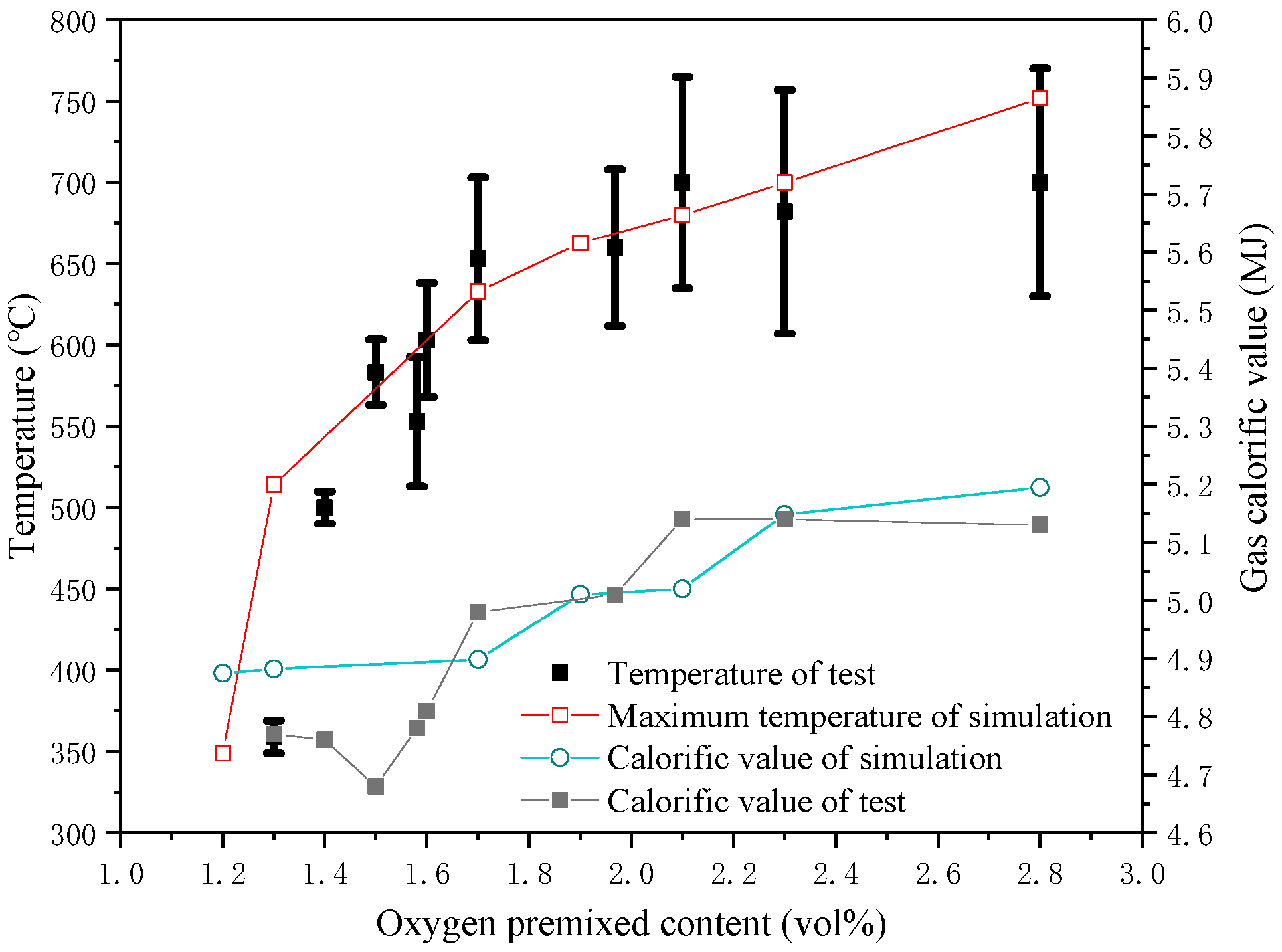
2.4. Model Verification
3. Results and Analysis
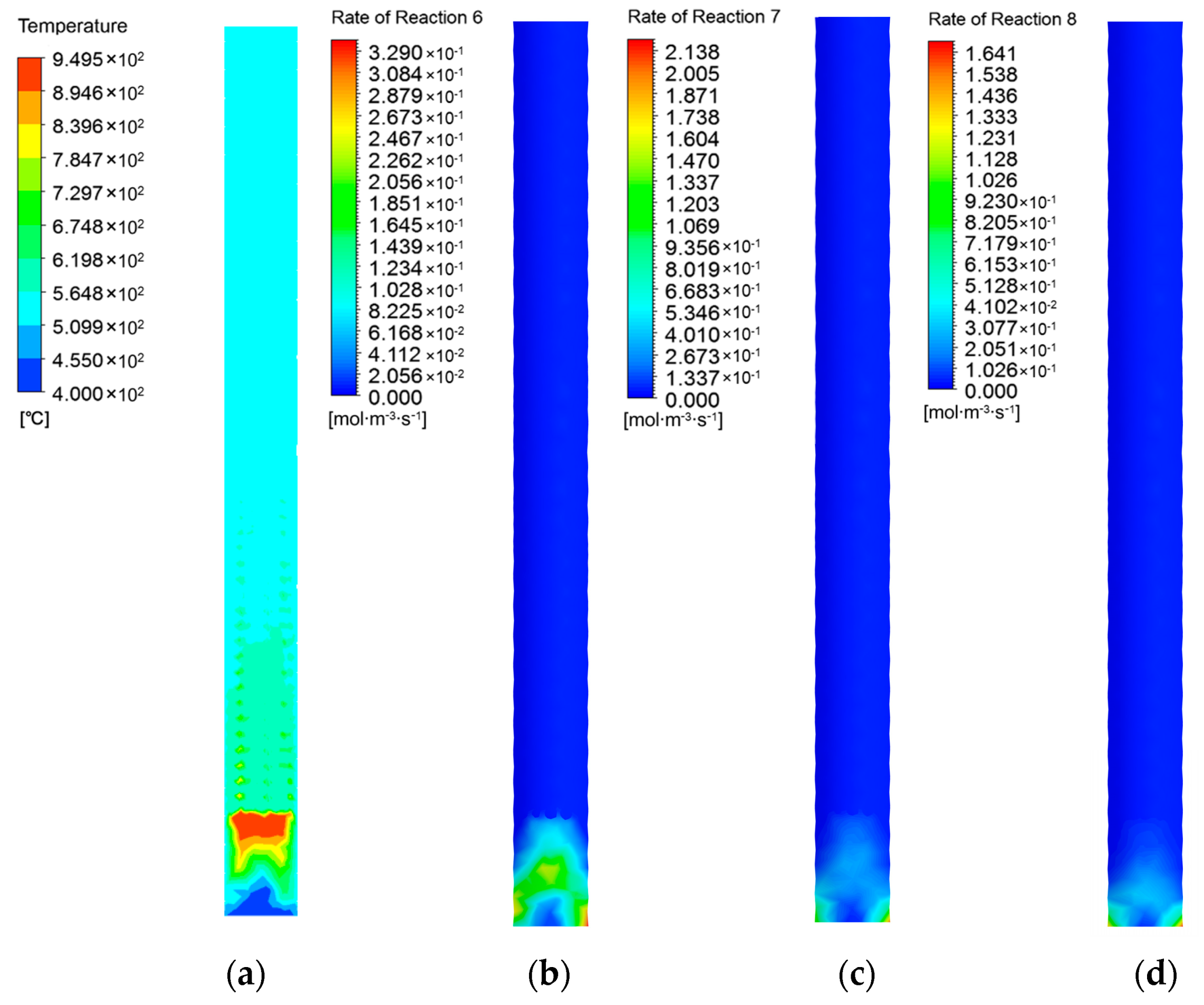
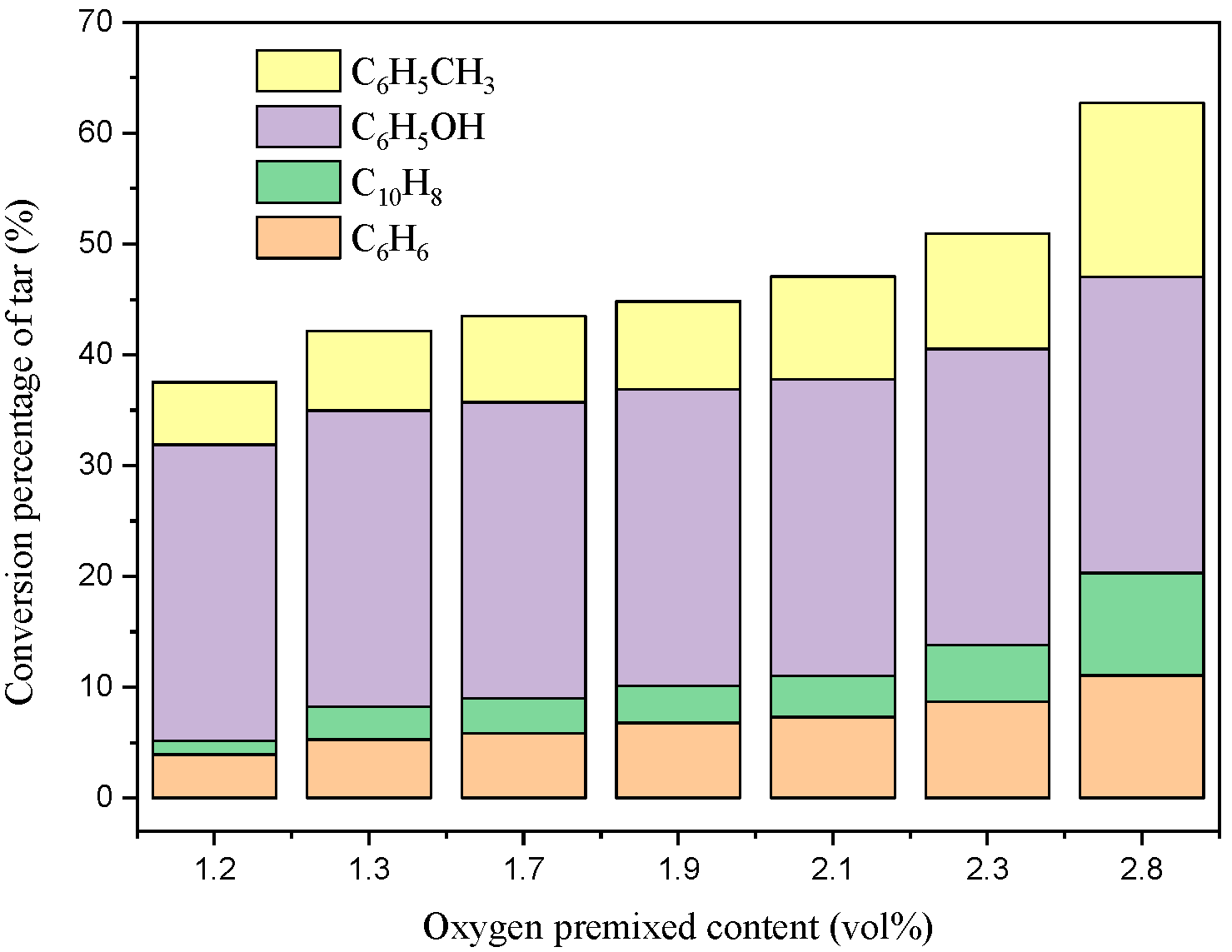
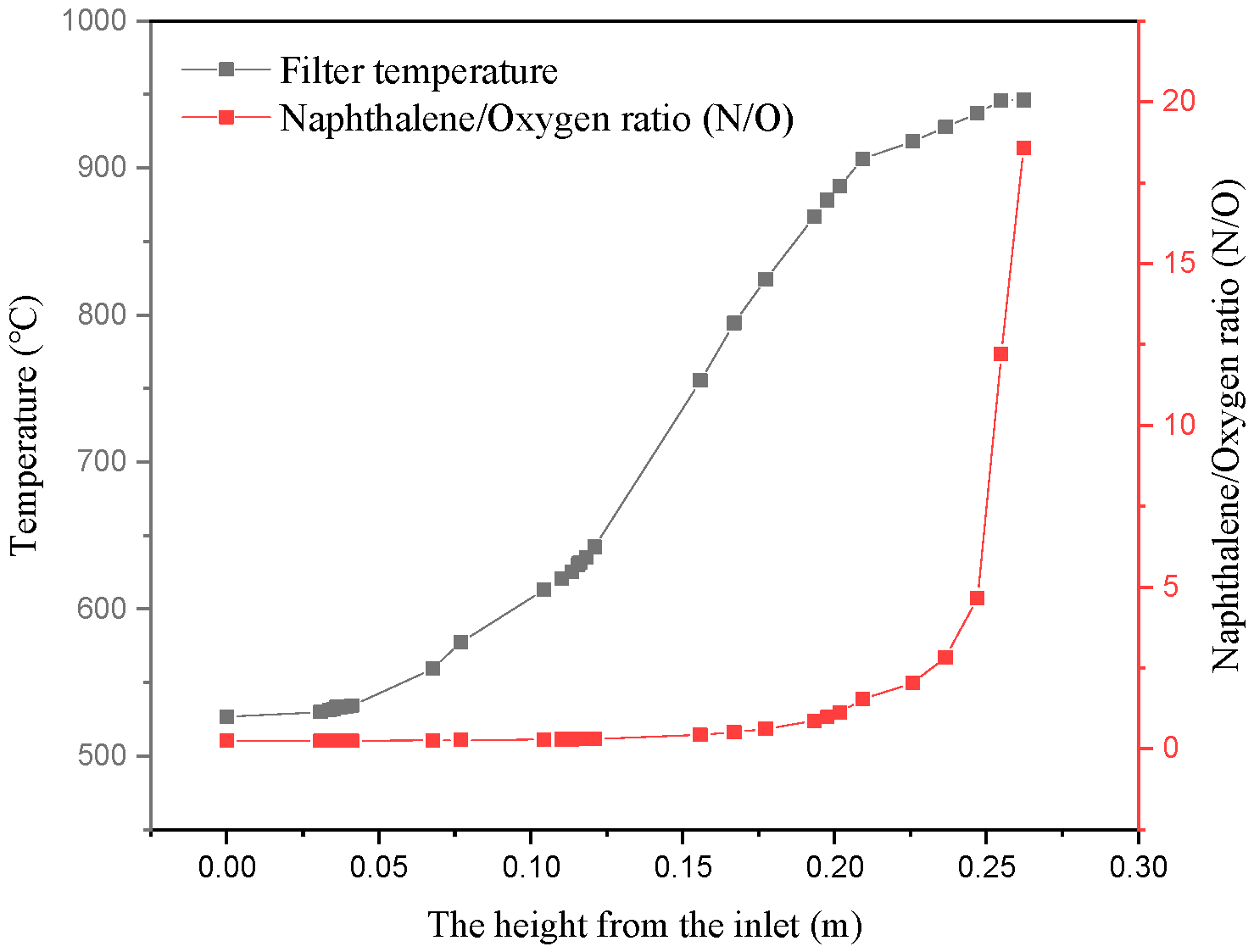
3.1. Tar Conversion and Soot Formation
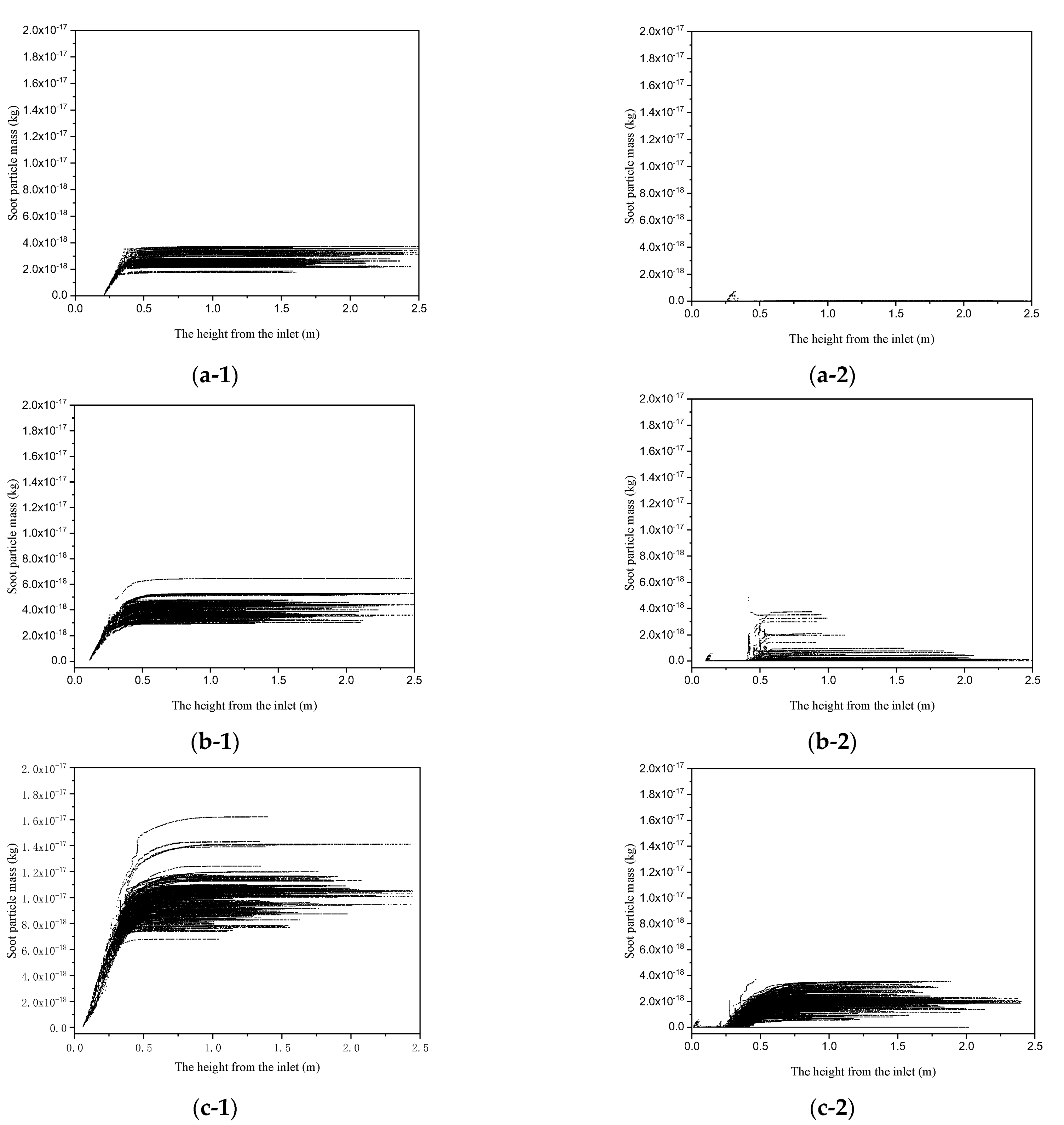
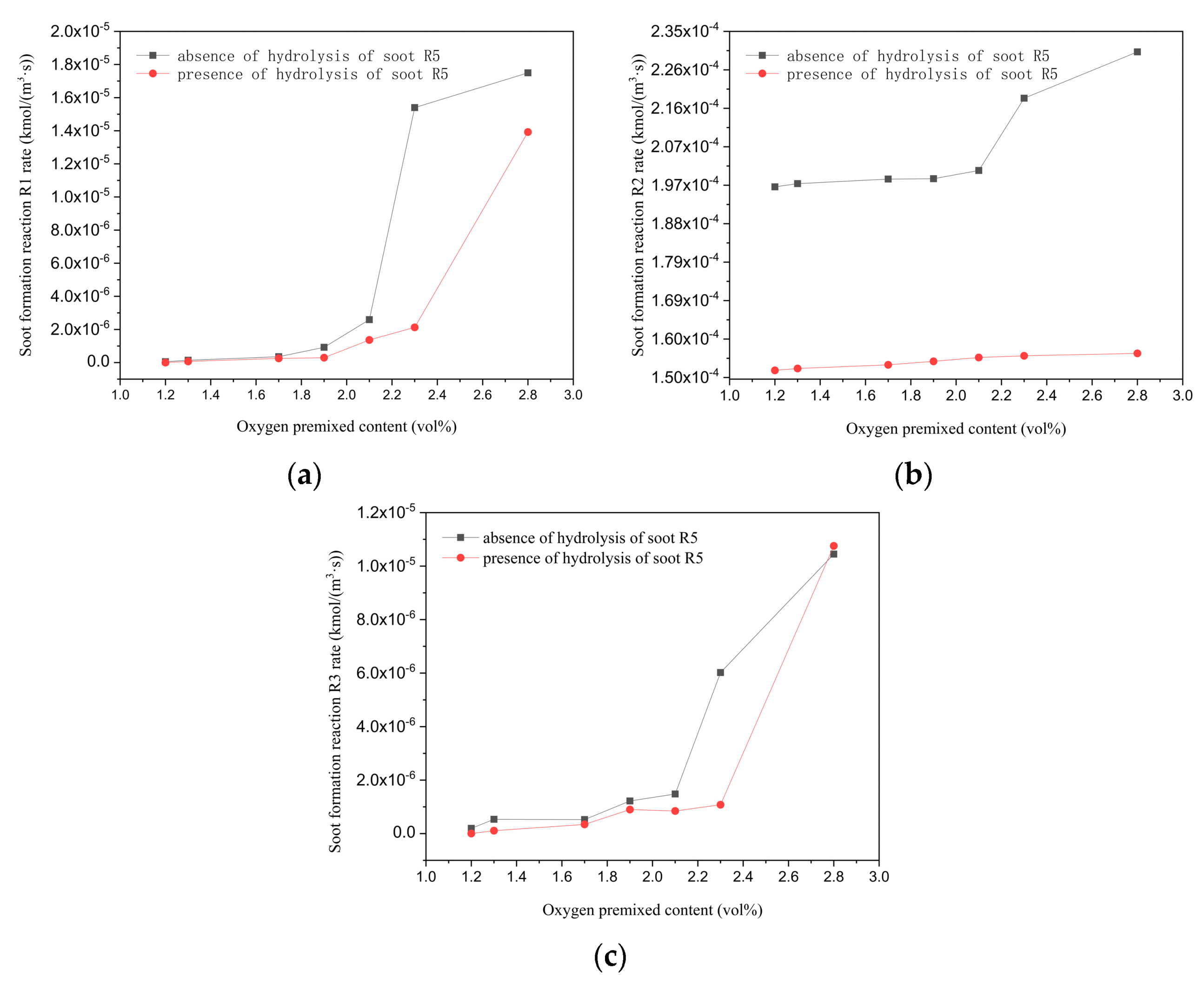
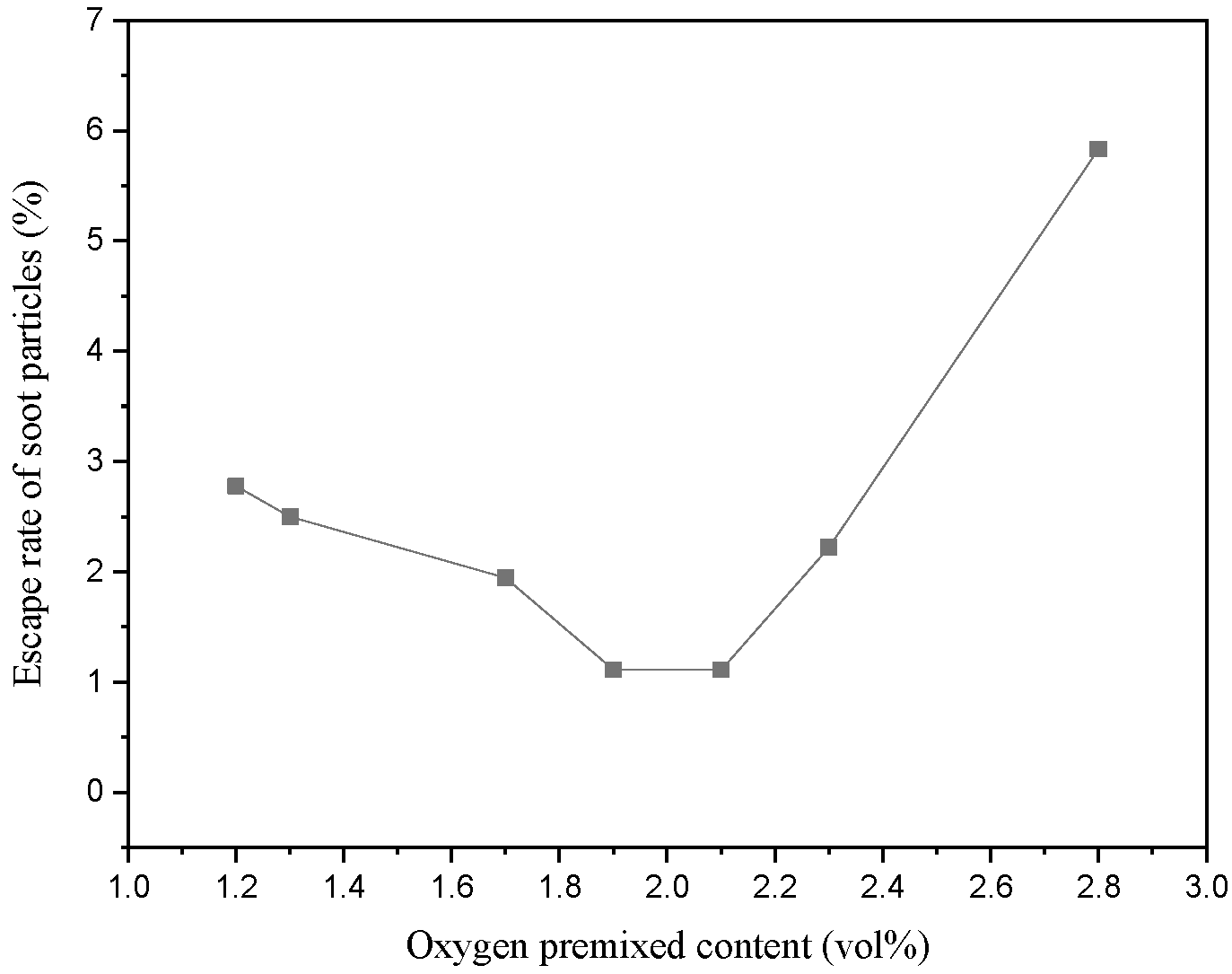
3.2. Inhibition on Growth of Soot, Filtration, and Interception
4. Conclusions
- (1)
- Although the partial oxidation method enhanced the conversion percentage of tar in the filtration process of biomass hot gas, the tar still generated soot through a polymerization reaction. This was mainly because the heat released by the gas oxidation reaction increased the temperature, and there was a higher naphthalene/oxygen ratio under oxygen-limited conditions.
- (2)
- Although the mass of soot particles increased with the increase in the oxygen content, the addition of oxygen inhibited the growth of soot particles. The inhibition was specifically embodied, with a lower increasing magnitude of the soot particle mass in the oxidation reaction and a reduction in the polymerization rate of tar due to the soot gasification reaction.
- (3)
- Partial oxidation led to the inevitable formation and growth of soot in the hot-gas filtration. Nevertheless, the presence of a low escape rate of soot provided a scientific basis to optimize the premixed oxygen content and further develop the application potential of partial oxidation-assisted hot-gas filtration.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sansaniwal, S.K.; Rosen, M.A.; Tyagi, S.K. Global challenges in the sustainable development of biomass gasification: An overview. Renew. Sustain. Energy Rev. 2017, 80, 23–43. [Google Scholar] [CrossRef]
- Díaz González, C.A.; Pacheco Sandoval, L. Sustainability aspects of biomass gasification systems for small power generation. Renew. Sustain. Energy Rev. 2020, 134, 110180. [Google Scholar] [CrossRef]
- Situmorang, Y.A.; Zhao, Z.; Yoshida, A.; Abudula, A.; Guan, G. Small-scale biomass gasification systems for power generation (<200 kW class): A review. Renew. Sustain. Energy Rev. 2020, 117, 109486. [Google Scholar]
- Singh Siwal, S.; Zhang, Q.; Sun, C.; Thakur, S.; Kumar Gupta, V.; Kumar Thakur, V. Energy production from steam gasification processes and parameters that contemplate in biomass gasifier—A review. Bioresour. Technol. 2020, 297, 122481. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Alam, M.T.; Krishna, B.B.; Bhaskar, T.; Perkins, G. A review on the production of renewable aviation fuels from the gasification of biomass and residual wastes. Bioresour. Technol. 2020, 312, 123596. [Google Scholar] [CrossRef]
- Rakesh, N.; Dasappa, S. A critical assessment of tar generated during biomass gasification—Formation, evaluation, issues and mitigation strategies. Renew. Sustain. Energy Rev. 2018, 91, 1045–1064. [Google Scholar] [CrossRef]
- He, Q.; Guo, Q.; Umeki, K.; Ding, L.; Wang, F.; Yu, G. Soot formation during biomass gasification: A critical review. Renew. Sustain. Energy Rev. 2021, 139, 110710. [Google Scholar] [CrossRef]
- Feng, D.; Guo, D.; Shang, Q.; Zhao, Y.; Zhang, L.; Guo, X.; Cheng, J.; Chang, G.; Guo, Q.; Sun, S. Mechanism of biochar-gas-tar-soot formation during pyrolysis of different biomass feedstocks: Effect of inherent metal species. Fuel 2021, 293, 120409. [Google Scholar] [CrossRef]
- Han, J.; Kim, H. The reduction and control technology of tar during biomass gasification/pyrolysis: An overview. Renew. Sustain. Energy Rev. 2008, 12, 397–416. [Google Scholar] [CrossRef]
- Heidenreich, S. Hot gas filtration—A review. Fuel 2013, 104, 83–94. [Google Scholar] [CrossRef]
- Li, S.; Baeyens, J.; Dewil, R.; Appels, L.; Zhang, H.; Deng, Y. Advances in rigid porous high temperature filters. Renew. Sustain. Energy Rev. 2021, 139, 110713. [Google Scholar] [CrossRef]
- Andersson, K.J.; Skov-Skjøth Rasmussen, M.; Højlund Nielsen, P.E. Industrial-scale gas conditioning including Topsoe tar reforming and purification downstream biomass gasifiers: An overview and recent examples. Fuel 2017, 203, 1026–1030. [Google Scholar] [CrossRef]
- Frenklach, M.; Clary, D.W.; Gardiner, W.C., Jr.; Stein, S.E. Detailed kinetic modeling of soot formation in shock-tube pyrolysis of acetylene. Symp. (Int.) Combust. 1985, 20, 887–901. [Google Scholar] [CrossRef]
- Frenklach, M. Reaction mechanism of soot formation in flames. Phys. Chem. Chem. Phys. 2002, 4, 2028–2037. [Google Scholar] [CrossRef]
- Stanmore, B.R.; Brilhac, J.F.; Gilot, P. The oxidation of soot: A review of experiments, mechanisms and models. Carbon 2001, 39, 2247–2268. [Google Scholar] [CrossRef]
- Nourbakhsh, H.; Rahbar Shahrouzi, J.; Zamaniyan, A.; Ebrahimi, H.; Jafari Nasr, M.R. A thermodynamic analysis of biogas partial oxidation to synthesis gas with emphasis on soot formation. Int. J. Hydrog. Energy 2018, 43, 15703–15719. [Google Scholar] [CrossRef]
- Josephson, A.J.; Gaffin, N.D.; Smith, S.T.; Fletcher, T.H.; Lignell, D.O. Modeling Soot Oxidation and Gasification with Bayesian Statistics. Energy Fuels 2017, 31, 11291–11303. [Google Scholar] [CrossRef]
- Lang, L.; Yang, W.; Xie, J.; Yin, X.; Wu, C.; Lin, J.Y.S. Oxidative filtration for flyash & tar removal from 1.0 MWth fixed-bed biomass air gasification. Biomass Bioenergy 2019, 122, 145–155. [Google Scholar]
- Su, Y.; Luo, Y.; Chen, Y.; Wu, W.; Zhang, Y. Experimental and numerical investigation of tar destruction under partial oxidation environment. Fuel Process. Technol. 2011, 92, 1513–1524. [Google Scholar] [CrossRef]
- Na, L.; Lang, L.; Huacai, L. Isothermal partial oxidative pyrolysis mechanisms of solid particles from biomass gasification. J. Fuel Chem. Techonlogy 2018, 46, 290–297. [Google Scholar]
- Valencia-López, A.M.; Bustamante, F.; Loukou, A.; Stelzner, B.; Trimis, D.; Frenklach, M.; Slavinskaya, N.A. Effect of benzene doping on soot precursors formation in non-premixed flames of producer gas (PG). Combust. Flame 2019, 207, 265–280. [Google Scholar] [CrossRef]
- Svensson, H.; Tunå, P.; Hulteberg, C.; Brandin, J. Modeling of soot formation during partial oxidation of producer gas. Fuel 2013, 106, 271–278. [Google Scholar] [CrossRef]
- Ku, X.; Jin, H.; Lin, J. Comparison of gasification performances between raw and torrefied biomasses in an air-blown fluidized-bed gasifier. Chem. Eng. Sci. 2017, 168, 235–249. [Google Scholar] [CrossRef]
- Gerun, L.; Paraschiv, M.; Vîjeu, R.; Bellettre, J.; Tazerout, M.; Gøbel, B.; Henriksen, U. Numerical investigation of the partial oxidation in a two-stage downdraft gasifier. Fuel 2008, 87, 1383–1393. [Google Scholar] [CrossRef]
- Ismail, T.M.; Abd El-Salam, M.; Monteiro, E.; Rouboa, A. Fluid dynamics model on fluidized bed gasifier using agro-industrial biomass as fuel. Waste Manag. 2018, 73, 476–486. [Google Scholar] [CrossRef]
- Loha, C.; Chattopadhyay, H.; Chatterjee, P.K. Three dimensional kinetic modeling of fluidized bed biomass gasification. Chem. Eng. Sci. 2014, 109, 53–64. [Google Scholar] [CrossRef]
- Shin, D.; Choi, S. The combustion of simulated waste particles in a fixed bed. Combust. Flame 2000, 121, 167–180. [Google Scholar] [CrossRef]
- Blasi, C.D. Dynamic behaviour of stratified downdraft gasifiers. Chem. Eng. Sci. 2000, 55, 2931–2944. [Google Scholar] [CrossRef]
- Jeong, H.J.; Hwang, I.S.; Park, S.S.; Hwang, J. Investigation on co-gasification of coal and biomass in Shell gasifier by using a validated gasification model. Fuel 2017, 196, 371–377. [Google Scholar] [CrossRef]
- Taralas, G.; Kontominas, M.G.; Kakatsios, X. Modeling the Thermal Destruction of Toluene (C7H8) as Tar-Related Species for Fuel Gas Cleanup. Energy Fuels 2003, 17, 329–337. [Google Scholar] [CrossRef]
- Morf, P.; Hasler, P.; Nussbaumer, T. Mechanisms and kinetics of homogeneous secondary reactions of tar from continuous pyrolysis of wood chips. Fuel 2002, 81, 843–853. [Google Scholar] [CrossRef]



| Number | Chemical Reaction | Reaction Type | A | β | E/(J·kmol−1) | a | b |
|---|---|---|---|---|---|---|---|
| R1 | C6H6 + 2H2O → 1.5C(s) + 2.5CH4 + 2CO | Polymerization of tar/formation of soot | 3.39 × 1016·H−0.4 | 0 | 4.43 × 108 | 1.3 | 0.2 |
| R2 | C6H5OH + 3H2O → 2CO+ CO2 + 2.95CH4 + 0.05C(s) + 0.1H2 | 1 × 108 | 0 | 1 × 108 | 1 | — | |
| R3 | C10H8 → 7.38C(s) + 0.275C6H6 + 0.97CH4 + 1.235H2 | 3.39 × 1014·H−0.4 | 0 | 3.5 × 108 | 1.6 | −0.5 | |
| R4 | 2C(s) + O2 → 2CO | Oxidation of soot | 592·T | 0 | 2 × 108 | — | 1 |
| R5 | C(s) + H2O → CO + H2 | Gasification of soot | 3.6×1012 | 0 | 3.1 × 108 | 1 | 1 |
| Number | Chemical Reaction | A | β | E/(J·kmol−1) | a | b |
|---|---|---|---|---|---|---|
| R6 | 2CO + O2 → 2CO2 | 1.3 × 1011·H2O0.5 | 0 | 1.2567 × 108 | 1 | 0.5 |
| R7 | 2H2 + O2 → 2H2O | 1 × 1014 | 0 | 4.2 × 107 | 1 | 1 |
| R8 | CH4 + 1.5O2 → CO + 2H2O | 4.4 × 1012 | 0 | 1.2552 × 108 | 0.5 | 1.25 |
| Number | Chemical Reaction | A | β | E/(J·kmol−1) | a | b |
|---|---|---|---|---|---|---|
| R9 | C6H5CH3 + H2 → CH4 + C6H6 | 3.3 × 1010 | 0 | 2.5 × 108 | 1 | 0.5 |
| R10 | 2C6H5CH3 + 21H2O → 7CO2 + 29H2 + 7CO | 2.323 × 1015 | 0 | 3.56 × 108 | 1 | 0 |
| R11 | C6H5CH3 + 3.5O2 → 7CO + 4H2 | 3.8 × 107 | 0.5 | 5.545 × 107 | 1 | 1 |
| R12 | C6H6 + 4.5O2 → 6CO + 3H2O | 2.4 × 1011 | 0 | 1.2552 × 108 | −0.1 | 1.85 |
| R13 | C6H5OH + 4O2 → 6CO + 3H2O | 9.2 × 106·T | 1 | 8 × 107 | 0.5 | 1 |
| R14 | C6H5OH → CO + 0.4C10H8 + 0.15C6H6 + 0.1CH4 + 0.75H2 | 1 × 107 | 0 | 1 × 108 | 1 | — |
| R15 | C10H8 + 7O2 → 10CO + 4H2O | 9.2 × 106·T | 1 | 8 × 107 | 0.5 | 1 |
| Components and Caloric Value of Inlet Hot Gas | Caloric Value 4.9–5.1 |
|---|---|
| Inlet oxygen content | 1.2 vol%~2.8 vol% |
| Inlet initial temperature | 400 °C |
| Inlet tar content | Total tar, accounting for 2.5 vol%–3 vol% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Jin, Z.; Gao, W. The Growth and Evolution of Biomass Soot in Partial Oxidation-Assisted Hot Gas Filtration. Energies 2023, 16, 4233. https://doi.org/10.3390/en16104233
Tian L, Jin Z, Gao W. The Growth and Evolution of Biomass Soot in Partial Oxidation-Assisted Hot Gas Filtration. Energies. 2023; 16(10):4233. https://doi.org/10.3390/en16104233
Chicago/Turabian StyleTian, Lin, Zixuan Jin, and Wenran Gao. 2023. "The Growth and Evolution of Biomass Soot in Partial Oxidation-Assisted Hot Gas Filtration" Energies 16, no. 10: 4233. https://doi.org/10.3390/en16104233
APA StyleTian, L., Jin, Z., & Gao, W. (2023). The Growth and Evolution of Biomass Soot in Partial Oxidation-Assisted Hot Gas Filtration. Energies, 16(10), 4233. https://doi.org/10.3390/en16104233








