Highly Conductive Cerium- and Neodymium-Doped Barium Zirconate Perovskites for Protonic Ceramic Fuel Cells
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Electrolyte Powder Synthesis and Characterizations
2.3. XRD Characterization
2.4. Fabrication of the Electrolyte Pellets and Conductivity Measurements
3. Results and Discussion
3.1. Structural Analysis
3.2. Conductivity and Activation Energy Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bonanos, N. Transport properties and conduction mechanism in high-temperature protonic conductors. Solid State Ion. 1992, 53–56, 967–974. [Google Scholar] [CrossRef]
- Fabbri, E.; Bi, L.; Pergolesi, D.; Traversa, E. Towards the next generation of solid oxide fuel cells operating below 600 °C with chemically stable proton-conducting electrolytes. Adv. Mater. 2012, 24, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Savchyn, V.; Popov, A.; Aksimentyeva, O.; Klym, H.; Horbenko, Y.Y.; Serga, V.; Moskina, A.; Karbovnyk, I. Cathodoluminescence characterization of polystyrene-BaZrO3 hybrid composites. Low Temp. Phys. 2016, 42, 597–600. [Google Scholar] [CrossRef]
- Gupta, S.K.; Abdou, H.; Segre, C.U.; Mao, Y. Excitation-Dependent Photoluminescence of BaZrO3: Eu3+ Crystals. Nanomaterials 2022, 12, 3028. [Google Scholar] [CrossRef]
- Medvedev, D.; Murashkina, A.; Pikalova, E.; Demin, A.; Podias, A.; Tsiakaras, P. BaCeO3: Materials development, properties and application. Prog. Mater. Sci. 2014, 60, 72–129. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kawasaki, Y.; Ito, N.; Enoki, M.; Ishihara, T. Relation between electrical conductivity and chemical stability of BaCeO3-based proton conductors with different trivalent dopants. Electrochem. Solid-State Lett. 2007, 10, 77–80. [Google Scholar] [CrossRef]
- Ryu, K.H.; Haile, S.M. Chemical stability and proton conductivity of doped BaCeO3–BaZrO3 solid solutions. Solid State Ion. 1999, 125, 355–367. [Google Scholar] [CrossRef]
- Bae, K.; Jang, D.Y.; Choi, H.J.; Kim, D.; Hong, J.; Kim, B.K.; Lee, J.H.; Son, J.W.; Shim, J.H. Demonstrating the potential of yttrium-doped barium zirconate electrolyte for high-performance fuel cells. Nat. Commun. 2017, 8, 14553. [Google Scholar] [CrossRef]
- Yilmaz, S.; Cobaner, S.; Yalaz, E.; Amini Horri, B. Synthesis and Characterization of Gadolinium-Doped Zirconia as a Potential Electrolyte for Solid Oxide Fuel Cells. Energies 2022, 15, 2826. [Google Scholar] [CrossRef]
- Fabbri, E.; D’Epifanio, A.; Di Bartolomeo, E.; Licoccia, S.; Traversa, E. Tailoring the chemical stability of Ba(Ce0.8−xZrx)Y0.2O3−δ protonic conductors for Intermediate Temperature Solid Oxide Fuel Cells (IT-SOFCs). Solid State Ion. 2008, 179, 558–564. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton-Conducting Oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Bae, K.; Noh, H.S.; Jang, D.Y.; Hong, J.; Kim, H.; Yoon, K.J.; Lee, J.H.; Kim, B.K.; Shim, J.H.; Son, J.W. High-performance thin-film protonic ceramic fuel cells fabricated on anode supports with a non-proton-conducting ceramic matrix. J. Mater. Chem. A 2016, 4, 6395–6404. [Google Scholar] [CrossRef]
- Bae, K.; Kim, D.H.; Choi, H.J.; Son, J.W.; Shim, J.H. High-Performance Protonic Ceramic Fuel Cells with 1 µm Thick Y:Ba(Ce, Zr)O3 Electrolytes. Adv. Energy Mater. 2018, 8, 1801315. [Google Scholar] [CrossRef]
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H.I.; Haile, S.M. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 2018, 3, 202–210. [Google Scholar] [CrossRef]
- Lyagaeva, J.; Danilov, N.; Vdovin, G.; Bu, J.; Medvedev, D.; Demin, A.; Tsiakaras, P. A new Dy-doped BaCeO3-BaZrO3 proton-conducting material as a promising electrolyte for reversible solid oxide fuel cells. J. Mater. Chem. A 2016, 4, 15390–15399. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.; Zhu, H.; Sullivan, N.; Zhu, L.; Bian, L.; Jennings, D.; O’Hayre, R. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 2019, 4, 230–240. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, F.; Zhang, L.; Chen, F. Synthesis of BaCe0.7Zr0.1Y0.1Yb0.1O3-δ proton conducting ceramic by a modified Pechini method. Solid State Ion. 2012, 213, 29–35. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Ran, R.; Shao, Z. A new neodymium-doped BaZr0.8Y0.2O3−δ as potential electrolyte for proton-conducting solid oxide fuel cells. J. Membr. Sci. 2012, 415–416, 391–398. [Google Scholar] [CrossRef]
- Su, X.T.; Yan, Q.Z.; Ma, X.H.; Zhang, W.F.; Ge, C.C. Effect of co-dopant addition on the properties of yttrium and neodymium doped barium cerate electrolyte. Solid State Ion. 2006, 177, 1041–1045. [Google Scholar] [CrossRef]
- Lyagaeva, Y.G.; Medvedev, D.A.; Demin, A.K.; Yaroslavtseva, T.V.; Plaksin, S.V.; Porotnikova, N.M. Specific features of preparation of dense ceramic based on barium zirconate. Semiconductors 2014, 48, 1353–1358. [Google Scholar] [CrossRef]
- Boultif, A.; Louër, D. Powder pattern indexing with the dichotomy method. J. Appl. Crystallogr. 2004, 37, 724–731. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Nath, D.; Singh, F.; Das, R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles- a comparative study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Mote, V.; Purushotham, Y.; Dole, B. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 2012, 6, 6. [Google Scholar] [CrossRef]
- Gazda, M.; Jasinski, P.; Kusz, B.; Bochentyn, B.; Gdula-Kasica, K.; Lendze, T.; Lewandowska-Iwaniak, W.; Mielewczyk-Gryn, A.; Molin, S. Perovskites in solid oxide fuel cells. Solid State Phenom. 2012, 183, 65–70. [Google Scholar] [CrossRef]
- Ishihara, T. Development of new fast oxide ion conductor and application for intermediate temperature Solid Oxide Fuel Cells. Bull. Chem. Soc. Jpn. 2006, 79, 1155–1166. [Google Scholar] [CrossRef]
- Imashuku, S.; Uda, T.; Nose, Y.; Taniguchi, G.; Ito, Y.; Awakura, Y. Dependence of Dopant Cations on Microstructure and Proton Conductivity of Barium Zirconate. J. Electrochem. Soc. 2009, 156, B1. [Google Scholar] [CrossRef]
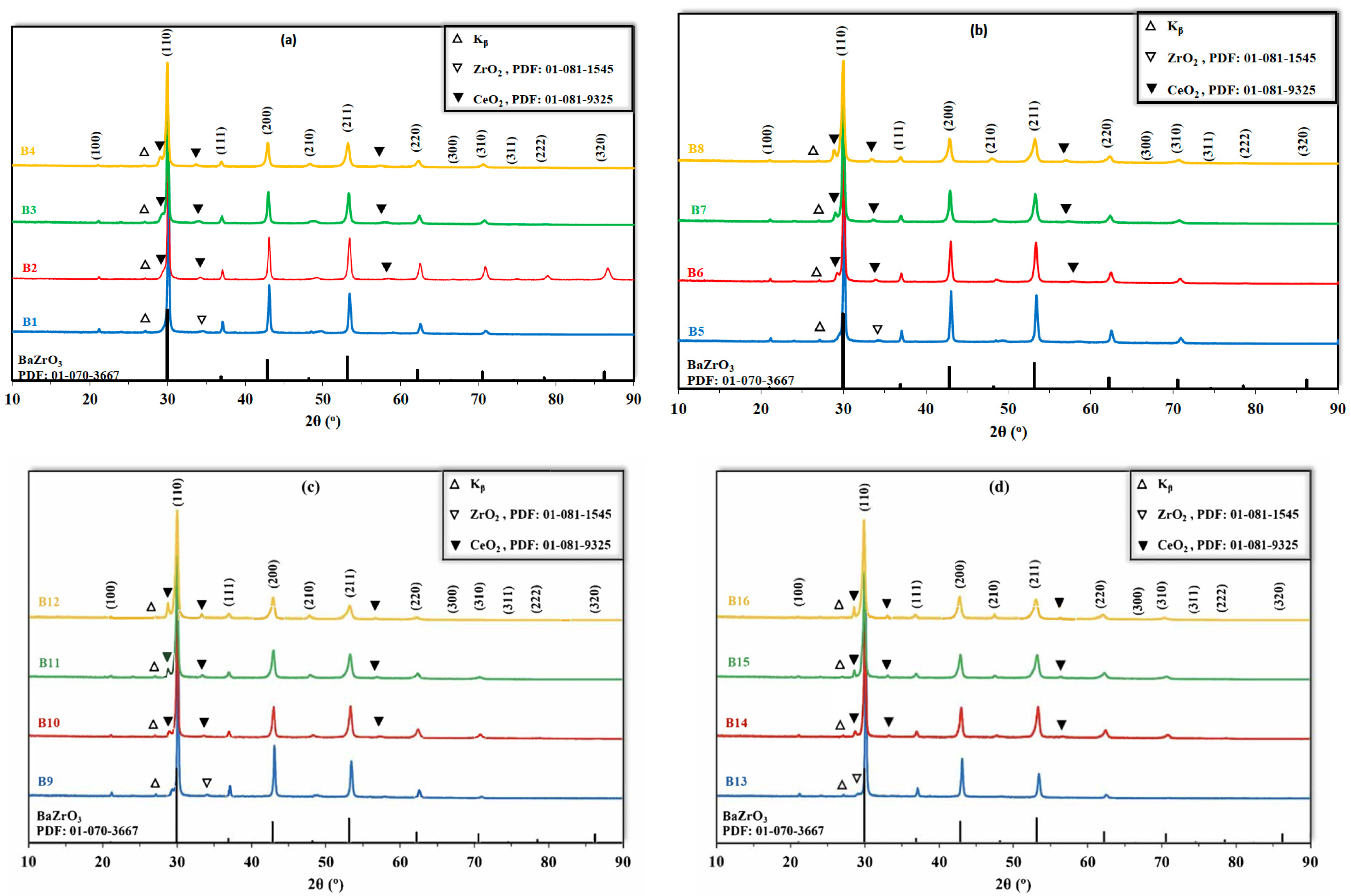


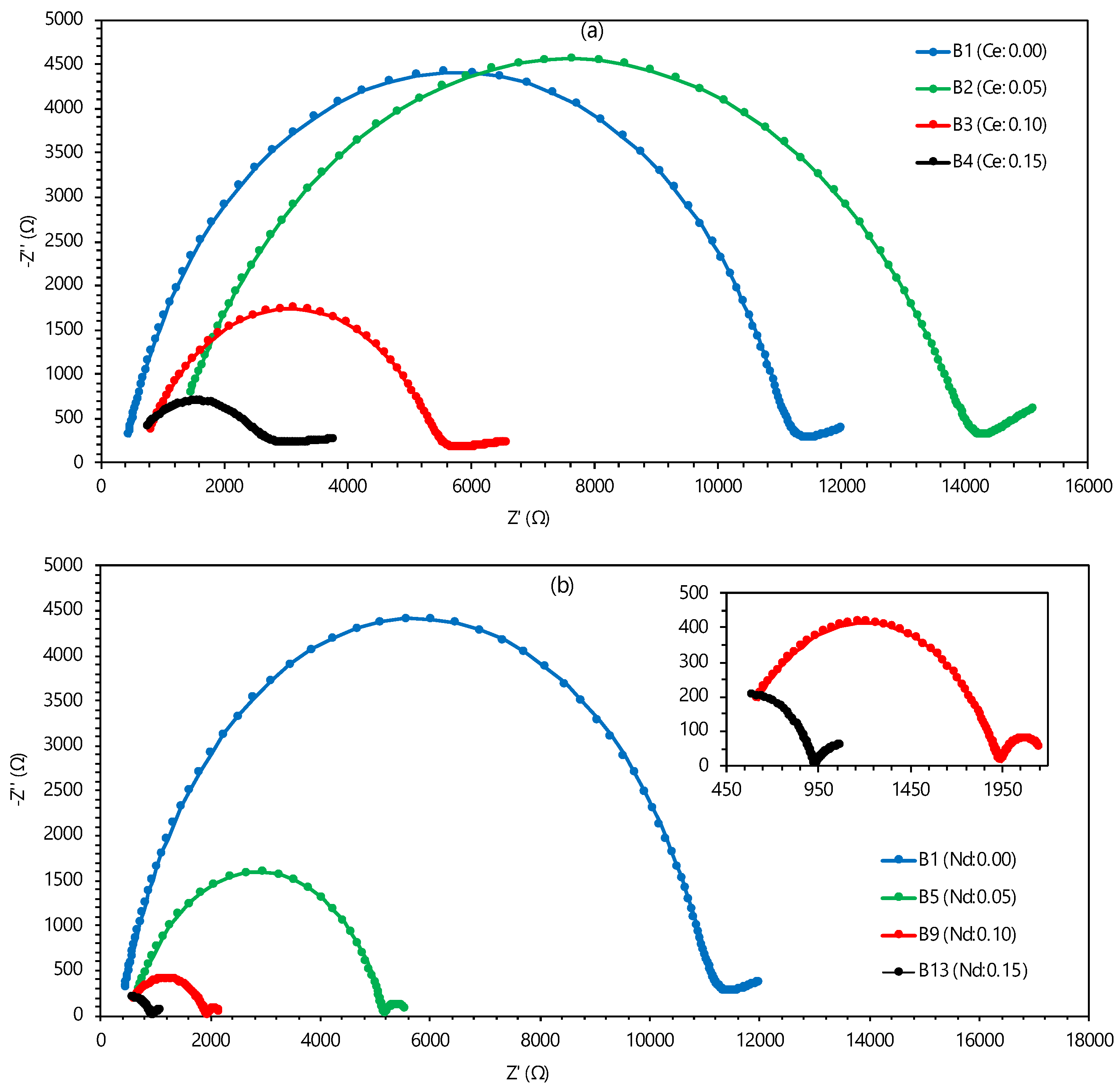

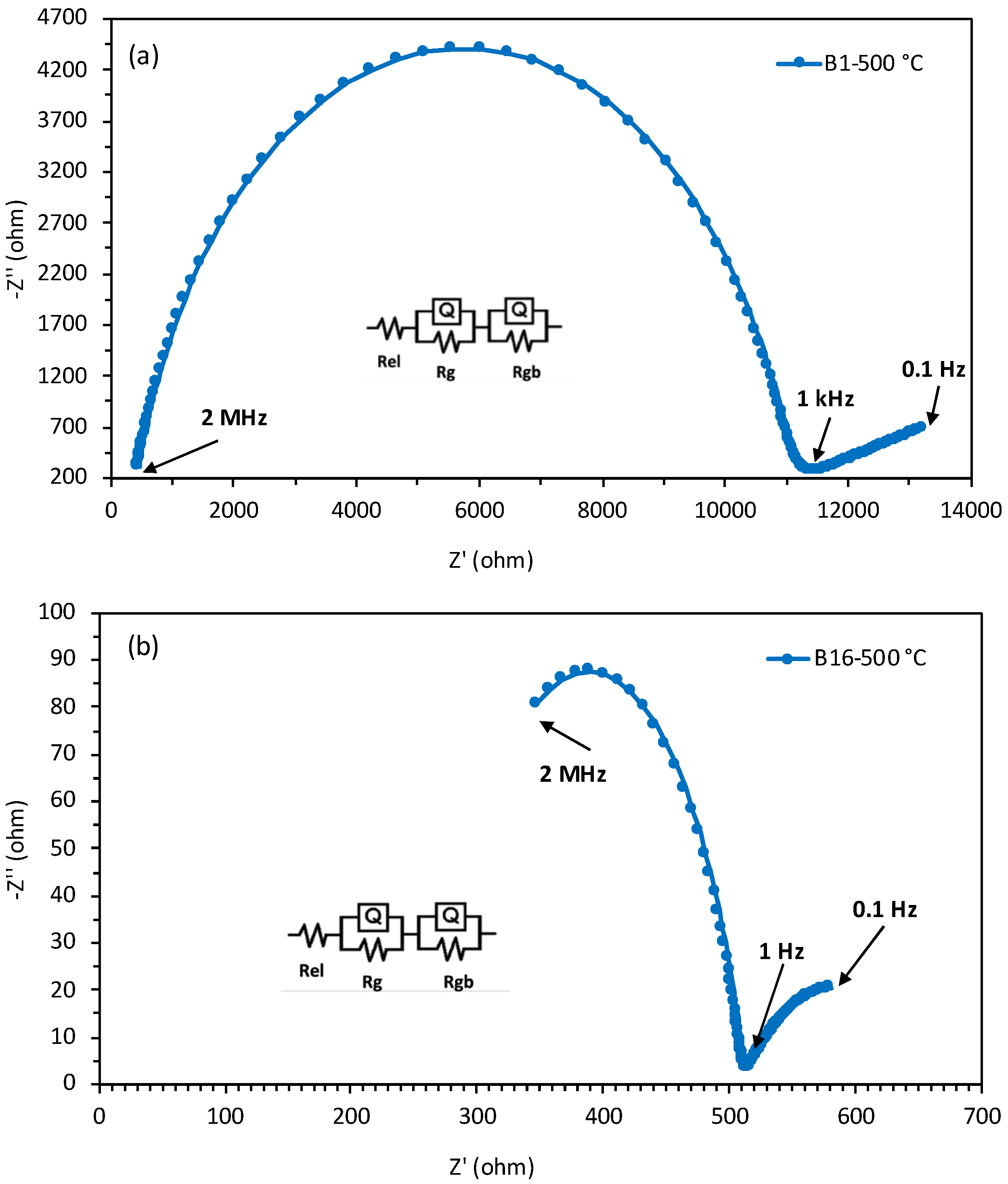

| Notations | BaZr(0.80-x-y-z)CexYyYbzO3-δ | Cex (mol) | Yy (mol) | Ybz (mol) | ||
| Counter samples | A1 | BaZrO3 | ||||
| A2 | BaZr0.80Y0.20O3 | 0.20 | ||||
| A3 | BaZr0.10Ce0.70Y0.20O3 | 0.70 | 0.20 | |||
| A4 | BaZr0.10Ce0.70Y0.10Yb0.10O3 | 0.70 | 0.10 | 0.10 | ||
| A5 | BaZr0.40Ce0.40Y0.10Yb0.10O3 | 0.40 | 0.10 | 0.10 | ||
| Notations | BaZr(0.80-x-y)CexNdyY0.10Yb0.10O3-δ | Cex (mol) | Ndy (mol) | Y0.10 (mol) | Yb0.10 (mol) | |
| BZCNYYO | B1 | BaZr0.80(Y0.10Yb0.10)O3 | 0 | 0 | 0.10 | 0.10 |
| B2 | BaZr0.75(Ce0.05Y0.10Yb0.10)O3 | 0.05 | ||||
| B3 | BaZr0.70(Ce0.10Y0.10Yb0.10)O3 | 0.10 | ||||
| B4 | BaZr0.65(Ce0.15Y0.10Yb0.10)O3 | 0.15 | ||||
| B5 | BaZr0.75(Nd0.05Y0.10Yb0.10)O3 | 0 | 0.05 | 0.10 | 0.10 | |
| B6 | BaZr0.70(Ce0.05Nd0.05Y0.10Yb0.10)O3 | 0.05 | ||||
| B7 | BaZr0.65(Ce0.10Nd0.05Y0.10Yb0.10)O3 | 0.10 | ||||
| B8 | BaZr0.60(Ce0.15Nd0.05Y0.10Yb0.10)O3 | 0.15 | ||||
| B9 | BaZr0.70(Nd0.10Y0.10Yb0.10)O3 | 0 | 0.10 | 0.10 | 0.10 | |
| B10 | BaZr0.65(Ce0.05Nd0.10Y0.10Yb0.10)O3 | 0.05 | ||||
| B11 | BaZr0.60(Ce0.10Nd0.10Y0.10Yb0.10)O3 | 0.10 | ||||
| B12 | BaZr0.55(Ce0.15Nd0.10Y0.10Yb0.10)O3 | 0.15 | ||||
| B13 | BaZr0.65(Nd0.15Y0.10Yb0.10)O3 | 0 | 0.15 | 0.10 | 0.10 | |
| B14 | BaZr0.60(Ce0.05Nd0.15Y0.10Yb0.10)O3 | 0.05 | ||||
| B15 | BaZr0.55(Ce0.10Nd0.15Y0.10Yb0.10)O3 | 0.10 | ||||
| B16 | BaZr0.50(Ce0.15Nd0.15Y0.10Yb0.10)O3 | 0.15 |
| First Step | Second Step | Third Step | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PVAsol (g) | Water (mL) | Time (min) | T (°C) | n-Prop. (mL) | Time (min) | T (°C) | Powder (g) | Time (h) | T (°C) |
| Proper Amount | 10 | 15 | 85 | 20 | 30 | 85 | BZCNYYO | 2 | room |
| Sample | a (Å) | ε (%) |
|---|---|---|
| B1 | 4.1989 | 0.19 |
| B2 | 4.2024 | 0.23 |
| B3 | 4.2115 | 0.26 |
| B4 | 4.2265 | 0.38 |
| B5 | 4.2016 | 0.17 |
| B6 | 4.2115 | 0.26 |
| B7 | 4.2150 | 0.32 |
| B8 | 4.2190 | 0.53 |
| B9 | 4.1938 | 0.20 |
| B10 | 4.2020 | 0.25 |
| B11 | 4.2071 | 0.30 |
| B12 | 4.2190 | 0.69 |
| B13 | 4.1934 | 0.19 |
| B14 | 4.2148 | 0.30 |
| B15 | 4.2269 | 0.54 |
| B16 | 4.2296 | 0.72 |
| B1 | B2 | B3 | B4 | B5 | B6 | B7 | B8 | B9 | B10 | B11 | B12 | B13 | B14 | B15 | B16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t (mm) | 1.2 | 1.2 | 1.1 | 1.2 | 1.2 | 1.2 | 1 | 0.9 | 1 | 0.8 | 1.5 | 1.1 | 1.2 | 1.1 | 1.2 | 1 |
| S (cm2) | 0.478 | 0.499 | 0.509 | 0.448 | 0.501 | 0.472 | 0.46 | 0.435 | 0.466 | 0.403 | 0.409 | 0.475 | 0.856 | 0.626 | 0.537 | 0.513 |
| T (°C) | Conductivity, σ (Scm−1) | |||||||||||||||
| 700 | 3.47 × 10−4 | 3.78 × 10−4 | 4.40 × 10−4 | 5.80 × 10−4 | 5.51 × 10−4 | 6.11 × 10−4 | 6.66 × 10−4 | 1.08 × 10−3 | 8.87 × 10−4 | 1.16 × 10−3 | 1.35 × 10−3 | 1.59 × 10−3 | 7.62 × 10−4 | 8.47 × 10−4 | 1.12 × 10−3 | 1.82 × 10−3 |
| 650 | 2.02 × 10−4 | 2.32 × 10−4 | 2.34 × 10−4 | 3.66 × 10−4 | 3.14 × 10−4 | 3.74 × 10−4 | 4.14 × 10−4 | 6.84 × 10−4 | 5.48 × 10−4 | 7.60 × 10−4 | 9.78 × 10−4 | 1.13 × 10−3 | 5.08 × 10−4 | 5.64 × 10−4 | 7.48 × 10−4 | 1.24 × 10−3 |
| 600 | 1.02 × 10−4 | 1.22 × 10−4 | 1.33 × 10−4 | 2.37 × 10−4 | 1.74 × 10−4 | 2.13 × 10−4 | 2.45 × 10−4 | 4.27 × 10−4 | 3.33 × 10−4 | 4.77 × 10−4 | 6.61 × 10−4 | 7.81 × 10−4 | 3.36 × 10−4 | 3.79 × 10−4 | 4.97 × 10−4 | 8.43 × 10−4 |
| 550 | 4.93 × 10−5 | 5.72 × 10−5 | 6.90 × 10−5 | 1.40 × 10−4 | 8.72 × 10−5 | 1.18 × 10−4 | 1.48 × 10−4 | 2.50 × 10−4 | 1.95 × 10−4 | 2.97 × 10−4 | 4.37 × 10−4 | 5.34 × 10−4 | 2.25 × 10−4 | 2.51 × 10−4 | 3.23 × 10−4 | 5.62 × 10−4 |
| 500 | 2.16 × 10−5 | 2.72 × 10−5 | 3.66 × 10−5 | 7.54 × 10−5 | 4.64 × 10−5 | 6.69 × 10−5 | 8.39 × 10−5 | 1.60 × 10−4 | 1.12 × 10−4 | 1.77 × 10−4 | 2.71 × 10−4 | 3.26 × 10−4 | 1.49 × 10−4 | 1.70 × 10−4 | 2.10 × 10−4 | 3.77 × 10−4 |
| 450 | 8.14 × 10−6 | 1.08 × 10−5 | 1.67 × 10−5 | 3.67 × 10−5 | 2.09 × 10−5 | 3.41 × 10−5 | 4.55 × 10−5 | 7.81 × 10−5 | 5.82 × 10−5 | 9.98 × 10−5 | 1.55 × 10−4 | 1.82 × 10−4 | 9.66 × 10−5 | 1.09 × 10−4 | 1.30 × 10−4 | 2.40 × 10−4 |
| 400 | 2.83 × 10−6 | 3.62 × 10−6 | 7.85 × 10−6 | 2.17 × 10−6 | 8.74 × 10−6 | 1.51 × 10−5 | 2.07 × 10−5 | 4.24 × 10−5 | 2.83 × 10−5 | 5.26 × 10−5 | 8.84 × 10−5 | 1.03 × 10−4 | 6.20 × 10−5 | 6.86 × 10−5 | 8.05 × 10−5 | 1.39 × 10−4 |
| 350 | 8.85 × 10−7 | 8.49 × 10−7 | 3.06 × 10−6 | 8.01 × 10−6 | 2.76 × 10−6 | 5.78 × 10−6 | 8.25 × 10−6 | 1.64 × 10−5 | 1.15 × 10−5 | 2.47 × 10−5 | 4.07 × 10−5 | 4.63 × 10−5 | 3.39 × 10−5 | 3.66 × 10−5 | 4.08 × 10−5 | 7.08 × 10−5 |
| 300 | 1.96 × 10−7 | 1.88 × 10−7 | 8.98 × 10−7 | 2.10 × 10−7 | 1.87 × 10−7 | 1.99 × 10−7 | 1.70 × 10−7 | 1.62 × 10−7 | 1.68 × 10−7 | 1.55 × 10−7 | 1.44 × 10−5 | 1.67 × 10−5 | 1.42 × 10−5 | 1.49 × 10−5 | 1.49 × 10−5 | 2.68 × 10−5 |
| A1 | A2 | A3 | A4 | A5 | |
|---|---|---|---|---|---|
| t (cm) | 0.16 | 0.155 | 0.115 | 0.16 | 0.095 |
| S (cm2) | 0.531 | 0.563 | 0.754 | 0.503 | 0.554 |
| T (°C) | Conductivity, σ (Scm−1) | ||||
| 700 | 8.57 × 10−7 | 3.64 × 10−5 | 1.49 × 10−3 | 1.58 × 10−3 | 8.41 × 10−4 |
| 650 | 3.73 × 10−7 | 1.62 × 10−5 | 1.07 × 10−3 | 1.04 × 10−3 | 5.58 × 10−4 |
| 600 | 1.52 × 10−7 | 8.18 × 10−6 | 7.11 × 10−4 | 6.75 × 10−4 | 3.55 × 10−4 |
| 550 | 8.23 × 10−8 | 3.79 × 10−6 | 5.03 × 10−4 | 6.13 × 10−4 | 2.32 × 10−4 |
| 500 | 2.70 × 10−8 | 1.63 × 10−6 | 3.77 × 10−4 | 4.04 × 10−4 | 1.54 × 10−4 |
| 450 | 4.28 × 10−9 | 6.90 × 10−7 | 2.99 × 10−4 | 2.45 × 10−4 | 1.45 × 10−4 |
| 400 | 9.18 × 10−10 | 2.37 × 10−7 | 1.83 × 10−4 | 1.35 × 10−4 | 7.78 × 10−5 |
| 350 | 1.86 × 10−10 | 7.43 × 10−8 | 9.32 × 10−5 | 6.84 × 10−5 | 3.68 × 10−5 |
| 300 | 2.97 × 10−11 | 1.64 × 10−9 | 4.05 × 10−5 | 3.08 × 10−5 | 1.66 × 10−5 |
| Ea (eV) | 1.25 | 1.07 | 0.45 | 0.4 | 0.44 |
| Mol | Nd:0 | Nd:0.05 | Nd:0.10 | Nd:0.15 | ||||
|---|---|---|---|---|---|---|---|---|
| Ce:0 | B1 | 0.90 eV | B5 | 0.78 eV | B9 | 0.64 eV | B13 | 0.45 eV |
| Ce:0.05 | B2 | 0.77 eV | B6 | 0.69 eV | B10 | 0.57 eV | B14 | 0.46 eV |
| Ce:0.10 | B3 | 0.73 eV | B7 | 0.65 eV | B11 | 0.53 eV | B15 | 0.48 eV |
| Ce:0.15 | B4 | 0.63 eV | B8 | 0.61 eV | B12 | 0.53 eV | B16 | 0.47 eV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, S.; Kavici, B.; Ramakrishnan, P.; Celen, C.; Amini Horri, B. Highly Conductive Cerium- and Neodymium-Doped Barium Zirconate Perovskites for Protonic Ceramic Fuel Cells. Energies 2023, 16, 4318. https://doi.org/10.3390/en16114318
Yilmaz S, Kavici B, Ramakrishnan P, Celen C, Amini Horri B. Highly Conductive Cerium- and Neodymium-Doped Barium Zirconate Perovskites for Protonic Ceramic Fuel Cells. Energies. 2023; 16(11):4318. https://doi.org/10.3390/en16114318
Chicago/Turabian StyleYilmaz, Serdar, Bekir Kavici, Prakash Ramakrishnan, Cigdem Celen, and Bahman Amini Horri. 2023. "Highly Conductive Cerium- and Neodymium-Doped Barium Zirconate Perovskites for Protonic Ceramic Fuel Cells" Energies 16, no. 11: 4318. https://doi.org/10.3390/en16114318







