Abstract
NaOH, dolomite and NiCl2 were used as catalysts to examine their effects on co–pyrolysis with waste bicycle tires (WT) and waste engine oil (WEO). The pyrolysis behaviors with catalysts were investigated by thermogravimetric analysis. The activation energy of the catalytic main reaction stage was derived by the Kissinger–Akahira–Sunose (KAS) method under four different heating rates conditions. The calculations show that all three catalysts can reduce the activation energy of the reaction. Co–pyrolysis of WT and WEO with different catalysts was performed in a self–made lab bench at 600 °C to explore the impact on the distribution of three–phase products. The properties of gas and oil products were characterized by FTIR and Py–GC/MS (Agilent 7890B, Santa Clara, CA, USA). With the mixing of catalysts, activation energy (Eα) decreased by 15–30% in the main reaction process. NaOH and dolomite increased the yield of gas by 7% and 10%. NaOH can significantly improve the yield of CH4. The proportion of limonene in pyrolysis oil increased to 19.65% with 10% NaOH. This article provides a new method for efficiently producing limonene by mixing WT and WEO with NaOH.
1. Introduction
With the continuous development of the transport industry and the rising living standards of humans, the retention of waste tires (WT) has increased dramatically. WT is a common type of hazardous waste. It contains a large amount of volatile organic compounds which are very damaging to the environment and has a high calorific value (33–35 MJ/kg) [1]. The high calorific value of tires is related to its carbon content. Typically, tires have a carbon content of 75–80%. It is difficult to degrade under natural conditions. The WT need to be disposed properly, otherwise they can cause waste of resources and easily form new “black pollution”, and cause “secondary damage” to the environment [2]. The WT piled up in the open air create an ideal breeding ground for pests and can also easily cause fires. In recent years, the application of pyrolysis technology to solid waste to recover energy and chemicals has become a good solution to reduce environmental pollution [3]. It can turn waste into value–added alternative energy sources. Pyrolysis gas is a reliable energy sources for the pyrolysis reaction [4]. The oil produced by pyrolysis is fractionated to give a variety of fuel oils [5]. It can also obtain chemical raw materials such as limonene and benzene compounds recovered through secondary reaction [6]. Limonene is a kind of cycloterpene. It is mainly derived from the natural rubber (NR) contained in tires. It can be used as a formula for industrial products (solvents, resins, adhesives). Limonene has broad application prospects and high economic value. It is widely used in the production of foods, spices chemicals, and pharmaceuticals. It can be used as antioxidant, preservative, and perfume additive, and also to synthesize chemical perfume. It has also shown advantages in the fight against cancer. The estimated output of this compound is 50–75 million kilograms per year [7]. According to different purity levels, the market price of limonene is about 1500–2500 dollars/ton [8]. The traditional method of obtaining limonene is to extract it from citrus or orange peel. Its extraction process is complex and inefficient.
Recently, the content of limonene in WT pyrolysis oil has attracted widespread attention. This provides a new technological route for the efficient production of limonene. A fixed bed reactor was studied to obtain the maximum yield of limonene at a pyrolysis temperature of 475 °C and a heating rate of 20 °C/min, with a value of 7.62 wt.% [9]. Photothermal cracking helps to increase the yield of limonene. The yield can reach up to 8.98 wt.% at 600 °C, and the relative peak areas of gas chromatography–mass spectrometry (GC–MS) results are about 30% [10]. WT started to degrade at about 120 °C less than polyolefins due to the presence of double bonds in the chains of rubbers resulting in weaker bonds in the β position. Due to the obstacles caused by the huge phenyl of SBR, rubber is a poor conductor of heat transfer [11]. During its heating process, it is easy to generate large temperature gradients and local high temperatures, resulting in coking. Previous studies have demonstrated the effect of co–pyrolysis of WT and WEO. The efficiency of heat transfer have been significantly improved. The main pyrolysis temperature has decreased by 50 °C. The generation rate of pyrolysis oil increased. When 20% WEO was added, the yields of H2 and limonene reached their maximum, increasing by 5.6% and 1.85%, respectively [12]. However, the composition of pyrolysis products is very complex. How to optimize the distribution of products to enhance their utilization value deserves more attention [13]. Many studies have explored the effect of catalysts on the products of WT pyrolysis as Table 1. The effect of NaOH on the properties of waste tires was studied under vacuum conditions. The addition of NaOH greatly improved the yield of pyrolysis oil. Adding 3 wt.% NaOH powder results in a maximum content of 12.39 wt.% limonene in pyrolysis oil [14]. Alkaline catalysts have good catalytic properties and can promote decarbonization and deoxygenation reactions, thereby improving the quality of pyrolysis oil [15]. S Miskah used 25% NaOH and 75% zeolite as catalysts to pyrolysis tires at 400 °C for 3 h. The product obtained an octane number of 113 and a maximum calorific value of 10.2 kcal/g [16]. Nickel–dolomite catalyst can increase the hydrogen production of waste tires. Dolomite is an important low–cost catalyst for tar destruction. NiTiO3 was used to catalyze the production of oil rich in high–value hydrocarbons in WT, resulting in a 50% increase in the yield of oxidized ilmenite [17]. Based on this, this article proposes using catalysts (NaOH, NiCl2 and dolomite) to improve the co–pyrolysis efficiency of WT and WEO in order to optimize the yield of limonene and some high value–added products.

Table 1.
The product distribution with different catalyst in previous study.
The purpose of this study is to investigate the effects of different catalysts on the co–pyrolysis of WT with 20%WEO, and to explore the enrichment of limonene and other high calorific value gases. In order to explore the influence of catalysts on co–pyrolysis process, thermogravimetric analyzer were employed to investigate the thermal behaviors. The activation energy (Eα) was calculated using the Kissinger–Akahira–Sunose (KAS) method based on the data obtained from thermogravimetric analysis to determine the effect of the catalyst on the change in activation energy. Pyrolysis experiments were conducted on a small pyrolysis reactor to analyze the changes in the distribution of three–phase products. The effect of catalysts on the yield of pyrolysis gas were explored by using FTIR. Quantitative and semi qualitative analysis of catalytic pyrolysis products was conducted using Py–GC/MS, and the peak area normalization method was used to compare and analyze the concentration and content of limonene. This study proposes a new approach to extract limonene and CH4 from WT and WEO, providing reference for the industrial application of this technology.
2. Materials and Methods
2.1. Materials
The WT samples in this article were collected from a reprocessing factory of City Qingdao, Shandong Province, China. The main components of tires are NR and synthetic rubber (SR) (50–60%), carbon black (15–25%), and steel (15%). Tires are divided into three types based on their purpose: light vehicle tires (bicycle tires and motorcycle tires), medium vehicle tires (car tires), and heavy vehicle tires (tires for trucks, buses, and large machinery) [24]. Their difference is mainly reflected in the different content of NR and SR. The NR content of light vehicle tires can reach up to 50%. Medium vehicle tires have the lowest NR content. The NR content of heavy vehicle tires is about 30%. The limonene in WT pyrolysis oil mainly comes from NR cracking. In order to obtain more limonene, this article uses bicycle tires as raw materials. First, WT are crushed to powder. The tire powder is placed in a 105 °C drying oven for 24 h. Then the WT powder was screened through a 60–80 mesh screen and sealed in the drying dish for storage. The average particle diameter is 0.2 mm. WEO samples come from a repair factory. The WEO samples were filtered to remove impurities and dried in a drying oven at 70 °C for 24 h. The mixture samples were prepared according to the weight of WEO accounting for 20% of the total weight of the mixture. The amount of catalyst added is 10% by weight of the mixture sample.
2.2. Apparatus and Procedure
2.2.1. TG–FTIR Measurement
The TG–FTIR measurement was carried out using a thermogravimetric (TG) analyzer from Netzsch, Bayern, Germany (Netzsch 209F3) connected with a Fourier transform infrared spectrometer from Thermo Fisher (Nicolet iS20), Waltham, MA, USA. The TG technique provides a powerful analytical tool, combining the quantitative analysis capability of TG with the qualitative analysis capability of FTIR. The sample is subjected to programmed pyrolysis in the TG analyzer and its gaseous products are fed directly into the FTIR spectrometer via a connected tube in order to obtain FTIR spectra [25]. To avoid gas condensation, the connected tube was continuously heated to maintain the temperature. The parameter settings for the experiment are shown in Table 2. Each experiment was repeated three times to exclude errors.

Table 2.
Operating conditions of TG–FTIR.
2.2.2. Pyrolysis Furnace
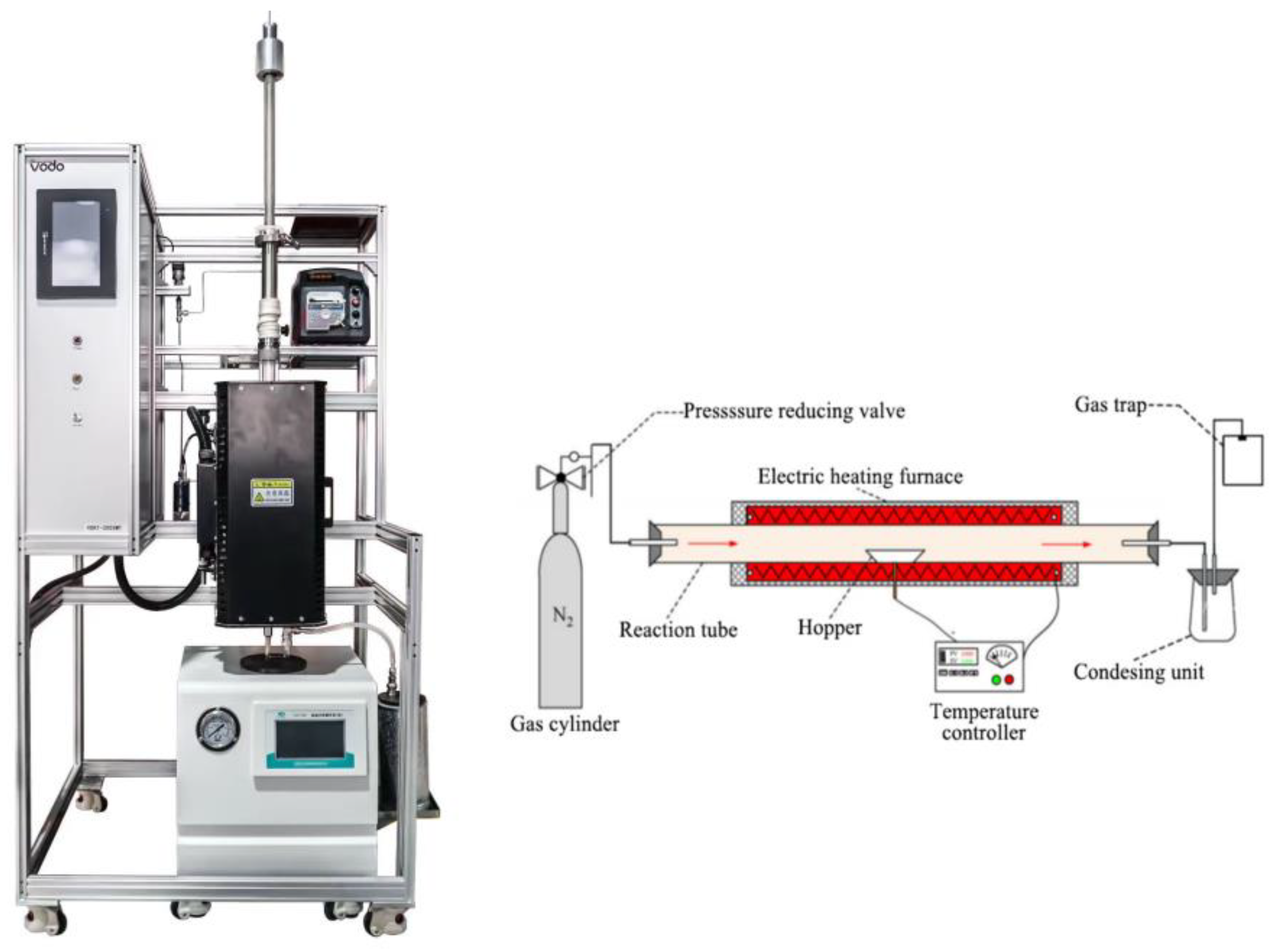
Pyrolysis experiments were carried out in a self–made pyrolysis furnace and the three–phase products were collected. The schematic diagram and the physical presentation of the pyrolysis furnace is shown in Figure 1. Before the experiment starts, N2 is passed through to expel the gas from the tube. The furnace is then programmed to heat up to 600 °C and this temperature was held. The container filled with material is quickly pushed into the furnace to allow it to pyrolysis. The reaction is finished when no more gas is produced. The sample is 10 g mixture and 1 g catalyst.
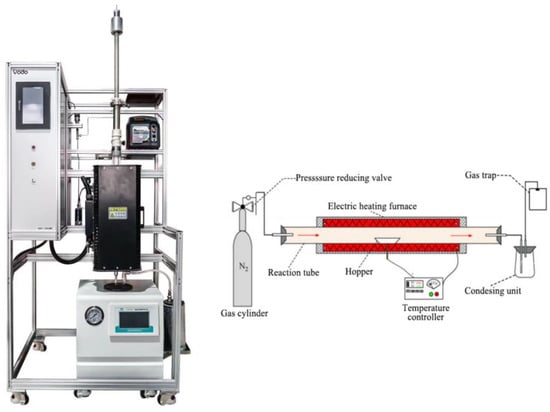
Figure 1.
Pyrolysis furnace.
2.2.3. Py–GC/MS Measurement
A pyrolyzer (Frontier EGA/PY3030D, Koriyama, Japan) combined with a gas chromatograph and mass spectrometer (GC/MS, Agilent 7890B, Santa Clara, CA, USA) was used for rapid pyrolysis and product analysis experiments. The experiment parameters are shown in Table 3. The semi–quantitative analysis of each component in pyrolysis oil was carried out using the peak area normalization method. For example, the limonene content is the ratio of the area of the limonene peak as determined by chromatography–mass spectrometry to the total peak area of the pyrolysis oil.

Table 3.
Operating conditions of Py–GC/MS.
2.3. Kinetic Analysis
Due to the difference of structural and chemical property, different catalysts have different catalytic mechanisms. In this study, the KAS model free method were used to compare the effect of catalysts on Eα. The overall reaction is described by Equation (1) as follows:
According to Arrhenius theory, the reaction rate equation of solid materials can be expressed as Equation (2):
where α is the rate of conversion, t is the reaction time (s), k(T) is the rate constant of reaction depending on the temperature, f(α) is the mechanism function, A is the pre–exponential factor (min−1), Eα is activation energy(kJ/mol), R is the ideal gas constant (8.314 J/mol∙K), T is the absolute temperature (K).
The conversion rate α can calculated by:
where m0 is the initial mass of sample, mt is the mass at a certain time, and m∞ is the final mass. Substituting the heating rate β = dT/dt into Equation (4), the kinetic equation of solid matter under non–isothermal conditions can be obtained:
Equation (4) has no exact solution; various approximation models were used to solve the complicated part of this equation.
Substituting the Coats–Redfern approximation of the temperature integral into (4), we get the following:
Activation energy can be obtained by drawing a graph between 1/T and ln[β/T2] and by obtaining slope from drawn straight line.
3. Results and Discussions
The results of proximate and elemental analyses and the predicted HHV (higher heating value) are shown in Table 4. The percentage content of volatile is higher than 60%, especially the content of WLO reaches 97%. This is favorable for ignition. This reduces the amount of heat required for thermochemical reactions to occur and increases the HHV. The yield of pyrolyzed oil and gas can also be promoted. The percentage content of ash is lower than 5–6%. It can be considered as a cleaner fuel. Based on the sustainability concept of biological waste management, zero waste discharge can be achieved.

Table 4.
Elemental and proximate analysis of feedstock.
3.1. Thermogravimetric Analysis
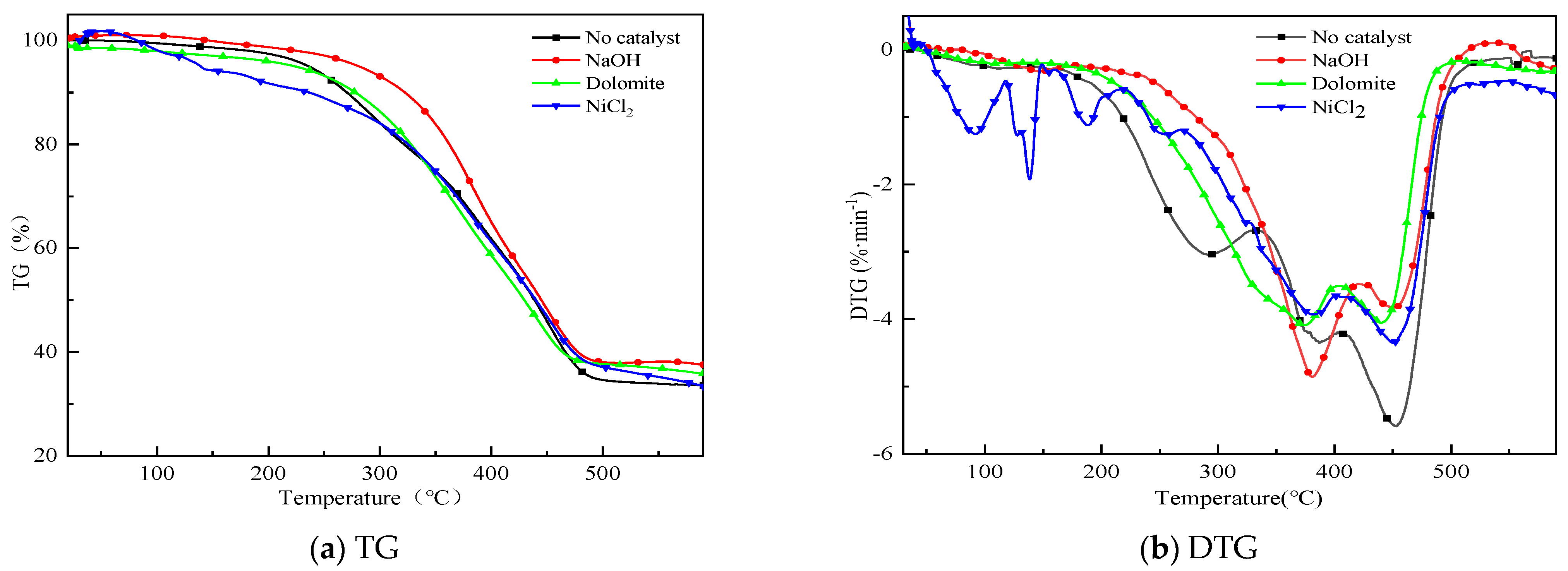
Figure 2 shows the TG and DTG curves of sample pyrolysis with different catalyst (No catalyst, NaOH, Dolomite, NiCl2). Catalysts have different effects on pyrolysis, that resulted in different weight loss. It is found in Figure 2 that the weight loss of the case without catalyst is the largest due to the influence of catalyst residue. The influence difference of three catalysts on weight loss is 5%. That means the above catalysts have little significance in promoting the pyrolysis of residual carbon. Pyrolytic char contains more ash. The sample started decomposition at 130 °C and became appreciable at 230 °C. Pyrolysis mainly occurs between 230 and 460 °C. There are two main stages in the pyrolysis process when no catalyst is mixed. The peak temperatures are 280 °C and 450 °C. The first stage started from 230 °C and ends at 340 °C caused by pyrolysis of NR. The second stage from 340 °C to 500 °C mainly caused by pyrolysis of styrene–butadiene rubber. Information shows the pyrolysis of WEO occurred in the active pyrolysis zone from 250 °C to 450 °C where the dissociation of heavier H/C mainly takes place to produce lower molecular compounds and gases. Catalysts changed the co–pyrolysis process of WT and WEO. The catalysts raised the first peak temperature to 380 °C. NaOH enhanced the weight loss rate of sample. The temperature corresponding to the second weight loss peak is not affected by the catalyst. However, the corresponding weight loss rate decreased. This may be due to the synergistic interaction between small molecular gases produced by co–pyrolysis.
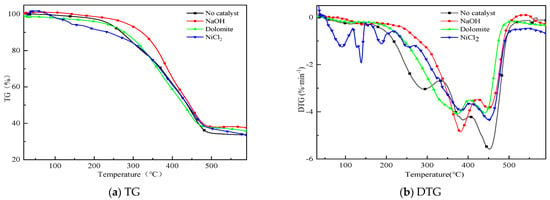
Figure 2.
TG and DTG curves of co–pyrolysis with different catalysts.
3.2. Kinetic Analysis of Catalytic Co–Pyrolysis
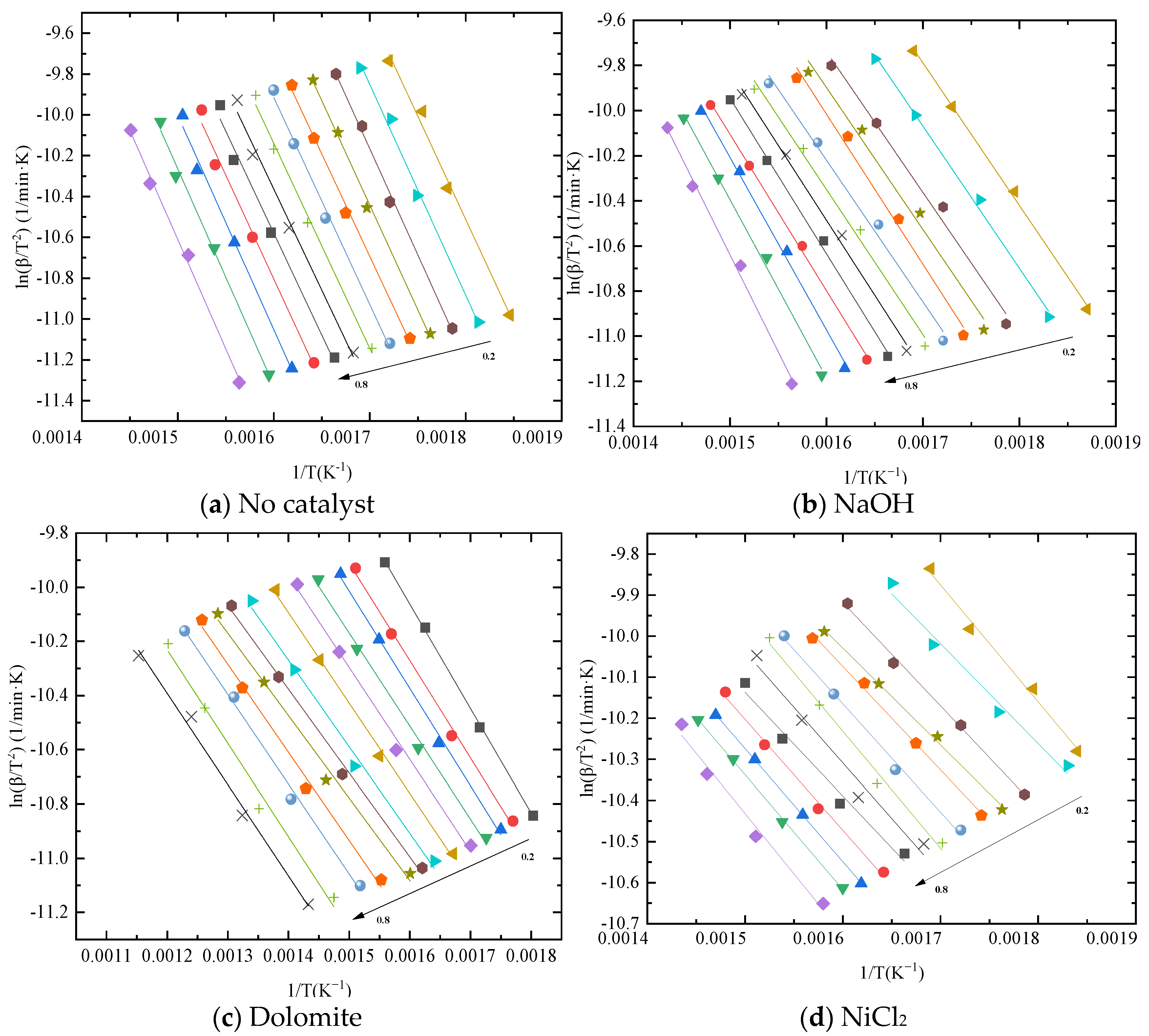
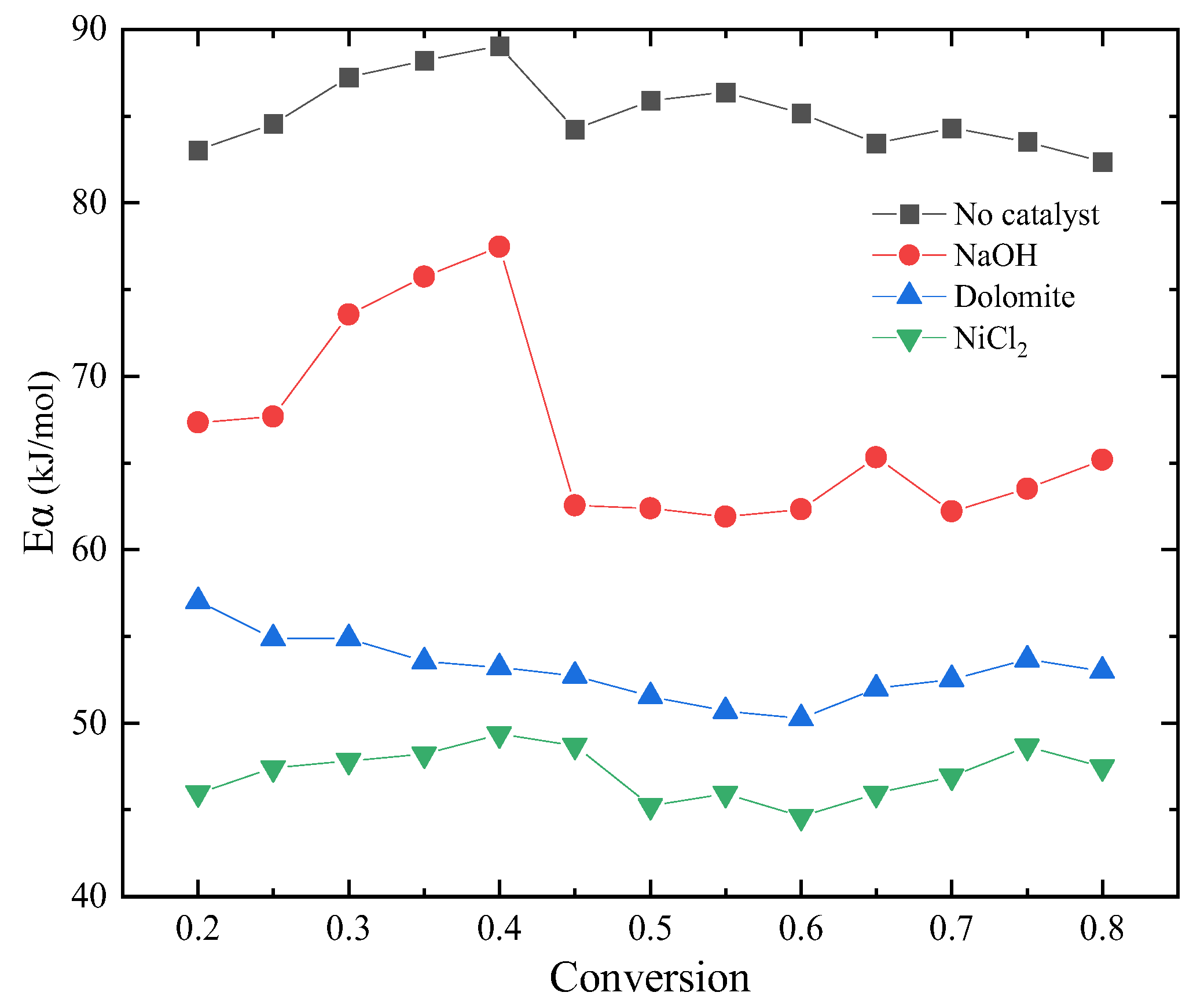
Kinetics of blends catalytic co–pyrolysis were determined by using KAS method at the selected degrees of conversion ranging from 0.2 to 0.8. Figure 3 shows the Arrhenius plot of blends with catalysts at different degrees from KAS method. It can be seen from the figure that the catalyst has different effects on the reaction process. Moreover, the variation in activation energy with conversion is revealed in Figure 4. From the comparison of activation energy values, the three catalysts all reduced the Eα required for the reaction process. Among them, the reduction effect of dolomite and NiCl2 is the most obvious. The Eα of sample without catalyst was within the range of 82–89 kJ/mol. The Eα of sample with NaOH was within the range of 65–78 kJ/mol. The difference is that when the conversion is between 0.3 and 0.4, the Eα increased by 10%. This indicates that the reaction resistance is large at this stage. Dolomite and NiCl2 make the Eα reduce to 55–60 kJ/mol and 45–50 kJ/mol, respectively.
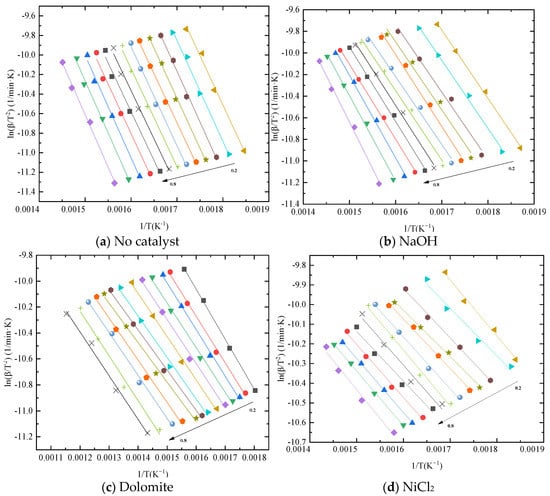
Figure 3.
Kinetic plots of blends with different catalysts.
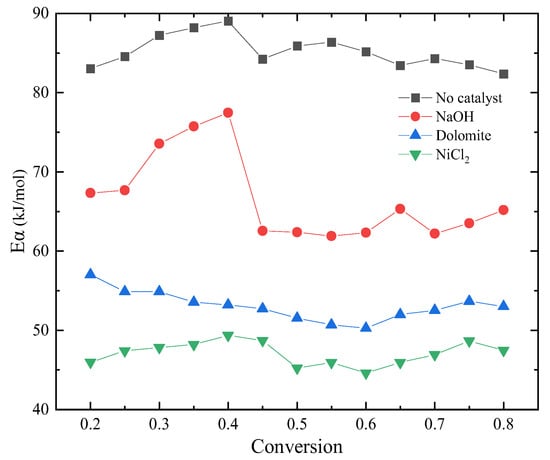
Figure 4.
Variation of Eα with conversion.
3.3. Distribution of Co–Pyrolysis Products
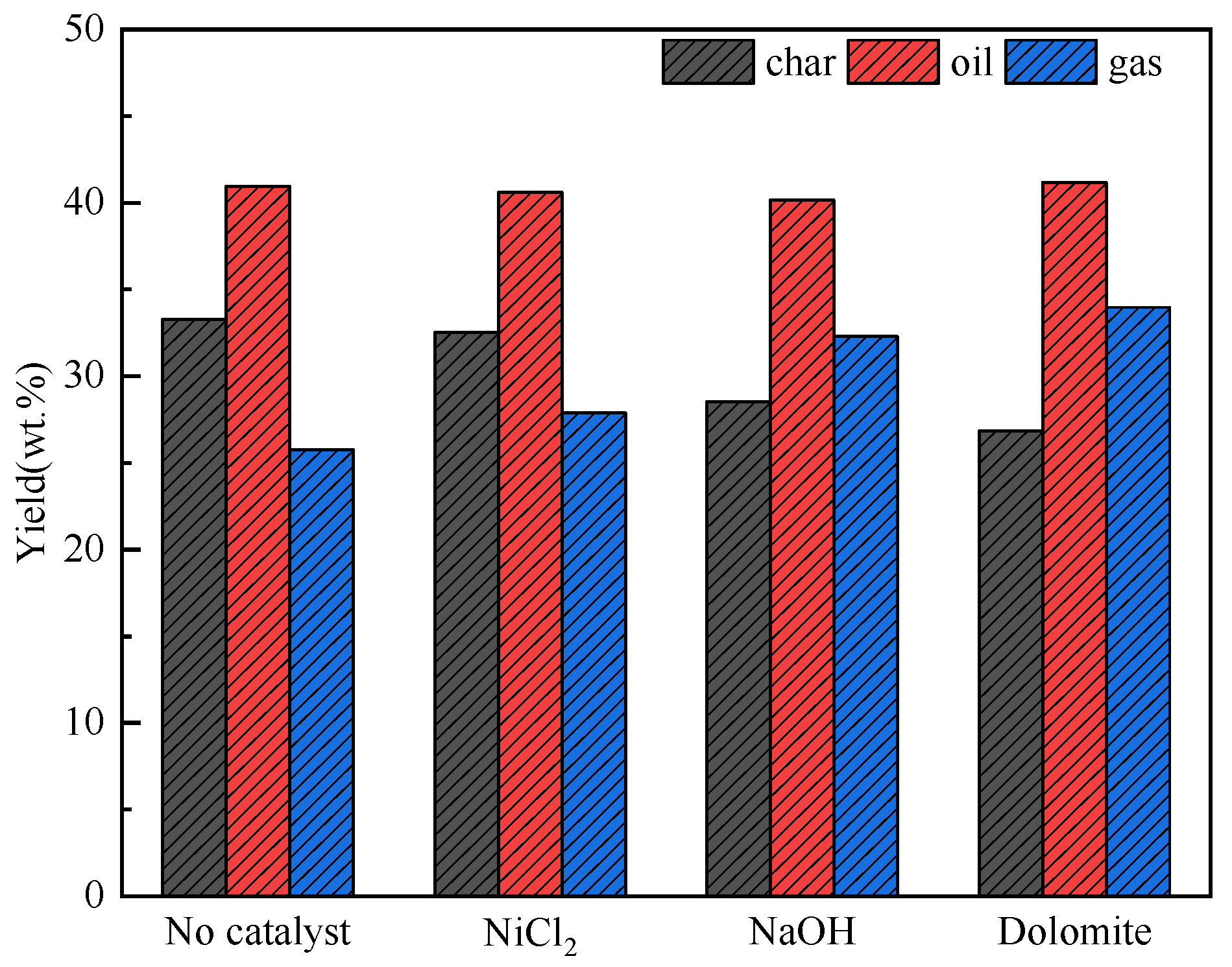
As shown in Figure 5, the yield of oil remains at 41 wt% with different catalysts. So, it can be judged that the catalysts has almost no effect on the yield of pyrolysis oil. Mixing dolomite and NaOH increased the yield of gas. The yield of char decreased. This proves that NaOH and dolomite can promote pyrolysis to generate more gases. When there is no catalyst mixing, the yield distribution of pyrolysis products are char 33.6%, oil 40.8%, and gas 25.6%. NiCl2 has little effect on product distribution. The yield of gas increased by 3%, 7%, and 10%, respectively.
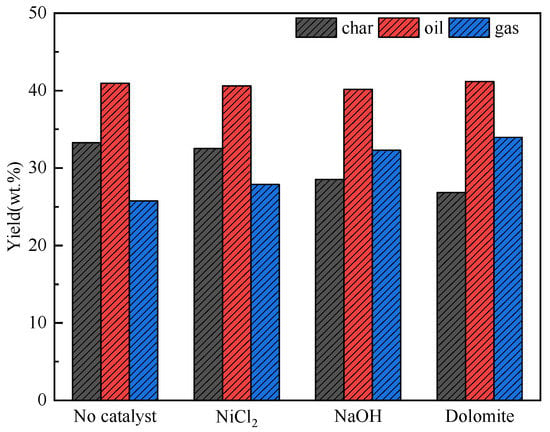
Figure 5.
Product distribution of co–pyrolysis with catalyst.
3.4. FTIR Analysis
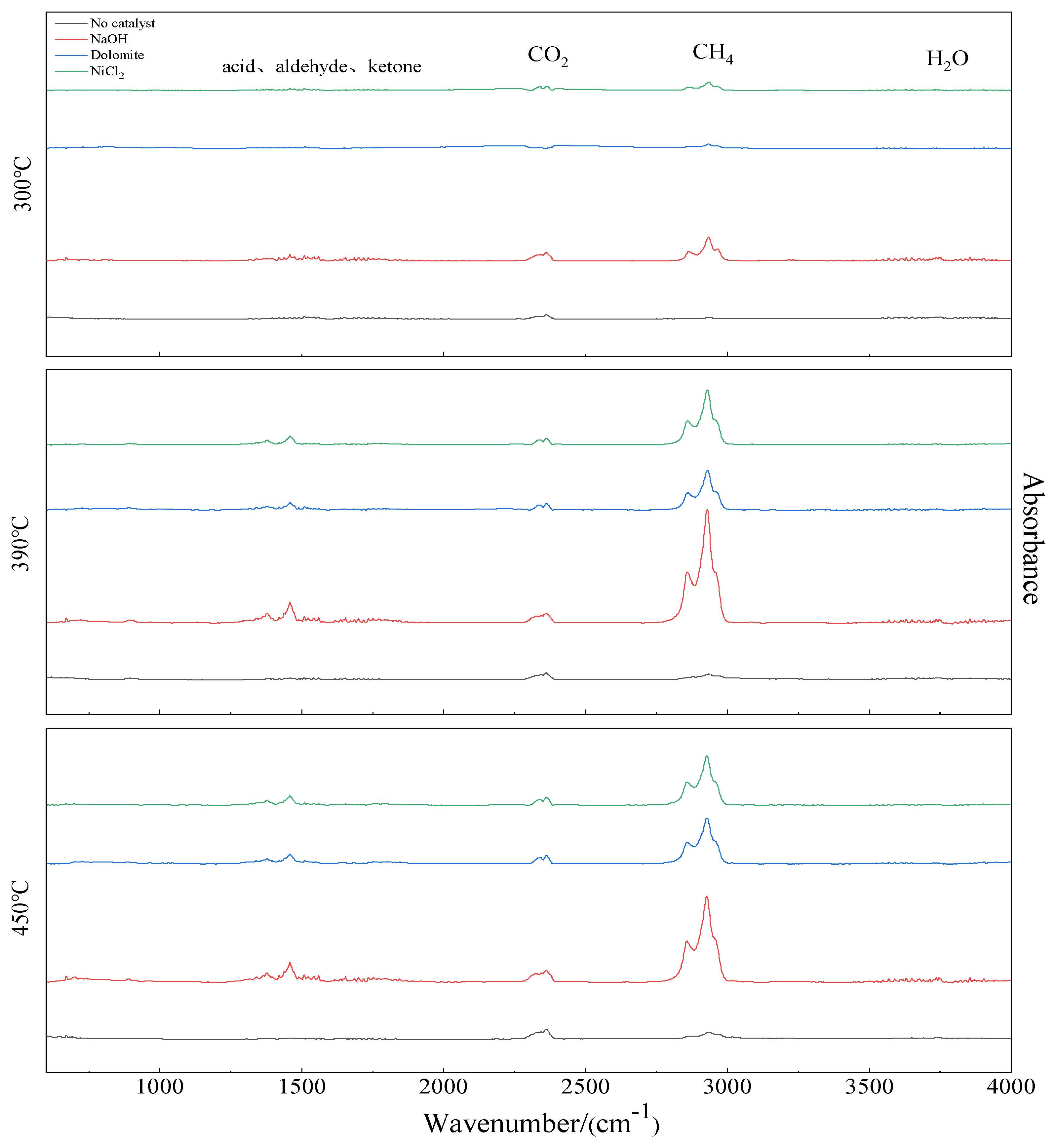
Based on the DTG curves of Figure 2, the pyrolysis process has three peak temperature values, 300, 390, and 450 °C. The corresponding FTIR spectrogram for the gaseous products at peak temperature values are shown in Figure 6. The IR spectrum of samples with catalysts show absorbance in the range of 1410–1580, 2350–2450, 2750–3000, and 3740–3950 cm. It indicates the main substances are C=O, CO2, CH4, and H2O [25,26,27]. The largest relative peak area is CH4. Next is CO2. At high temperatures, gases containing C=O functional groups such as acids, aldehydes, and ketones precipitate. Comparing with the curve of no catalyst, the absorption peak of CH4 significant increase with catalyst. NaOH has a great influence on gas release. A small amount of H2O is also observed.
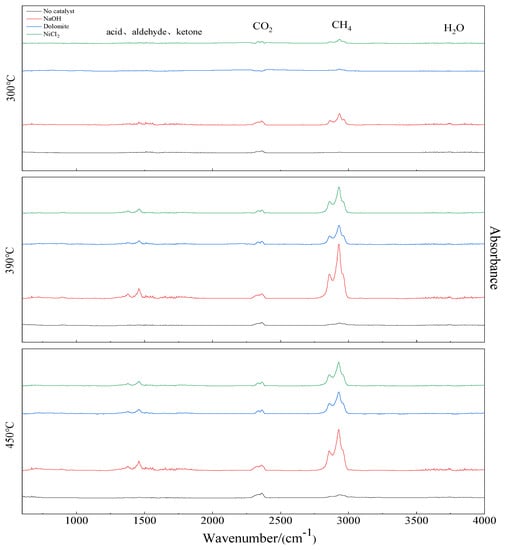
Figure 6.
FTIR spectrogram of co–pyrolysis gas at different temperatures.
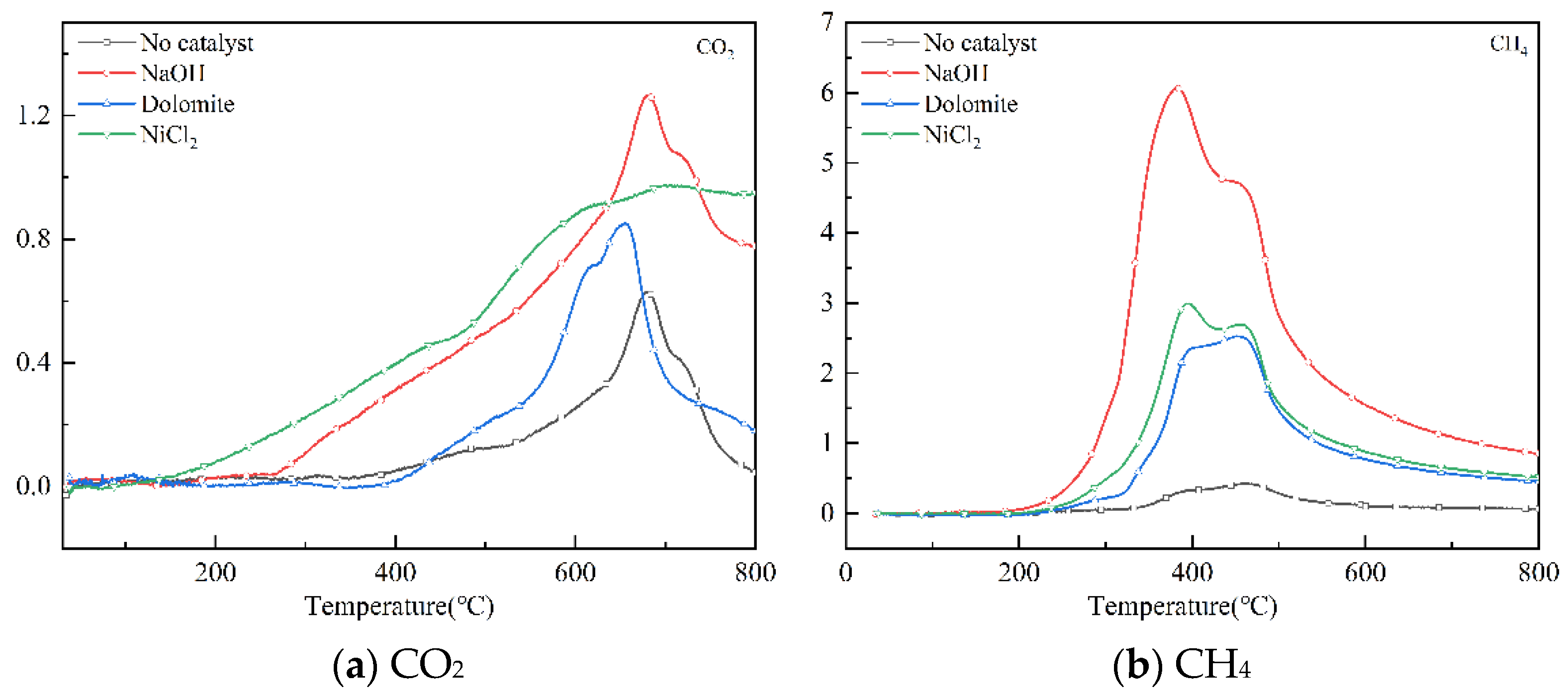
CH4 and CO2 are the main recyclable products in pyrolysis gas. They are the main raw materials for producing synthetic gas through methane drying and reforming method [28]. Figure 7 shows the infrared spectrum of CO2 and CH4 from 20–800 °C with different catalysts. Where CH4 is detected and decomposition starts from 280 °C to 530 °C. CO2 is released at 260–800 °C. The maximum CO2 intensity released by NaOH catalytic pyrolysis was at 600 °C. The maximum strength of CH4 formation is at 400 °C. All the catalysts can enhance the production of CH4. NaOH shows the most significant effect on the release of CO2 and CH4. The NaOH catalyst doubled the relative intensity of CH4 and CO2 peaks. From the perspective of manufacturing synthetic gas, NaOH is a better catalyst.
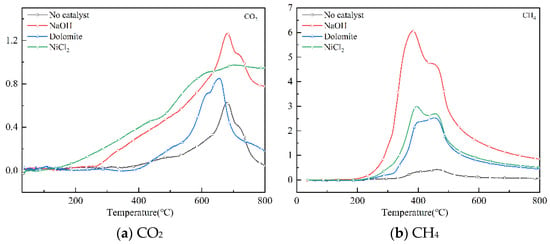
Figure 7.
The strength of CO2 and CH4 varies with temperature.
3.5. Py–GC/MS Analysis
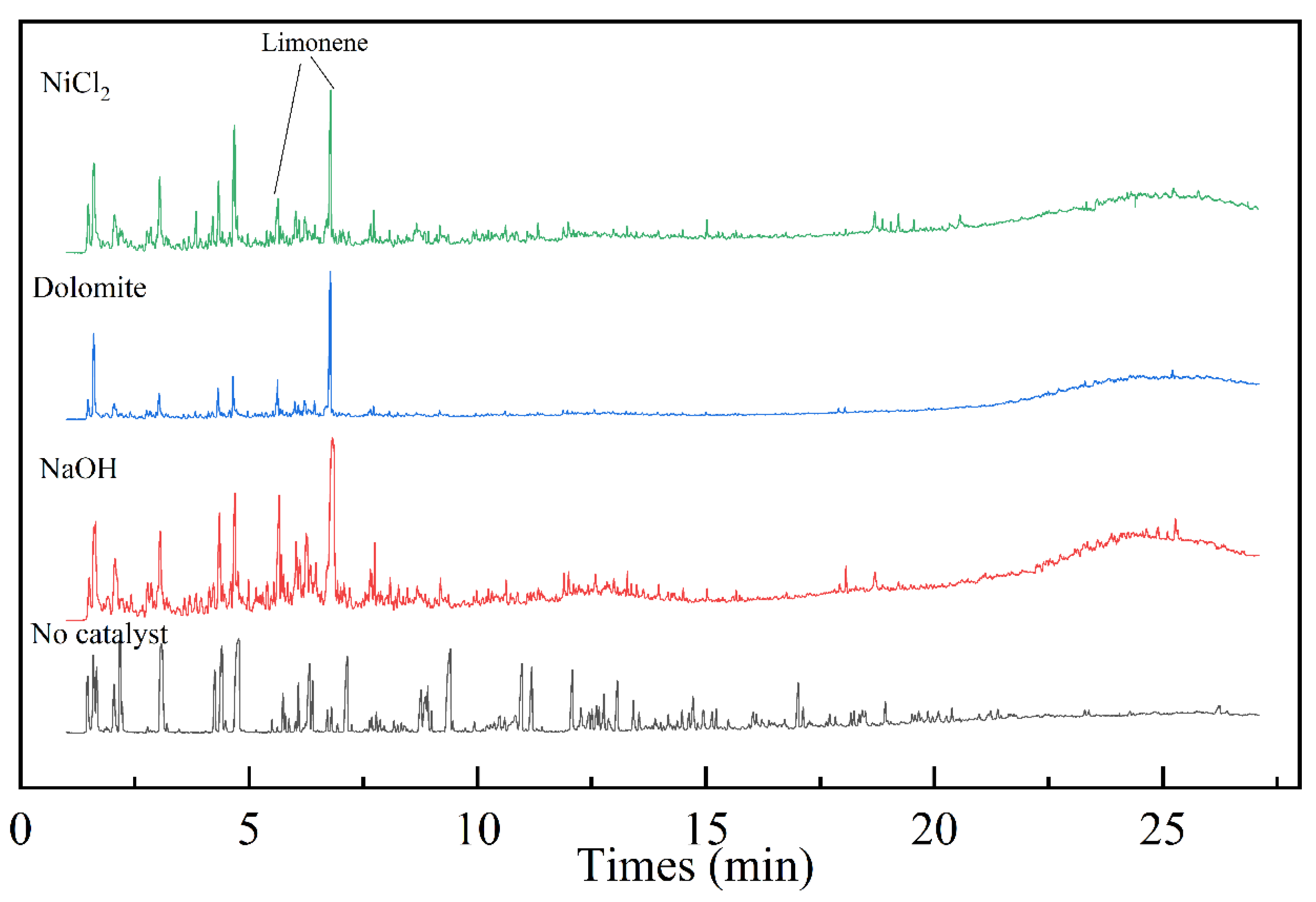
Figure 8 shows the total ion chromatogram of Py–GC/MS with different kinds of catalysts. It can be seen that the pyrolysis products of blending are mainly composed of macromolecular. The products are mainly acid organics, and macromolecule acids. The content of olefins, alkanes, and other organic compounds are relatively low. Compared with the co–pyrolysis oil without catalysts, the components detected after using the catalysts was small molecule unsaturated alkenes. This indicates that the three catalysts can effectively promote the decomposition of macromolecular substances into small molecular substances. Compared to the peak plots without catalysts, the peaks of limonene in the plots with catalysts are all enhanced. Table 5 compares the content of limonene in pyrolysis oil and the yield of pyrolysis oil under different operating conditions. By comparison, NaOH has the most significant effect on improving the yield of limonene in pyrolysis oil. The yield reaches 19.65% when with 10% NaOH. Moreover, the yield of oil did not decrease. Compared to previous studies, the yield of limonene has also increased significantly. It provides a reference pathway for the production of limonene.
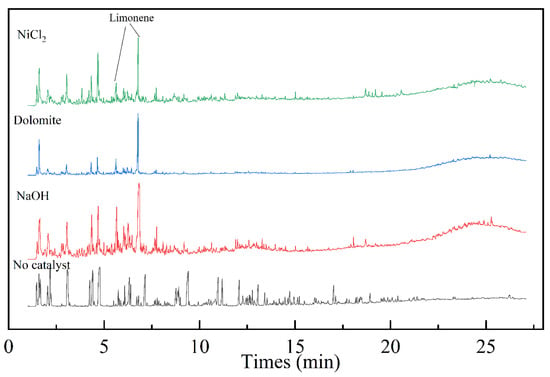
Figure 8.
Py–GC/MS chromatograms with different kinds of catalyst.

Table 5.
The yield of oil and limonene compared with previous studies.
4. Conclusions
In this study, NaOH, dolomite, and NiCl2 are used as the catalysts for co–pyrolysis of WT and WEO to explore improving the yield of limonene. Analysis of three–phase products obtained from pyrolysis revealed that all three catalysts can improve the yield of pyrolysis gas. Adding catalysts could reduce the value of Eα by 15–30%. NaOH significantly enhances the infrared spectral intensity of CH4 and CO2. This is beneficial for the development of synthetic gas. Pyrolysis of WT with 20% WEO and 10% NaOH under conventional conditions can obtain pyrolysis oil containing 19.65% limonene. Compared to other conventional methods, the yield of limonene obtained in this study increased by 70%. The absorption intensity of CH4 has doubled. This not only enables the utilization of WT and WEO, but also provides an effective way to generate limonene and synthetic gas. However, this article just focused on the differences in results and did not complete the exploration of the essence of the catalytic mechanism. In future research, it is recommended to explore the pyrolysis mechanism of the catalyst.
Author Contributions
Conceptualization, S.L. and Z.Z.; methodology, S.L.; software, X.D.; validation, X.D.; formal analysis, J.W.; Investigation, J.W.; resources, J.W.; data curation, J.W.; writing—original draft preparation, J.W.; writing—review and editing, J.W.; visualization, J.W.; supervision, J.W.; project administration, J.W.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the 2020 Science and Technology Project of Qingdao West Coast New Area (2020-99).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kositkanawuth, K.; Bhatt, A.; Sattler, M.; Dennis, B. Renewable energy from waste: Investigation of Co-pyrolysis between Sargassum macroalgae and polystyrene. Energy Fuels 2017, 31, 5088–5096. [Google Scholar] [CrossRef]
- Kai, X.; Yang, T.; Shen, S.; Li, R. TG-FTIR-MS study of synergistic effects during co-pyrolysis of corn stalk and high-density polyethylene (HDPE). Energy Convers. Manag. 2019, 181, 202–213. [Google Scholar] [CrossRef]
- Hoang, A.T.; Le, V.V.; Al-Tawaha, A.R.M.; Nguyen, D.N.; Noor, M.M.; Pham, V.V. An absorption capacity investigation of new absorbent based on polyurethane foams and rice straw for oil spill cleanup. Pet. Sci. Technol. 2019, 36, 361–370. [Google Scholar] [CrossRef]
- Tarifa, J.M.; Ruiz, C.P.A.; Gomes, V.L.A.; Araújo, J.S. Coke deposition during electromagnetic heating. Liq. Fuels Technol. 2017, 35, 1–8. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Y.; Duan, P.; Wang, F.; Xu, Z.-X. Thermo-chemical conversion of scrap tire waste to produce gasoline fuel. Waste Manag. 2019, 86, 1–12. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Ming, X.; Jiang, Y.; Hao, J.; Qiao, Y.; Tian, Y. TG-FTIR and Py-GC/MS study on pyrolysis mechanism and products distribution of waste bicycle tire. Energy Convers. Manag. 2018, 175, 288–297. [Google Scholar] [CrossRef]
- Januszewicz, K.; Kazimierski, P.; Suchocki, T.; Kardaś, D.; Lewandowski, W.; Klugmann-Radziemska, E.; Łuczak, J. Waste Rubber Pyrolysis: Product Yields and Limonene Concentration. Materials 2020, 13, 4435. [Google Scholar] [CrossRef]
- Song, Z.; Liu, L.; Yang, Y.; Sun, J.; Zhao, X.; Wang, W.; Mao, Y.; Yuan, X.; Wang, Q. Characteristics of limonene formation during microwave pyrolysis of scrap tires and quantitative analysis. Energy 2018, 142, 953–961. [Google Scholar] [CrossRef]
- Mkhize, N.M.; van der Gryp, P.; Danon, B.; Gorgens, J.F. Effect of temperature and heating rate on limonene production from waste tyre pyrolysis. J. Anal. Appl. Pyrolysis 2016, 120, 314–320. [Google Scholar] [CrossRef]
- Gang, Y.; Pan, L.; Niu, M.; Zhang, X.; Li, D.; Li, W. Catalytic hydrogenation of Low temperature coal tar into jet fuel by using two-reactors system. J. Anal. Appl. Pyrolysis 2018, 134, 202–208. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, J.; Hu, S.; Ma, S.; Huang, R.; Su, S.; Wang, Y.; Jiang, L.; Xu, J.; Xiang, J. Novel photothermal pyrolysis on waste tire to generate high-yield limonene. Fuel 2022, 329, 125482. [Google Scholar] [CrossRef]
- Lanteigne, J.-R.; LaViolette, J.-P.; Tremblay, G.; Chaouki, J. Predictive Kinetics Model for an Industrial Waste Tire Pyrolysis Process. Energy Fuels 2013, 27, 1040–1049. [Google Scholar] [CrossRef]
- Wang, J.; Qi, X.; Dong, X.; Luo, S.; Feng, Y.; Feng, M.; Guo, X. The co-pyrolysis of waste tires and waste engine oil. Energy Sources Part A Recover. Util. Environ. Eff. 2022, 44, 9764–9778. [Google Scholar] [CrossRef]
- Khalil, U.; Vongsvivut, J.; Shahabuddin, M.; Samudrala, S.P.; Srivatsa, S.C.; Bhattacharya, S. A study on the performance of coke resistive cerium modified zeolite Y catalyst for the pyrolysis of scrap tyres in a two-stage fixed bed reactor. Waste Manag. 2019, 102, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, T.; Ma, L.; Chang, J. Vacuum pyrolysis of waste tires with basic additives. Waste Manag. 2008, 28, 2301–2310. [Google Scholar] [CrossRef]
- Kabir, G.; Hameed, B. Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals. Renew. Sustain. Energy Rev. 2017, 70, 945–967. [Google Scholar] [CrossRef]
- Miskah, S.; Aprianti, T.; Moeksin, R. Potential use of waste rubber tires containing polystyrene to produce gasoline-like hydrocarbon by thermal catalytic cracking. IOP Conf. Series Earth Environ. Sci. 2019, 298, 012014. [Google Scholar] [CrossRef]
- Thomas, B.S.; Gupta, R.C. A comprehensive review on the applications of waste tire rubber in cement concrete. Renew. Sustain. Energy Rev. 2016, 54, 1323–1333. [Google Scholar] [CrossRef]
- Shah, J.; Jan, M.R.; Mabood, F. Catalytic conversion of waste tyres into valuable hydrocarbons. J. Polym. Environ. 2007, 15, 207–211. [Google Scholar] [CrossRef]
- Shah, J.; Rasul, M.; Mabood, F. Catalytic Pyrolysis of Waste Tyre Rubber into Hydrocarbons Via Base Catalysts. Iran. J. Chem. Chem. Eng.-Int. Engl. Ed. 2008, 27, 103–109. [Google Scholar] [CrossRef]
- Shah, J.; Jan, M.; Mabood, F. Recovery of value-added products from the catalytic pyrolysis of waste tyre. Energy Convers. Manag. 2009, 50, 991–994. [Google Scholar] [CrossRef]
- Ahoor, A.H.; Zandi-Atashbar, N. Fuel production based on catalytic pyrolysis of waste tires as an optimized model. Energy Convers. Manag. 2014, 87, 653–669. [Google Scholar] [CrossRef]
- Palza, H.; Aravena, C.; Colet, M. Role of the Catalyst in the Pyrolysis of Polyolefin Mixtures and Used Tires. Energy Fuels 2017, 31, 3111–3120. [Google Scholar] [CrossRef]
- Elbaba, I.F.; Williams, P.T. High yield hydrogen from the pyrolysis–catalytic gasification of waste tyres with a nickel/dolomite catalyst. Fuel 2013, 106, 528–536. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Jana, A.; Mondal, S.; Jana, B.; Sadhukhan, A.K.; Gupta, P. Pyrolysis of three different categories of automotive tyre wastes: Product yield analysis and characterization. J. Anal. Appl. Pyrolysis 2018, 135, 379–389. [Google Scholar] [CrossRef]
- Luo, W.; Wan, J.; Fan, Z.; Hu, Q.; Zhou, N.; Xia, M.; Song, M.; Qi, Z.; Zhou, Z. In-situ catalytic pyrolysis of waste tires over clays for high quality pyrolysis products. Int. J. Hydrogen Energy 2020, 46, 6937–6944. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Thermally exfoliated graphene oxide for hydrogen storage. Mater. Chem. Phys. 2019, 239, 122102. [Google Scholar] [CrossRef]
- Zhao, X.; Ning, Z.; Li, Z.; Zou, W.; Li, B.; Huang, Y.; Cao, F.; Sun, J. Evolved gas analysis of PEP-SET sand by TG and FTIR. J. Anal. Appl. Pyrolysis 2017, 127, 490–495. [Google Scholar] [CrossRef]
- Liao, C.-H.; Horng, R.-F. Experimental study of syngas production from methane dry reforming with heat recovery strategy. Int. J. Hydrogen Energy 2017, 42, 25213–25224. [Google Scholar] [CrossRef]
- Choi, G.-G.; Oh, S.-J.; Kim, J.-S. Clean pyrolysis oil from a continuous two-stage pyrolysis of scrap tires using in-situ and ex-situ desulfurization. Energy 2018, 141, 2234–2241. [Google Scholar] [CrossRef]
- Li, S.-Q.; Yao, Q.; Chi, Y.; Yan, J.-H.; Cen, K.-F. Pilot-Scale Pyrolysis of Scrap Tires in a Continuous Rotary Kiln Reactor. Ind. Eng. Chem. Res. 2004, 43, 5133–5145. [Google Scholar] [CrossRef]
- Mkhize, N.; Danon, B.; Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M.; van der Gryp, P.; Görgens, J. Influence of reactor and condensation system design on tyre pyrolysis products yields. J. Anal. Appl. Pyrolysis 2019, 143, 104683. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).